Abstract
Background
F2-isoprostanes (F2-IsoP) are oxidant stress biomarkers that are higher in HIV-infected women than men. We explored whether the effect of hemoglobin (Hgb), serum iron, or anemia on F2-IsoP is different between HIV-infected women and men.
Methods
Plasma F2-IsoP were quantified by gas chromatography/mass spectrometry; clinical and laboratory data were collected at enrollment or from the medical record. Multivariable linear regression was used to assess associations between F2-IsoP and Hgb, anemia as a dichotomous variable, and serum iron with adjustment for age, sex, race, body-mass index, CD4+ lymphocyte count, self-reported current smoking status, and antiretroviral therapy.
Results
Compared to men, women had lower Hgb (median [IQR] 12.7 [11.8-13.9] vs. 14.9 [13.7-15.8] g/dL, P<0.001), lower iron levels (75 [47-97] vs. 90 [69-121] μg/dL, P=0.004), more anemia (29% vs. 10%, P<0.001), and higher levels of F2-IsoP (42 [32-62] vs. 36 [25-46] pg/mL, P<0.001). The relationship between iron and F2-IsoP differed significantly between men and women (interaction P=0.02). Men had a 21% (95% CI: 8%-36%) increase in F2-IsoP per interquartile increase in iron (P=0.001); while no relationship was seen among women (-4% [-17%-13%], P=0.65).
Conclusions
Although women have overall higher F2-IsoP than men, a relationship between circulating F2-IsoP and iron levels was observed in men but not in women with HIV infection. The association between female sex and higher F2-IsoP is not explained by iron or Hgb levels as the association persists when controlling for these factors. The role of iron in oxidant stress and sex-specific differences among HIV-infected individuals require further study.
Keywords: HIV, isoprostanes, iron, hemoglobin
Introduction
In the era of potent antiretroviral therapy (ART), human immunodeficiency virus (HIV) infection has become a treatable chronic disease. The most common causes of death among ART-treated persons are no longer directly related to acquired immunodeficiency syndrome (AIDS) but rather to co-morbid hepatic, cardiovascular, and pulmonary diseases, as well as non-AIDS malignancies.1 This is thought to be due in part to a state of increased inflammation and immune activation related to factors including: ongoing HIV replication; microbial translocation via impaired gut-associated lymphoid tissue; co-infections; and chronic exposure to antiretroviral medications.2 Chronic inflammation is also related to oxidant stress,3 cellular damage mediated by reactive oxygen species (ROS), and oxidant stress has long been felt to play a role in HIV pathogenesis.4,5
The F2-isoprostanes (F2-IsoP) are prostaglandin-like compounds produced by ROS-induced peroxidation of cell membrane lipids, and are accurate and reliable markers of in vivo oxidant stress in humans.6,7,8 Among HIV-infected persons, F2-IsoP levels have been found to be higher in persons with suppressed plasma HIV-1 RNA on ART compared to untreated individuals,9 and among those on treatment, higher F2-IsoP levels have been found in those with treatment-associated lactic acidosis and lipoatrophy.4
Our group previously examined the association between several clinical factors and plasma F2-IsoP levels in a cross-sectional study of HIV-infected adults,10 and in a smaller longitudinal analysis of the association between clinical factors and urine F2-IsoP.11 Both studies found that female sex was independently associated with higher F2-IsoP levels, an association also seen in HIV-uninfected populations.12,13 Determining factors that explain higher F2-IsoP levels in HIV-infected women compared to men could lead to a better understanding of unique risks for HIV-infected women and provide guidance in management of these patients. Glesby, et al. examined plasma F2-IsoP in a cross-sectional analysis within the Women's Interagency HIV Study cohort, identifying homocysteine concentration, as well as hepatitis C virus (HCV) infection, abdominal obesity and aspartate aminotransferase level as independent predictors of increased plasma F2-IsoP.14
Anemia has been shown to be a predictor of mortality among HIV-infected persons in a wide range of clinical settings, independent of markers of disease progression such as CD4+ lymphocyte count and HIV-1 RNA level.15,16 Anemia is also more common in HIV-infected women compared to men,17 and is associated with exposure to the nucleoside reverse transcriptase inhibitor (NRTI), zidovudine (AZT). Although anemia has been associated with increased F2-IsoP in HIV-uninfected persons,3 treatment of anemia with intravenous iron sucrose or dextran was associated with an increase in F2-IsoP among HIV-uninfected hemodialysis patients.18 However, the effect of endogenous iron may differ from intravenous administration of iron. A trend towards correlation has been shown between iron levels and increased urinary F2-IsoP in healthy postmenopausal women. However, this association was not statistically significant.19 The aim of this study was to determine whether hemoglobin (Hgb) level, the presence of anemia, and serum iron level were associated with increased oxidant stress as measured by F2-IsoP levels. The interaction between Hgb, anemia, and iron level were separately assessed with sex to evaluate for differences in association with F2-IsoP between HIV-infected men and women.
