Abstract
Restrictive dietary interventions exert significant beneficial physiological effects in terms of aging and age-related disease in many species. Every other day feeding (EOD) has been utilized in aging research and shown to mimic many of the positive outcomes consequent with dietary restriction. This study employed long living Ames dwarf mice subjected to EOD feeding to examine the adaptations of the oxidative phosphorylation and antioxidative defense systems to this feeding regimen. Every other day feeding lowered liver glutathione (GSH) concentrations in dwarf and wild type (WT) mice but altered GSH biosynthesis and degradation in WT mice only. The activities of liver OXPHOS enzymes and corresponding proteins declined in WT mice fed EOD while in dwarf animals, the levels were maintained or increased with this feeding regimen. Antioxidative enzymes were differentially affected depending on the tissue, whether proliferative or post-mitotic. Gene expression of components of liver methionine metabolism remained elevated in dwarf mice when compared to WT mice as previously reported however, enzymes responsible for recycling homocysteine to methionine were elevated in both genotypes in response to EOD feeding. The data suggest that the differences in anabolic hormone levels likely affect the sensitivity of long living and control mice to this dietary regimen, with dwarf mice exhibiting fewer responses in comparison to WT mice. These results provide further evidence that dwarf mice may be better protected against metabolic and environmental perturbations which may in turn, contribute to their extended longevity.
Keywords: Ames dwarf, glutathione, catalase, hormones, every other day feeding, methionine, oxidative phosphorylation
Introduction
Restrictive dietary interventions have been shown to alter life span in many species. Rodents, in particular, have been examined using numerous dietary intervention protocols that have varied the age of onset of the restrictive intervention, the length of time restrictive intervention was applied, the level of restriction and the type of nutrient restriction using either a percent reduction in calories or every other day feeding (EOD). In many cases, these variations have resulted in reduced age-related pathologies and life span extension (Ingram and Reynolds, 1987; Weindruch and Walford, 1988; Weindruch et al., 1986; Goodrick et al., 1990; Anson et al., 2003; Shimokawa et al., 2003; Caro et al., 2008; Pearson et al., 2008; Tatsumi et al., 2008; Cleary et al., 2007; Zha et al., 2008; Masoro, 2009).
Every other day feeding is a treatment protocol that can be readily applied in a variety of settings including studies in mice and humans. This type of feeding protocol has been shown to increase longevity in mice despite modest changes in total food intake and body weight (Ingram and Reynolds, 1987; Goodrick et al. 1990; Anson et al., 2003). In addition, EOD has been shown to exert similar beneficial effects to standard dietary restriction (DR) in which daily 20–40% decreases in food intake are imposed. The mechanisms underlying the ability of DR and EOD feeding to extend health span and life span are still under debate and include potential alterations in mitochondrial metabolism, glucose metabolism, stress resistance, cancer incidence and immune function among others.
Ames dwarf mice are growth hormone (GH), prolactin and thyrotropin-deficient and live more than 50% longer than their normal, WT siblings (Brown-Borg et al. 1996). Based on the known functions of GH in particular, the Ames dwarf mouse differs significantly from normal animals in a variety of important metabolic parameters including the plasma levels of insulin-like growth factor-1 (IGF1), insulin and glucose levels resulting in increased life time insulin sensitivity (Borg et al., 1995; reviewed in Bartke, 2011). Numerous aspects of enhanced resistance to oxidative stress and other stressors have also been observed in dwarf mice when compared to WT mice at young and old ages (Salmon et al., 2005; Bartke et al., 2000; Bokov et al., 200; Brown-Borg, 2009). These in vivo and in vitro observations of cellular stress resistance are clearly supported by data showing enhanced levels of antioxidative enzymes, glutathione, detoxification enzymes, heat shock proteins, and metal chelators among others (reviewed in Brown-Borg, 2007).
Restrictive dietary interventions have been also been shown to enhance resistance to stressors in rodents and humans (Yu and Mattson, 2001; Duan et al., 2001; 2003; Berryman et al., 2008; Weiss and Fontana, 2011; Lee and Longo, 2011). It is unclear how aspects of metabolism involving antioxidative defense, glutathione, methionine or components of mitochondrial oxidative phosphorylation (OXPHOS) are affected by a protocol of every other day feeding. Therefore, we conducted this study to examine the effects of short-term EOD on aspects of metabolism known to be involved in stress resistance, mitochondrial function and ultimately, in life span extension. Our working hypothesis was that Ames mice would exhibit differential responses to EOD feeding compared to WT mice.
Materials and Methods
Ames dwarf mice were bred and maintained at the University of North Dakota’s Center for Biomedical Research under controlled conditions of photoperiod (12 h light:12 h dark) and temperature (22 ± 1°C) with ad libitum access to food (8640 Teklad 22/5 rodent diet with 22.6% crude protein, 5.2% fat, Harlan Laboratories) and water (standard laboratory conditions) until the feeding experiment started. The Ames dwarf (df/df) mice used in this study were derived from a closed colony with a heterogeneous background (over 25 years). Dwarf mice were generated by mating either homozygous (df/df) or heterozygous (df/+) dwarf males with carrier females (df/+). All procedures involving animals were reviewed and approved by the UND Institutional Animal Care and Use Committee.
At five-six months of age, dwarf (male and female) and WT mice (male) were subjected to an EOD feeding paradigm. At the beginning of the study, food was removed from the hopper late afternoon and returned approximately 24 hours later. This pattern of food removal and return took place daily for six weeks. Water was available ad libitum throughout the study. Two repetitions were conducted due to the amounts of tissue necessary for the assays listed below. Seven to 11 mice/genotype/treatment were utilized in each of the repetitions. Female dwarf mice do not exhibit estrus cyclicity and thus are sterile. We have combined female and male dwarf mice in multiple studies and have found no significant differences between genders on a large variety of parameters (Brown-Borg and Rakoczy, 2003; Sharma et al., 2010a; 2011; Brown-Borg et al, 2011). No differences were noted in these experiments, therefore the male and female dwarf data were combined. Body weights were assessed weekly. After six weeks of EOD feeding, liver, heart, kidney, brain and hind limb skeletal muscle tissues were collected from dwarf and WT mice (between 8:00 and 11:00 a.m. following a day when food was present), rapidly frozen and stored at −80°C until analysis. Whole tissues were used to determine the levels of GSH metabolic components and antioxidative enzymes. For measurement of OXPHOS activities and proteins, mitochondria were isolated from liver and hind limb skeletal muscle using standard techniques (Trounce et al., 1996; Brown-Borg et al., 2011). Ten to 50 μg of mitochondrial protein were used in each assay depending on the enzyme examined. Protein concentrations were determined using the Bradford assay (Bradford, 1976).
Glutathione (GSH) and oxidative metabolism
For these assays, tissue samples were homogenized on ice in buffer (20 mM MOPS, 300 mM sucrose, 0.1 mM EDTA at pH 7.2). The homogenate was centrifuged (30 minutes at 13000g) and the supernatant fraction used for analysis. The activities of GSH peroxidase (GPX), GSH reductase (GR), γ-glutamyl transpeptidase (GGT) and GSH S-transferase (GST) were determined as previously described (Brown-Borg et al., 1999; Brown-Borg and Rakoczy, 2000; 2005). Antioxidative enzyme activities measured included catalase and GPX (as described in publications above). The cellular oxidation state was determined by measuring the ratio of a specific reduction/oxidation pair, GSH/glutathione disulfide (GSSG) using procedures described previously (Brown-Borg and Rakoczy, 2005).
Protein expression was evaluated using standard immunoblotting procedures with chemiluminescence and densitometry (Brown-Borg et al., 2005; 2011). Mouse glutamate-cysteine ligase [GCL; heavy (catalytic) subunit; Neomarkers; Fremont, CA], human erythrocyte catalase (Calbiochem, LaJolla, CA), GPX1 (Biogenesis, England) and SOD2 (Oxis International; Portland, OR) antibodies were used to detect protein levels within each treatment group. The antibodies for OXPHOS proteins were obtained from Molecular Probes (Eugene, OR): 39 kDa subunit of Complex I (NADH dehydrogenase), 70 kDa subunit of Complex II (Succinate dehydrogenase), Core 2 subunit of Complex III (50 kDa; Cytochrome bc1), 57 kDa mitochondrially-encoded subunit I of Complex IV (Cytochrome c oxidase), 20 kDa nuclear-encoded subunit IV of Complex IV and α-subunit of Complex V (55 kDa; ATP synthase). Ponceau-S staining of membranes was used to evaluate equal loading of protein.
OXPHOS Protein Activities
The activities of the OXPHOS enzymes were evaluated in liver and hindlimb skeletal muscle mitochondrial preparations. The activity of complex IV (cytochrome c oxidase, COX) was measured following the oxidation of reduced cytochrome c at 550 nm as previously described (Cooperstein and Lazorow, 1951; Brown-Borg et al., 2011). Complex I+III (NADH-cytochrome c oxidoreductase) and complex II+III (succinate-cytochrome c reductase) activities were evaluated utilizing linked assays (Kuznetsov et al., 1996; Brown-Borg et al., 2011). For all enzyme assays, the appropriate controls and blanks were utilized.
Gene Expression
Gene expression was evaluated in liver and skeletal muscle using standard RT-PCR techniques (Brown-Borg et al., 2009; 2011). Total RNA was extracted from tissues and equal amounts of RNA for the gene of interest and the reference gene, β2 microglobulin (β2M; Lupberger et al., 2002) were utilized to perform one-step real-time quantitative PCR using a QuantiTect SYBR Green RT-PCR kit (Qiagen). The gene specific forward and reverse primers utilized are listed in Table 1. Gene expression was quantified using the comparative CT (threshold cycle) method (Heid et al., 1996). The amount of target DNA was normalized to the endogenous reference gene and compared relative to the control group (WT mice fed group).
Table 1.
