Abstract
The number of neurons in the geniculate ganglion that are available to innervate taste buds is regulated by neurotrophin-4 (NT-4) and brain-derived neurotrophic factor (BDNF). Our goal for the current study was to examine the timing and mechanism of NT-4-mediated regulation of geniculate neuron number during development. We discovered that NT-4 mutant mice lose 33% of their geniculate neuronal cells between E10.5 and E11.5. By E11.5, geniculate axons have just reached the tongue and do not yet innervate their gustatory targets; thus, NT-4 does not function as a target-derived growth factor. At E11.5, no difference was observed in proliferating cells or the rate at which cells exit the cell cycle between NT-4 mutant and wild type ganglia. Instead, there was an increase in TUNEL-labeling, indicating an increase in cell death in Ntf4−/− mice compared with wild types. However, activated caspase-3, which is up-regulated in the absence of BDNF, was not increased. This finding indicates that cell death initiated by NT-4-removal occurs through a different cell death pathway than BDNF-removal. We observed no additional postnatal loss of taste buds or neurons in Ntf4−/− mice. Thus, during early embryonic development, NT-4 produced in the ganglion and along the projection pathway inhibits cell death through an activated caspase-3 independent mechanism. Therefore, compared to BDNF, NT-4 plays distinct roles in gustatory development; differences include timing, source of neurotrophin, and mechanism of action.
Keywords: Neurotrophin-4, Gustatory/geniculate neurons, Taste development, Activated caspase-3
Introduction
Like most sensory ganglia, taste neurons of the geniculate ganglion make precise connections with their peripheral and central targets. For example, the number of neurons innervating a specific taste bud is tightly controlled and is related to the eventual size of a taste bud (Krimm and Hill, 1998). Much of this regulation occurs during early embryonic development (Altman and Bayer, 1982; Kandel et al., 2000) when the total number of geniculate neurons available to innervate taste buds is determined. The geniculate ganglion undergoes expansion via rapid proliferation, and production peaks at embryonic day 12 (E12; E10–E11 in mice) (Altman and Bayer, 1982). The geniculate ganglion initially overproduces neurons and reduces these numbers through developmental cell death (Carr et al., 2005). In rats, cell death in the geniculate ganglion appears to peak at two time points, E16.5 (approximately E14–E15 in mice), which is during target innervation, and earlier at E12.5 (around E10–E11 in mice), which is before target innervation (Carr et al., 2005). In general, there is considerable overlap between the periods of cell proliferation and programmed cell death in this ganglion (Carr et al., 2005). Thus, throughout much of embryonic development, the geniculate ganglion contains a mixed population of cells including proliferating, differentiating, and dying neurons and precursors.
The final number of neurons in the geniculate ganglion is regulated by the neurotrophins brain-derived neurotrophic factor (BDNF) and neurotrophin-4 (NT-4) (Conover et al., 1995; Liu et al., 1995). While the role of BDNF in embryonic gustatory development is fairly well established (Hoshino et al., 2010; Lopez and Krimm, 2006a; Ma et al., 2009; Mistretta et al., 1999; Nosrat et al., 1997; Oakley et al., 1998; Patel and Krimm, 2010; Patel et al., 2010), the role of NT-4 is much less clear. Neurotrophins are capable of influencing numerous different aspects of neuronal development, including proliferation and survival of progenitors, neuronal differentiation, successful target innervation, and survival (Farinas et al., 1996, 2002; Huang and Reichardt, 2001). For example, NT-4 prevents neuron loss in the nodose ganglion before target innervation during the initial formative stages (ElShamy and Ernfors, 1997), but also induces trigeminal ganglion neuron cell death (Agerman et al., 2000). NT-4 can also influence neuronal differentiation. Specifically, BDNF-dependent mechanoreceptors become neurotrophin 3 (NT-3)-dependent proprioceptors in the absence of NT-4 (Liebl et al., 2000). Furthermore, NT-4 is required for the postna-tal development and maintenance of specific somatosensory receptors called D-hair receptors (Stucky et al., 1998). Thus, the timing and role of NT-4 during development differs considerably across different systems, and remains unclear for neurons of the geniculate (taste) ganglion.
It has been determined that, in the absence of NT-4, half of the geniculate neurons are missing at birth (Conover et al., 1995; Liu et al., 1995; Patel et al., 2010) resulting in a reduction in fungiform papillae and taste buds (Liebl et al., 1999; Patel et al., 2010). In this study, we examined the timing and mechanism by which NT-4 influences geniculate neuron numbers during development. We conclude that NT-4 produced in the geniculate ganglion and along the projection pathways prevents geniculate ganglion cell death early in development before target innervation. Unlike with BDNF, NT-4 removal does not result in the activation of caspase 3, indicating that BDNF and NT-4 function through different cell death pathways. Furthermore, we established that NT-4 has no role in the postnatal development of gustatory neurons.
Material and methods
Animals
All NT-4 mutant (Ntf4−/−) and wild-type mice were on a 129S4/ SvJae background. Homozygous Ntf4−/− embryos were obtained by breeding heterozygous mice with targeted mutations of the Ntf4 gene. The heterozygous Ntf4−/+ mice were acquired from Jackson Laboratories (Bar Harbor, Maine, USA; #002266). Animals were genotyped using polymerase chain reaction, as described in protocols provided by the Jackson Laboratory (http://jaxmice.jax//index.html). Embryonic mice were obtained from timed breeding of females that were examined for plugs the following morning. The day a plug was positively identified was designated embryonic day 0.5 (E0.5). Animals were cared for and used in accordance with the guidelines of the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and the NIH Guide for the Care and Use of Laboratory Animals.
β-III tubulin labeling and quantification of geniculate ganglion neurons
Embryos aged 14.5 days (E14.5) and older were immediately transcardially perfused using chilled 4% paraformaldehyde (PFA). Animals E13.5 and younger were fixed by immersion in 4% PFA. All embryos were fixed overnight in 4% PFA. Following fixation, the embryo heads were moved to 70% ethanol and processed for paraffin embedding. Immuno-detection of the cytoskeletal element class III β-tubulin (β-III tubulin) was used to visualize geniculate ganglion neurons. Serial sections (5 μm) of paraffin-embedded embryo heads were collected on Fischer SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA, USA). The paraffin was removed by immersion in CitriSolv (Fisher). Sectioned tissues were rehydrated in a graded series of alcohols followed by incubation in phosphate-buffered saline (PBS), pH 7.4. Endogenous peroxidase activity was quenched by treatment for 15 min in a solution of 10% methanol and 3% hydrogen peroxide in PBS, and the slides were then washed in distilled water (dH2O) (3×5 min each). For antigen retrieval, the slides were boiled for 15 min in citrate buffer (0.1 M citric acid, 0.1 M sodium citrate, dH2O; pH 6) and allowed to cool for 10 min at room temperature (RT). The sections were washed in PBS, blocked for 1 h in blocking buffer (PBS, 5% goat serum, 0.25% Triton X-100), and were incubated overnight at 4 °C in blocking buffer containing a 1:1000 dilution of monoclonal mouse anti-β-III tubulin antibody (Covance, Princeton, NJ, USA; catalog #MMS-435P). On the following day, the sections were washed in PBS (3×5 min), incubated for 2 h at RT in blocking buffer containing a 1:200 dilution of biotinylated anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA, USA; #BA-2000), and visualized with an ABC diaminobenzidine reaction. The following numbers of mouse embryos were used for quantification: wild type, n=3 for E12.5 and E14.5 and n=4 for E11.5, E16.5, and E18.5; Ntf4−/−, n=3 for E11.5, E12.5, E14.5, E16.5, and E18.5.
Neurons in the geniculate ganglion were quantified in transverse serial sections of the mouse embryo head. Monoclonal mouse anti-β-III tubulin antibodies (TUJ-1) were used to identify and count neuron profiles only in sections where the nucleus was visible (Fig. 1G, arrowheads). Neuronal profiles were counted in six sections per geniculate ganglion. At E11.5 non-neuronal profiles were also quantified. The area containing the geniculate ganglion was measured for each section and multiplied by the section thickness (5 μm) to derive the volume, and the volumes for all the sections were summed to derive the volume for the entire ganglion. The total number of neuron profiles in the ganglion was estimated as the product of the number of profiles per volume of the counted section×the total volume of the entire ganglion. The total number of neurons per ganglion was estimated by multiplying the number of total neuron profiles by a correction factor to compensate for the presence of a nucleus in multiple sections (Abercrombie, 1946). The correction factor was calculated according to the formula: N=n×[T/(T×D)], where N is the estimated total number of neurons, n is the number of nuclear profiles, T is the measured section thickness, and D is the average diameter of the nuclei. This estimate was calculated separately for each ganglion based on the average diameter of 50 neuronal nuclei per ganglion, which were calculated from area measurements for each nucleus. This is the approach that has been used most successfully to examine changes in ganglion neuron numbers following neurotrophin manipulations (Agerman et al., 2003; ElShamy and Ernfors, 1997; Erickson et al., 1996, 2001; Carr et al., 2005 #204; Ernfors et al., 1995; Farinas et al., 1996; Krimm et al., 2001) and allows the greatest comparison with the literature.