Methods
Study population
This cross-sectional study enrolled a convenience sample of ambulatory HIV-infected adults from the Comprehensive Care Center (CCC) in Nashville, Tennessee from 2003 to 2005. Specific inclusion/exclusion criteria were described in detail elsewhere.10 Participants on ART were all considered adherent based on primary provider opinion but no other adherence assessments were performed. The study was approved by the Vanderbilt Institutional Review Board and all subjects provided written informed consent.
Clinical assessments
At enrollment, blood samples were obtained, and study personnel collected demographic and clinical data in a standardized fashion. A questionnaire was administered to assess ART and current antioxidant medication use. Smoking status was self-reported as number of cigarettes per day and categorized as non-current smoker, current non-heavy smoker (<20 cigarettes/day), or current heavy smoker (≥20 cigarettes/day). Antioxidant use was self-reported as use of any “alternative therapies and/or dietary supplements, including antioxidants”, and categorized as current use or no current use. Data on specific agents or doses were not routinely collected. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Clinical diagnoses of liver failure/hepatotoxicity/cirrhosis, chronic active hepatitis B, chronic HCV, and coronary artery disease/myocardial infarction were extracted from the medical record using ICD-9 codes and, in the case of chronic HCV, laboratory confirmation of serologic or virologic evidence of infection in the medical record.
Laboratory data
Laboratory data were obtained by collection of a blood sample at the time of study enrollment. Assays for HIV-1 RNA and CD4+ lymphocytes were performed at a commercial laboratory (LabCorp, Louisville, KY). F2-IsoP levels were quantified using immediate processing and cryopreservation of plasma, and gas chromatography/mass spectrometry techniques described previously.20 Additional laboratory data including Hgb levels, serum iron levels, and HCV status were obtained from the electronic medical record using the results within six months before or after the study enrollment date. Iron levels were available during this time period on 212 (74%) study participants. Anemia was defined according to the World Health Organization (WHO) definition of Hgb<12.0 g/dL for women and <13.0 g/dL for men.
Statistical analyses
Descriptive statistics were presented as frequencies and percentages (%) for categorical variables and mean with standard deviation (SD), or median with interquartile range (IQR), according to the distribution of the continuous variables. Demographic and clinical factors were compared between gender using Wilcoxon rank sum test or a Pearson Chi-square test, as appropriate.
Spearman correlation tests were used to assess the relationship between Hgb or iron and plasma F2-IsoP levels. Three separate multivariable linear regression models were used to evaluate independent associations between F2-IsoP level and Hgb, anemia, or iron level with adjustment for age, sex, race, BMI, CD4+ lymphocyte count, self-reported current smoking status (non-smoker versus non-heavy smoker versus heavy smoker), current use of any ART, and specifically current use of AZT. These factors were chosen a priori based on clinical significance and possible effect on oxidant stress. F2-IsoP level was natural log-transformed to provide normality in the regression residuals. Beta-coefficients were back-transformed indicating percent increase in F2-IsoP by one unit increase in corresponding covariate.
Assessment of whether sex modified the effects of Hgb, anemia, and iron on plasma F2-IsoP levels was conducted by including the cross-product terms of these factors and sex individually in each separate model while controlling for age, race, BMI, smoking, and current use of ART and AZT. Regression diagnostics were performed to verify assumptions of normality of residuals. All analyses used a 2-sided 5% significance level. Data management and analyses were performed using R statistical software (version 2.15.1; available at: http://www.rproject.org), STATA IC (version 10.1; Stata Corporation) and SPSS (version 18; SPSS: An IBM Company).
Results
Characteristics of the 285 patients are shown in Table 1 stratified by gender. Median age was 41 (IQR 34-47) years, 69 (24%) were women, 37% were African-American, 68% were receiving ART, and 36% were receiving AZT. Women were significantly less likely to be on ART (52% vs. 73%, P=0.001) but there was no significant difference in AZT use between men and women (29% vs. 38%, P=0.16). Women were significantly more likely to be of non-white race (64% vs. 36%, P<0.001) compared to men. Women also smoked fewer cigarettes per day (median [IQR] 0 [0, 10] vs. 3 [0, 20] cigarettes/day, P=0.10), were less likely to have a history of coronary artery diseases (0% vs. 2%, P=0.45), and were more likely to be on statins (6% vs. 2%, P=0.08) than men, but these differences were not statistically significant. Median (IQR) iron levels were significantly higher among those on AZT compared to those not on AZT (97.5 [70.0-139.2] vs. 81.5 [60.0-104.8] μg/dL, P=0.005). AZT use was not significantly associated with either Hgb (P=0.56) or anemia (P=0.15). Seventy-three (26%) subjects with missing iron levels (30% of men; 12% of women) had lower BMI (P=0.04), were more likely to use ART (P=0.003), and had a lower plasma HIV-1 RNA level (P=0.001) than those with iron levels.