Primer sets (5′-3′) used for real time PCR analysis of gene expression.
| Gene of Interest | Forward Primer | Reverse Primer |
|---|---|---|
| Complex I (ND1) mitochondrially-encoded | caggatgagcctcaaactcc | |
| Complex I (ND2) | agggatcccactgcacatag | |
| Complex II (SDHC) nuclear-encoded | acaaatggtctcttcctatggca | cccctccactcaaggctattc |
| Complex III (cytb) nuclear-encoded | acgtccttccatgaggacaa | gaggtgaacgattgctaggg |
| Complex IV (cox1a) mitochondrially-encoded | cttttatcctcccaggatttgg | gctaaatactttgacaccgg |
| Complex IV (cox5a) nuclear-encoded | ctttaaatgaattgggaatctccac | gcccatcgaagggagtttaca |
| Complex V (atp6) | aattacaggcttccgacacaaac | tggaattagtgaaattggagttcct |
| Peroxisome proliferator-activated receptor γ coactivator 1 alpha | caatgaatgcagcggtctta | gtgtgaggagggtcatcgtt |
| Adenine nucleotide transporter 1 | acttcgccttcaaagacaagtaca | gcgccagaactgcttatgg |
| Methionine adenosyltransferase 1a (Mat1a) | ctgaggcgctctggtgtc | tcctgcatgtactgaactgttacc |
| Glycine N-methyltransferase (GNMT) | gctggacgtagcctgtgg | cacgctcatcacgctgaa |
| S-adenosylhomocysteine hydrolase (Ahcy) | ctgttggggttcacttcctg | acattcagcttgcccaggt |
| Cystathionine β-synthase (CBS) | cgcacaggaaggactgcta | agccttcacagccacagc |
| Cystathionase (Cth) | gagtctggctgagcttcca | cgagggtagctctgtccttc |
| 5-methyltetrahydrofolate-homocysteine methyltransferase (Mtr) | gcagatgtggccagaaaag | gccacaaacctcttgactcc |
| 5,10-methylenetetrahydrofolate reductase (Mthfr) | agcttgaagccacctggactgtat | agactagcgttgctgggtttcaga |
| Glutamylcysteine ligase catalytic subunit (gclc) | ggaggcgatgttcttgagac | cagagggtcggatggttg |
| Glutamylcysteine ligase modifier subunit (gclm) | gactcacaatgacccgaaaga | gatgctttcttgaagagcttcct |
| β2-microglobulin | aagtatactcacgccaccca | aagaccagtccttgctgaag |
Statistical Analysis
In each experiment, differences between means was assessed utilizing Prism (Graphpad, San Diego, CA). For activity assays, protein and gene expression data, two-way analyses of variance (ANOVA) were employed and when appropriate, Bonferroni post-hoc testing was used to determine differences among means. Data are reported as means ± SEM.
Results
Body and liver weights
Six weeks of EOD feeding reduced body weights of dwarf and wild type mice (Table 2) with dwarf mice losing 1.7 grams of body weight on average and WT mice losing on average 7.3 grams. Small but significantly lower liver weights were also observed in mice subjected to EOD (Table 2).
Table 2.
Body and liver weights in Ames dwarf and wild type mice at the beginning (body weight only) and following six weeks of ad libitum or every other day feeding.
| genotype/treatment | Dwarf Ad libitum n=14 | Wild Type Ad libitum n=14 | Dwarf EOD n=20 | Wild Type EOD n=20 | P value | ||
|---|---|---|---|---|---|---|---|
| genotype | treatment | interaction | |||||
| Beginning Body weight (g) | 10.79 ± 0.44 | 31.78 ± 1.05 | 11.26 ± 0.26 | 30.82 ± 0.91 | <.0001 | .7370 | .3399 |
| End Body weight (g) | 10.91 ± 0.51 | 31.86 ± 1.19 | 9.20 ± 0.22 | 23.55 ± 0.61 | <.0001 | <.0001 | <.0001 |
| % change | +1.1% | +0.3% | −18.3% | −23.6% | |||
| Liver weight (g) | 0.41 ± 0.01 | 1.30 ± 0.04 | 0.36 ± 0.01 | 0.95 ± 0.04 | <.0001 | <.0001 | <.0001 |
Values represent mean ± SEM. P values represent results of a two-way ANOVA.
Antioxidative Defense and EOD Feeding
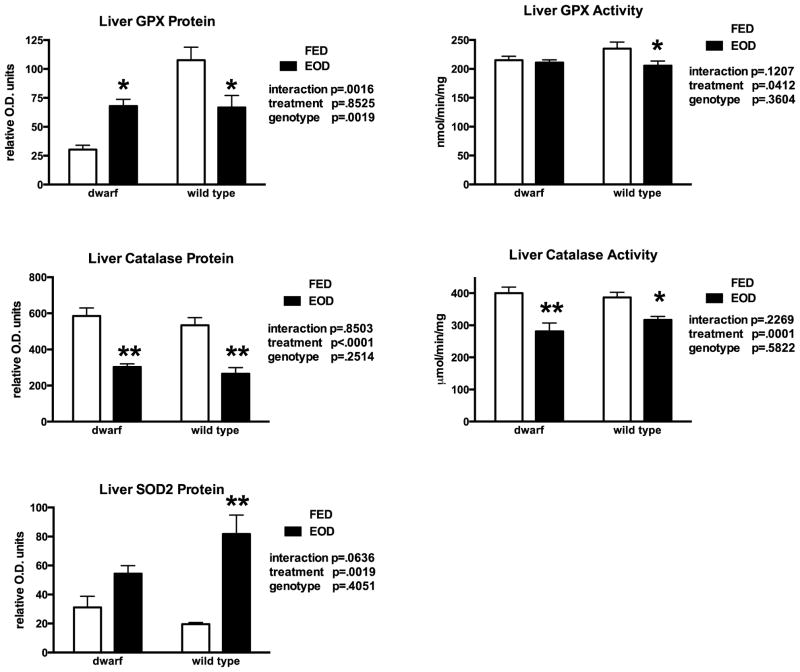
The effect of EOD on the antioxidative defense capacity of dwarf and WT mice was evaluated in several tissues. In the liver, GSH levels declined in Ames dwarf and WT mice while oxidized GSH (GSSG) levels were unaffected by the EOD treatment (Table 3). These differences resulted in GSH:GSSG ratios decreasing 25% in Ames mice and 46% in WT mice following EOD. The enzyme responsible for the biosynthesis of GSH is glutamate cysteine ligase, which is composed of catalytic and modifier subunits. No differences in liver gene expression were observed for either subunit (data not shown). However, the protein levels of GCL in wild type liver rose 45% following EOD feeding (p<0.05; Table 4). Glutathione S-transferase utilizes GSH to detoxify endogenous and exogenous compounds. In EOD fed WT mice, GST activity decreased (38%; Table 4). The enzyme activity of γ-glutamyl transpeptidase, which degrades GSH, was increased in WT liver tissue from the EOD group when compared to ad libitum fed mice (Table 4). Protein levels of GPX were differentially affected by genotype following EOD resulting in a significant interaction (Figure 1). The activity of liver GPX was maintained in EOD dwarf mice as compared to ad libitum fed mice while in WT mice, GPX declined (18%; Figure 1). Liver catalase protein decreased by half resulting in 20–30% decreases in activity levels following EOD in both genotypes (Figure 1). The levels of SOD2 protein increased in both genotypes (75% dwarf; >300% WT) that were fed EOD (Figure 1).
Table 3.
Glutathione (GSH; nmol GSH/mg tissue), glutathione disulfide (GSSG; nmol GSH/2/mg tissue) and GSH/GSSG ratios in tissues from Ames dwarf and wild type mice following six weeks of ad libitum or every other day feeding.
| genotype/treatment | Dwarf Ad libitum | Wild Type Ad libitum | Dwarf EOD | Wild Type EOD | P value | ||
|---|---|---|---|---|---|---|---|
| tissue | genotype | treatment | interaction | ||||
| Liver GSH | 951.2 ± 27 | 1236.2 ± 45 | 682.7 ± 28 | 722.5 ± 29 | <.0001 | <.0001 | .0008 |
| GSSG | 286.4 ± 10.6 | 291.8 ± 8.0 | 273.3 ± 6.4 | 314.6 ± 10.1 | .0147 | .5959 | .0147 |
| GSH:GSSG | 3.34 ± 0.13 | 4.24 ± 0.13 | 2.50 ± 0.11 | 2.28 ± 0.09 | .0051 | <.0001 | <.0001 |
| Kidney GSH | 93.9 ± 8.6 | 69.5 ± 6.1 | 76.5 ± 4.7 | 78.3 ± 5.7 | .0800 | .4966 | .0447 |
| GSSG | 27.3 ± 2.1 | 21.9 ± 0.9 | 22.9 ± 1.5 | 19.6 ± 0.8 | .0048 | .0247 | .4610 |
| GSH:GSSG | 3.64 ± 0.5 | 3.27 ± 0.4 | 3.51 ± 0.4 | 4.07 ± 0.4 | .8186 | .4137 | .2645 |
| Heart GSH | 390.8 ± 8.7 | 425.4 ± 24.0 | 367.2 ± 12.7 | 367.6 ± 15.6 | .2809 | .0167 | .2940 |
| GSSG | 48.5 ± 2.8 | 38.7 ± 1.2 | 46.9 ± 3.0 | 34.5 ± 0.4 | <.0001 | .1951 | .5628 |
| GSH:GSSG | 8.19 ± 0.4 | 11.11 ± 0.8 | 8.14 ± 0.7 | 10.65 ± 0.4 | <.0001 | .6695 | .7305 |
| Brain GSH | 706.1 ± 16.2 | 728.3 ± 26.2 | 767.7 ± 9.8 | 732.7 ± 9.1 | .6824 | .0420 | .0754 |
| GSSG | 131.1 ± 0.8 | 132.1 ± 1.5 | 133.6 ± 0.9 | 133.4 ± 1.1 | .7146 | .1052 | .5989 |
| GSH:GSSG | 5.39 ± 0.13 | 5.51 ± 0.16 | 5.75 ± 0.07 | 5.50 ± 0.09 | .5589 | .1238 | .1040 |
| Skeletal Muscle GSH | 83.2 ± 4.8 | 90.7 ± 5.9 | 69.9 ± 3.6 | 62.3 ± 5.0 | .9906 | <.0001 | .1076 |
| GSSG | 24.1 ± 0.8 | 24.9 ± 0.9 | 24.0 ± 0.2 | 23.0 ± 0.6 | .8175 | .1406 | .1916 |
| GSH:GSSG | 3.49 ± 0.2 | 3.67 ± 0.3 | 2.91 ± 0.2 | 2.68 ± 0.2 | .9116 | .0005 | .3017 |
Values represent mean ± SEM (n=7–9). P values represent results of a two-way ANOVA.
