Abstract
Socioeconomic disadvantage confers risk for ill health. Historically, the pathways by which socioeconomic disadvantage may affect health have been viewed from epidemiological perspectives emphasizing environmental, behavioral, and biopsychosocial risk factors. Such perspectives, however, have yet to integrate findings from emerging neuroscience studies demonstrating that indicators of socioeconomic disadvantage relate to patterns of brain morphology and functionality that have been associated with aspects of mental, physical, and cognitive health over the lifecourse. This commentary considers findings from one such study appearing in the current issue of Psychosomatic Medicine. It reports that an area-level indicator of socioeconomic disadvantage relates to cortical morphology in brain regions important for language, executive control, and other cognitive and behavioral functions—possibly via a systemic inflammatory pathway. These findings are put into context by discussing broader questions and challenges that need to be addressed in order for neuroscience approaches to (i) become better integrated with existing epidemiological perspectives and (ii) more fully advance our understanding of the pathways by which socioeconomic disadvantage becomes embodied by the brain in relation to health.
Keywords: health disparities, neuroimaging, neuroscience, socioeconomic status
Nearly four decades ago Patricia R. Barchas, a pioneer in biobehavioral medicine and social neuroscience, observed that “There is empirical evidence that position in a social structure alters brain events in a patterned way, mediated by psychological variables.” (Patricia R. Barchas, 1976) (1).”
Since the time of Barchas’ early work, we have learned much about the psychological variables and pathways by which our ‘position in a social structure’ – or our socioeconomic position (SEP) more precisely – may also relate to or alter our health and longevity (2–4). In a separate development over this same timespan, the growth of neuroscience and the surge in neuroimaging research have led to an ever-deepening understanding of the brain and its complex relationship to factors that affect and are affected by health and disease over the lifecourse (5, 6). However, the integration of neuroscience and neuroimaging research with the study of the socioeconomic health disparities has been slow to develop. Consequently, most epidemiological and conceptual models of the factors and pathways linking SEP to health have generally omitted a formal consideration of the role of the brain.
In this editorial, some of the implications of this historical omission are considered in the context of findings from a new neuroimaging study of socioeconomic disadvantage by Krishnadas and colleagues, reported in this issue of Psychosomatic Medicine (7). Specifically, we consider how key findings from this study (and others with a similar focus) might add to our understanding of socioeconomic health disparities by raising these questions:
How can neuroscience and neuroimaging approaches contribute to the understanding of the pathways linking SEP to health?