Table 1.
Demographic and clinical characteristics of the study population by sex.
| Characteristics | Study population N=285 | Men N=216 | Women N=69 | P* |
|---|---|---|---|---|
| Age, median (IQR), years | 41 (34-46) | 41 (34-47) | 39 (30-45) | 0.12 |
| Race, no. (%) | <0.001 | |||
| White | 163 (57) | 138 (64) | 25 (36) | |
| African-American | 106 (37) | 66 (31) | 40 (58) | |
| Hispanic | 8 (3) | 5 (2) | 3 (4) | |
| Asian | 5 (2) | 5 (2) | 0 | |
| Other | 3 (1) | 2 (1) | 1 (1) | |
| CD4+ lymphocyte count, median (IQR), cells/mm3 | 428 (269-621) | 425 (260-635) | 434 (288-589) | 0.71 |
| HIV-1 RNA, median (IQR), log10 copies/mL | 3.0 (1.8-4.0) | 2.9 (1.8-4.1) | 3.3 (1.8-4.0) | 0.55 |
| Hemoglobin, median (IQR), g/dL | 14.5 (13.2-15.6) | 14.9 (13.7-15.8) | 12.7 (11.8-13.9) | <0.001 |
| Anemia, no. (%) | 42 (15) | 22 (10) | 20 (29) | <0.001 |
| **Iron, median (IQR), μg/dL | 83 (62-118) | 90 (68-121) | 75 (47-97) | 0.004 |
| HAART use, no. (%) | 194 (68) | 158 (73) | 36 (52) | 0.001 |
| AZT use, no. (%) | 103 (36) | 83 (38) | 20 (29) | 0.16 |
| Antioxidant use, no. (%) | 10 (4) | 9 (4) | 1 (1) | 0.29 |
| Aspirin use, no. (%) | 9 (3) | 8 (4) | 1 (1) | 0.35 |
| NSAIDs use, no. (%) | 15 (5) | 10 (5) | 5 (7) | 0.4 |
| Statin use, no. (%) | 8 (3) | 4 (2) | 4 (6) | 0.08 |
| Smoking category, no. (%) | 0.60 | |||
| Never smoker | 98 (34) | 71 (33) | 27 (39) | |
| Prior smoker | 37 (13) | 28 (13) | 9 (13) | |
| Current smoker | 150 (53) | 117 (54) | 33 (48) | |
| Heavy smoker | 78 (27) | 65 (30) | 13 (19) | 0.07 |
| BMI, median (IQR), m2/kg | 26.0 (23.0-30.0) | 26.0 (22.0-29.0) | 28.5 (24.3-34.0) | <0.001 |
| Hepatitis C, no. (%) | 54 (19) | 39 (18) | 15 (22) | 0.50 |
| Hepatitis B, no. (%) | 25 (9) | 22 (10) | 3 (4) | 0.14 |
| Liver failure/hepatotoxicity/cirrhosis, no. (%) | 9 (3) | 6 (3) | 3 (4) | 0.52 |
| Coronary artery disease, no. (%) | 5 (2) | 5 (2) | 0 (0) | 0.45 |
Note: IQR: interquartile range. HAART: highly active antiretroviral therapy. NSAID: non-steroidal anti-inflammatory drugs. BMI: body mass index.
Continuous variables were compared using Wilcoxon test. Categorical variables were compared using Pearson Chi-square test.
There were no clinical iron levels within 6 months of the F2-IsoP level in 73 (26%) participants, 65 (30%) men, 8 (12%) women.
Median (IQR) plasma F2-IsoP among women was statistically significantly elevated compared to men (42 [32-62] vs. 36 [25-46] pg/mL, P<0.001). Female patients also had lower Hgb and iron concentrations: 12.7 (11.8-13.9) vs. 14.9 (13.7-15.8) g/dL, P<0.001 and 75 (47-97) vs. 90 (69-121) μg/dL, P=0.004, respectively. Twenty (29%) women and 22 (10%) men were anemic (P<0.001). Overall, plasma F2-IsoP concentrations were not significantly correlated with Hgb (rho=-0.04, P=0.55) or iron levels (rho=0.03, P=0.68) in univariate analyses, nor was there any significant correlation in unadjusted sex-stratified analyses. F2-IsoP also did not differ significantly between patients with anemia and patients without anemia (P=0.62).