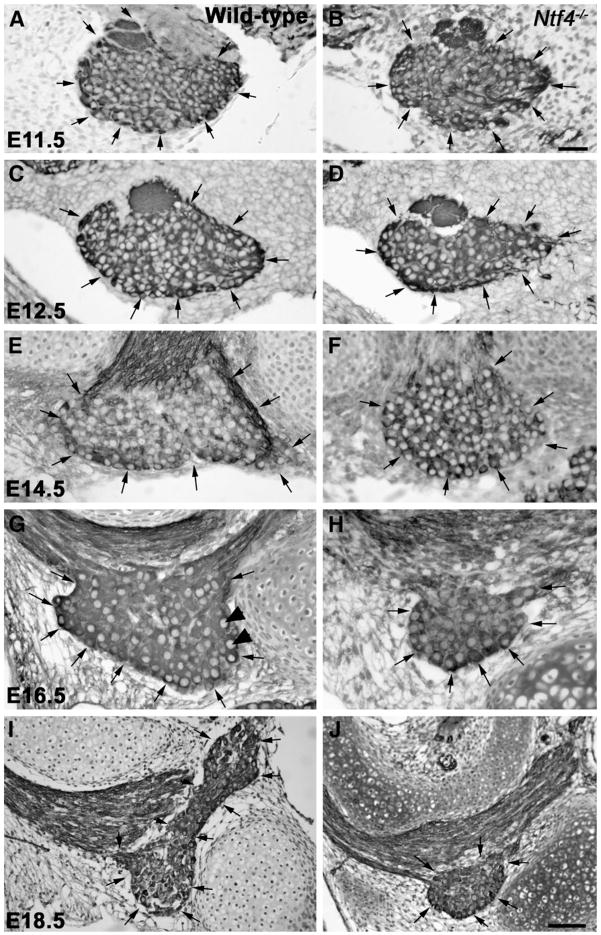
Fig. 1.
Anti-TUJ-1-labeled geniculate ganglia (arrows) from Nft4−/− mice (B, D, F, H, J) are smaller than those of wild type mice (A, C, E, G, I) beginning at E12.5. At E11.5, there is no obvious difference in the size or appearance of the geniculate ganglion between wild type (A) and Ntf4−/− mice (B). Beginning at E12.5, the Ntf4−/− geniculate ganglion (D) is noticeably smaller than the wild type (C) ganglion. These differences were also observed at E14.5 (E, F), E16.5 (G, H) and E18.5 (I, J). Note that the ganglion is much larger at E18.5 (J) compared to E16.5 (H) for wild type and Ntf4−/− mice, and, thus, imaged at a lower magnification. TUJ1 labeled neurons with a large pale nucleus were quantified as neurons (arrowheads in G). Scale bar in B=20 μm (applies to A–H); inset in H, scale bar=5 μm; J=50 μm (applies to I and J).
Stereology
Time-bred mouse embryos at E11.5 (wild type, n=3; Ntf 4−/−, n=3) were euthanized and immersion fixed in 4% PFA in 0.1 M PB (pH 7.4) and transferred into 30% sucrose until the tissue sank to the bottom of the jar. The heads were then transferred to a 1:1 mixture of 30% sucrose and optimal cutting temperature compound (OCT; Tissue Tek) and allowed to equilibrate for 1–2 h. The heads were subsequently frozen in OCT embedding medium and stored at −80 °C. The embryo heads were cut in transverse serial sections (60 μm), air-dried on a slide warmer for 3 h, and stained with cresyl violet. To maintain section thickness, tissues were not dehydrated and were mounted using an aqueous mounting medium designed to reduce shrinkage (Glycergel, Dako, Carpinteria, CA). The neurons were then quantified using Stereo Investigator (version 7) software (MBF Bioscience, Williston, VT, USA). An experimenter blinded to the genotype of the animals traced a contour around the geniculate ganglion under low magnification (20×). Every section containing the ganglion was traced for a volume measurement, and the cells were quantified in three of those sections. The counting parameters were optimized so that a section with at least 300 cells was counted. The computer determined the placement of the counting frames randomly within each traced contour of the geniculate ganglion. The depth (z-axis) of the counting frame was equal to the minimal thickness of the section minus a total guard zone of 6 μm (3 μm from the top and bottom of the section). At 100× magnification, the geniculate ganglion neurons were counted in the volume designated by each counting frame (25 μm2). The cells were counted only when they first came into focus (cell top) so that each cell was counted only once. Based on these measurements, the total volume of the ganglion was estimated using the Cavalieri method (Garcia-Finana et al., 2003; Gundersen et al., 1999), and the total number of cells was estimated for the entire volume of the ganglion using the optical fractionator probe (MBF Bioscience).
Immunohistochemistry for cell cycle analysis
Neuronal cell cycle in the geniculate ganglion was analyzed using a method previously described in detail (Vega and Peterson, 2005). Briefly, pregnant mice were injected with equimolar amounts of chlorodeoxyuridine (CldU) and iododeoxyuridine [IdU; CldU (17 mg/ml) at E10 followed by IdU (23 mg/ml) at E11.5, both at 2.5 mg/kg]. Two hours following the IdU treatment, the embryos were removed, immersion fixed in 4% paraformaldehyde overnight, and the embryo heads were then transferred to 30% sucrose, 0.1 M phosphate buffer (PB) overnight for cryoprotection. The heads were then frozen on dry ice in OCT embedding medium. The embedded embryo heads, n=3 for wild type and Ntf4−/− embryos at E11.5, were sectioned transversely (16 μm), mounted on SuperFrost/Plus slides (Fisher), and allowed to air-dry. The fixed mounted sections were treated with 2 N HCl and heated to 37 °C for 10 min. After several PBS washes, the sections were incubated overnight in mouse anti-BrdU (Becton Dickinson #347580; clone B44) at 1:500 for IdU and rat anti-BrdU (ABDSerotec #OBT-0030; clone BU1/75) for CldU at 1:250. After being washed in PBS (3×5 min), the sections were incubated for 2 h at RT in the appropriate secondary antibody solutions [anti-rat Alexa-647 or anti-mouse Alexa 555 (A21244; Invitrogen, Carlsbad, CA, USA)] at a dilution of 1:200. The slides were then dehydrated in a graded series of alcohols and cover-slipped in CitriSolv with DPX mounting medium (Sigma-Aldrich, St. Louis, MO, USA). Immuno-labeled tissues were visualized using a fluorescence confocal microscope. Antigen-positive cells were quantified in each section and the area of each section of ganglion was measured. The volume was calculated by multiplying each area by the total thickness of the section and summing the volumes for each section. The density of labeled cells was calculated by dividing the total number of labeled cells by the volume.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)
Apoptotic cell death was detected by the TUNEL method using an ApopTag peroxidase in situ apoptosis detection kit (Millipore #S7100.). Timed-bred embryos (E11.5) were fixed in paraformaldehyde in 0.1 M PB, washed in PB, transferred to 30% sucrose, frozen in embedding compound, and stored at −80 °C. The embryos were serially sectioned in the transverse plane through the head at a thickness of 16 μm. Sections containing the geniculate ganglion were stained according to the manufacturer’s instructions. Briefly, sections were postfixed in 1% paraformaldehyde in PBS (pH=7.4) for 10 min followed by precooled ethanol:acetate 2:1 for 5 min at −20 °C. Endogenous peroxidase activity was quenched with 0.3% hydrogen peroxidase in PBS for 5 min. Tissue sections were incubated in TdT enzyme for 1 h at RT followed by anti-digoxigenin, which was visualized with a standard peroxidase reaction. The tissues were counter-stained with cresyl violet, and the total number of tunnel positive cells were quantified for wild type (n=4) and Ntf4−/− (n=4) embryos.
Triple immunohistochemistry for activated caspase-3, BrdU and neurofilaments
Pregnant females were injected 2 h before sacrifice with 50 mg/kg (intraperitoneal) of 2′-bromo-5′-deoxyuridine (BrdU; 5 ml/kg of a 10 mg/ml stock solution in 0.1 M Tris–HCl buffer, pH 7.5). The embryos were immersion-fixed overnight in 4% PFA in PBS, pH 7.4. They were then placed in 30% sucrose in PBS overnight for cryoprotection and frozen on dry ice in OCT embedding medium. The embedded embryo heads were sectioned transversely (16 μm), mounted on Super-Frost Plus slides (Fisher), and allowed to air-dry. The fixed mounted sections were treated with 2 N HCl, heated to 37 °C for 30 min, washed in PBS (3×5 min), and blocked in PBS with 5% goat serum and 0.25% Triton X-100 for 30 min. The sections were incubated overnight in primary antibodies: rat anti-BrdU antibody (Accurate Chemicals, Westbury, NY, USA; #OBT0030), mouse anti-neurofilament antibody (Millipore, Billerica, MA, USA; #MAB5266), and rabbit cleaved anti-caspase-3 (Asp175) antibody (Cell Signaling, Beverly, MA, USA; #9661) at dilutions of 1:200 in the blocking buffer described above. After being washed in PBS (3×5 min), sections were incubated for 2 h at RT in the secondary antibody solutions: anti-rat Alexa 488 (A11006), anti-mouse Alexa 546 (A11030), and anti-rabbit Alexa-647 (A21244; Invitrogen, Carlsbad, CA, USA) at a dilution of 1:200. The slides were dehydrated in a graded series of alcohols and cover slipped in CitriSolv with DPX mounting medium (Sigma-Aldrich, St. Louis, MO, USA). The immuno-labeled tissues were visualized using a fluorescence confocal microscope. Antigen-positive cells were quantified from wild type and Ntf4−/− embryos at E11.5 (n=3).