Which barriers interfere with the integration of neuroscience approaches in general and neuroimaging measures in particular into research on socioeconomic health disparities?
What are some of the conceptual challenges engendered by conducting neuroscience research on the role of the brain in the study of socioeconomic health disparities?
After considering these questions, we offer suggestions for ways to better integrate neuroscience and neuroimaging approaches into the study of socioeconomic health disparities.
(1) Neuroscience and the pathways linking SEP to health
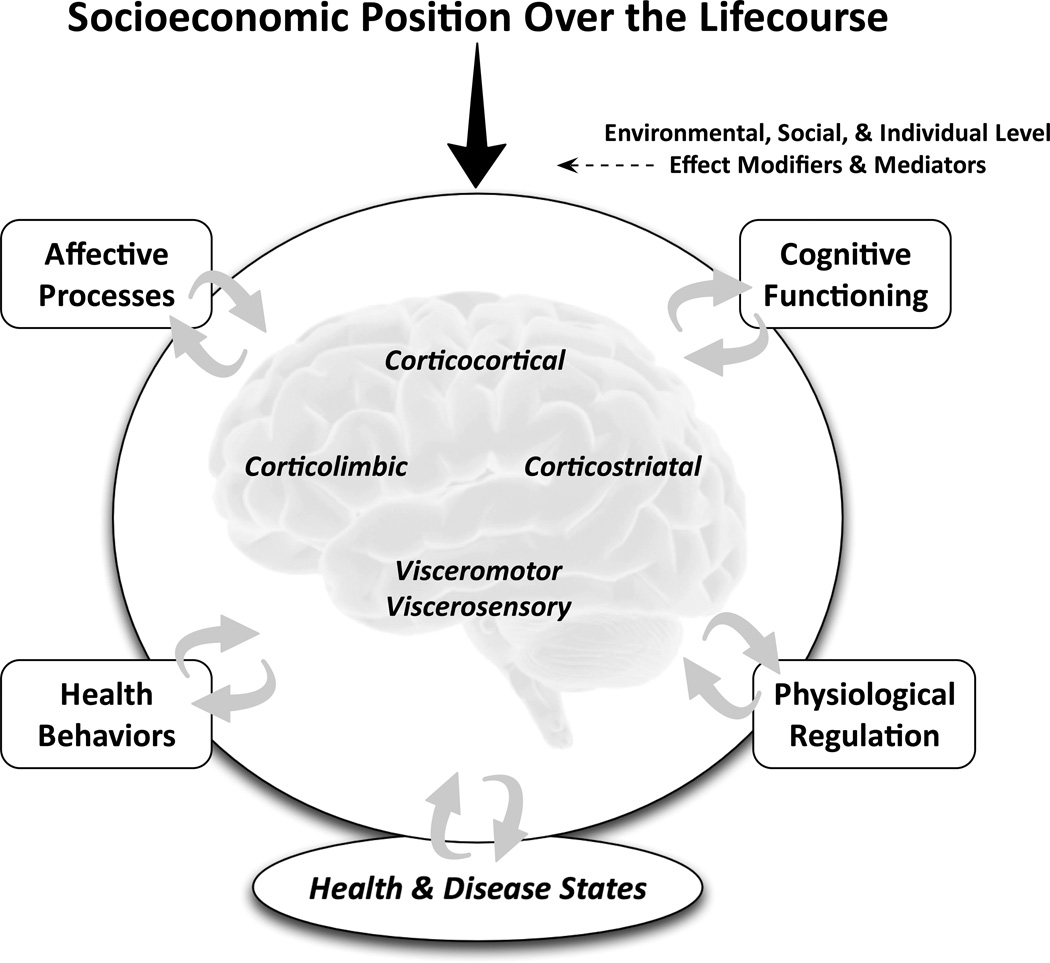
To provide an initial context, it is helpful to consider some common features of conceptual models guiding epidemiological research on the pathways linking SEP to health (e.g., 3, 4, 8, 9). Across many of these models, it is proposed that educational, occupational, financial and other dimensions of socioeconomic disadvantage – whether measured at the level of the person, family, household, neighborhood, etc. – can adversely influence aspects of health over the lifecourse through pathways that encompass protective or damaging environmental exposures, social, psychological, and cognitive processes, and health behaviors. In turn, the interrelated factors encompassed by such pathways are thought to affect biological aspects of physiological, cellular, and gene regulatory mechanisms that proximally relate to the development and progression of health-related endpoints that track a socioeconomic gradient. For example, it has been proposed that psychological factors related to the regulation of positive and negative emotions and moods are encompassed by a pathway by which socioeconomic disadvantage influences biological and behavioral factors that themselves proximally influence risk for the development of chronic illnesses, such as atherosclerotic coronary heart disease (e.g., 3, 10). As illustrated in Figure 1, we propose that a neuroscience perspective in general, and neuroimaging research approaches specifically, can build upon such conceptualizations. Concretely, the first step toward building upon such conceptualizations would be to consider the particular brain regions and networks that would plausibly relate to the factors and processes encompassed by the pathways emphasized in such models of socioeconomic health disparities.
Fig. 1.
Schematic illustration conveying the notion that aspects of socioeconomic position (SEP) over the lifecourse can come to affect health and disease states via neurobiological pathways. Affective, cognitive, behavioral, and physiological factors linking different dimensions of SEP to health and disease states can be viewed as bidirectionally related to multiple brain networks, amenable to study by neuroimaging approaches. The links between dimensions of SEP and the function and structure of different brain networks can be further conceptualized as subject to effect modification and mediation by environmental, social, and individual level factors that can influence downstream pathways to health and disease.