After adjustment for age, race, sex, BMI, CD4+ T-cell count, smoking status, current use of ART, and current use of AZT, female sex was associated with a 24% (95%CI: 7%-43%, P=0.004) higher F2-IsoP. This relationship did not notably change after additional adjustment for Hgb, anemia, and iron in three separate models as F2-IsoP was 27% (95% CI 12%-43%, P=0.001), 22% (7%-37%, P=0.004), and 25% (8%-42%, P=0.004) higher in females than in males, respectively in the three models. Smoking ≥1 pack of cigarettes was associated with 21% (95% CI 6%-35%, P=0.01), 21% (7%-35%, P=0.008), and 22% (5%-39%, P=0.03) higher F2-IsoP as compared to non-smokers in the Hgb, anemia, and iron models, respectively; however, F2-IsoP did not differ among those who smoked <1 pack of cigarettes per day compared to non-smokers. Current ART use was also associated with 16% (95% CI 2%-31%, P=0.03), 18% (3%-32%, P=0.02), and 20% (3%-38%, P=0.02) higher F2-IsoP in the Hgb, anemia, and iron models, respectively. Current AZT use tended to be associated with lower F2-IsoP when adjusted for Hgb (P=0.08) or anemia (P=0.07), and was associated with 19% (95% CI 37%-2%; P=0.03) lower F2-IsoP levels when adjusted for serum iron levels. Table 2 shows results of the multivariate model which included iron; results of multivariate models including Hgb (see Supplemental Digital Content 1) and anemia (see Supplemental Digital Content 2) are shown in the supplemental tables.
Table 2. Adjusted relationship between iron levels and F2-IsoP.
| Covariate | Adjusted β-coefficient | 95% CI | P-value |
|---|---|---|---|
| Iron (per interquartile increase) | 1.14 | 1.04-1.24 | 0.008 |
| Sex (female vs. male) | 1.25 | 1.08-1.42 | 0.004 |
| Age (per 10 year increase) | 1.01 | 0.91-1.11 | 0.38 |
| Race (white vs. non-white) | 0.97 | 0.82-1.13 | 0.74 |
| CD4 lymphocyte count (per 100 cell/mm3 increased) | 0.96 | 0.89-1.03 | 0.23 |
| BMI (increased from 25-30 kg/m2) | 1.12 | 1.04-1.20 | 0.003 |
| Heavy Smoker (≥20 cigarettes/day vs. none) | 1.22 | 1.05-1.39 | 0.01 |
| Smoker (<20 cigarettes/day vs. none) | 0.99 | 0.82-1.17 | 0.95 |
| Current ART use | 1.20 | 1.03-1.38 | 0.02 |
| Current AZT use | 0.81 | 0.63-0.98 | 0.03 |
Adjusted β-coefficient values from multivariate linear regression model which included iron, shown as percent change in F2-IsoP for a unit change in covariate. As body mass index (BMI) was included in the model as a nonlinear effect using restricted cubic spline, the β-coefficient shown for BMI corresponds to percent change in F2-isop as BMI increase from 25-30 kg/m2. ART = antiretroviral therapy; AZT = zidovudine; CI = confidence interval; F2-IsoP = F2-isoprostane.
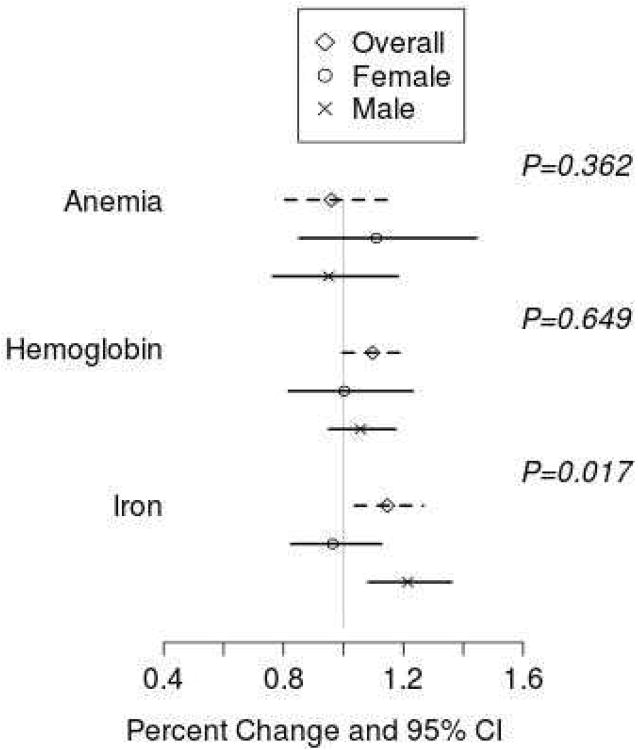
After adjustment for age, race, CD4 count, smoking status, BMI, ART and AZT use, the relationship between F2-IsoP and either Hgb or anemia did not differ significantly between sexes (interaction p-value=0.65 and 0.36, respectively); however, the relationship between iron level and F2-IsoP was significantly different among men and women (interaction P=0.02). Among men there was a 21% (95% CI 8%-36%, P=0.001) increase in F2-IsoP per interquartile increase in iron level (Figure 1; for example, an increase in iron from 75 to 97 ug/dL was associated with a 21% increase in F2-IsoP); among women there was no significant association between F2-IsoP and iron (-4% [-17%-13%], P=0.65, Figure 1).