Table 4.
Protein levels of the glutathione biosynthetic enzyme (glutamate cysteine ligase, GCL; relative optical density units) and activities of the glutathione degrading enzyme (γ-glutamyltranspeptidase, GGT; nmol/mg protein except kidney – nmol/min/mg) and glutathione S-transferase (GST; μmol/min/mg) in tissues from Ames dwarf and wild type mice following six weeks of ad libitum or every other day feeding.
| genotype/treatment | Dwarf Ad libitum | Wild Type Ad libitum | Dwarf EOD | Wild Type EOD | P value | ||
|---|---|---|---|---|---|---|---|
| tissue/protein or activity | genotype | treatment | interaction | ||||
| Liver GCL | 89.9 ± 6.0 | 70.7 ± 11.4 | 93.5 ± 7.2 | 112.4 ± 15.9 | .5574 | .0186 | .2170 |
| GGT | 70.4 ± 3.1 | 82.0 ± 3.6 | 84.5 ± 3.1 | 95.9 ± 3.1 | .0077 | .0017 | .9890 |
| GST | 2.22 ± 0.11 | 2.45 ± 0.14 | 2.03± 0.07 | 1.52 ± 0.25 | .4162 | .0023 | .0351 |
| Kidney GCL | 35.6 ± 3.3 | 61.3 ± 6.1 | 38.1 ± 1.6 | 44.5 ± 2.5 | .0029 | .0962 | .0341 |
| GGT | 0.36 ± 0.02 | 0.55 ± 0.04 | 0.30 ± 0.05 | 0.51 ± 0.04 | <.0001 | .2246 | .9235 |
| GST | 0.64 ± 0.02 | 0.37 ± 0.02 | 0.46 ± 0.01 | 0.42 ± 0.03 | <.0001 | .0049 | <.0001 |
| Heart GCL | 163.4 ± 19.6 | 64.0 ± 12.9 | 178.4 ± 27.0 | 124.0 ± 27.5 | .0052 | .1221 | .3380 |
| GGT | 117.8 ± 10.6 | 116.0 ± 5.8 | 150.4 ± 13.9 | 142.9 ± 12.6 | .6812 | .0140 | .7941 |
| GST | 0.51 ± 0.03 | 0.29 ± 0.02 | 0.44 ± 0.02 | 0.39 ± 0.03 | <.0001 | .4744 | .0009 |
| Skeletal Muscle GCL | 19.2 ± 0.5 | 31.1 ± 7.5 | 24.5 ± 4.1 | 5.6 ± 1.5 | .4482 | .0467 | .0067 |
| GGT | 32.9 ± 3.4 | 44.0 ± 4.1 | 18.9 ± 2.6 | 37.9 ± 4.0 | .0022 | .0305 | .3748 |
| GST | 0.32 ± 0.01 | 0.23 ± 0.02 | 0.28 ± 0.01 | 0.29 ± 0.02 | .0284 | .3290 | .0073 |
| Brain GCL | 29.5 ± 3.5 | 44.2 ± 4.9 | 28.3 ± 5.5 | 35.3 ± 7.9 | .0662 | .3605 | .4836 |
| GGT | 81.0 ± 2.7 | 96.8 ± 3.1 | 71.0 ± 13.0 | 87.4 ± 6.7 | .0062 | .0811 | .9525 |
| GST | 0.32 ± 0.02 | 0.27 ± 0.01 | 0.34 ± 0.02 | 0.34 ± 0.03 | .3331 | .0372 | .2891 |
Values represent mean ± SEM (n=3–9). P values represent results of a two-way ANOVA.
Figure 1.
Liver protein and activity levels of glutathione peroxidase (GPX), catalase and superoxide dismutase levels in dwarf and wild type mice following six weeks of every other day feeding (EOD) or ad libitum feeding (FED). N=7–11 mice/genotype/treatment. *p<0.05, **p<0.01.
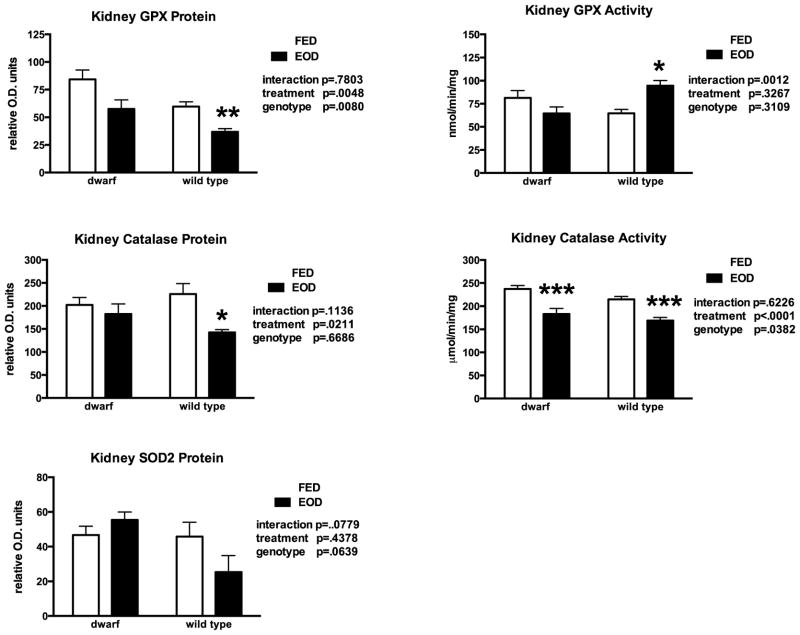
In the kidney, EOD feeding lowered GSH levels in dwarf mice. However, the statistical comparison between genotype and feeding resulted in a significant interaction (Table 3). While GSSG levels declined slightly in both genotypes fed EOD, the ratio of GSH:GSSG did not change significantly. Protein levels of GCL in WT kidney tissues appeared to decrease with EOD when compared to ad lib feeding but the interaction was significant (Table 4). Every other day feeding did not affect GR activity in kidney tissue although WT mice maintained higher overall levels (25–30%) compared to dwarf mice (data not shown). GST activity was higher in dwarf kidney tissues but declined in the EOD group compared to ad lib fed group (Table 4). Kidney GGT activity was not affected by EOD but WT mice exhibited greater GGT levels compared to dwarf mice (Table 4) as previously shown. GPX protein levels were lower in WT mice compared to dwarf mice and EOD decreased them further (38%) when compared to ad lib feeding (Figure 2). Following EOD, WT kidney GPX activity increased 32% but a significant interaction was also observed (Figure 2). Feeding EOD induced a decline in catalase protein levels that resulted in a significant decrease in catalase activity in both genotypes fed EOD (Figure 2).
Figure 2.
Kidney protein and activity levels of glutathione peroxidase (GPX), catalase and superoxide dismutase levels in dwarf and wild type mice following six weeks of every other day feeding (EOD) or ad libitum feeding (FED). N=7–11 mice/genotype/treatment. *p<0.05, **p<0.01, ***p<0.001.
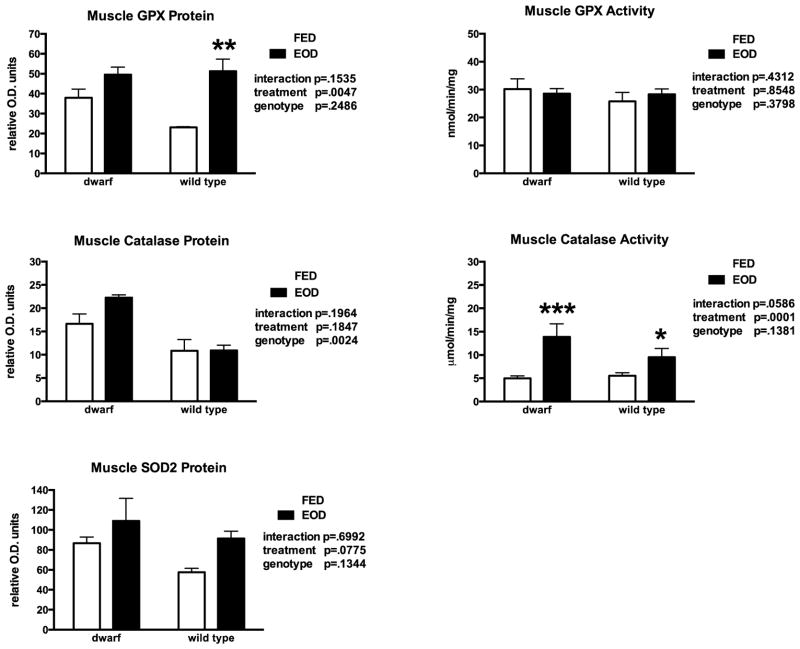
In hindlimb skeletal muscle, EOD feeding decreased the GSH:GSSG ratio in wild type mice (Table 3), resulting from lower GSH levels when compared to animals that were fed ad libitum. Protein levels of GCL were differentially affected by genotype and feeding treatment decreasing markedly in WT mice fed EOD (Table 4). Feeding EOD increased GST detoxification activity in WT mice only (Table 4). GGT activity was suppressed by EOD feeding in both genotypes and higher in WT compared to dwarf mice (Table 4). Protein levels of GPX detected in WT mice increased (120%) with this altered feeding treatment (Figure 3) but not change in activity was observed. Conversely, although muscle catalase protein was higher in dwarf mice and was not affected by treatment, the catalase activity levels increased in both genotypes following EOD (Figure 3).
Figure 3.
Hindlimb skeletal muscle protein and activity levels of glutathione peroxidase (GPX), catalase and superoxide dismutase levels in dwarf and wild type mice following six weeks of every other day feeding (EOD) or ad libitum feeding (FED). N=7–11 mice/genotype/treatment. *p<0.05, **p<0.01, ***p<0.001.