To revisit the example above, psychological factors encompassing aspects of emotion and mood regulation and physiological factors considered as biological mediators or ‘biomediators’ of disease risk (e.g., 11) can be conceptualized as relating to the structure and function of particular brain regions; namely, networked cortical and limbic regions that are involved in (i) detecting and appraising salient stimuli in the environment that signal safety or threat, (ii) generating and regulating emotional reactions, mood states and behavioral or cognitive coping strategies, and (iii) controlling peripheral neuroendocrine, autonomic, and immune functions (12). In addition, disadvantageous health behaviors may be another pathway connecting socioeconomic disadvantage to health (e.g., smoking, alcohol overconsumption, etc.) and these health behaviors may also be related to the structure and function of cortical and striatal networks involved in self-regulatory decision-making, habit formation and impulse control, and sensitivity to rewarding stimuli in the environment (10, 13). Finally, there is increasing human evidence complementing research in animal models that aspects of peripheral neuroendocrine, autonomic, and immune functions that have been the focus of much research on socioeconomic and social health disparities (e.g., 14, 15) are bi-directionally related to brain function and structure through efferent (or top-down) and afferent (or bottom-up) mechanisms as mediated by visceromotor and viscerosensory networks encompassing brainstem and forebrain cell groups (16–18).
What then is the potential added value of a neuroscience approach to the study of socioeconomic health disparities? As noted elsewhere (19–22), it allows us to better specify and refine the particular pathways connecting distal to proximal factors affecting different health-related outcomes. For example, with a neuroscience approach we can ask: Are disadvantageous health behaviors (e.g., smoking) that are associated with socioeconomic disadvantage also associated with the function and structure of networked cortical and striatal brain regions? If so, might such associations reflect top-down processes wherein such behaviors are emergent endpoints mediated by corticostriatal regions? Also, might such associations partly reflect bottom-up processes wherein disadvantageous health behaviors target and affect the structure and function of corticostriatal regions (e.g. through systemic inflammatory states affecting the central nervous system)? And, could such kinds of association in part account for observed links between SEP, health behaviors, and health-related outcomes? In other words, where and how do particular brain networks fit into the widely studied pathways linking SEP to health? In addition to enabling the above lines of questioning, a neuroscience approach in general and neuroimaging methodologies in particular could arguably allow for a greater refinement of lifecourse models and studies of the emergence, persistence, and change in socioeconomic health disparities during early childhood development and later aging processes (e.g., 11, 23), while also potentially providing novel neural measures of risk and resilience that may be clinically useful for detection and intervention (22).
The study by Krishnadas and colleagues in this issue of Psychosomatic Medicine offers an example of a neuroscience approach to the study of health disparities, insofar as it combines neuroimaging techniques with the assessment and mediation modeling of socioeconomic factors and peripheral physiological correlates of disease risk. At issue in this study is the question of whether an area-level indicator of socioeconomic disadvantage related to aspects of brain morphology – namely, cortical volume, thickness, and surface area – in networks that support several cognitive and behavioral functions. These functions primarily include those related to language and executive control processes that are known to relate to SEP. To address this question, the authors recruited men between the ages of 35 and 64 from the least and most deprived regions of Glasgow, Scotland as part of a larger cross-sectional study on determinants of ill health, the PSoBiD study (24). Noteworthy is the appreciable 29-year life expectancy difference between men residing in the least and most deprived areas of Glasgow (25), reflecting substantial socioeconomic health disparities in this sample. The authors report that area-level disadvantage (or deprivation) is associated with smaller cortical surface areas in the parietal and fusiform cortices, which are brain regions important for executive functions, such as selective attention and working memory. The authors also report that area-level disadvantage was associated with reduced cortical thickness in a brain region important for language processing, Wernicke’s area in the left hemisphere (as well as its right hemisphere homologue). Finally, cross-sectional mediation analyses suggest that a composite variable reflecting systemic inflammation explains part of the association of area-level disadvantage with cortical thickness in Wernicke’s area. These findings broadly agree with those of studies employing neuropsychological measures, electrophysiological recording methods, and neuroimaging approaches documenting SEP-related cognitive differences and differences in brain regions supporting language and executive functions (20). The findings also add to an emerging body of studies that has begun to establish that cortical morphology relates to indicators of SEP both in childhood and adulthood (19, 26), and that the relationship between disadvantage and morphology may be linked to factors associated with systematic inflammation (27). However, the findings and approach of Krishnadas and colleagues raise several issues and questions that are central to address in neuroscience studies of socioeconomic health disparities. The first issue we consider pertains to the barriers to incorporating neuroscience research into the study of health disparities. The next issue pertains to the conceptual and inferential implications of these barriers.