Taste bud quantification
Mice were perfused trans-cardially at birth and postnatal days 10 and 60 with ice cold 4% PFA. The tongue was dissected out and immersion-fixed for another two hours in 4% PFA. The circumvallate papilla of adult mice was separated from the front of the tongue containing the fungiform field, and the front of the tongue was placed in 30% sucrose in phosphate buffer overnight. The following day, the tissue was embedded in OCT (Sakura Finetek USA, Inc., #4583). Serial sagittal sections of the tongue were collected onto SuperFrost Plus slides (Fisher). For antigen retrieval, sections were heat dried overnight, rehydrated, placed into citrate buffer (pH 6.0), heated for 15 min in a boiling water bath, and incubated for 10 min at RT. The slides were washed in PBS and incubated overnight at RT in 1:100 rat anti-TROMA-I antibodies (Developmental Studies Hybridoma Bank, http://dshb.biology.uiowa.edu) in PBS. The next day, the slides were rinsed in PBS (3×5 min), and the tissue was incubated in anti-rat Alexa 555 secondary antibodies (Molecular Probes) for two hours at RT. After washing in PBS (3×5 min), the slides were dehydrated, cleared in Citrisolv (a xylene substitute), and cover-slipped using DPX mounting medium (Fluka). The sections were examined in order, and the taste buds were followed across sections so that each taste bud was only counted once.
Adult circumvallate papillae were embedded in paraffin, serially sectioned in the coronal plane at 8 μm, and stained with hematoxylin and eosin. Each section containing the circumvallate papilla was examined for taste buds that contain pores. The total number of taste bud profiles and the number of taste buds with pores were recorded for each section. Taste buds were followed across sections so that a pore split between sections was only recorded one time. The number of taste buds with pores was totaled across all the sections for each animal to determine total number of circumvallate taste buds. The experimenter was blind to the genotype of the animal during quantification.
Data analysis
The total neuron numbers and total volumes were compared between genotypes on embryonic days E11.5, E12.5, E14.5, E16.5, and E18.5 using a two-way analysis of variance (ANOVA). The cell cycle, cell death, and fungiform taste bud data were analyzed using a two-way repeated measures ANOVA (one factor repetition). A Bonferroni correction was used to compare individual means as necessary. The circumvallate taste bud numbers were compared using t-tests. The alpha levels were set at p≤0.05 for all statistical comparisons. The data are described as mean±S.E.M. in the test and figures.
Results
NT-4 dependence begins early in embryonic development
In the absence of NT-4, the geniculate ganglia are missing approximately half of their neurons at birth (Liu et al., 1995). To gain insight into the developmental period when these neurons initially become NT-4 dependent, we quantified the total number of neurons in the ge-niculate ganglion from E10.5 until E18.5 for wild type and Ntf4−/− mice (Fig. 1). A TUJ1-labeled neuronal cell body with a clear nucleus was used as the criteria to identify neurons (Fig. 1G). For geniculate ganglia developing in the absence of NT-4, the losses began early. By E11.5, Ntf4−/− mice lost 33% of the geniculate ganglion neurons compared to wild type mice (p≤0.02, Fig. 2A). To determine whether the cell losses were specific to neurons we quantified non-neuronal cells at this same age. We found no difference in the number of non-neurons between wild type (482±77) and Ntf4−/− (532.5± 84) embryos on E11.5 of development. This indicates that NT4 does not influence the development of neuronal precursors or neural crest derived glia precursors or glia, both of which should be present in the ganglia at this age (Harlow et al., 2011). The neuronal losses which were first observed at E11.5 were verified using a stereological counting method, the optical fractionator. This approach has the advantage of being considered more reliable because fewer assumptions are made about the tissue (Coggeshall, 1992). However, it also has the disadvantage in that the TUJ1 antibody for labeling neurons could not penetrate the tissue at thicknesses required for stereology. Instead, neurons were identified based on morphological and Nissl staining characteristics, which may be less reliable for identifying neurons than a specific label. Using this method, 19% of the geniculate ganglion neurons were lost by E11.5 in Ntf4−/− (1371.0±60) mice compared to wild type mice (1684.2±55; p≤0.02). This finding confirms that neurons within the geniculate ganglion are lost by E11.5 in the Ntf4−/− ganglia. Neuronal numbers continued to decrease in the absence of NT-4 until E16.5, when a total loss of 55% of the geniculate ganglion neurons was observed compared to wild type littermates (p≤0.001; Fig. 2A), and a 63% loss was observed compared to E11.5 Ntf4−/− mice (p≤0.001). At E18.5, there was a 46% loss in the number of geniculate ganglion neurons compared to wild type littermates (p≤0.001, Fig. 2A). There was not a significant difference in the total neuronal cell numbers in Ntf4 mutant ganglia between E16.5 and E18.5, therefore, neuron loss in Ntf4−/− mice has ceased by E16.5. Together these findings demonstrate that neurons of the geniculate are lost continually between E11.5 and E16.5 in the absence of NT-4.
Fig. 2.
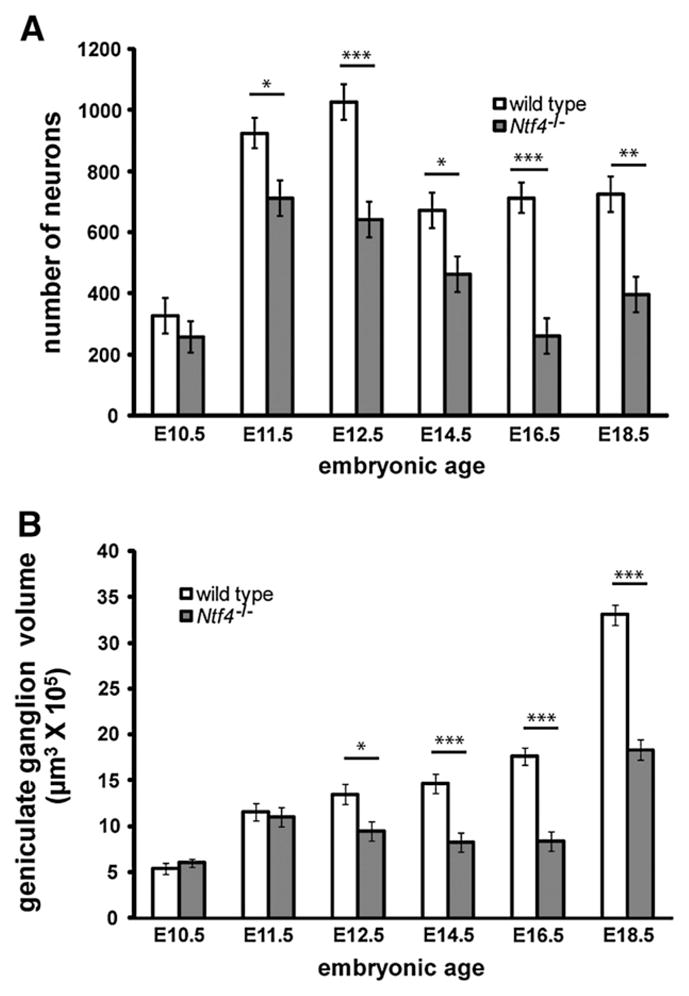
Ntf4−/− mice lose geniculate ganglion neurons between E10.5 and E11.5, and these losses continue to increase until E18.5 of development. Total neuron numbers were plotted at E11.5, E12.5, E14.5, E16.5 and E18.5 in Ntf4−/− and wild type mice (A). The first significant loss in neuron number in Ntf4−/− mice occurred at E11.5, and losses continued until E16.5 of development. The reduction in geniculate ganglion volume in Ntf4−/− compared with wild type mice did not occur until E12.5; however, following this age, reductions in geniculate volume reflected the losses in neuron number (B) versus wild type. *p≤0.05, **p≤0.01 and ***p≤0.001.