Figure 1. Multivariable analysis of factors potentially associated with plasma F2-IsoP levels.
Percent change in F2-IsoP associated with the presence of anemia and per interquartile increase in Hgb and iron are shown stratified by gender with 95% confidence intervals. Interaction P-values are shown for interaction between covariate and sex.
Discussion
Differences in F2-IsoP between men and women in HIV-negative populations,12,13 and also initially described in this HIV-infected study population10 raise questions regarding the etiology of the higher F2-IsoP levels in women and their clinical significance. Since women have been shown to have lower Hgb and iron levels than men and are at greater risk for anemia in HIV-infected populations, Hgb, anemia, and iron were specifically targeted in this analysis.17 Elevated iron stores have also been associated with disease progression and increased mortality among HIV-infected patients or patients with AIDS who are not on highly active ART, but so have anemia and iron deficiency.15-17,21-23 Increased oxidative stress is a potential contributing mechanism to these negative outcomes. In an HIV-uninfected population of patients with hemochromatosis, those with iron overload were found to have increased urinary excretion of 8-iso-prostaglandin F2α, the urinary form of F2-IsoP, and F2-IsoP was corrected with removal of excess iron by phlebotomy.24
Although our study population did not have elevated iron stores, some studies have found a positive correlation between serum ferritin, a measure of body iron stores, and urinary excretion of the oxidative stress biomarker 8-hydroxydeoxyguanosine in men who were mildly dyslipidemic25 and in working men and women in Japan26 suggesting that increased oxidative stress may be associated with iron even at non-iron-overload levels. We sought to examine whether either Hgb or iron levels among women were associated with oxidant stress, measured by levels of F2-IsoP in plasma. This is the first time this question has been addressed since previous studies of F2-IsoP in HIV-infected populations did not include measurement of Hgb or iron levels.
As expected, Hgb and iron levels were significantly lower among women than among men and, accordingly, there was a significantly greater prevalence of anemia among women versus men. Iron levels were found to be significantly higher among those on AZT, which is likely due to the clinical avoidance of AZT in patients with iron deficiency. In multivariate analysis there was a trend towards association between F2-IsoP and Hgb however, this did not reach statistical significance. The direction of this trend was interesting, as F2-IsoP were marginally associated with higher rather than lower Hgb. Anemia was not associated with F2-IsoP and, thus, did not appear to be related to increased oxidant stress in this population. Iron was positively and significantly correlated with F2-IsoP in multivariate analysis, but including Hgb or iron in the multivariate models did not attenuate the relationship between sex and F2-IsoP. The point estimate of a 24% relative increase in F2-IsoP among females10 was no different when Hgb was added to the model (27%) or when iron was added to the model (25%). Thus, our results do not suggest that the difference in F2-IsoP between men and women is accounted for by sex differences in Hgb or iron levels.
Detailed information was collected regarding ART regimen and analyzed previously.10 For the multivariate models in this study, current ART use was included as a dichotomous variable to avoid overfitting the model. AZT use was also included separately as a dichotomous variable, given that anemia is a common side effect of AZT. Current ART use was found to be associated with higher F2-IsoP. However, iron was found to have a significant relationship with F2-IsoP while controlling for current treatment, thus indicating that the association between iron and F2-IsoP is independent of treatment related oxidant stress or AZT-induced anemia.
Evaluation of effect modification showed that sex did not modify relationships between Hgb or anemia and F2-IsoP, but did modify the relationship between iron and F2-IsoP. The association between iron and F2-IsoP was limited to men; men with higher iron levels had higher F2-IsoP than men with lower iron. This association was not seen among women. Although the cross-sectional design of this study limits the conclusions that can be made regarding the nature of the iron-F2IsoP association in men, several possibilities should be considered. Effects of sex hormones on F2-IsoP are not well characterized. In a study by Basu, et al., HIV-negative women had lower urinary F2-IsoP than men after adjustment for smoking status, creatinine, and country of origin (p<0.02).27 However, several other studies of HIV-negative subjects found that women had higher levels of oxidant stress.28,29 Endogenous sex hormones (estrogen in particular) have been positively correlated with levels of isoprostanes among pre-menopausal women in some studies,30,31 while in other studies this relationship was detected in postmenopausal women only.32 Most of the women in our study were probably premenopausal, but menopausal status was not confirmed, and sex hormone levels were not measured.