(2) Barriers to neuroscience research on socioeconomic health disparities
One barrier to integrating neuroscience approaches into socioeconomic health disparities research is the financial cost of its methodologies that are optimal for human research, particularly structural and functional neuroimaging. Another barrier pertains to the limited (or lack of) availability of neuroimaging facilities in geographical regions with entrenched socioeconomic health disparities - but without large, academic institutions and medical centers where such facilities are typically concentrated. The study by Krishnadas and colleagues, in part, deals with at least one of these barriers by using an extreme groups and relatively cost-effective approach to selecting a small sample of individuals from the least and most deprived regions in Glasgow that were enrolled in a larger, population-based study. This approach benefits from the rigorous characterization of the larger sample done as part of epidemiological assessment strategies. Hence, Krishnadas and colleagues illustrate how neuroscience research can be ‘embedded’ within larger-scale epidemiological study frameworks. However, an extreme groups approach relying upon small samples (which is often the case in neuroimaging research) raises several potential concerns – especially in this context. In a small sample, it is difficult if not impossible to dissociate area-level disadvantage from individual-level SEP or even different dimensions of SEP across different levels of analysis. Moreover, with small samples comparing extreme groups, there is a salient barrier to explicating the particular environmental exposures, social, psychological, and cognitive processes, and health behaviors linking SEP indicators to particular health outcomes vis-à-vis the brain. This barrier extends to the challenge of identifying and studying the potential impact of effect modifiers (such as genetic factors, sex, ethnicity, and environmental exposures). Finally, an extreme group approach makes it difficult to examine linear or non-linear associations between different indicator variables and particular neuroimaging measures across individuals from a diverse socioeconomic spectrum.
The study of small samples and extreme groups in this area of research may also influence the significance, stability, reliability, and generalizability of underlying or true effects of interest. For example, small samples can affect the statistical power to detect significant effects in neuroimaging research, particularly when between-individual effects are of interest and when many statistical tests are conducted that require correction for multiple comparisons across the brain. This issue can result in effect sizes that are typically considered moderate-to-large (such as a Cohen’s d of 0.75), but that are not statistically significant. Statistically significant effect sizes may also be inflated and unlikely to reflect the true magnitude of underlying effects (28, 29). Such issues also extend to mediation analyses, which require larger sample sizes that are sufficiently powered to detect mediated effects (30) and unlikely to result in model mis-specification (31). Thus, small samples in neuroimaging research on socioeconomic health disparities may enable cost-effective preliminary and foundational work to be done. However, for future research in this area, we would caution against a reliance on small samples and extreme group approaches while also emphasizing a need for replication when such approaches are employed.
The work by Krishnadas and colleagues is based on a cross-sectional design that leaves unclear the directions of association and temporal ordering between study variables—raising a justification for longitudinal work. Notwithstanding such justification, however, another notable barrier in this area of study is the difficulty of conducting longitudinal brain imaging research, especially in young and diverse samples of people among whom developmental and aging processes are at play. Within a broader context, it has become increasingly clear that early life SEP and developmental factors are important for long-term health (4, 10), and socioeconomic health disparities are understood to relate to complex and interacting factors that operate throughout the lifespan (19). Moreover, the brain exhibits a remarkable degree of plasticity throughout life as a function of social and other factors of relevance for disparities research (32, 33). Consequently, a complete test of a conceptual model of socioeconomic health disparities requires longitudinal studies informed by developmental and lifecourse perspectives. Although this is not a unique issue for neuroscience approaches, there are particular challenges that arise when employing a longitudinal neuroimaging approach. These include the limited number of straightforward, easily interpretable structural and functional neuroimaging measures that are reliable and measured equivalently across ages, unresolved methodological questions about analyzing longitudinally collected images, and uncertainties in how to interpret age-related differences or changes in brain activity and morphology (34–37). Moreover, rapidly changing technologies for image acquisition and analysis make it difficult to conduct longitudinal neuroimaging studies that benefit from the state-of-the-art methodologies and conceptual frameworks, as technology employed during the first wave of data collection may be outdated by the time analyses are conducted after the final wave of data collection is complete. Accordingly, there are multiple hurdles to effectively implementing developmental and lifecourse perspectives with longitudinal neuroimaging to inform our understanding of emergent health disparities across time.