The volume of the geniculate ganglion was also reduced in embryonic Ntf4−/− mice. As with cell counts, we measured the volume at E11.5 using two different methods: in TUJ-1 labeled paraffin sections and with a stereological method. At E11.5, there was no significant difference in volume of the geniculate ganglion (p≤0.69) between wild type and Ntf4−/− mice in the TUJ-1 paraffin sectioned ganglion (Fig. 2B). However, we observed a 22% decrease in total geniculate ganglion volume by E11.5 in Ntf4−/− (27.3±1.5 μm3×105) compared to wild type mice (35±1.6 μm3×105; p≤0.02) using the Cavalieri estimation. Because the Cavalieri estimation requires the use of minimally dehydrated tissue, the estimated volumes using this method are closer to the actual volumes. Beginning at E12.5, the geniculate ganglion appeared noticeably smaller (Fig. 1C and D), it existed in fewer sections per embryo head, and the ganglia were 30% smaller in volume (p≤0.01, Fig. 2B) compared to wild type littermates. Similar to neuron numbers, the size of the geniculate ganglion is substantially reduced in Ntf4−/− mice at E16.5 (8.3± 0.3 μm3×105) with a 53% loss in volume compared to wild types. By E18.5, the geniculate ganglia in Ntf4−/− embryos were visibly larger than the knockout ganglia at E16.5 (Fig. 1J) with a 55% increase in volume (Fig. 2B; p≤0.001). This increase in ganglion size is likely due to increases in the size of neurons between these ages. Although there was an increase in Ntf4−/− ganglia volume between E16.5 and E18.5, the E18.5 ganglia of Ntf4−/− mice were still 45% smaller than those of their wild type littermates (Fig. 2B; p≤0.001). Generally, geniculate volume losses appear to reflect the substantial neuron loss observed in Ntf4−/− mice.
To determine whether a particular size range of cells was preferentially lost in the absence of NT-4, we measured the neuronal cell sizes in wild type and Ntf4−/− ganglia at E18.5. No size difference was observed for the surviving geniculate neurons in Ntf4−/− mice (133±6.1 μm2) compared with neurons in the wild type ganglia (123±4.1 μm2). These findings suggest that NT-4 dependence begins early in ganglion development and is equally distributed among various geniculate ganglion cell size populations.
NT-4 removal regulates cell death independent of caspase-3 activation
NT-4 regulates neurons during peak geniculate neuron proliferation (Altman and Bayer, 1982) and before peak cell death (Carr et al., 2005). Because neurotrophins are capable of regulating proliferation and early exit from the cell cycle (Farinas et al., 1996, 2002; Huang and Reichardt, 2001) in addition to cell death, we wanted to determine if NT-4 could have this function in the geniculate ganglion. To test whether NT-4 influences neuronal precursor proliferation or cell cycle exit in the geniculate ganglion, we injected pregnant mice with two BrdU analogues that could be independently detected (Fig. 3; Vega and Peterson, 2005). Because CldU was injected at E10.0 and IdU was injected at E11.5, the single-labeled cells indicate the rate of proliferation at these two ages, while double-labeled cells are those that remained in the cell cycle at both time points. We found no differences in either the number CldU-labeled neurons in Ntf4−/− mice (304±31/μm3×10−6) compared with wild types (252±10/μm3×10−6; p≤0.25) or in the number of IdU-labeled cells in Ntf4−/− mice (226±41/μm3×10−6) compared with wild types (203±20/μm3×10−6). This finding indicates that proliferation in the geniculate ganglion was unaffected by the removal of NT-4 at both E10 and E11.5. Furthermore, we observed no difference in the number of double-labeled cells between Ntf4−/− mice (85±28/ μm3×10−6) and wild type (60±1.3/μm3×10−6) littermates, indicating that the rate of exit from the cell cycle is unaffected by NT-4 removal at these ages. Therefore, we conclude that neither proliferation nor early exit from the cell cycle is responsible for the neuronal loss observed by E11.5 in Ntf4−/− mice.
Fig. 3.
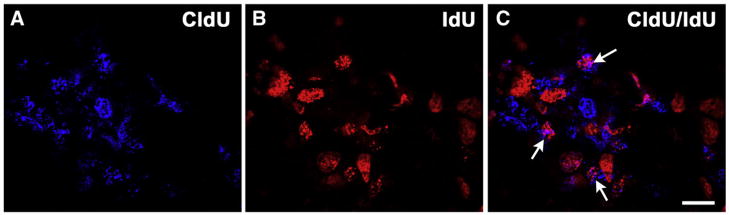
Cells labeled with CldU (A) are dividing at E10.5, while cells labeled with IdU (B) are dividing at E11.5. Double-labeled cells (C, arrows) were those cells that were dividing at E10.5 and remained in the cell cycle at E11.5. Scale bar in C=10 μm.
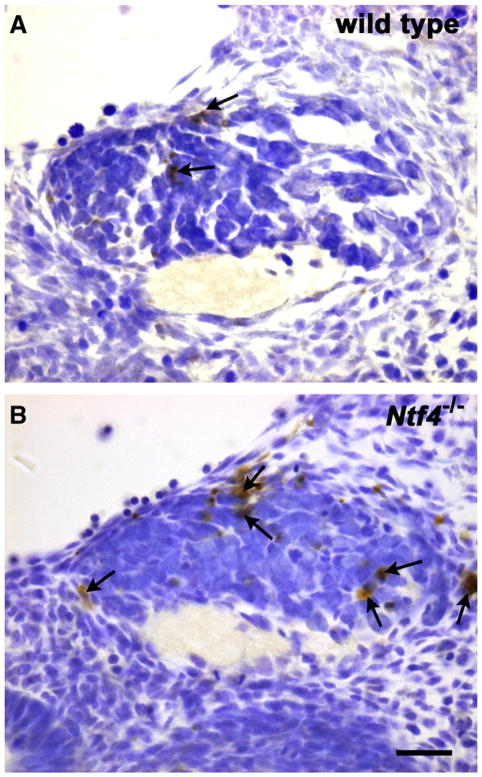
Because the neuronal loss in the geniculate ganglion by E11.5 in Ntf4−/− mice was not due to reduced proliferation or early exit from the cell cycle, we hypothesized that the cell loss was the result of increased cell death. To test this hypothesis, we quantified the number of TUNEL-labeled cells in the geniculate ganglion at E11.5 (Fig. 4). The geniculate ganglion of Ntf4−/− mice had substantially more TUNEL-labeled profiles (195±31/μm2×10−6) than wild type littermates (78±9/μm2×10−6; p≤0.01), indicating that mice lacking NT-4 had substantially increased cell death in this region. Thus, NT-4 functions to rescue neurons during the earliest wave of cell death (when cell numbers are also being regulated by proliferation in the ganglion) and before the second wave of cell death, which is associated with target innervation.
Fig. 4.
TUNEL-labeled neurons (arrows) indicate dying cells in wild type (A) and Ntf4−/−(B) geniculate ganglia on E11.5 of development. Ntf4−/− geniculate ganglia appear to contain more dying cells than wild type ganglia.
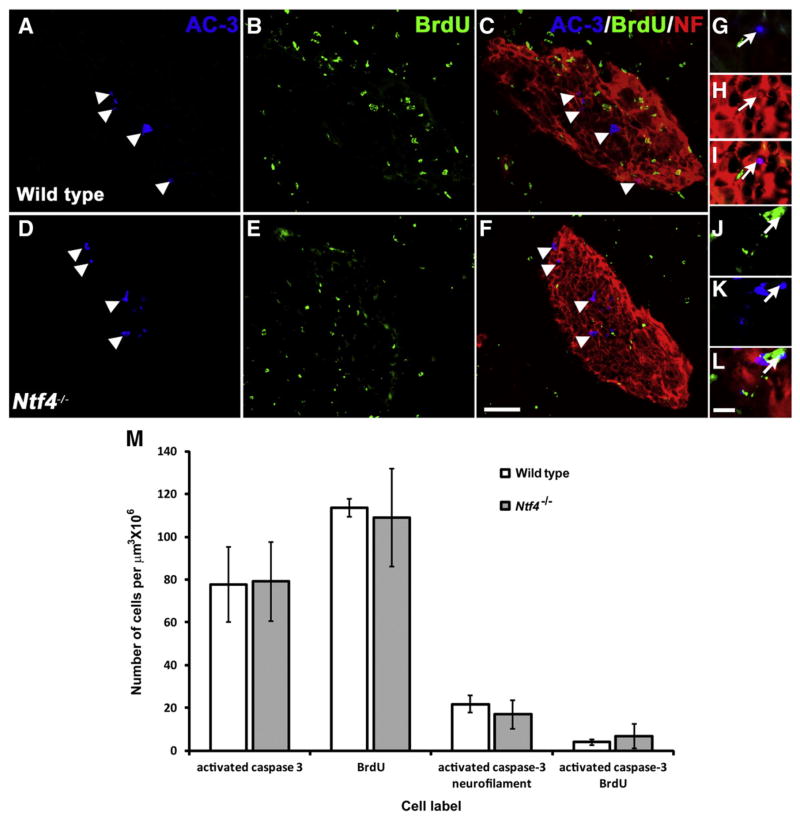
BDNF influences cell survival by preventing caspase-3 activation in differentiated neurons (Patel and Krimm, 2010). Caspase-3 is a member of the pro-apoptotic caspase family that is synthesized as an inactive pro-enzyme and is processed to its active form only in cells undergoing apoptosis. Thus, it functions as a marker for developmental cell death (De Zio et al., 2005). To determine if NT-4 also regulates cell survival by inhibiting caspase-3 activation, we examined the number of cells containing activated caspase-3 in Ntf4−/− mice compared to wild type mice (Fig. 5). Differentiated neurons were visualized by labeling with anti-neurofilament, which is an intermediate filament found specifically in neurons (Debus et al., 1982; Shaw et al., 1984). Ganglion cells actively dividing and forming the precursor pool were labeled and quantified using BrdU, a cell proliferation marker. In the absence of NT-4, there was no significant change in the total number of geniculate ganglion cells positively labeled for activated caspase-3 compared to wild type littermates (p≤0.9, Fig. 5M). In addition, there was no difference between the total numbers of BrdU positive cells in Ntf4−/− ganglia compared to wild type embryos (p≤0.8, Fig. 5M). Unlike at E13.5 (Patel and Krimm, 2010), at E11.5 there was some co-labeling for activated caspase-3 and BrdU, indicating that neuronal precursors were dying (Fig. 5J, K and L). In addition, we observed activated caspase-3 co-localized with anti-neurofilament staining, which indicated that early differentiated neurons were also in the process of dying (Fig. 5G, H and I); however, the number of double-labeled cells were not significantly different between Ntf4−/− and wild type ganglia (p≤0.79 for both). In addition, no cells co-labeled for neurofila-ment and BrdU, indicating that dividing cells do not contain neurofilaments in the geniculate ganglion at this age. Together, these findings suggest that NT-4 does not regulate cell survival by inhibiting caspase-3 activation and, therefore, must inhibit a different cell death-signaling pathway to mediate neuronal survival during development.
