Abstract
Abnormal brain activity during the processing of simple sounds is evident in individuals with increased genetic liability for schizophrenia; however, the diagnostic specificity of these abnormalities has yet to be fully examined. Because recent evidence suggests that schizophrenia and bipolar disorder may share aspects of genetic etiology the present study was conducted to determine whether individuals with heightened genetic liability for each disorder manifested distinct neural abnormalities during auditory processing. Utilizing a dichotic listening paradigm, we assessed target tone discrimination and electrophysiological responses in schizophrenia patients, first-degree biological relatives of schizophrenia patients, bipolar disorder patients, first-degree biological relatives of bipolar patients and nonpsychiatric control participants. Schizophrenia patients and relatives of schizophrenia patients demonstrated reductions in an early neural response (i.e. N1) suggestive of deficient sensory registration of auditory stimuli. Bipolar patients and relatives of bipolar patients demonstrated no such abnormality. Both schizophrenia and bipolar patients failed to significantly augment N1 amplitude with attention. Schizophrenia patients also failed to show sensitivity of longer-latency neural processes (N2) to stimulus frequency suggesting a disorder specific deficit in stimulus classification. Only schizophrenia patients exhibited reduced target tone discrimination accuracy. Reduced N1 responses reflective of early auditory processing abnormalities are suggestive of a marker of genetic liability for schizophrenia and may serve as an endophenotype for the disorder.
Keywords: Schizophrenia, Audition, Bipolar, Endophenotype, Relatives, N1
1. Introduction
Aberrant brain activity during the processing of sound may reflect a genetically determined predisposition for psychosis (Ahveninen et al., 2006; Frangou et al., 1997). Investigations have shown that individuals who carry genetic liability for schizophrenia exhibit abnormal brain electrical responses during sensory gating and identification of simple auditory stimuli (Bramon et al., 2005; Freedman et al., 1997; Hall et al., 2007; Schreiber, Stolz-Born, Kornhuber, & Born, 1992; Waldo, 1999). Individuals who develop schizophrenia also manifest anomalous attentional modulation of neural responses to simple sounds (Umbricht, Bates, Lieberman, Kane, & Javitt, 2006). Nevertheless, it has yet to be determined whether auditory processing abnormalities are diagnostically specific to genetic liability for schizophrenia (Turetsky et al., 2007). Because recent evidence suggests that some genes may create vulnerability for both schizophrenia and bipolar disorder it is necessary to determine whether etiologic mechanisms are shared between the two disorders, and examine the possibility that the disorders, as clinically-defined, may not conform to the genetic nosology of severe psychopathology (Badner & Gershon, 2002). We used a dichotic listening task to study auditory processing in schizophrenia and bipolar disorder patients, first-degree biological relatives of both patient groups, and nonpsychiatric comparison participants. The study design allowed us to determine whether neural abnormalities during auditory processing were consistent with a diagnostically specific genetic liability for schizophrenia and whether the abnormal brain responses were modulated by attention. To our knowledge this is the first published study to investigate multiple event-related potential components in relatives of schizophrenia patients and relatives of bipolar patients.
Deficient sensory registration and impaired attentional modulation of auditory input may reflect etiologic mechanisms in schizophrenia (Clementz & Blumenfeld, 2001; Freedman et al., 1997; Heinrichs & Zakzanis, 1998). The negative event-related potential (ERP) that occurs approximately 100 milliseconds after the onset of an auditory stimulus (N1 or N100) is elicited in the absence of task demands but is modulated by voluntary attention (Neelon, Williams, & Garell, 2006). Several studies have revealed reduced N1 amplitudes in individuals with schizophrenia with reductions apparently present regardless of illness chronicity (Brown, Gonsalvez, Harris, Williams, & Gordon, 2002; Bruder et al., 1999; Shelley, Silipo, & Javitt, 1999; Wood, Potts, Hall, Ulanday, & Netsiri, 2006). Direct recordings from the cortex have provided evidence that the N1 potential derives from the upper superior temporal gyrus, a cortical region shown to be of reduced volume in schizophrenia patients and first-degree relatives of schizophrenia patients (Goghari, Rehm, Carter, & Macdonald, 2006; McCarley et al., 2002; Neelon et al., 2006). N1 peak amplitude is highly heritable and reductions in N1 peak amplitude appear to be a function of genetic relatedness in monozygotic and dizygotic twins discordant for schizophrenia (Ahveninen et al., 2006; Anokhin, Vedeniapin, Heath, Korzyukov, & Boutros, 2006). Thus, decremented auditory N1 may serve as a functional manifestation of superior temporal gyrus anomalies that are evident in schizophrenia, relate to genetic liability for the disorder, and operate as an endophenotype (Gottesman & Gould, 2003).