(3) Conceptual challenges
The first clear-cut conceptual challenge we discuss relates to the fundamental relevance of measures of brain structure and function for specific health outcomes, as well as the clarity of their mapping onto intermediary factors and pathways of interest. In Krishanadas et al, area-level disadvantage was associated with differences in cortical surface areas in the parietal and fusiform cortices and cortical thickness in Wernicke’s area, which the authors argue are important for executive functions and language, respectively. However, the mapping of these distinct components of brain structure to cognitive performance outcomes is not entirely clear, as it is not established that these morphological differences reliably predict neurocognitive performance or mediate the effects of SEP on such performance. In addition, what is especially needed at the outset of conducting the kind of neuroimaging study illustrated by Krishanadas et al. is a theoretical model or conceptual rationale that articulates how specific morphological (or functional) brain measures map onto more complex factors encompassed by the pathways connecting indicators of SEP to disease-related outcomes. There is thus a need for researchers to develop clearer conceptual models from which to derive a priori hypotheses about how specific morphological or functional brain measures will relate to SEP, as well as to intermediary pathway factors and downstream health outcomes.
An additional conceptual challenge in neuroscientific studies of socioeconomic health disparities is the same encountered in any correlational study of SEP that relies upon mediation analysis; namely, there are limitations to what can be inferred concerning the causal direction of reported associations and the mechanisms underlying these associations. Hence: do differences in SEP lead to differences in brain structure or function? Do differences in brain structure or function lead to differences in SEP? Is some unmeasured ‘third variable’ responsible for observed and spurious associations through indirect selection processes? Such weighty questions are unlikely to ever be resolved by cross-sectional or even longitudinal neuroimaging studies. However, there may be some utility to future study designs employing a combination of interventions, quasi-experiments, and observational methods that use mediation and multi-level modeling. Even so, mediation analyses with respect to SEP, such as those reported by Krishnadas et al., can still present conceptual challenges because putative mediators are typically correlated with one another and others that remain unmeasured (38). These issues render it difficult to meet the assumption that no unmeasured candidate mediators have been omitted from a given model (39), raising questions about the validity, specificity, and reliability of mediation findings when only a few mediators are examined. As one example, strong candidate mediators of SEP-related differences in cortical morphology in language and executive function areas are social and environmental exposures in childhood and adolescence, such as cognitive stimulation in the home, differences in parenting practices, and family stress (20, 23). Thus, it is not clear if the mediation results reported by Krishnadas would change if such potentially correlated variables were measured and included in their analyses. In sum, the use of a neuroscience approach in disparities research, particularly with respect to mediation analyses, would benefit from multidimensional measurement of both socioeconomic indicators across levels of analysis and measurement of intermediary factors across development and the lifecourse.