Fig. 5.
Triple immuno-fluorescence labeling was used to identify dying cells (blue: rabbit polyclonal anti-activated caspase-3 (AC-3)), dividing cells (green: rat monoclonal anti-BrdU), and differentiated neurons (red: anti-neurofilament (NF)) in the developing geniculate ganglia of wild type (A–C) and Ntf4−/− (D–F) mice at E11.5. Several cells are positively labeled for the cell death marker, AC-3 (white arrowheads, A, C, D, F). A composite image for all three antigens allowed for the study of co-labeled populations (C, F). A high magnification image of a cell double-labeled for NF and AC-3 is shown at the magnification used for counting (white arrows, G–I). A few cells were labeled with both BrdU and AC-3 (white arrows, J–L) in wild type and Ntf4−/− mice, indicating that precursors were dying at E11.5. No differentiated neuronal cells were still dividing. Scale bar in F=50 μm and applies to A–F; scale bar in L=10 μm and applies to applies to G–I. Quantification of these labels demonstrates that NT-4 does not influence cell death by activating caspase-3 at E11.5 (M). Number of geniculate ganglion cells positively labeled for the antigens AC-3, BrdU and double-labeled for NF and AC-3 was plotted for wild type and Ntf4−/− embryos. The same number of cells was positively labeled for AC-3 in Ntf4−/− compared to wild type ganglia. No significant difference was observed in the number of cells positively labeled for BrdU or double-labeled with BrdU and AC-3 between wild type and Ntf4−/− ganglia. Thus, the neurotrophin NT-4 regulates geniculate ganglion cell death without activating caspase-3 at E11.5.
NT-4 does not function as a target derived neurotrophic factor
Neurotrophins can influence neuron development from multiple locations, the ganglion itself, along the projection pathway and from the target. At E12.5 NT-4 is expressed in most of these locations (Huang and Krimm, 2010). To determine the extent to which axons innervate the tongue when neurons become NT-4 dependent, we examined the axonal projections of the geniculate ganglion on E11.5. The innervation pattern in the tongue was observed using the neuronal marker anti-neurofilament in whole mount tongues and in sections (Fig. 6C). By E11.5, the axons from invading neurons were just entering the base and the lateral edge of the tongue (Fig. 6A and B). A few fibers were adjacent to the lingual epithelial surface at the lateral edges of the tongue (Fig. 6C). The final targets for these neurons are the fungiform placodes, which line up along the mid-line and are clustered at the tongue tip. We saw no evidence that nerve fibers come anywhere near the locations where fungiform placodes will develop by E13.5. This pattern of innervation is similar to what has been observed in rats at an equivalent age (Rochlin and Farbman, 1998; Rochlin et al., 2000). Thus, during the time when geniculate neurons become NT-4 dependent, the source of NT-4 may be the geniculate ganglion itself, the projection pathway to the tongue, the mesenchyme of the tongue, or even the lingual epithelium. However, the source of NT-4 is not the fungiform placodes. These findings demonstrate that NT-4 does not function as a classic target-derived neuro-trophin at this age, but instead influences neurons in the ganglion itself or along the projection pathway before target innervation.
Fig. 6.
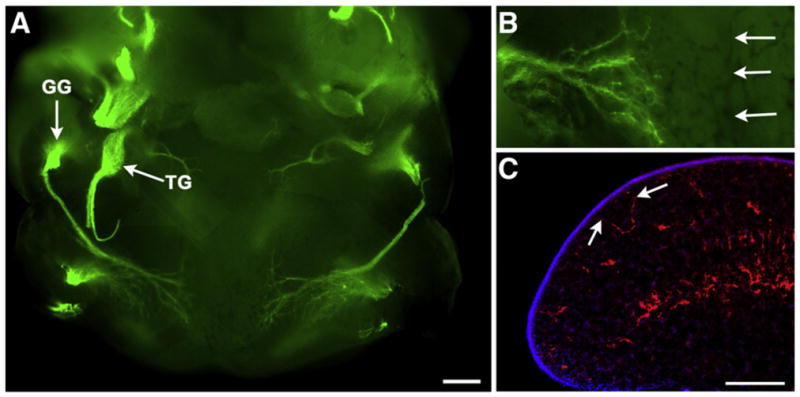
Lower jaw whole mount from an E11.5 mouse embryo labeled with anti-neurofilament (green, A, B). The geniculate ganglion (GG) and the trigeminal ganglion (TG) are brightly labeled green. Fibers from the geniculate ganglion can be seen entering the lateral edges of the tongue. At higher magnification we did not see any fibers nearing the tongue midline (B, arrows). In sections through the lateral edges of the tongue (C), some labeled fibers (anti-neurofilament, red) were seen nearing the epithelial surface (arrows, DAPI, blue). Scale bar in A=300 μm; scale bar in C=200 μm and also applies to B.
NT-4 does not regulate postnatal geniculate neuron or taste bud development
A previous study demonstrated a postnatal loss of fungiform papillae in Ntf4−/− mice (Liebl et al., 1999) and, therefore, postulated that there may be a second postnatal wave of NT-4-dependent geniculate neuron loss. Because this decrease in papilla number was found to occur between postnatal days 20 and 60, we quantified geniculate neuron numbers using optical dissector at these two ages in Ntf4−/− and wild type mice. At postnatal day 20, the geniculate ganglion of Ntf4−/− mice had 48% fewer geniculate neurons than the wild type littermates (Ntf4−/−, 566±29 vs. wild type, 1092±124, p≤0.01). In addition, on postnatal day 60, there was a 40% decrease in the number of geniculate ganglion neurons between Ntf4−/− (815±71) and wild type (1358±260) mice. Although neuron numbers in the geniculate ganglion may increase postnatally, the relative losses in Ntf4−/− mice ranged between 40% and 48% from E18.5 until postnatal day 60 compared to wild type mice. There do not appear to be any additional postnatal losses in geniculate neuron numbers in Ntf4−/− mice compared with wild type controls.
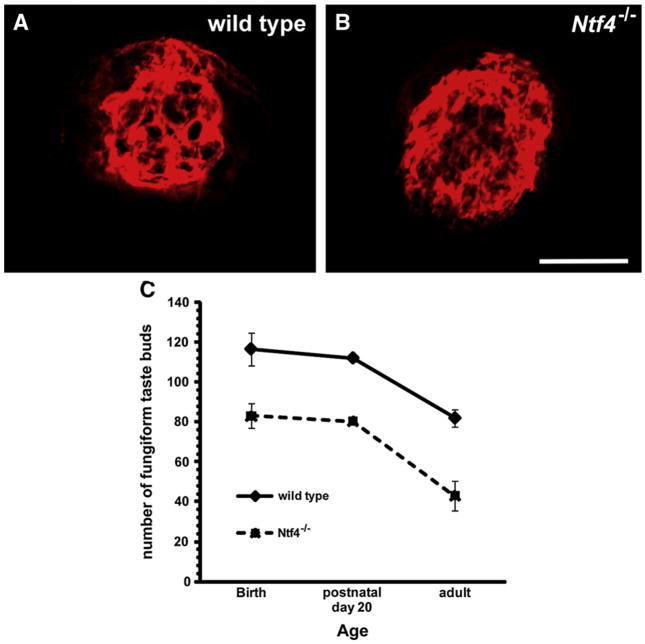
A postnatal loss of fungiform papillae (Liebl et al., 1999), could be indirect evidence of a postnatal loss in fungiform taste buds. To examine this possibility we quantified anti-TROMA-I-labeled fungiform taste buds at birth, postnatal day 20, and in adulthood (Fig. 7A and B). At birth, we observed fewer taste buds in Ntf4−/− mice than in wild type mice (p≤0.001). Interestingly, this difference was maintained throughout postnatal development, but did not increase as development continued. Furthermore, wild type (p≤0.02) and Ntf4−/− mice (p≤0.002) lost taste buds between postnatal day 20 and adulthood. Because this decrease occurred to an equivalent degree in both genotypes, this loss was not attributed to the absence NT-4. Instead, it is another developmental phenomenon that occurs to an equivalent degree regardless of the presence or absence of NT-4. We conclude that the loss of taste buds at birth in Ntf4−/− mice is likely caused by a loss of gustatory innervation due to the early loss of gustatory neurons from the geniculate ganglion and that NT-4 does not regulate the taste system during postnatal development.