Although no studies have directly examined whether early abnormal neural responses (i.e., N1 and N2, [i.e., N200]) to tones are specific to liability for schizophrenia, two investigations examined early auditory processing in schizophrenia and bipolar disorder patients. One study documented that N1 peak amplitude was reduced in the schizophrenia patients but not in individuals with bipolar disorder (O’Donnell, Vohs, Hetrick, Carroll, & Shekhar, 2004). Both studies reported that only schizophrenia patients exhibited diminished longer-latency components (P2 [i.e., P200] and N2), while both patient groups exhibited decremented P3 (i.e., P300) amplitude (Muir, St Clair, & Blackwood, 1991; O’Donnell et al., 2004). Also, a recent investigation of auditory responding in schizophrenia revealed diminished late ERP components (i.e., N2 and the P3) in first episode schizophrenia patients and chronic patients but abnormal preattentive components (i.e. MMN) only in chronic patients (Umbricht et al., 2006) suggesting that several mechanisms comprise auditory processing and that they may be differentially affected over the course of the disorder. Recordings during a dichotic listening task requiring shifts in directed attention may provide insight into abnormal mechanisms of volitional attention in biological relatives of patients with schizophrenia. Although attention and orienting abnormalities are observed outside the auditory domain in schizophrenia (e.g., Gouzoulis-Mayfrank et al., 2007) biological indices employed in the context of a family study involving more than one severe mental disorder allows determination of which elements of the auditory response are abnormal, influenced by volitional attention, and specific to liability for schizophrenia.
To carry out the first direct test of whether auditory processing abnormalities are possibly specific to genetic liability for schizophrenia, we collected electrophysiological data from schizophrenia and bipolar disorder outpatients, first-degree biological relatives of individuals with each disorder, and nonpsychiatric control participants during a dichotic listening task. The study was designed to address 1) whether early auditory processing abnormalities (N1) showed evidence of specificity to genetic liability for schizophrenia, and 2) whether auditory processing abnormalities in the disorder were modified by directed attention. As researchers have found N2 and P3 abnormalities in individuals with schizophrenia, these components were subjected to exploratory analyses (Brown et al., 2002; Mathalon, Ford, & Pfefferbaum, 2000). Due to the study not being a twin design we were unable to directly test the amount of genetic contribution to electrophysiological abnormalities.
2. Methods and Materials
2.1. Participants
Table 1 presents the characteristics of participants. Stable psychiatric outpatients were recruited from the Minneapolis VA Medical Center and community mental health agencies and screened for exclusion criteria. Patients were identified through application of exclusion criteria during reviews of clinic rosters by clinicians, chart reviews, or screening interviews with individuals expressing interest in study participation. We excluded potential participants if they had English as a second language, charted IQ less than 70 or a diagnosis of mental retardation, current alcohol or drug abuse, past drug dependence, a current or past central nervous system disease or condition, a medical condition or disease with likely significant central nervous system effects, history of head injury with skull fracture or loss of consciousness of greater than 20 min, a physical problem that would render study measures difficult or impossible to administer or interpret (e.g., blindness, hearing impairment, paralysis in upper extremities, etc.), an age less than 18 or greater than 59, significant tardive dyskinesia as indicated by a Dyskinesia Identification System: Condensed User Scale (DISCUS), or been adopted. Research staff identified first-degree biological relatives of patients by completing a pedigree from the patient’s report. Interested relatives completed a telephone interview to determine their demographic and medical characteristics and were excluded if they had a physical problem that would render study measures impossible to measure, or were younger than age 18 or older than age 68. Control participants were solicited through postings in the medical center, community libraries, fitness centers, and fraternal organization newsletters. Study staff screened potential control participants via a telephone interview using the same age range as relatives and the same exclusion criteria as schizophrenia participants. Additionally, staff excluded control participants if they had a personal history of, or a first-degree biological relative with a likely history of psychotic symptoms or an affective disorder as defined by the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) (American-Psychiatric-Association, 1994).
Table 1.