Considerations for future neuroscience research on socioeconomic health disparities
This editorial considered questions and challenges that need to be addressed in order for neuroscientific studies, such as those of Krishnadas and colleagues, to add to our understanding of how socioeconomic disadvantage relates to health over the lifespan. We do not suggest that what we need now is a new subfield of ‘neurodisparities’ research. To the contrary, we suggest that the next steps to move neuroscience research on socioeconomic health disparities forward include the following: First, more elaborate conceptual models are needed that can be used to derive a priori and empirically testable predictions about how socioeconomic factors in health disparities are related to measures of brain function and structure. Such models need to specifically incorporate multilevel and distal indicators of SEP to more proximal factors and processes associated with particular health outcomes. This could be accomplished in several ways: by modifying, for example, existing conceptual models of disparities driving particular studies to include specific brain networks and neurobiological components along the pathways hypothesized to link SEP to health. Second, such conceptual advancement should also incorporate the notion that particular brain regions and networks can be viewed as both mediators and targets of health-related processes and risk factors for disease via top-down and bottom-up influences, respectively. Third, future work on the neuroscience of socioeconomic health disparities should adopt neurodevelopmental and lifecourse perspectives. Such perspectives are needed in order to fully address questions regarding when SEP-health relationships emerge in life and how such relationships affect later life outcomes. Fourth, the functional implications of selected neurobiological and neuroimaging measures need to be interpreted carefully, particularly with respect to how they map onto or relate to pathways, processes, outcomes, and effect modifiers associated with health endpoints patterned by SEP. Fifth, it is important to capitalize on and exploit existing large-scale epidemiological studies and populations where there is dense assessment of SEP, health, and intermediary factors – especially where recruitment of larger samples of individuals across a broad range of SEP levels for neuroimaging research is feasible. Finally, a neuroscience approach and neuroimaging methodologies may inform interventions and policies designed to address socioeconomic health disparities. Hence, the structure and function of different brain networks related to environmental exposures, social, psychological, and cognitive processes, and health behaviors may themselves become novel foci of risk reduction approaches that could range from nutritional approaches to curriculum development to behavioral interventions (20). In this regard, neuroscientific integration with socioeconomic health disparities research could plausibly advance our understanding of the specificity and generality of intervention mechanisms and add to the evidence base supporting current approaches.
In sum, we propose that current models of socioeconomic health disparities could in fact be informed by considering the potential role of the brain to more fully explain and clarify the intermediary pathways linking and modifying the associations of SEP and health. The work by Krishnadas and colleagues illustrates the promise of such a consideration, but much more needs to be done.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01 HL089850 and T32 MH018269. We thank Drs. Anna Marsland and Willem Kop for their constructive comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors declare no conflict of interest.
References
- 1.Barchas PR. Physiological sociology: interface of sociological and biological processes. Ann Rev Sociol. 1976;2:299–333. [Google Scholar]
- 2.Adler NE, Stewart J. In: The Biology of Disadvantage: Socioeconomic Status and Health. Adler NE, Stewart J, editors. New York: The New York Academy of Sciences; 2010. [DOI] [PubMed] [Google Scholar]
- 3.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Ann Rev Psychol. 2011;62:501–530. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen E, Miller GE. Socioeconomic status and health: mediating and moderating factors. Ann Rev Clin Psychol. 2013;9:723–749. doi: 10.1146/annurev-clinpsy-050212-185634. [DOI] [PubMed] [Google Scholar]
- 5.Lane RD, Waldstein SR, Chesney MA, Jennings JR, Lovallo WR, Kozel PJ, Rose RM, Drossman DA, Schneiderman N, Thayer JF, Cameron OG. The rebirth of neuroscience in psychosomatic medicine, part I: historical context, methods and relevant basic science. Psychosom Med. 2009;71:117–134. doi: 10.1097/PSY.0b013e31819783be. [DOI] [PubMed] [Google Scholar]
- 6.Lane RD, Waldstein SR, Critchley HD, Derbyshire SW, Drossman DA, Wager TD, Schneiderman N, Chesney MA, Jennings JR, Lovallo WR, Rose RM, Thayer JF, Cameron OG. The rebirth of neuroscience in psychosomatic medicine, part II: clinical applications and implications for research. Psychosom Med. 2009;71:135–151. doi: 10.1097/PSY.0b013e318198a11f. [DOI] [PubMed] [Google Scholar]