Fig. 7.
By adulthood, anti-TROMA-1-labeled taste buds do not appear different in size between wild type (A) and Ntf4−/− (B) tongues. However, fewer taste buds remained in Ntf4−/− mice at every developmental age examined (C). Scale bar=20 μm and applies to A and B.
Previous reports have shown little effect of NT-4 removal on the circumvallate papillae, suggesting that NT-4 regulates geniculate but not petrosal taste neurons (Liebl et al., 1999). To examine this assertion, we quantified the size of the circumvallate papilla in Ntf4−/− mice compared with wild type controls. We found that Ntf4−/− mice had shorter (length, Ntf4−/− =405±6, wild type=468±25, p≤0.03) and narrower (width, Ntf4−/−=365±7, wild type=414± 10, p≤0.004) circumvallate papillae than those in wild type mice. These smaller circumvallate papillae also contained fewer taste buds (Ntf4−/− =187±7, wild type=307±12, p≤0.004). Therefore, it is likely that the neurons previously shown to be lost in the petrosal ganglion in Ntf4−/− mice (Erickson et al., 1996) are gustatory.
Discussion
NT-4 is required for the survival of half of the geniculate ganglion neurons by birth (Liu et al., 1995). Here, we demonstrate that NT-4 influences geniculate neuron numbers by E11.5, which is early in ge-niculate ganglion development. Neurons of the geniculate ganglion arise from the epibranchial placodes, which de-laminate around E9 in the mouse (Baker and Bronner-Fraser, 2001). By E11.5 in the mouse, the ganglion shows some cytological differentiation and becomes a spherical structure (E13.5 in rat; Altman and Bayer, 1982). Peak proliferation in the geniculate ganglion occurs at approximately E10–E11 in mice, based on rat data (Altman and Bayer, 1982); the proliferation at this age is consistent with the dramatic increase we observed in neuron number between E10.5 and E11.5, just after peak proliferation. An early wave of geniculate ganglion cell death also appears to immediately follow and overlaps this early period of proliferation (Carr et al., 2005). Terminal target innervation occurs later (between E13.5 and E15) in mice (Harada et al., 2000; Lopez and Krimm, 2006b; Mbiene and Mistretta, 1997). Therefore, NT-4 is essential for cell survival during the first wave of cell death, which occurs during peak cell proliferation in the ganglion and before target innervation.
Using whole mount immunohistochemistry we illustrate that by E11.5 fibers from the chorda tympani have not yet reached the developing placodes, this rules out the fungiform placodes as a source of NT-4 at this stage of development. Consistent with this observation BDNF is expressed in developing placodes, while NT-4 expression cannot be localized to the placode (Nosrat and Olson, 1995; Nosrat et al., 1996). During this early developmental period, NT-4 levels are relatively high in the epithelium but also in the tongue mesenchyme (Huang and Krimm, 2010). The chorda tympani fibers grow through the tongue mesenchyme and have already branched toward the epithelial surface at the lateral edge of the tongue by E11.5. As these fibers grow through the tongue they come in contact with various tissue fields each with a different neurotrophin content (Al-Hadlaq et al., 2003). NT-4 is present in the tongue mesenchyme (Huang and Krimm, 2010) and cooperates with BDNF to encourage chorda tympani branching to the tongue surface (Ma et al., 2009); therefore, NT-4 from the mesenchyme may also be preventing neuron cell death in the ganglion. However, NT-4 is also expressed at high levels in the epithelium and the geniculate ganglion itself and all of these NT-4 sources may function synergistically to support geniculate neuron survival.
In addition to depending on NT-4 for survival during development, geniculate neurons are also supported by BDNF (Conover et al., 1995; Hoshino et al., 2010; Ito and Nosrat, 2009; Ito et al., 2010; Krimm et al., 2001; Mistretta et al., 1999; Nosrat and Olson, 1998; Nosrat et al., 2004; Oakley et al., 1998; Patel et al., 2010; Sun and Oakley, 2002); however, the timing of this support is different. BDNF begins to support geniculate neuron survival as gustatory axons are innervating fungiform placodes. The mechanism also appears to be different; BDNF removal results in an increase in activated caspase-3 labeled cells during target innervation (Patel and Krimm, 2010), while NT-4 removal did not affect the number of activated caspase-3-labeled cells. This implies that NT-4 regulates cell survival through a different mechanism than BDNF. Both BDNF and NT-4 function via the same receptors to regulate gustatory development (Fritzsch et al., 1997; Krimm, 2006), TrkB and p75. The TrkB receptor is particularly important for geniculate neuron survival (Fritzsch et al., 1997) and is expressed at high steady levels in the geniculate ganglion throughout embryonic development (Huang and Krimm, 2010) and adulthood (Matsumoto et al., 2001). Because BDNF and NT-4 regulate cell death through a different mechanism, these two neurotrophins may be capable of activating different signaling pathways via the same receptors (Minichiello et al., 1998).
There are a number of different possible pathways by which NT-4 could influence geniculate neuron death (Vaghefi et al., 2004; Yu et al., 2003; Yuan and Kroemer, 2010; Zheng et al., 2000). More than one of these pathways involves the activation of caspases (Zheng et al., 2000). In the mitochondrial dependent pathway, caspase activation involves a mitochondria release of cytochrome c, which activates caspases resulting in cell death (Zheng et al., 2000). Caspase 3 activation frequently underlies cell death in this pathway. However, when caspase 3 is blocked in vitro (Vaghefi et al., 2004) or eliminated by mutation in vivo (Oppenheim et al., 2001; Yaginuma et al., 2001) cell death still occurs indicating that other cell death mechanisms can compensate for caspase 3 activation. Caspase 6 is also activated following NGF withdrawal and is important for neuronal cell death (Monnier et al., 2011; Troy et al., 2002). Caspases 2 and 7 have also been shown to result in cell death following neurotrophin withdrawal (Yu et al., 2003). Specifically, following GDNF deprivation, sympathetic neurons die using a different pathway than for NGF-deprivation. Sympathetic neurons do not die because of cytochrome c release from mitochondria or activation of caspase 3; instead, caspases 2 and 7 were activated. Thus, a number of different caspases may substitute for one another to cause developmental cell death, NT-4 likely prevents activation of caspases other than caspase 3 (Monnier et al., 2011; Vaghefi et al., 2004). However, it is also the case that in vitro that application of a broad-spectrum of caspase inhibitors also does not eliminate cell death (Vaghefi et al., 2004) suggesting that non-caspase-dependent pathways also regulate cell death, and could regulate geniculate neuron cell death in Ntf4−/− mice.
The role of NT-4 appears to vary considerably in different sensory systems. For example, while we have shown here that NT-4 prevents early cell death in the geniculate ganglion, NT-4 maintains a class of hair follicle receptors postnatally (Stucky et al., 1998). Similarly, it has been postulated that NT-4 has a role in regulating the gustatory system later in development (Liebl et al., 1999). However, we found that neurons and taste buds were lost only during embryonic development, demonstrating that there is no postnatal role for NT-4 in the fungiform taste system. All of these studies examined the role on NT-4 using full knockouts which means that NT-4 is not present during development. It is possible that postnatal effects of NT-4 removal are blocked by the early neuronal loss seen in NT-4 knockouts. We think this is unlikely because NT-4 expression is high early in embryonic gustatory system and then decreases rapidly to practically non-detectable levels by birth (Huang and Krimm, 2010). However, a conditional mutant allowing NT-4 removal in adulthood would be required to address this issue. In summary, NT-4 supports early embryonic survival for the developing gustatory system. Given that removal of NT-4 has very different effects in other sensory systems, the taste system may be the only peripheral system where NT-4 functions to regulate early neuron survival.
In the geniculate ganglion, it has been well-established that the cells lost after NT-4 removal are gustatory neurons that innervate the fungiform field of the tongue and the palate (Patel et al., 2010). The circumvallate papilla of the tongue is innervated by the petrosal ganglion and neurons in the petrosal also require NT-4 for survival (Conover et al., 1995; ElShamy and Ernfors, 1997). Although NT-4 supports gustatory neurons in the geniculate ganglion, it is unclear whether the neurons lost in the petrosal ganglion are gustatory. Although NT-4 deletion has been reported to have minimal effect on the circumvallate papilla (Liebl et al., 1999), no one has actually quantified circumvallate taste buds in Ntf4−/− mice. Here, we observed a reduction in the circumvallate size and a loss of taste buds in Ntf4−/− mice, indicating that NT-4 supports the development of gustatory neurons in the petrosal ganglion. Non-gustatory petrosal neurons are likely not supported by NT-4 (Erickson et al., 1996).