Characteristics of Participants.
| Variable | Schizophrenia Patients | Relatives of Schizophrenia Patients | Controls | Bipolar Patients | Relatives of Bipolar Patients |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| n=19 Mean (SD) |
n=37 Mean (SD) |
n=36 Mean (SD) |
n=18 Mean (SD) |
n=25 Mean (SD) |
|
| Age (Years) | 41.3 (10.3) | 49.8 (10.3) | 47.5 (15.1) | 44.8 (9.8) | 45.7 (17) |
| % Female | 21a,b | 68 | 42 | 22c,d | 36 |
| Education | 13.1 (3.1)a,b | 14.9 (2.5) | 15.3 (2.8) | 16 (2.6) | 14.3 (3.5) |
| Estimated IQ | 96.6 (12.6)a,b | 108.7 (10.3) | 111.9 (10.8) | 112.8 (14.4) | 111.1 (13.4) |
| BPRS Total | 46.9 (9.2) | NA | NA | 36 (8.9) | NA |
| SPQ Total | NA | 14.7 (10.8) | 10.0 (6.7) | NA | 14.9 (13.8) |
Note. SD=Standard Deviation. IQ=Intelligence Quotient. Estimated IQ was derived from a formula using Vocabulary and Block Design subtests (Brooker & Cyr, 1986). BPRS=Brief Psychiatric Rating Scale (Ventura et al. 1993). NA=not applicable. SPQ=Schizotypal Personality Questionnaire (Raine, 1991).
Schizophrenia Patients different from Control Group mean, p < .05.
Schizophrenia Patients different from Relatives of Schizophrenia Group mean, p < .05.
Bipolar Patients different from Control Group mean, p < .05.
Bipolar Patients different from Relatives of Bipolar Group mean, p < .05.
To obtain diagnostic information a trained doctoral-level clinical psychologist completed the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994) (DIGS) with each patient. From the clinical interview the psychologist rated current symptomatology using the Scale for the Assessment of Negative Symptoms (Andreasen, 1983a) (SANS) the Scale for the Assessment of Positive Symptoms (Andreasen, 1983b) (SAPS), and the 24-item version of the Brief Psychiatric Rating Scale (Lukoff, Nuechterlein, & Ventura, 1986) (BPRS). The psychologist utilized all available clinical information to apply the Operational Criteria for Psychotic Illness (McGuffin, Farmer, & Harvey, 1991) (OPCRIT) to determine the DSM-IV (American-Psychiatric-Association, 1994) diagnosis. A second psychologist or advanced doctoral psychology student reviewed all the available material and completed a second OPCRIT for the participants. Any diagnostic disagreement was resolved through review of OPCRIT items. See a previously published report for full information regarding clinical assessment of relatives and control participants (Sponheim, McGuire, & Stanwyck, 2006).
A minority of relatives had DSM-IV diagnoses and relatives of both patient groups had similar levels of diagnosed psychopathology. Of the relatives of schizophrenia patients, one was diagnosed with schizophrenia and another with schizoaffective disorder, ten were diagnosed with an affective disorder though all but three were in full remission, and one had a history of alcohol dependence. For cluster A personality disorders, one relative met the criteria for schizotypal personality disorder and three met criteria for schizoid personality disorder. Of the relatives of bipolar patients, one relative was diagnosed with delusional disorder, nine were diagnosed with an affective disorder with five in remission, one had comorbid alcohol dependence and one met criteria for past alcohol and cannabis dependence. For cluster A personality disorders one relative met criteria for schizotypal personality disorder and two met criteria for paranoid personality disorder. Of the 36 control participants, two were diagnosed with past alcohol dependence and one participant had a current eating disorder. Of the schizophrenia patients, three were diagnosed with past alcohol dependence, one of which was also diagnosed with past cannabis dependence. For the patients with bipolar disorder, there were no past or current substance dependence diagnoses. The exclusion of relatives with a current or past DSM-IV diagnosis on either Axis I or Axis II had no significant effect on the dependent variables of interest. There were virtually no medication effects on dependent variables. The few identified effects indicated that medications tended to normalize responses of patients and thus findings are unlikely to be an artifact of medication status. See supplemental material for detail on analyses of medication effects. All participants completed an informed consent process and the Minneapolis VA Medical Center and University of Minnesota Institutional Review Boards approved the study protocol.