Plasma F2-IsoP in women may also be less sensitive to changes in iron than men, perhaps due to sex differences in iron absorption and metabolism. Animal models have shown greater peak concentration of serum iron among female rats compared to males following oral administration of toxic levels of iron, suggesting increased absorption by the females. This resulted in increased mortality among prepubertal female rats, and earlier death among pubertal and adult female rats compared to males.33 A human study of erythrocyte incorporation of an orally administered iron isotope has also supported increased absorption of iron among prepubertal girls compared to boys. Because ferritin levels were similar among the girls and boys, the authors of that study hypothesized that hormonal effects on iron absorption caused this difference.34 Thus, there is evidence of hormone-related differences in iron absorption, but it is unknown whether hormonal differences are responsible for the relationship we observed between iron and oxidant stress in men but not in women. An additional interpretation may be that other unmeasured and/or unknown factors, such as estrogen levels, which are associated with increased F2-IsoP in HIV-infected women30,32 overwhelms any relationships between iron and oxidant stress, but in men (with lower overall F2-IsoP levels), this relationship is more easily detected.
The BMI was significantly higher in women than in men, and higher BMI was associated with higher F2-IsoP, consistent with prior studies.12,19,35 Female sex remained significantly associated with higher F2-IsoP after controlling for BMI. Having suppressed HIV RNA on ART has been associated with higher F2-IsoP,9 and there was a significant association between higher F2-IsoP and current ART treatment in this population. HIV RNA and F2-IsoP were not correlated in overall or sex-stratified univariate analysis (data not shown). Studies to date have not been of sufficient size to determine if specific ART classes or drugs are associated with increased F2-IsoP. Heavy smokers in our study also had significantly higher F2-IsoP, consistent with data in HIV-negative populations36 and other studies in HIV-infected populations.11 However, women were less likely than men to be on ART, less likely to report heavy smoking, and adjusted models included both of these covariates. Thus, the relationship between sex and F2-IsoP was independent of ART and smoking status.
This study has limitations related in part to its cross-sectional design. Although associations were detected between iron and F2-IsoP levels in men, the nature of this relationship, such as a causal one, cannot be inferred. In addition, serum iron and Hgb levels were not consistently measured in samples collected at the same time as those in which F2-IsoP were measured. The latter was collected at study enrollment, while Hgb and iron were collected during routine clinical care. It is possible that a relationship between F2-IsoP and Hgb is more immediate, and would only be identified by simultaneous sampling. It should be noted, however, that 91% of Hgb values were collected within one day of the F2-IsoP level. Iron levels were obtained in a majority of subjects, and as expected and noted in the Results, these subjects differed in several ways from those who did not have iron levels, including having a lower BMI, being more likely to be on ART, and having a lower plasma HIV-1 RNA level. These factors were included in multivariate models, but it is possible that other unmeasured confounders, such as inflammation and estrogen levels, were different in those subjects with missing iron levels. We did not account for renal function or endogenous erythropoietin levels; these will be important considerations for future studies. Also, as serum iron levels were collected during clinical care, the levels are non-fasting, and we do not have accurate data on use of iron supplements or iron-containing multi-vitamins. We did not quantify markers of oxidant stress other than F2-IsoP, and we did not assess measures of central adiposity, or inflammation. For these reasons, and because both iron deficiency and iron excess have been linked to adverse outcomes in HIV-infected populations, our findings should also be interpreted with some caution.
This was an otherwise healthy HIV-infected population with a median CD4+ lymphocyte count of >400 cells/mm3, and the vast majority of patients with anemia (90%) had only mild anemia (hemoglobin 10.0-11.9 g/dL for women and 10.0-12.9 g/dL for men). There may be associations between more severe anemia and/or iron deficiencies and oxidant stress that were not observed in this population. Finally, we do not have information regarding the menopausal status of women in this study or whether menstrual status at the time serum iron levels were collected may have impacted iron measurements. Despite potentially earlier age at menopause onset among HIV-infected women,37 a median age of 39 years among these female subjects means most were likely to be pre-menopausal. Although we cannot stratify by menopausal status or menstrual status, adjusting for age did not significantly alter associations between F2-IsoP, sex and Hgb or iron. This will be an important question to address in future studies.
In conclusion, higher iron levels were found to be independently associated with higher F2-IsoP in HIV-infected men, a new finding in this population. The same association was not seen in women in this sample. The clinical significance of this observation is unknown, however, as higher F2-IsoP have not been associated with adverse outcomes in HIV infection and are also associated with ART use. In addition, lower Hgb, iron levels, and anemia do not appear to explain increased oxidant stress (as measured by plasma F2-IsoP) in HIV-infected women. Female sex remained independently associated with higher F2-IsoP in multivariate analysis after controlling for Hgb, iron, and the presence of anemia, in addition to other covariates potentially influencing oxidant stress and F2-IsoP such as ART and smoking. The reason for higher F2-IsoP among HIV-infected women compared to HIV-infected men remains unclear. Prospective, sex-stratified studies that include careful assessments of menopausal status and inflammation are needed to better characterize the mechanisms, etiologies, epidemiology, and outcomes of increased oxidant stress in HIV-infected persons.