Consistent with what we observed for the geniculate ganglion, in the petrosal ganglion NT-4 mutant mice also show an earlier loss of neurons compared with bdnf−/− mice (ElShamy and Ernfors, 1997). However, these losses occur much later in the petrosal for both mutants than they do for the geniculate ganglion. This difference could be due to a difference in time of the development and innervation for the petrosal compared with the geniculate, but it could also be that these neurotrophins have slightly different roles in the development of this ganglion. Consistent with this second idea, proliferation is decreased in the petrosal ganglion of Ntf4−/− mice compared to wild type mice (ElShamy and Ernfors, 1997). In two separate experiments we did not observe any difference in proliferation in the geniculate ganglion following NT4 removal. Thus, even for these two gustatory ganglia, NT-4 may function differently during embryonic development.
Here we have determined the time course (Fig. 8) and mechanism of NT-4 regulation of the geniculate ganglion. These findings add substantially to the literature which focuses on BDNF (Al-Hadlaq et al., 2003; Harlow et al., 2011; Hoshino et al., 2010; Krimm et al., 2001; Liebl et al., 1999; Lopez and Krimm, 2006b; Ma et al., 2009; Mistretta et al., 1999; Nosrat and Olson, 1995; Nosrat et al., 1997, 2004; Patel and Krimm, 2010; Patel et al., 2010; Sun and Oakley, 2002; Vilbig et al., 2004; Yamout et al., 2005; Zhang et al., 1997) and together these studies provide a clearer picture of the role of these neurotrophins during embryonic gustatory development. Both BDNF and NT-4 are expressed at high levels in the early embryonic ganglion (Harlow et al., 2011; Huang and Krimm, 2010) and ganglionic BDNF influences the rate of development of gustatory neurons (Harlow et al., 2011). By E11.5, geniculate axons are growing through the tongue mesenchyme, where they come in contact with NGF, BDNF, NT-3 and NT-4 (Huang and Krimm, 2010; Nosrat et al., 2001). These neurotrophins influence the development of the physiological characteristics of the neurons (Al-Hadlaq et al., 2003) and BDNF and NT-4 function interchangeably to support branching toward the tongue surface (Ma et al., 2009). At this early age (E11.5), NT-4 in the geniculate ganglion, mesenchyme, or even the lateral embryonic epithelium, but not the target, supports neuron survival during an early wave of cell death (Carr et al., 2005). Unlike target-derived neurotrophins, NT-4 does not regulate survival by preventing activated caspase-3 activation. Although proliferation within the ganglion is still robust at the onset of NT-4-dependency, NT-4 does not influence cell proliferation.
Fig. 8.
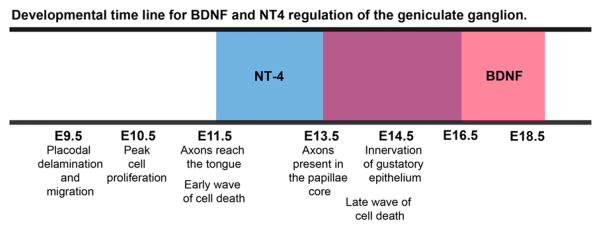
A developmental time line describing when BDNF and NT-4 influence geniculate ganglion development. Below the time line are descriptions of developmental changes occurring within the geniculate ganglion at each age. The time period during which geniculate neurons are NT-4 dependent is represented by the blue box and the time period during which geniculate neurons are BDNF dependent is represented by the red box. BDNF and NT-4 support of the geniculate ganglion overlaps between E13.5 and E16.5. Notice that NT-4 starts early before target innervation unlike BDNF which begins its influence around the time of target innervation. Also, both BDNF and NT-4 have a similar duration of influence on development of the taste system.
As gustatory axons innervate their targets, NT-4 is down regulated (Huang and Krimm, 2010) and gustatory neurons come in contact with substantial amounts of BDNF from the target (Huang and Krimm, 2010; Nosrat and Olson, 1995; Nosrat et al., 1996). BDNF is chemoattractive (Hoshino et al., 2010) and its normal pattern of expression is required for target innervation (Lopez and Krimm, 2006a; Ma et al., 2009; Ringstedt et al., 1999). BDNF from the target supports gustatory neuron survival by preventing Bax and activated caspase-3 dependent cell death beginning at E13.5 (Patel and Krimm, 2010). NT-4 and BDNF both support the survival of geniculate neurons during target innervation between E13.5 and E16.5. Therefore, geniculate neurons do not simply switch from NT-4 to BDNF dependence at a specific age (Fig. 8). Instead, they are dependent on both neurotrophins for a period of embryonic development. NT-4 is required for normal development of the taste system until E16.5 but has no obvious influence on postnatal maintenance of taste bud or gustatory neurons, while the postnatal role for BDNF in gustatory development is still unclear.
Acknowledgments
We thank Michelle E. Smith for her technical support. This work was supported by the National Institutes of Health Grant DC007176 (RFK).
References
- Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Agerman K, Baudet C, Fundin B, Willson C, Ernfors P. Attenuation of a caspase-3 dependent cell death in NT4- and p75-deficient embryonic sensory neurons. Mol Cell Neurosci. 2000;16:258–268. doi: 10.1006/mcne.2000.0875. [DOI] [PubMed] [Google Scholar]
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–1491. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- Al-Hadlaq SM, Bradley RM, MacCallum DK, Mistretta CM. Embryonic genic-ulate ganglion neurons in culture have neurotrophin-specific electrophysiological properties. Neuroscience. 2003;118:145–159. doi: 10.1016/s0306-4522(02)00814-x. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Adv Anat Embryol Cell Biol. 1982;74:1–90. doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Carr VM, Sollars SI, Farbman AI. Neuronal cell death and population dynamics in the developing rat geniculate ganglion. Neuroscience. 2005;134:1301–1308. doi: 10.1016/j.neuroscience.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;15:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, et al. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- De Zio D, Giunta L, Corvaro M, Ferraro E, Cecconi F. Expanding roles of programmed cell death in mammalian neurodevelopment. Semin Cell Dev Biol. 2005;16:281–294. doi: 10.1016/j.semcdb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Debus E, Flugge G, Weber K, Osborn M. A monoclonal antibody specific for the 200 K polypeptide of the neurofilament triplet. EMBO J. 1982;1:41–45. doi: 10.1002/j.1460-2075.1982.tb01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElShamy WM, Ernfors P. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 complement and cooperate with each other sequentially during visceral neuron development. J Neurosci. 1997;17:8667–8675. doi: 10.1523/JNEUROSCI.17-22-08667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci. 1996;16:5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primary sensory neurons in vivo. J Neurosci. 2001;21:581–589. doi: 10.1523/JNEUROSCI.21-02-00581.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Farinas I, Yoshida CK, Backus C, Reichardt LF. Lack of neurotrophin-3 results in death of spinal sensory neurons and premature differentiation of their precursors. Neuron. 1996;17:1065–1078. doi: 10.1016/s0896-6273(00)80240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Cano-Jaimez M, Bellmunt E, Soriano M. Regulation of neurogenesis by neurotrophins in developing spinal sensory ganglia. Brain Res Bull. 2002;57:809–816. doi: 10.1016/s0361-9230(01)00767-5. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Sarai PA, Barbacid M, Silos-Santiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int J Dev Neurosci. 1997;15:563–576. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Finana M, Cruz-Orive LM, Mackay CE, Pakkenberg B, Roberts N. Comparison of MR imaging against physical sectioning to estimate the volume of human cerebral compartments. NeuroImage. 2003;18:505–516. doi: 10.1016/s1053-8119(02)00021-6. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology—reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Harada S, Yamaguchi K, Kanemaru N, Kasahara Y. Maturation of taste buds on the soft palate of the postnatal rat. Physiol Behav. 2000;68:333–339. doi: 10.1016/s0031-9384(99)00184-5. [DOI] [PubMed] [Google Scholar]
- Harlow DE, Yang H, Williams T, Barlow LA. Epibranchial placode-derived neurons produce BDNF required for early sensory neuron development. Dev Dyn. 2011;240:309–323. doi: 10.1002/dvdy.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino N, Vatterott P, Egwiekhor A, Rochlin MW. Brain-derived neurotrophic factor attracts geniculate ganglion neurites during embryonic targeting. Dev Neurosci. 2010;32:184–196. doi: 10.1159/000313902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Krimm RF. Developmental expression of Bdnf, Ntf4/5, and TrkB in the mouse peripheral taste system. Dev Dyn. 2010;239:2637–2646. doi: 10.1002/dvdy.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Nosrat CA. Gustatory papillae and taste bud development and maintenance in the absence of TrkB ligands BDNF and NT-4. Cell Tissue Res. 2009;337:349–359. doi: 10.1007/s00441-009-0833-7. [DOI] [PubMed] [Google Scholar]
- Ito A, Nosrat IV, Nosrat CA. Taste cell formation does not require gustatory and somatosensory innervation. Neurosci Lett. 2010;471 (3):189–194. doi: 10.1016/j.neulet.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. 4. McGraw-Hill; New York: 2000. [Google Scholar]