- 7.Krishnadas R, McLean J, Batty DG, Burns H, Deans KA, Ford I, McConnachie A, McGinty A, McLean JS, Millar K, Packard CJ, Sattar N, Shiels P, Tannahill C, Velupillai YN, Cavanagh J. Socio-economic deprivation and cortical morphology: Psychological, social and biological determinants of ill health study. Psychosom Med. 2013 doi: 10.1097/PSY.0b013e3182a151a7. [DOI] [PubMed] [Google Scholar]
- 8.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 9.Evans G, Kantrowitz E. Socioeconomic status and health: The potential role of environmental risk exposure. Annu Rev Public Health. 2002;23:303–331. doi: 10.1146/annurev.publhealth.23.112001.112349. [DOI] [PubMed] [Google Scholar]
- 10.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen E, Miller GE, Lachman ME, Gruenewald TL, Seeman TE. Protective factors for adults from low-childhood socioeconomic circumstances: the benefits of shift-and-persist for allostatic load. Psychosom Med. 2012;74:178–186. doi: 10.1097/PSY.0b013e31824206fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianaros PJ, Manuck SB, Sheu LK, Kuan DC, Votruba-Drzal E, Craig AE, Hariri AR. Parental education predicts corticostriatal functionality in adulthood. Cereb Cortex. 2011;21:896–910. doi: 10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallo LC, Fortmann AL, de Los Monteros KE, Mills PJ, Barrett-Connor E, Roesch SC, Matthews KA. Individual and neighborhood socioeconomic status and inflammation in Mexican American women: what is the role of obesity? Psychosom Med. 2012;74:535–542. doi: 10.1097/PSY.0b013e31824f5f6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King KE, Morenoff JD, House JS. Neighborhood context and social disparities in cumulative biological risk factors. Psychosom Med. 2011;73:572–579. doi: 10.1097/PSY.0b013e318227b062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slavich GM, Cole SW. The emerging field of social genomics. Clin Psychol Sci. 2013 doi: 10.1177/2167702613478594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- 19.Gianaros PJ, Manuck SB. Neurobiological pathways linking socioeconomic position and health. Psychosom Med. 2010;72:450–461. doi: 10.1097/PSY.0b013e3181e1a23c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Reviews Neuroscience. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raizada RD, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front Hum Neurosci. 2010;4:3. doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 23.Loucks EB, Almeida ND, Taylor SE, Matthews KA. Childhood family psychosocial environment and coronary heart disease risk. Psychosom Med. 2011;73:563–571. doi: 10.1097/PSY.0b013e318228c820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velupillai YN, Packard CJ, Batty GD, Bezlyak V, Burns H, Cavanagh J, Deans K, Ford I, McGinty A, Millar K, Sattar N, Shiels P, Tannahill C. Psychological, social and biological determinants of ill health (pSoBid): study protocol of a population-based study. BMC Public Health. 2008;8:126. doi: 10.1186/1471-2458-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanlon P, Walsh D, Whyte B. Let Glasgow flourish: a comprehensive report on health and its determinants in Glasgow and west central Scotland: Glasgow Centre for Population Health. 2006 [Google Scholar]
- 26.Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLoS ONE. 2011;6:e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex. 2013 doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 29.Yarkoni T. Big correlations in little studies: Inflated fMRI correlations reflect low statistical power— Commentary on Vul et al. (2009) Perspect Psychol Sci. 2009;4:294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 30.Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preacher KJ, Rucker DD, MacCallum RC, Nicewander WA. Use of the extreme groups approach: a critical reexamination and new recommendations. Psychol Methods. 2005;10:178–192. doi: 10.1037/1082-989X.10.2.178. [DOI] [PubMed] [Google Scholar]
- 32.Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–695. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aubert-Broche B, Fonov VS, Garcia-Lorenzo D, Mouiha A, Guizard N, Coupe P, Eskildsen SF, Collins DL. A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood. Neuroimage. 2013;82C:393–402. doi: 10.1016/j.neuroimage.2013.05.065. [DOI] [PubMed] [Google Scholar]
- 35.Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Ann N Y Acad Sci. 2010;1191:133–155. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- 36.Luna B, Velanova K, Geier CF. Methodological approaches in developmental neuroimaging studies. Hum Brain Mapp. 2010;31:863–871. doi: 10.1002/hbm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poldrack RA. Interpreting developmental changes in neuroimaging signals. Hum Brain Mapp. 2010;31:872–878. doi: 10.1002/hbm.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans GW. The environment of childhood poverty. Am Psychol. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 39.MacKinnon DP. Introduction to statistical mediation analysis. Mahwah, NJ: Erlbaum; 2006. [Google Scholar]