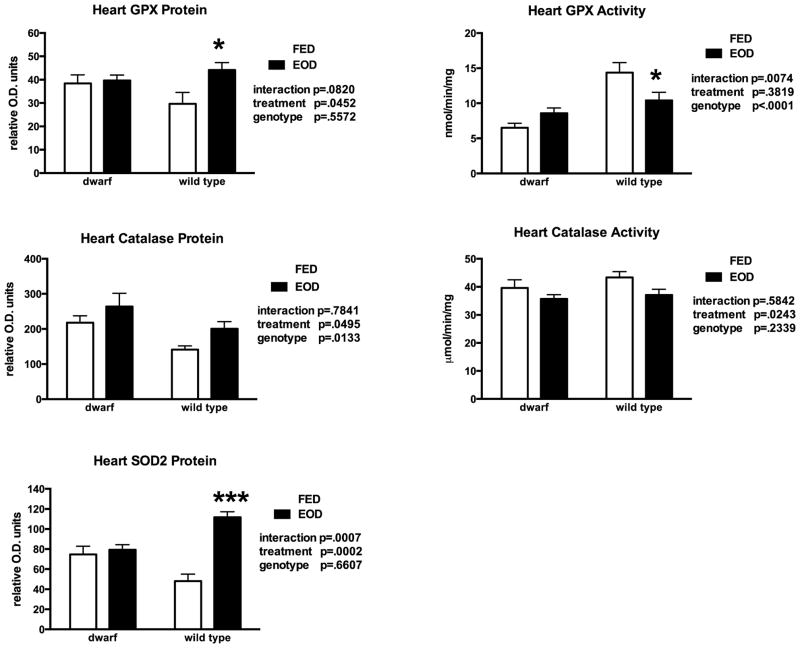
Heart muscle tissue was evaluated for the effects of EOD on antioxidative defense. Glutathione levels decreased slightly in WT mice but did not change in Ames mice fed EOD (Table 3). Overall, the GSH:GSSG ratio remained unchanged by treatment but the ratio remained higher in WT mice. Protein levels of GCL, were higher in dwarf mice compared to WT mice regardless of treatment (Table 4). Every other day feeding did not alter heart GR activity but WT mice exhibited greater activity (15–39%) compared to dwarf mice (data not shown). GST activity was differentially affected by genotype and feeding treatment leading to an interaction and higher activity following EOD in WT mice (Table 4). Activity of GGT in heart tissue increased in both genotypes fed EOD compared to ad lib feeding (Table 4). Protein levels of GPX increased 49% in WT mice on EOD however activity was differentially affected depending on genotype and feeding paradigm (Figure 4). Every other day feeding induced an increase in catalase protein in both Ames and WT mouse heart tissue whereas activity was decreased (Figure 4). Feeding EOD increased SOD2 protein levels in WT mouse heart tissue by 130% (Figure 4).
Figure 4.
Heart protein and activity levels of glutathione peroxidase (GPX), catalase and superoxide dismutase levels in dwarf and wild type mice following six weeks of every other day feeding (EOD) or ad libitum feeding (FED). N=7–11 mice/genotype/treatment. *p<0.05, ***p<0.001.
Whole brains were also collected to evaluate the antioxidative and metabolic responses to EOD in dwarf and WT mice. Brain GSH levels increased slightly in EOD fed dwarf mice (Table 3). Brain GR activity decreased 31% in dwarf and 36% in WT mice following six weeks of EOD (p=0.0064; data not shown). The activity of GST was stimulated by EOD feeding (Table 4). Brain GGT enzyme activity was higher in WT mice compared to dwarf mice (p=0.0062; Table 4) similar to that found in other tissues.
Oxidative Phosphorylation and EOD Feeding
Components of the OXPHOS system were examined in liver (proliferative) and skeletal muscle (post-mitotic) mitochondria following six weeks of EOD feeding. The activity of liver complex I+III decreased 44% in WT mice following EOD when compared to their ad libitum fed counterparts while no differences were observed in dwarf mice (Table 5). Complex II+III activity was also lower in the EOD-fed WT mice (29%) while the activity in dwarf mice increased with this feeding treatment (57%). Similarly, the activities of complex IV in dwarf mice increased (36%) with EOD while in WT mice activity decreased (Table 5).
Table 5.
Complex I+III, II+III and Complex IV (cytochrome c oxidase) activities (μmol/min/mg) in liver and muscle tissue mitochondria from Ames dwarf and wild type mice following six weeks of every other day or ad libitum feeding.
| genotype/treatment | Dwarf Ad libitum | Wild Type Ad libitum | Dwarf EOD | Wild Type EOD | P value | ||
|---|---|---|---|---|---|---|---|
| activity tissue | genotype | treatment | interaction | ||||
| Liver I+III | 0.480 ± 0.05 | 0.925 ± 0.05 | 0.468 ± 0.04 | 0.522 ± 0.02 | <.0001 | <.0001 | <.0001 |
| II+III | 0.161 ± 0.01 | 0.321 ± 0.02 | 0.253 ± .04 | 0.230 ± .04 | .0677 | .9872 | .0168 |
| IV | 1.299 ± .07 | 1.706 ± .13 | 1.762 ± .31 | 1.305 ± .11 | .9095 | .8848 | .0508 |
| Skeletal Muscle I+III | 0.307 ± 0.03 | 0.732 ± 0.05 | 0.207 ± 0.04 | 0.375 ± 0.07 | <.0001 | .0006 | .0390 |
| II+III | .088 ± .006 | .241 ± .034 | .274 ± .063 | .211 ± .024 | .2240 | .0391 | .0054 |
| IV | 1.55 ± .09 | 2.894 ± .32 | 2.179 ± .26 | 2.378 ± .18 | .0028 | .8129 | .0220 |
Values represent mean ± SEM (n=7–11). P values represent results of a two-way ANOVA.
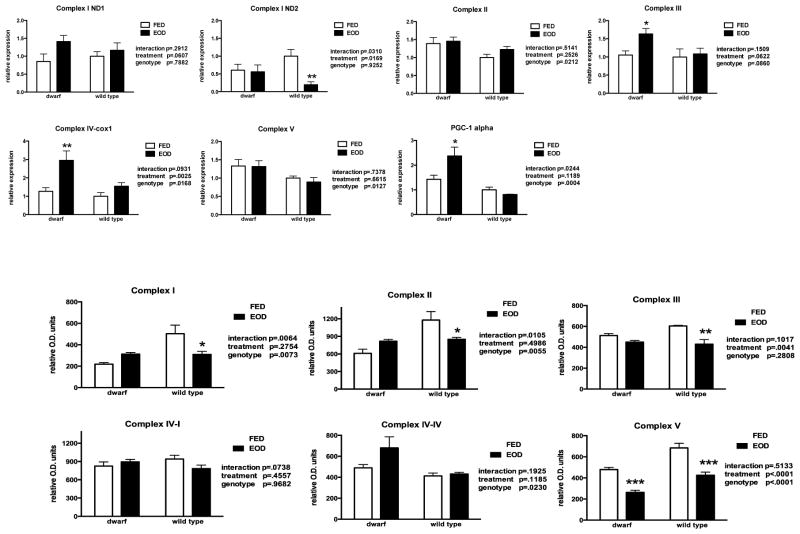
Liver protein levels of complexes I and III decreased (38% and 29%, respectively) in WT mice matching the decrease in activity of complexes I+III (Figure 5). Similarly, complex II protein decreased in WT mice by 28% following six weeks of EOD feeding. As observed with complex I, the protein levels in complex II matched that detected in activity level of complex II+III for both genotypes. Ames dwarf mice appeared to exhibit higher levels of the nuclear-encoded complex IV subunit protein compared to WT mice (Figure 5). Protein levels of the α-subunit of complex V were decreased in dwarf and WT mice after EOD.
Figure 5.
Liver oxidative phosphorylation gene expression (relative expression) and protein (relative O.D. units) levels of OXPHOS complexes and PGC-1α in dwarf and WT mice following six weeks of every other day feeding (EOD) or ad libitum feeding (FED). N=7–11 mice/genotype/treatment. *p<0.05, **p<0.01, ***p<0.001.
Gene expression of OXPHOS components was also evaluated in liver and skeletal muscle in this study. In liver, mitochondrially-encoded ND1 gene expression tended to be higher following EOD. The ND2 subunit decreased (81%) in EOD fed WT mice (Figure 5). These data support the difference in both proteins and activities of complexes found in this study. No difference in liver complex II mRNA was observed due to feeding treatment but dwarf mice exhibited greater levels when compared to WT mice. Complex III gene expression was elevated in dwarf mice following EOD compared to the ad lib fed group. Both treatment and genotype differences were observed in complex IV-cox1a subunit expression (Figure 5) with EOD feeding increasing in dwarf liver by 130%. Dwarf mice exhibited elevated levels of complex V mRNA compared to WT mice (p=0.0127). Gene expression of PGC-1α was markedly elevated (66%) in dwarf mice fed EOD compared to ad libitum fed dwarfs and was also higher than WT mice fed ad libitum (Figure 5).
Mitochondria isolated from the hind limb skeletal muscles of dwarf and WT mice showed several similarities with liver tissue in OXPHOS activities and protein. Every other day feeding decreased complex I+III activities in WT (49%) compared to ad libitum fed mice (Table 5). Complex II+III activity in muscle tissue of dwarf mice increased over 200%. Muscle mitochondria from dwarf mice also exhibited elevated complex IV activity (40%) while WT mouse muscle had 18% less activity with EOD resulting in a positive interaction (Table 5). These activity levels were reflected in protein differences described below. Wild type mice had elevated skeletal muscle complex I protein but the feeding treatment did not affect levels of this protein (Figure 6). Protein levels of complex II were decreased 110% in WT mice fed EOD. The differences in complexes III, IV-I and V protein levels were also confounded (significant treatment X genotype interactions) but similar to complex II, levels of each protein declined in WT mice fed EOD (Figure 6). Muscle complex I (ND1 subunit) gene expression tended to be higher in dwarf mice (WT fed 1.0 ± 0.30; WT EOD 0.49 ± 0.29; dwarf fed 1.56 ± 0.80; dwarf EOD 2.59 ± 0.56; genotype p=0.06) but no effect of EOD feeding was observed. Complex II mRNA was elevated in dwarf and WT muscle tissue following EOD (WT fed 0.47 ± 0.18; WT EOD 1.67 ± 0.81; dwarf fed 0.58 ± 0.20; dwarf EOD 1.51 ± 0.57; p=0.05) compared to ad libitum fed mice.
Figure 6.