- Krimm RF. Mice lacking the p75 receptor fail to acquire a normal complement of taste buds and geniculate ganglion neurons by adulthood. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1294–1302. doi: 10.1002/ar.a.20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm RF, Hill DL. Innervation of single fungiform taste buds during development in rat. J Comp Neurol. 1998;398:13–24. [PubMed] [Google Scholar]
- Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial over-expression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Dev Biol. 2001;232:508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Mbiene JP, Parada LF. NT4/5 mutant mice have deficiency in gustatory papillae and taste bud formation. Dev Biol. 1999;213:378–389. doi: 10.1006/dbio.1999.9385. [DOI] [PubMed] [Google Scholar]
- Liebl DJ, Klesse LJ, Tessarollo L, Wohlman T, Parada LF. Loss of brain-derived neurotrophic factor-dependent neural crest-derived sensory neurons in neurotrophin-4 mutant mice. Proc Natl Acad Sci U S A. 2000;97:2297–2302. doi: 10.1073/pnas.040562597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Epithelial overexpression of BDNF and NT4 produces distinct gustatory axon morphologies that disrupt initial targeting. Dev Biol. 2006a;292:457–468. doi: 10.1016/j.ydbio.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Refinement of innervation accuracy following initial targeting of peripheral gustatory fibers. J Neurobiol. 2006b;66:1033–1043. doi: 10.1002/neu.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lopez GF, Krimm RF. Epithelial-derived brain-derived neurotrophic factor is required for gustatory neuron targeting during a critical developmental period. J Neurosci. 2009;29:3354–3364. doi: 10.1523/JNEUROSCI.3970-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto I, Emori Y, Ninomiya Y, Abe K. A comparative study of three cranial sensory ganglia projecting into the oral cavity: in situ hybridization analyses of neurotrophin receptors and thermosensitive cation channels. Brain Res Mol Brain Res. 2001;93:105–112. doi: 10.1016/s0169-328x(01)00129-2. [DOI] [PubMed] [Google Scholar]
- Mbiene JP, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae: nerves follow distinctive and spatially restricted pathways. Acta Anat (Basel) 1997;160:139–158. doi: 10.1159/000148006. [DOI] [PubMed] [Google Scholar]
- Minichiello L, Casagranda F, Tatche RS, Stucky CL, Postigo A, Lewin GR, Davies AM, Klein R. Point mutation in trkB causes loss of NT4-dependent neurons without major effects on diverse BDNF responses. Neuron. 1998;21:335–345. doi: 10.1016/s0896-6273(00)80543-7. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Goosens KA, Farinas I, Reichardt LF. Alterations in size, number, and morphology of gustatory papillae and taste buds in BDNF null mutant mice demonstrate neural dependence of developing taste organs. J Comp Neurol. 1999;409:13–24. [PMC free article] [PubMed] [Google Scholar]
- Monnier PP, D’Onofrio PM, Magharious M, Hollander AC, Tassew N, Szydlowska K, Tymianski M, Koeberle PD. Involvement of caspase-6 and caspase-8 in neuronal apoptosis and the regenerative failure of injured retinal ganglion cells. J Neurosci. 2011;31:10494–10505. doi: 10.1523/JNEUROSCI.0148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrat CA, Olson L. Brain-derived neurotrophic factor mRNA is expressed in the developing taste bud-bearing tongue papillae of rat. J Comp Neurol. 1995;360:698–704. doi: 10.1002/cne.903600413. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Olson L. Changes in neurotrophin-3 messenger RNA expression patterns in the prenatal rat tongue suggest guidance of developing somatosensory nerves to their final targets. Cell Tissue Res. 1998;292:619–623. doi: 10.1007/s004410051092. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Ebendal T, Olson L. Differential expression of brain-derived neurotrophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates roles in gustatory and somatosensory innervation. J Comp Neurol. 1996;376:587–602. doi: 10.1002/(SICI)1096-9861(19961223)376:4<587::AID-CNE7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, MacCallum DK, Mistretta CM. Distinctive spatiotemporal expression patterns for neurotrophins develop in gustatory papillae and lingual tissues in embryonic tongue organ cultures. Cell Tissue Res. 2001;303:35–45. doi: 10.1007/s004410000271. [DOI] [PubMed] [Google Scholar]
- Nosrat IV, Agerman K, Marinescu A, Ernfors P, Nosrat CA. Lingual deficits in neurotrophin double knockout mice. J Neurocytol. 2004;33:607–615. doi: 10.1007/s11068-005-3330-2. [DOI] [PubMed] [Google Scholar]
- Oakley B, Brandemihl A, Cooper D, Lau D, Lawton A, Zhang C. The morphogenesis of mouse vallate gustatory epithelium and taste buds requires BDNF-dependent taste neurons. Brain Res Dev Brain Res. 1998;105:85–96. [PubMed] [Google Scholar]
- Oppenheim RW, Flavell RA, Vinsant S, Prevette D, Kuan CY, Rakic P. Programmed cell death of developing mammalian neurons after genetic deletion of caspases. J Neurosci. 2001;21:4752–4760. doi: 10.1523/JNEUROSCI.21-13-04752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AV, Krimm RF. BDNF is required for the survival of differentiated geniculate ganglion neurons. Dev Biol. 2010;340:419–429. doi: 10.1016/j.ydbio.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AV, Huang T, Krimm RF. Lingual and palatal gustatory afferents each depend on both BDNF and NT-4, but the dependence is greater for lingual than palatal afferents. J Comp Neurol. 2010;518:3290–3301. doi: 10.1002/cne.22400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstedt T, Ibanez CF, Nosrat CA. Role of brain-derived neurotrophic factor in target invasion in the gustatory system. J Neurosci. 1999;19:3507–3518. doi: 10.1523/JNEUROSCI.19-09-03507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin MW, Farbman AI. Trigeminal ganglion axons are repelled by their presumptive targets. J Neurosci. 1998;18:6840–6852. doi: 10.1523/JNEUROSCI.18-17-06840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin MW, O’Connor R, Giger RJ, Verhaagen J, Farbman AI. Comparison of neurotrophin and repellent sensitivities of early embryonic geniculate and trigeminal axons. J Comp Neurol. 2000;422:579–593. [PubMed] [Google Scholar]
- Shaw G, Debus E, Weber K. The immunological relatedness of neurofilament proteins of higher vertebrates. Eur J Cell Biol. 1984;34:130–136. [PubMed] [Google Scholar]
- Stucky CL, DeChiara T, Lindsay RM, Yancopoulos GD, Koltzenburg M. Neu-rotrophin 4 is required for the survival of a subclass of hair follicle receptors. J Neurosci. 1998;18:7040–7046. doi: 10.1523/JNEUROSCI.18-17-07040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Oakley B. Development of anterior gustatory epithelia in the palate and tongue requires epidermal growth factor receptor. Dev Biol. 2002;242:31–43. doi: 10.1006/dbio.2001.0526. [DOI] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons. Role of caspases. J Biol Chem. 2002;277:34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- Vaghefi H, Hughes AL, Neet KE. Nerve growth factor withdrawal-mediated apoptosis in naive and differentiated PC12 cells through p53/caspase-3-dependent and -independent pathways. J Biol Chem. 2004;279:15604–15614. doi: 10.1074/jbc.M311500200. [DOI] [PubMed] [Google Scholar]
- Vega CJ, Peterson DA. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat Methods. 2005;2:167–169. doi: 10.1038/nmeth741. [DOI] [PubMed] [Google Scholar]
- Vilbig R, Cosmano J, Giger R, Rochlin MW. Distinct roles for Sema3A, Sema3F, and an unidentified trophic factor in controlling the advance of geniculate axons to gustatory lingual epithelium. J Neurocytol. 2004;33:591–606. doi: 10.1007/s11068-005-3329-8. [DOI] [PubMed] [Google Scholar]
- Yaginuma H, Sato N, Homma S, Oppenheim RW. Roles of caspases in the programmed cell death of motoneurons in vivo. Arch Histol Cytol. 2001;64:461–474. doi: 10.1679/aohc.64.461. [DOI] [PubMed] [Google Scholar]
- Yamout A, Spec A, Cosmano J, Kashyap M, Rochlin MW. Neurotrophic factor receptor expression and in vitro nerve growth of geniculate ganglion neurons that supply divergent nerves. Dev Neurosci. 2005;27:288–298. doi: 10.1159/000086708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LY, Jokitalo E, Sun YF, Mehlen P, Lindholm D, Saarma M, Arumae U. GDNF-deprived sympathetic neurons die via a novel nonmitochondrial pathway. J Cell Biol. 2003;163:987–997. doi: 10.1083/jcb.200305083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Kroemer G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010;24:2592–2602. doi: 10.1101/gad.1984410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Brandemihl A, Lau D, Lawton A, Oakley B. BDNF is required for the normal development of taste neurons in vivo. Neuroreport. 1997;8:1013–1017. doi: 10.1097/00001756-199703030-00039. [DOI] [PubMed] [Google Scholar]
- Zheng TS, Hunot S, Kuida K, Momoi T, Srinivasan A, Nicholson DW, Lazebnik Y, Flavell RA. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat Med. 2000;6:1241–1247. doi: 10.1038/81343. [DOI] [PubMed] [Google Scholar]