Supplementary Material
Supplemental Digital Content 1. Table that shows the results of the multivariate analysis which included hemoglobin in the model. docx
Supplemental Digital Content 2. Table that shows the results of the multivariate analysis which included anemia in the model. docx
Acknowledgments
We acknowledge Stephanie Sanchez and Kedria Reed-Walker for assisting with laboratory assays; Ravi Misra, MD, Milica Markovic, Ikwo Oboho, and the Vanderbilt ACTC staff (Vicki Bailey, RN, Brenda Jackson, RN, Michael Morgan, FNP, Janet Nicotera, RN, and Fred Nicotera,) for assisting with subject recruitment; and the staff, providers, and study participants from the Comprehensive Care Center who made this study possible.
Source of Funding: These studies were supported in part by research grants from Boehringer-Ingelheim Pharmaceuticals and the Bristol-Myers Squibb Company. Additional support was provided by NIH/NCCAM grant K23 AT002508 (T.H.), NIH/NIMH grant 1R01 MH095621 (A.K.), the Vanderbilt-Meharry Center for AIDS Research grant P30 AI54999 (A.S., D.W.H.), and the Vanderbilt University School of Medicine Emphasis Program (L.A.D.). The funding agencies were not involved in data collection, analysis, or manuscript preparation.
Footnotes
Presentation: Parts of the data were presented at IDWeek in San Diego, CA October 17-21.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: T.H. has received research funding from Merck & Co. D.W.H. has received research grants from Bavarian Nordic, Boehringer-Ingelheim, and the Bristol-Myers Squibb Company, Gilead Sciences, Merck, Tanox, and Tibotec. He is a Scientific Advisory Board member for GlaxoSmithKline. GM has provided consultancy work for Roche USA.
References
- 1.Palella FJ, Baker AK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Gil L, Perez D, Tapanes R, et al. Does mitochondrial dysfunction during antiretroviral therapy in human immunodeficiency virus infection suggest antioxidant supplementation as a beneficial option? Redox Rep. 2005;10(3):113–9. doi: 10.1179/135100005X38905. [DOI] [PubMed] [Google Scholar]
- 3.Wiswedel I, Peter D, Gardemann A, et al. Serum Concentrations of F2-Isoprostanes and 4-Hydroxynonenal in Hemodialysis Patients in Relation to Inflammation and Renal Anemia. Biomark Insights. 2008;3:419–428. doi: 10.4137/bmi.s363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McComsey GA, Morrow JD. Lipid oxidative markers are significantly increased in lipoatrophy but not in sustained asymptomatic hyperlactatemia. J Acquir Immune Defic Syndr. 2003;34:45–9. doi: 10.1097/00126334-200309010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gil L, Tarinas A, Hernandez D, et al. Altered oxidative stress indexes related to disease progression marker in human immunodeficiency virus infected patients with antiretroviral therapy. Biomedicine and Aging Pathology. 2011;1(1):8–15. doi: 10.1016/j.biopha.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 7.Morrow JD. Quantification of isoprostanes as indices of oxidant stress and the risk of atherosclerosis in humans. Arterioscler Thromb Vasc Biol. 2005;25:279–86. doi: 10.1161/01.ATV.0000152605.64964.c0. [DOI] [PubMed] [Google Scholar]
- 8.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins and DNA markers of CCl4 poisoning? Free Radical Biology and Medicine. 2005;38:698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Hulgan T, Morrow J, D'Aquila RT, et al. Oxidant stress is increased during treatment of human immunodeficiency virus infection. Clin Infect Dis. 2003;37:1711–7. doi: 10.1086/379776. [DOI] [PubMed] [Google Scholar]
- 10.Redhage LA, Shintani A, Haas DW, et al. Clinical factors associated with plasma F2-isoprostane levels in HIV-infected adults. HIV Clin Trials. 2009;10:181–92. doi: 10.1310/hct1003-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boger MS, Bian A, Shintani A, et al. Sex differences in urinary biomarkers of endothelial function, inflammation, and oxidant stress in HIV-infected persons receiving antiretroviral therapy. Antivir Ther. doi: 10.3851/IMP1990. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 13.Gross M, Steffes M, Jacobs DR, Jr, et al. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA Study. Clin Chem. 2005;51:125–31. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 14.Glesby MJ, Hoover DR, Raiszadeh F, et al. Oxidant stress in HIV-infected women from the Women's Interagency HIV Study. Antivir Ther. 2009;14(6):763–9. doi: 10.3851/1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(1):29–33. doi: 10.1097/00042560-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lundgren JD, Mocroft A. Anemia and survival in human immunodeficiency virus. Clin Infect Dis. 2003;37(Suppl 4):S297–S303. doi: 10.1086/376909. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan PS, Hanson DL, Chu SY, et al. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91(1):301–8. [PubMed] [Google Scholar]