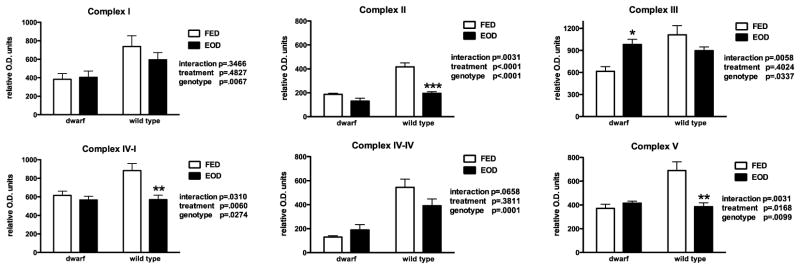
Skeletal muscle oxidative phosphorylation proteins (relative O.D. units) in dwarf and WT mice following six weeks of every other day feeding (EOD) or ad libitum feeding (FED). N=7–11 mice/genotype/treatment. *p<0.05, **p<0.01, ***p<0.001.
Methionine Metabolism and EOD Feeding
The methionine metabolic pathway is highly active in liver thus, gene expression was evaluated in this proliferative tissue. Skeletal muscle mRNA was also examined as a post-mitotic tissue in which the components of the MET pathway function at much lower levels. Similar to previous reports from our laboratory, gene expression of Mat1a, Gnmt, Ahcy, Cbs and Cth were significantly elevated in dwarf mice when compared to WT mice (Figure 7). However, the expression of Ahcy, which metabolizes s-adenosylmethionine to homocysteine, markedly increased in EOD fed dwarf mice but not in WT mice where the levels remained low. In addition, transcription of the transsulfuration pathway enzyme, Cbs, declined in EOD WT mice. Gene expression of Mtr and Mthfr, two enzymes that contribute to the recycling of homocysteine to methionine, were increased in EOD animals compared to ad libitum fed mice (Figure 7). In skeletal muscle tissue, Ames mice maintained higher basal levels of the genes in the MET pathway, as is known for liver tissue. The mRNA expression of Mat1a, Gnmt and Ahcy mRNA followed a similar pattern in response to EOD with dwarf mice expressing decreased levels and WT mice increasing or maintaining expression of these genes (Figure 8). The mRNA for enzymes recycling MET (Mtr, Mthfr) decreased with EOD as did Cbs in the transsulfuration pathway.
Figure 7.
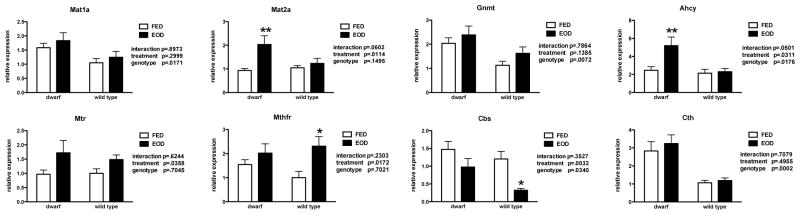
Gene expression (relative expression) of methionine metabolic enzymes in liver tissue from dwarf and WT mice following six weeks of every other day feeding (EOD) or ad libitum feeding (FED). N=7–11 mice/genotype/treatment. *p<0.05, **p<0.01.
Figure 8.
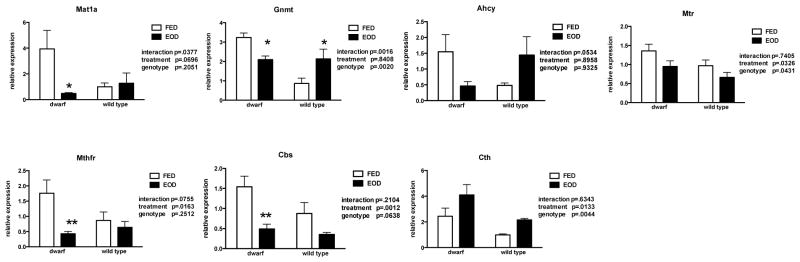
Gene expression (relative expression) of methionine metabolic enzymes in skeletal muscle tissue from dwarf and WT mice following six weeks of every other day feeding (EOD) or ad libitum feeding (FED). N=7–11 mice/genotype/treatment. *p<0.05, **p<0.01.
Discussion
The physiological effects of restrictive dietary interventions have been studied for more than 75 years. Those studies related to aging and age-related disease have reported primarily beneficial effects of restricting food intake (either a daily reduction in calories or limiting consumption every 24 hours) in terms of life span extension. Bartke and coworkers (2001) showed that 30% dietary restriction imposed on long living Ames mice further extended lifespan when compared to their ad libitum-fed dwarf counterparts (as well as both restricted and ad libitum-fed WT control mice). However, a 40% DR protocol did not affect longevity of these mice when the Prop-1 mutation was expressed on a C57Bl/6 background (Garcia et al., 2008). In addition, Ames mice subjected to nine months of EOD feeding, exhibited alterations in the expression of genes related to insulin and IGF1 signaling resulting in enhanced insulin sensitivity (Masternak et al., 2005). This EOD experiment demonstrated that both WT and dwarf mice responded to this feeding treatment although fewer changes were observed in dwarf mice. Aside from insulin sensitivity, however, little is known about the effects of EOD feeding on other key factors that contribute to health span and life span such as stress defense and mitochondrial function in long living mice.
We were interested in the tissue specific metabolic adaptions in antioxidative defense capacity and mitochondrial OXPHOS that occur in response to EOD feeding. Tissues from Ames dwarf mice have been shown to maintain higher levels of GSH compared to WT mice thus, our initial experiments focused on GSH metabolism and the enzymes that counter ROS. Cellular GSH is the most abundant of the redox pairs and is an excellent indicator of cellular redox state (Droge, 2002; Jones, 2002; Schafer and Buettner, 2001). Proteins involved in the maintenance of GSH levels include GCL and GGT, of which the liver and kidneys serve as the primary tissues responsible for de novo synthesis and degradation, respectively (Anderson and Meister, 1980). Importantly, changes in liver GSH metabolism alone have been shown to have widespread impact on the redox state of extrahepatic tissues (Anderson et al., 1980).
In the current study, WT mice appeared to be more sensitive metabolically to EOD feeding, while many parameters were unchanged in dwarf mice. Wild type animals responded to a reduction in the liver GSH redox ratio with an increase in GCL protein, a finding that is in agreement with previous reports of DR in normal mice (Pearson et al., 2008; Rebrin et al., 2011). The activities of GSH-related and antioxidative enzymes were also altered in liver tissue of WT mice in response to this feeding paradigm. Lower levels of GST and GPX mirrored the decline in GSH, while GGT increased perhaps to increase amino acid components (glutamine, glycine, cysteine) for de novo synthesis. It has been shown that aspects of GSH metabolism are modified by hormone status, also. For example, GH administration to GH-deficient mice increases GGT activity (Brown-Borg et al., 2005). This anabolic hormone is also altered by changes in nutrient intake. Serum GH increases during fasting and chronic DR, both of which are known to exert many metabolic changes (Thissen et al., 1994a; Buyse et al., 2000). Moreover, IGF1, downstream of GH, is regulated by nutrients and IGF1 plasma levels decrease in long term DR (Thissen et al., 1994a). In addition, DR increases the clearance and degradation of circulating IGF1. It has been demonstrated that GH and amino acids work together to regulate the production of liver IGF1 directly and exert indirect effects by regulating IGF binding protein 1 production (Thissen et al., 1994b). Furthermore, liver GH receptor expression is dependent on the level (severity) of nutritional insult therefore, taken together the evidence suggests that the lack of responsiveness to EOD feeding in Ames mice may be in part due directly to their GH and IGF1 deficiencies. Others have shown that both circulating GH and IGF1 levels rise with intermittent feeding, likely to stimulate amino acid uptake and attenuate nitrogen loss (Anson et al., 2003; 2005; Nir et al., 1983; Ekmay et al., 2010; Snyder et al., 1988; Kupfer et al., 1993). Dwarf mice may exhibit fewer changes in response to EOD feeding possibly due to their inability to alter plasma GH concentrations when faced with the absence of nutrients.
The expression of GSH-related and antioxidative genes (Gclc, Gclm, catalase, SOD2, GST and GPX) is controlled by Nrf2. Dwarf mice exhibit elevated Nrf2 levels in comparison to WT mice (Sharma et al., 2010b; Leiser and Miller, 2010; Sun et al., 2011) resulting in enhanced antioxidative enzyme activities (Brown-Borg and Rakoczy, 2000; Brown-Borg et al., 2001; 2003). When challenged with a stressor (kainic acid), dwarf mice do not need additional hippocampal Nrf2 support to maintain their antioxidative enzyme system (Sharma et al., 2010b). In agreement, Sun and colleagues (2011) reported that diquat increased dwarf Nrf2 levels and downstream Nrf2 responsive genes. Growth hormone administration (in vitro and in vivo) and overexpression have been shown to down regulate antioxidative enzymes (Brown-Borg et al., 1999; 2002; 2003; Brown-Borg and Rakoczy, 2000). Thus, if EOD feeding increased plasma GH as predicted, then the down regulation of these defense mechanisms in WT mice is projected. Therefore, it appears that dwarf tissues are less responsive to this diet manipulation and able to maintain their antioxidative defense system during this environmental challenge, in part, because they lack circulating GH. Liver GSH metabolism was strongly up regulated in a previous study utilizing EOD feeding in mice (Pearson et al., 2008) and other reports demonstrate that DR increases liver GST activity (Chen et al., 1994; Kim et al., 1996) with no effect on the liver GSH redox pair (Rebrin et al., 2003). The differences observed in WT mice in our study may reflect strain specificity and treatment length differences compared to other studies. Recent reports confirm that the background strain of a mouse affects its response to DR and EOD feeding in terms of lifespan extension and short-term effects on GSH metabolism (Liao et al., 2010; Rebrin et al., 2011; Garcia et al., 2008).
In this study, it appears that the decline in liver GSH in WT mice had a widespread impact on GSH levels and antioxidative defense in other tissues as suggested by Anderson and coworkers (1980). Similar to liver, a role for elevated GH in the WT kidney is suggested by the decline in protein levels of catalase, SOD2 and GPX following EOD feeding. In the heart, antioxidative enzyme activities decreased in WT mice. In contrast, the defense enzyme response to intermittent feeding was dampened in dwarf heart muscle. Some reports evaluating heart tissue have shown that EOD restored GSH redox ratios in old mice to levels observed in young mice in addition to reductions in ROS generation and oxidative damage (long term DR; Castello et al., 2010; Rebrin et al., 2003; Gredilla et al., 2001). Our results suggest that short-term EOD feeding exerts a greater effect on ROS defense in WT when compared to Ames mice.