- 18.Pai AB, Conner T, McQuade CR, et al. Non-transferrin bound iron, cytokine activation and intracellular reactive oxygen species generation in hemodialysis patients receiving intravenous iron dextran or iron sucrose. Biometals. 2011;24(4):603–13. doi: 10.1007/s10534-011-9409-6. [DOI] [PubMed] [Google Scholar]
- 19.Crist BL, Alekel DL, Ritland LM, et al. Association of oxidative stress, iron, and centralized fat mass in healthy postmenopausal women. J Womens Health (Larchmt) 2009;18:795–801. doi: 10.1089/jwh.2008.0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrow JD, Roberts LJ., II Mass spectrometric quantification of F2-isoprostanes as indicators of oxidant stress. Methods Mol Biol. 2002;186:57–66. doi: 10.1385/1-59259-173-6:57. [DOI] [PubMed] [Google Scholar]
- 21.Jacobus D. Randomization to iron supplementation of patients with advanced human immunodeficiency virus disease - an inadvertent but controlled study with results important for patient care. J Infect Dis. 1996;173:1044–45. doi: 10.1093/infdis/173.4.1044. [DOI] [PubMed] [Google Scholar]
- 22.Clark T, Semba R. Iron supplementation during human immunodeficiency virus infection: a double-edged sword? Med Hypotheses. 2001;57(4):476–9. doi: 10.1054/mehy.2001.1368. [DOI] [PubMed] [Google Scholar]
- 23.Mcdermid J, Jaye A, Schim van der Loeff M, et al. Elevated iron status strongly predicts mortailty in West African adults with HIV infection. J Acquir Immune Defic Syndr. 2007;46:498–507. doi: 10.1097/qai.0b013e31815b2d4b. [DOI] [PubMed] [Google Scholar]
- 24.Kom GD, Schwedhelm E, Nielsen P, et al. Increased urinary excretion of 8-iso-prostaglandin F2α in patients with HFE-related hemochromatosis: A case-control study. Free Radic Biol Med. 2006;40:1194–1200. doi: 10.1016/j.freeradbiomed.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Tuomainen T, Loft S, Nyyssonen K, et al. Body iron is a contributor to oxidative damage of DNA. Free Radic Res. 2007;41(3):324–8. doi: 10.1080/10715760601091642. [DOI] [PubMed] [Google Scholar]
- 26.Hori A, Mizoue T, Kasai H, et al. Body iron store as a predictor of oxidative DNA damage in healthy men and women. Cancer Science. 2010;101(2):517–22. doi: 10.1111/j.1349-7006.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu S, Helmersson J, Jarosinska D, et al. Regulatory factors of basal F(2)-isoprostane formation: population, age, gender and smoking habits in humans. Free Radic Res. 2009;43(1):85–91. doi: 10.1080/10715760802610851. [DOI] [PubMed] [Google Scholar]
- 28.Keaney JF, Jr, Larson MG, Vasan RS, et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arteriosclerosis Thrombosis Vascular Biol. 2003;23:434–39. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 29.Block G, Dietrich M, Norkus E, et al. Determinants of oxidative stress in human populations. Am J Epidemiol. 2002;156:274–85. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 30.Schisterman EF, Gaskins AJ, Mumford SL, et al. Influence of endogenous reproductive hormones on F2-isoprostane levels in premenopausal women: the BioCycle Study. Am J Epidemiol. 2010;172(4):430–9. doi: 10.1093/aje/kwq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy A, Danaher M, Mumford SL, et al. A Bayesian order-restricted model for hormonal dynamics during menstrual cycles of healthy women. Stat Med. 2012;31(22):2428–40. doi: 10.1002/sim.4419. [DOI] [PubMed] [Google Scholar]
- 32.Sowers M, McConnell D, Jannausch ML, et al. Oestrogen metabolites in relation to isoprostanes as a measure of oxidative stress. Clin Endocrinol. 2008;68(5):806–13. doi: 10.1111/j.1365-2265.2007.03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berkovitch M, Livne A, Lushkov G, et al. Acute iron intoxication: significant differences between sexes. Vet Hum Toxicol. 1997;39(5):265–7. [PubMed] [Google Scholar]
- 34.Woodhead JC, Drulis JM, Nelson SE, et al. Gender-related differences in iron absorption by preadolescent children. Pediatr Res. 1991;29(5):435–9. doi: 10.1203/00006450-199105010-00005. [DOI] [PubMed] [Google Scholar]
- 35.Davi G, Guagnano MT, Ciabatton G, et al. Platelet activation in obese women: Role of inflammation and oxidant stress. JAMA. 2002;288:2008–14. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 36.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 37.Kojic EM, Wang CC, Cu-Uvin S. HIV and menopause: a review. J Womens Health (Larchmt) 2007;16:1402–11. doi: 10.1089/jwh.2007.0345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Table that shows the results of the multivariate analysis which included hemoglobin in the model. docx
Supplemental Digital Content 2. Table that shows the results of the multivariate analysis which included anemia in the model. docx