Every other day feeding and dietary restriction have been shown to decrease markers of oxidative damage (Castello et al., 2010; Yu and Mattson, 1999; Bruce-Keller et al., 1999; Sohal and Weindruch, 1996; Merry, 2004; Marzetti et al., 2009). There are numerous examples of studies in which DNA, protein and lipid (peroxidation) oxidative damage were reduced following both EOD and DR (Caro et al., 2008; Merry, 2002; 2004; Barja, 2004; Heilbronn et al., 2006; Castello et al., 2005; Chiarpotto et al., 2006; Gredilla and Barja, 2005). These studies among many others conclude that these dietary manipulations reduce cumulative oxidative damage by altering ROS production and protective mechanisms (antioxidative enzymes, repair pathways). These protective pathways respond to the environment to allow the organism to adapt. In the Ames dwarf mouse, we have previously shown that the generation of liver ROS is lower and many of the protective mechanisms are up regulated. It may be that these mice have minimized ROS production and/or maximized their defense mechanisms such that an additional perturbation such as EOD feeding has less of an effect on these pathways when compared to WT mice.
Metabolites of methionine feed directly into the GSH pathway. Components of this essential amino acid pathway have been evaluated extensively in the Ames mouse and differ substantially between genotypes (Uthus and Brown-Borg, 2003, 2006; Brown-Borg et al., 2005). The EOD treatment may have altered the amount of substrate transferred or passing through to transsulfuration and that being recycled back to MET as an effect of feeding was observed in both genotypes in the MET recycling enzymes. These changes in turn, affect GSH levels and further MET metabolism. The basal MET enzyme levels in Ames mice are indicative of a heightened oxidative defense system. An overall decrease in oxidative defense capacity is predicted in WT mice fed EOD based on the decrease in transsulfuration, GPX and catalase and knowing that fasting and intermittent feeding increase GH secretion that in turn, suppresses antioxidative activities.
Growth hormone also regulates components of the MET pathway, in particular GNMT activity, and thus plays a role in the control of tissue SAM and SAH concentrations (Cook and Wagner, 1984; Wagner et al., 1985; Ogawa et al., 1997; Luka et al., 2009; Aida et al., 1997; Uthus and Brown-Borg, 2003; Brown-Borg et al., 2005). Sustained increases in transsulfuration activities in Ames mice over those in WT mice with and without EOD feeding suggest that the GH-deficient dwarf system is very responsive to additional changes in MET by maintaining high rates of catabolism. It is unclear why the levels of GSH declined in dwarf mice on EOD with the concurrent increase in transsulfuration, unless they have different requirements for GSH in the presence of an overall heightened antioxidative defense system.
Components of the OXPHOS system were evaluated in mitochondria in response to this feeding intervention. OXPHOS enzymes decreased in WT mice fed EOD. Dwarf mice were differentially affected exhibiting no change due to feeding treatment or an increase in complex activities. The activities of complex enzymes I through IV are closely related as complex I serves as the master regulator of the electron transport chain (ETC) and thus greatly affects the activities of the downstream complexes (Capaldi et al., 1988). In EOD fed WT mice, the decrease in complex I was replicated in the downstream ETC activities. Although, complex V is not coupled to the activity of the other complexes, it was also decreased by EOD feeding. The overall down regulation of OXPHOS in WT mice may reflect the energy-restricted state induced by the EOD regimen. Our previous work has shown that old Ames mice maintain higher complex activities compared to old WT mice suggesting better preservation of mitochondrial function with age. This finding holds true for dwarf mice subjected to EOD feeding supporting the protection of mitochondrial function during a metabolic stress such as energy restriction. Others have also observed significant reductions in liver OXPHOS proteins with EOD feeding in mice (Caro et al., 2008). A recent report found that intermittent feeding in rats decreased kidney complex I activity but increased complex IV activities in liver, kidney and heart (Singh et al., 2012). These investigators suggested that the differential response in activities was dependent upon whether the tissue was post-mitotic or proliferative. Feuers (1998) found that DR increased complex IV activity (muscle) and in agreement, brain complex IV increased in response to long-term EOD feeding (Olgun et al., 2002). We found that in proliferative tissue (liver), Ames mice maintained or increased OXPHOS activities while in post-mitotic tissue (skeletal muscle), electron flow through I+III decreased slightly but activities of downstream enzymes increased with EOD feeding. In contrast, complex enzyme proteins and activities declined in WT mice fed EOD in both mitotic and post-mitotic tissues perhaps leading to increased ROS production. Our report differs from others that showed no change (1 month DR) or increased OXPHOS expression (9 months DR; Estey et al., 2012; Sreekumar et al., 2002; Baker et al., 2006). Importantly, our study suggests that muscle mitochondria from dwarf mice are better protected against a metabolic stress in the form of EOD feeding, when compared to WT mice. This protection may contribute to overall aging and lifespan differences observed within these genotypes.
Reactive oxygen species generation is known to be tissue- and substrate-specific and altered by respiratory rate (Tahara et al., 2009; Chen et al., 2008). Inhibition of complex I or III activity leads to enhanced ROS release from mitochondria. In contrast, increasing the respiratory rate by promotion of OXPHOS decreases ROS generation. Although not measured in this study, it is predicted that mitochondrial ROS release would be higher in WT mice compared to dwarf mice based on the evidence that OXPHOS enzymes decline with EOD feeding in these wild type animals. In a previous study, we observed higher H2O2 production in liver mitochondria of wild type mice in comparison to age-matched dwarf mice (Brown-Borg et al., 2001).
Many reports demonstrate that DR and EOD feeding contribute to healthy aging by enhancing antioxidative defense, reducing ROS generation and increasing cellular resistance to stress and disease (Feuers, 1998; Liang et al., 2003; Mattson et al., 2004; Martin et al., 2006; Berryman et al., 2008; Kalaany and Sabatini, 2009). We did not observe this enhancement in WT mice in our study potentially due to the short period of intervention and that EOD in comparison to daily DR does not systematically reduce caloric intake. Some studies have shown that with EOD feeding, total food intake is not significantly reduced (Anson et al., 2003; Hipkiss, 2007; Cerqueira et al., 2010) indicating that EOD feeding is not truly calorie restriction but the periodic absence of food that may induce metabolic changes similar to DR. Traditional DR involves reducing the amount of food intake daily so that mice eat their allotted amount of food all at once. The remaining time each day is spent in an energy-restricted state. These periods of energy-restriction are similar to the alternate day feeding paradigm where animals eat ad libitum one day and spend the next 24 hours in an energy-restricted state (no food; Mootha et al., 2003).
Ames mice exhibit elevated liver PGC1-α levels in comparison to WT mice (ad libitum feeding conditions; reviewed in Corton and Brown-Borg, 2005; Amador-Noguez et al., 2004; Bartke et al., 2008; Brown-Borg and Bartke, 2012). In the current study, PGC-1α mRNA increased in EOD dwarf mice while no response was observed in WT mice. This increased energy-responsive gene expression may represent an additional short-term adaptation to reduced nutrient availability and explain in part, the maintenance or increases in ETC activities in dwarf mice. It is unclear why a similar increase was not observed in WT mice although we suspect that the GH responsiveness is at least partially involved. Tsuchiya and coworkers (2004) suggested that DR and disrupted growth factor signaling exert different but overlapping or additive effects on longevity via hepatic gene regulation. Their results differ somewhat from our study in dwarf mice fed EOD as we evaluated activity and protein and the Tsuchiya study examined mRNA expression under DR conditions. Several labs have shown that both short- and long-term DR and other changes in diet can induce PGC-1α and increase mitochondrial biogenesis and mitochondrial efficiency (Lopez-Lluch et al., 2006; Zhu et al., 2004; Corton and Brown-Borg, 2005; Muoio and Koves, 2007; Dunn et al., 1997; Kari et al., 1999; Breese et al., 1991; Ruggeri et al., 1989; Sell, 2003; Varady et al., 2007; Thissen et al., 1994; Wan et al., 2003). In contrast, Caro and coworkers (2008) reported decreased PGC-1α protein levels and mitochondrial oxidative stress in mouse liver using an EOD feeding paradigm. However, the strain of mice (C57Bl/6), the age at which EOD feeding started (eight weeks of age), the conditions of tissue collection as well as the measure of this protein was different (protein versus mRNA) compared to our study. Growth hormone receptor knock out (GHRKO) mice are long living and also exhibit undetectable plasma IGF1 levels due to the targeted disruption of the GH receptor. These animals do not respond to 30% DR or EOD feeding with extended mean or maximum lifespans or changes in insulin sensitivity (Bonkowski et al., 2006; Arum et al., 2009). Taken together, the evidence suggests that an intact GH signaling system is the key to respond to dietary restriction or EOD feeding of any type.
Overall, the decline in OXPHOS enzymes in WT mice subjected to EOD feeding may be due to increased ROS production during electron transport (Chen et al., 2007; Tahara et al., 2009). This in turn, reflects an energy-restricted state and suggests a greater overall need for mechanisms to counter ROS. Dwarf mice, on the other hand, adapt differently to the potential energy restriction with elevations in OXPHOS activities, potentially lowering ROS generation resulting in fewer effects on antioxidative enzymes and amino acid metabolism. These responses are indicative of the greater overall defense mechanisms in dwarf mice and the enhanced ability to adapt or cope with metabolic perturbations.
In summary, EOD feeding had less impact on antioxidative and OXPHOS enzymes of GH-deficient mice compared to WT animals. The differential response of the dwarf mouse reflects the lack of GH and it’s complex metabolic interactions with defense mechanisms and mitochondrial function. Dwarf mice live significantly longer and maintain lower redox ratios yet higher antioxidative defense capacities and lower oxidative damage accumulation to DNA and proteins (Brown-Borg et al., 2001; 2005; Romanick et al., 2004; Brown-Borg, 2009; Bokov et al., 2009; Choksi et al., 2007; Sanz, 2002). In the current study, dwarf mice were less sensitive to the lack of nutrients and energy restriction imposed by short term EOD feeding compared to WT mice. This study provides further evidence of the widespread protection from environmental and metabolic challenges that GH-deficiency invokes that may assist in health and lifespan extension in mammals.
Highlights.
Liver glutathione declines in long-living Ames dwarf and wild type mice but glutathione biosynthesis and degradation are altered in wild type animals only in response to every other day feeding
Ames mice maintain or increase liver OXPHOS components while wild type mice respond to EOD feeding with declines in OXPHOS
Selective antioxidative enzymes are not up regulated with EOD feeding in wild type mice
Ames dwarf mice appear to be less sensitive to the metabolic challenge of EOD feeding compared to wild type mice, findings at least partially explained by the lack of anabolic hormone stimulation
Acknowledgments
This work was supported by the NIH R15 AG022909, NIH RO1 AG034206, Glenn Foundation for Medical Research, Ellison Medical Foundation AG-SS-2376-09 and the University of North Dakota.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aida K, Tawata M, Negishi M, Onaya T. Mouse glycine N-methyltransferase is sexually dimorphic and regulated by growth hormone. Horm Metab Res. 1997;29:646–649. doi: 10.1055/s-2007-978982. [DOI] [PubMed] [Google Scholar]
- Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3:423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Bridges RJ, Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980;96:848–853. doi: 10.1016/0006-291x(80)91433-3. [DOI] [PubMed] [Google Scholar]
- Anderson ME, Meister A. Dynamic state of glutathione in blood plasma. J Biol Chem. 1980;255:9530–9533. [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Jones B, de Cabo R. The diet restriction paradigm: a brief review of the effects of every-other-day feeding. AGE. 2005;27:17–25. doi: 10.1007/s11357-005-3286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arum O, Bonkowski MS, Rocha JS, Bartke A. The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell. 2009;8:756–760. doi: 10.1111/j.1474-9726.2009.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Betik AC, Krause DJ, Hepple RT. No decline in skeletal muscle oxidative capacity with aging in long-term calorically restricted rates: effects are independent of mitochondrial DNA integrity. J Geront A Biol Med Sci. 2006;61:675–684. doi: 10.1093/gerona/61.7.675. [DOI] [PubMed] [Google Scholar]
- Barja G. Aging in vertebrates and the effect of calorie restriction: a mitochondrial free radical production-DNA damage mechanism? Biol Rev Camb Philos Soc. 2004;79:235–51. doi: 10.1017/s1464793103006213. [DOI] [PubMed] [Google Scholar]
- Bartke A. Pleiotropic effects of growth hormone signaling in aging. Trends Endocrin Metab. 2011;22:437–442. doi: 10.1016/j.tem.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Bonkowski M, Masternak M. How diet interacts with longevity genes. Hormones. 2008;7:17–23. doi: 10.14310/horm.2002.1111033. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg HM, Kinney B, Mattison J, Wright C, Hauck S, Coschigano K, Kopchick JJ. Growth hormone and aging. J Amer Aging Assoc. 2000;23:219–225. doi: 10.1007/s11357-000-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived Ames dwarf mice are resistant to chemical stressors. J Gerontol A Biol Sci Med Sci. 2009;64:819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg KE, Brown-Borg HM, Bartke A. Assessment of the primary adrenal cortical and pancreatic hormone basal levels in relation to plasma glucose and age in the unstressed Ames dwarf mouse. Proc Soc Exp Biol Med. 1995;210:126–133. doi: 10.3181/00379727-210-43931. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breese CR, Ingram RL, Sonntag WE. Influence of age and long-term dietary restriction on plasma insulin-like growth factor-1 (IGF-1), IGF-1 gene expression, and IGF-1 binding proteins. J Gerontol. 1991;46:B180–187. doi: 10.1093/geronj/46.5.b180. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. Hormonal regulation of longevity in mammals. Ageing Res Rev. 2007;6:28–45. doi: 10.1016/j.arr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM. Hormonal control of aging in rodents: the somatotropic axis. Mol Cell Endocrinol. 2009;299:64–71. doi: 10.1016/j.mce.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Bartke A. GH and IGF1: Roles in energy metabolism of long-living GH mutant mice. J Gerontol A Biol Sci Med Sci. 2012;67:652–660. doi: 10.1093/gerona/gls086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11:41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Johnson WT, Rakoczy S. Expression of oxidative phosphorylation components in mitochondria of long-living Ames dwarf mice. AGE. 2011;34:43–57. doi: 10.1007/s11357-011-9212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Johnson WT, Rakoczy SG, Kennedy MA, Romanick MA. Mitochondrial oxidant production and oxidative damage in Ames dwarf mice. J Am Aging Assoc. 2001;24:85–96. doi: 10.1007/s11357-001-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp Gerontol. 2000;35:199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Growth hormone administration to long-living dwarf mice alters multiple components of the antioxidative defense system. Mech Ageing Dev. 2003;124:1013–1024. doi: 10.1016/j.mad.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Glutathione metabolism in long-living Ames dwarf mice. Exp Gerontol. 2005;40:115–120. doi: 10.1016/j.exger.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin-like growth factor-1 on hepatocyte antioxidative enzymes. Exp Biol Med (Maywood) 2002;227:94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Sharma S, Bartke A. Long-living growth hormone receptor knock out mice: potential mechanisms of altered stress resistance. Exp Gerontol. 2009;44:10–19. doi: 10.1016/j.exger.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- Buyse J, Decuypere E, Darras VM, Vleurick LM, Kuhn ER, Veldhuis JD. Food deprivation and feeding of broiler chickens is associated with rapid and interdependent changes in the somatotrophic and thyrotrophic axes. Br Poult Sci. 2000;41:107–116. doi: 10.1080/00071660086493. [DOI] [PubMed] [Google Scholar]
- Capaldi RA, Halphen DG, Zhang YZ, Yanamura W. Complexity and tissue specificity of the mitochondrial respiratory chain. J Bioenerg Biomembr. 1988;20:291–311. doi: 10.1007/BF00769634. [DOI] [PubMed] [Google Scholar]
- Caro P, Gomez J, Lopez-Torres M, Sanchez I, Naudi A, Portero-Otin M, Pamplona R, Barja G. Effect of every other day feeding on mitochondrial free radical production and oxidative stress in mouse liver. Rejuvenation Res. 2008;11:621–629. doi: 10.1089/rej.2008.0704. [DOI] [PubMed] [Google Scholar]
- Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, Donati A, Bergamini E, Poli G, Chiarpotto E. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Rad Biol Med. 2010;48:47–54. doi: 10.1016/j.freeradbiomed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Cerqueira FM, Kowaltowski AJ. Commonly adopted caloric restriction protocols often involve malnutrition. Ageing Res Rev. 2010;7:552–560. doi: 10.1016/j.arr.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Chen LH, Hu N, Snyder DL. Effects of age and dietary restriction on liver glutathione transferase activities in Lobund-Wistar rats. Arch Gerontol Geriatics. 1994;18:191–205. doi: 10.1016/0167-4943(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Chen Q, Moghaddas S, Hoppel CL, Lefnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- Chiarpotto E, Castello I, Leonarduzzi G, Biasi F, Poli G. Role of 4-hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol Nutr Food Res. 2005;49:1044–49. doi: 10.1002/mnfr.200500090. [DOI] [PubMed] [Google Scholar]
- Choksi KB, Roberts LJ, 2nd, DeFord JH, Rabek JP, Papaconstantinou J. Lower levels of F2-isoprostanes in serum and livers of long-lived Ames dwarf mice. Biochem Biophys Res Commun. 2007;364:761–764. doi: 10.1016/j.bbrc.2007.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MP, Hu X, Grossmann ME, Juneja SC, Dogan S, Grande JP, Maihle NJ. Prevention of mammary tumorigenesis by intermittent caloric restriction: does caloric intake during refeeding modulate the response? Exp Biol Med (Maywood) 2007;232:70–80. [PubMed] [Google Scholar]
- Cook RJ, Wagner C. Glycine N-methyltransferase is a folate binding protein of rat liver cytosol. Proc Natl Acad Sci U S A. 1984;81:3631–3634. doi: 10.1073/pnas.81.12.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperstein SJ, Lazorow A. A microspectrophotometric method for the determination of cytochrome oxidase. J Biol Chem. 1951;189:665–670. [PubMed] [Google Scholar]
- Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Geron A Biol Sci Med Sci. 2005;60:1494–509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446–53. doi: 10.1210/en.2002-0113. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Mattson MP. Brain-derived neurotrophic factor mediates an excitoprotective effect of dietary restriction in mice. J Neurochem. 2001;76:619–26. doi: 10.1046/j.1471-4159.2001.00071.x. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC. Dietary restriction reduces insulin-like growth factor 1 levels, which modulates apoptosis, cell proliferation and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- Ekmay RD, de Beer M, Rosebrough RW, Richards MP, McMurtry JP, Coon CN. The role of feeding regimens in regulation metabolism of sexually mature broiler breeders. Poult Sci. 2010;89:1171–1181. doi: 10.3382/ps.2009-00465. [DOI] [PubMed] [Google Scholar]
- Estey C, Seifert EL, Aguer C, Moffat C, Harper M-E. Calorie restriction in mice overexpressing UCP3: evidence that prior mitochondrial uncoupling alters response. Exp Gerontol. 2012;47:361–371. doi: 10.1016/j.exger.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuers RJ. The effects of dietary restriction on mitochondrial dysfunction in aging. Ann NY Acad Sci. 1998;854:192–201. doi: 10.1111/j.1749-6632.1998.tb09902.x. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Busuttil RA, Calder RB, Dolle MET, Diaz V, McMahan CA, Bartke A, Nelson J, Reddick R, Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech Ageing Dev. 2008;129:528–33. doi: 10.1016/j.mad.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi: 10.1016/0047-6374(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G. Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146:3713–33. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G, Lopez-Torres M. Effect of short-term caloric restriction on H2O2 production and oxidative DNA damage in rat liver mitochondria and location of the free radical source. J Bioenerg Biomemb. 2001;33:279–287. doi: 10.1023/a:1010603206190. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–94. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E Pennington CALERIE Team. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipkiss AR. On why decreasing protein synthesis can increase lifespan. Mech Ageing Dev. 2007;128:412–414. doi: 10.1016/j.mad.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Reynolds MA. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. In: Woodhead AD, Thompson KH, editors. Evolution of longevity in animals. Plenum Plress; New York: 1987. pp. 247–280. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari FW, Dunn SE, French JE, Barrett JC. Roles for insulin-like growth factor-1 in mediating the anti-carcinogenic effects of caloric restriction. J Nutr Health Aging. 1999;3:92–101. [PubMed] [Google Scholar]
- Kim JD, McCarter RJ, Yu BP. Influence of age, exercise, and dietary restriction on oxidative stress in rats. Aging. 1996;8:123–129. doi: 10.1007/BF03339566. [DOI] [PubMed] [Google Scholar]
- Kupfer SR, Underwood LE, Baxter RC, Clemmons DR. Enhancement of the anabolic effects of growth hormone and insulin-like growth factor-1 by use of both agents simultaneously. J Clin Invest. 1993;91:391–396. doi: 10.1172/JCI116212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov AV, Clark JF, Winkler K, Kunz WS. Increase of flux control of cytochrome c oxidase in copper-deficient mottled brindled mice. J Biol Chem. 1996;2271:283–288. doi: 10.1074/jbc.271.1.283. [DOI] [PubMed] [Google Scholar]
- Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30:3305–3316. doi: 10.1038/onc.2011.91. [DOI] [PubMed] [Google Scholar]
- Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010;30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Masoro EJ, Nelson JF, Strong R, McMahan CA, Richardson A. Genetic mouse models of extended lifespan. Exp Gerontol. 2003;38:1353–1364. doi: 10.1016/j.exger.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetics efficiency. Proc Natl Acad Sci. 2007;103:1768–1773. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J Biol Chem. 2009;284:22507–22511. doi: 10.1074/jbc.R109.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupberger J, Kreuzer KA, Baskaynak G, Peters UR, le Coutre P, Schmidt CA. Quantitative analysis of beta-actin, beta-2-microglobulin and porphobilinogen deaminase mRNA and their comparison as control transcripts for RT-PCR. Mol Cell Probes. 2002;16:25–30. doi: 10.1006/mcpr.2001.0392. [DOI] [PubMed] [Google Scholar]
- Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Wohlgemuth SD, Anton SD, Bernabei R, Carter CS, Leewenburgh C. Cellular mechanisms of cardioprotection by calorie restriction. Clin Geriatri Med. 2009;25:715–732. doi: 10.1016/j.cger.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790:1040–1048. doi: 10.1016/j.bbagen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Al-Regaiey KA, Bonkowski MS, Panici JA, Bartke A. Effect of every other day feeding diet on gene expression in normal and in long lived Ames dwarf mice. Exp Gerontol. 2005;40:491–497. doi: 10.1016/j.exger.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroTherapeutics. 2004;1:111–16. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. 2002;34:1340–54. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- Merry BJ. Oxidative stress and mitochondrial function with aging-the effects of calorie restriction. Aging Cell. 2004;3:7–12. doi: 10.1046/j.1474-9728.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stalh E, Bolouri MS, Ray HN, Sihag S, Kamal M, Patterson N, Lander ES, Mann M. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell. 2003;115:629–640. doi: 10.1016/s0092-8674(03)00926-7. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl Physiol Nutr Metab. 2007;32:874–83. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- Nir I, Harvey S, Nitsan Z, Pinchasov Y, Chadwick A. Effect of intermittent feeding on blood plasma growth hormone and prolactin in chickens of a heavy breed. Br Poult Sci. 1983;24:63–70. doi: 10.1080/00071668308416714. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Gomi T, Imamura T, Kobayashi M, Huh N. Rat liver 4S-benzo[a]pyrene-binding protein is distinct from glycine N-methyltransferase. Biochem Biophys Res Commun. 1997;233:300–304. doi: 10.1006/bbrc.1997.6444. [DOI] [PubMed] [Google Scholar]
- Olgun A, Akman S, Serdar AM, Kutluay T. Oxidative phosphorylation enzyme complexes in caloric restriction. Exp Gerontol. 2002;37:639–645. doi: 10.1016/s0531-5565(02)00009-8. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metabolism. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Association between life span extension by caloric restriction and thiol redox state in two different strains of mice. Free Rad Biol Med. 2011;51:225–233. doi: 10.1016/j.freeradbiomed.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov D, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free Rad Biol Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanick MA, Rakoczy SG, Brown-Borg HM. Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech Ageing Dev. 2004;125:269–281. doi: 10.1016/j.mad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Ruggeri BA, Klurfeld DM, Kritchevsky D, Furlanetto RW. Caloric restriction and 7,12-dimethylbenz(a)anthracene-induced mammary tumor growth in rats: alterations in circulating insulin, insulin-like growth factors I and II, and epidermal growth factor. Cancer Res. 1989;49:4130–4134. [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metab. 2005;289:E23–29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Sanz A, Bartke A, Barja G. Long-lived Ames dwarf mice: oxidative damage to mitochondrial DNA in heart and brain. J Amer Aging Assoc. 2002;25:119–122. doi: 10.1007/s11357-002-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulphide/glutathione couple. Free Rad Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Sell C. Caloric restriction and insulin-like growth factors in aging and cancer. Horm Metab Res. 2003;35:705–711. doi: 10.1055/s-2004-814156. [DOI] [PubMed] [Google Scholar]
- Sharma S, Darland D, Lei S, Rakoczy S, Brown-Borg HM. NMDA and kainate receptor expression, long-term potentiation and neurogenesis in long-lived Ames dwarf mice. AGE. 2011;34:609–620. doi: 10.1007/s11357-011-9253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Haselton J, Rakoczy SG, Branshaw S, Brown-Borg HM. Spatial memory is enhanced in long-living Ames dwarf mice and maintained following kainic acid induced neuronal loss. Mech Ageing Dev. 2010a;131:422–435. doi: 10.1016/j.mad.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Rakoczy SG, Dahlheimer K, Brown-Borg HM. The hippocampus of Ames dwarf mice is resistant to kainic acid-induced oxidative stress. Exp Geront. 2010b;45:936–949. doi: 10.1016/j.exger.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa I, Jigami Y, Tsuchiya T, Otani H, Komatsu T, Chiba T, Yamaza H. Life span extension by reduction of the growth-hormone–insulin-like growth factor-1 axis: relation to caloric restriction. FAEB J. 2003;17:1108–1109. doi: 10.1096/fj.02-0819fje. [DOI] [PubMed] [Google Scholar]
- Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, Kaur M, Kaur G. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. AGE. 2012;34:917–933. doi: 10.1007/s11357-011-9289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DK, Clemmons DR, Underwood LE. Treatment of obese, diet-restricted subjects with growth hormone for 11 weeks: effects of anabolism, lipolysis and body composition. J Clin Endocrinol Metab. 1988;67:54–61. doi: 10.1210/jcem-67-1-54. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Short KR, Schimke J, Barazzoni R, Nair KS. Effects of caloric restriction on mitochondrial function and gene transcripts in rat muscle. Am J Physiol Endocrinol Metab. 2002;283:E38–E53. doi: 10.1152/ajpendo.00387.2001. [DOI] [PubMed] [Google Scholar]
- Sun LY, Bokov AF, Richardson A, Miller RA. Hepatic response to oxidative injury in long-lived Ames dwarf mice. FASEB J. 2011;25:398–408. doi: 10.1096/fj.10-164376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara EB, Navarete FD, Kowaltowski AJ. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med. 2009;46:1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Tatsumi S, Ito M, Asaba Y, Tsutsumi K, Ikeda K. Life-long caloric restriction reveals biphasic and dimorphic effects on bone metabolism in rodents. Endocrinology. 2008;149:634–641. doi: 10.1210/en.2007-1089. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994a;15:80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- Thissen JP, Pucilowska JB, Underwood LE. Differential regulation of insulin-like growth factor I (IGF-1) and IGF binding protein-1 messenger ribonucleic acids by amino acid availability and growth hormone in rat hepatocyte primary culture. Endocrinology. 1994b;134:1570–1576. doi: 10.1210/endo.134.3.7509741. [DOI] [PubMed] [Google Scholar]
- Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Dhahbi JM, Cui X, Mote PL, Bartke A, Spindler SR. Additive regulation of hepatic gene expression by dwarfism and caloric restriction. Physiol Genomics. 2004;17:307–315. doi: 10.1152/physiolgenomics.00039.2004. [DOI] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Methionine flux to transsulfuration is enhanced in the long living Ames dwarf mouse. Mech Ageing Dev. 2006 doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Altered methionine metabolism in long living Ames dwarf mice. Exp Gerontol. 2003;38:491–498. doi: 10.1016/s0531-5565(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Varady KA, Roohk DJ, Hellerstein MK. Dose effects of modified alternate-day fasting regimens on in vivo cell proliferation and plasma insulin-like growth factor-1 in mice. J Appl Physiol. 2007;103:547–551. doi: 10.1152/japplphysiol.00209.2007. [DOI] [PubMed] [Google Scholar]
- Wagner C, Briggs WT, Cook RJ. Inhibition of glycine N-methyltransferase activity by folate derivative: implications for regulation of methyl group metabolism. Biochem Biophys Res Commun. 1985;127:756–752. doi: 10.1016/s0006-291x(85)80006-1. [DOI] [PubMed] [Google Scholar]
- Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003;17:1133–1134. doi: 10.1096/fj.02-0996fje. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: CC Thomas; 1988. [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and life time energy intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301:H1205–H1219. doi: 10.1152/ajpheart.00685.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:803–9. [PubMed] [Google Scholar]
- Zha Y, Taguchi T, Nazneen A, Shimokawa I, Higami Y, Razzaque MS. Genetic suppression of GH-IGF-1 activity, combined with lifelong caloric restriction, prevents age-related renal damage and prolongs the life span in rats. Am J Nephrol. 2008;28:755–764. doi: 10.1159/000128607. [DOI] [PubMed] [Google Scholar]
- Zhu M, de Cabo R, Lane MA, Ingram DK. Caloric restriction modulates early events in insulin signaling in liver and skeletal muscle of rat. Ann NY Acad Sci. 2004;1019:448–452. doi: 10.1196/annals.1297.082. [DOI] [PubMed] [Google Scholar]