2.2. Auditory Processing Task
The task was a two-dimensional (space and pitch) dichotic listening task with an established design and akin to that used to investigate auditory abnormalities in children at risk for schizophrenia (Hillyard, Hink, Schwent, & Picton, 1973; Schreiber et al., 1992). Participants completed the task in a single session of four blocks consisting of 200 trials. Headphones were used to present 96dB tone pips over 55dB background white noise. Pips alternated between each ear and for each block participants were instructed to identify the high tone only in the attended ear (i.e., target), which for half of the trials was not the highest overall tone (i.e., higher pitched targets in the ear that was presented the lower pitched set of pips). They were instructed to respond as quickly as possible to the target tones in the attended ear with a single button press using the right thumb. The order of directed attention was: block 1-left, block 2-right, block 3-right, block 4-left. For the third and fourth blocks the headphones were reversed on the participant’s head to counterbalance stimulus delivery. Thus, each set of infrequent and frequent pips was delivered to each ear twice, once attended and once unattended. Participants completed 30 practice trials before the first and second blocks to ensure their ability to distinguish between tone pips.
The pips were of four different pitches and pseudo-randomized such that 10% were infrequent tones delivered to the attended ear (targets) and 10% were infrequent tones delivered to the unattended ear (unattended deviants). The remaining 80% of the pips were a half-octave lower than the corresponding infrequent pips (channel 1: 2400Hz infrequent and 1600Hz frequent; channel 2: 1200Hz infrequent and 800Hz frequent). As each set of pips were delivered to each ear twice, once attended and once unattended, participants only responded to infrequent pips (i.e., target was 1200Hz in the lower pitched set of pips and target was 2400Hz in the higher pitched set of pips) in the attended channel. Tone pips were 100msec in length with a 10msec rise/fall time, with a between-channel inter-stimulus interval varying pseudorandomly between 1120msec, 1220msec, 1330msec, 1420msec, and 1530msec.
2.3. Electrophysiological Data Collection and Processing
Electroencephalograms (EEG) were collected utilizing an elastic electrode cap with 27 tin electrodes placed on the scalp conforming to a subset of locations in the 10-10 International System (Chatrian, Lettich, & Nelson, 1988). Electrodes were filled with conductive gel and the sites were abraded to reduce impedances to less than 5 kΩ. Data were collected referenced to the left earlobe and digitized at the rate of 500 Hz with .05-Hz low-frequency and 100-Hz high-frequency filters and a 60 Hz notch filter. To reduce horizontal eye movements during the task participants were instructed to focus their eyes on an arrow at center one meter away. The arrow reminded participants of the ear to which to attend. Electrodes above and below the right eye recorded the vertical electro-oculogram (VEOG) which was used to remove ocular artifact (Semlitsch, Anderer, Schuster, & Presslich, 1986). Offline EEG recordings were rereferenced to linked-ears and bandpass filtered with .05Hz low-frequency (48dB/octave roll-off) and 30Hz high-frequency (48dB/octave roll-off) filters. Data were epoched from 100ms pre-stimulus to 800ms post-stimulus. Epochs with voltages exceeding +/−75uV were automatically rejected and all remaining data were visually inspected for biolelectrical artifact including eye movements evident in the horizontal electro-oculogram. For each participant, trials were averaged by condition and grand averages were computed by averaging waveforms within conditions across participants. ERP component windows were defined through inspection of grand average waveforms and review of the literature. N1 and N2 amplitudes were defined as the maximal negative voltage occurring between 80 and 120ms, and 180 and 260ms, respectively. P3 amplitude was also analyzed and defined as the greatest positive voltage occurring between 300 and 430ms post-stimulus at midline sites. See supplemental material for extended results of the P3 component analyses and associations of clinical, demographic and behavioral indices with ERP components.
2.4. Statistical Analyses
To examine differences in task performance a repeated measures analysis of variances (ANOVAs) were computed on signal detection indices (d′ and β) (Swets & Green, 1966) and reaction time for correctly identified targets with a between-subjects factor of group (schizophrenia patients, relatives of schizophrenia patients, controls or bipolar patients, relatives of bipolar patients, controls) and within-subjects factors of pitch (high-tone set, low-tone set) and side (left ear, right ear). For ERP component analyses we examined scalp sites where the component of interest was most prominent. Peak amplitude of the N1 and N2 components were analyzed at site CZ. Separate repeated measures ANOVAs were computed for each ERP component. The analyses included the same between subjects factors as analyses of performance data as well as within- subjects factors of pitch (high-tone set, low-tone set), attention (attend, unattended), and probability (rare, frequent). Gender was not included as a factor in analyses of N1 and N2 as there were no differences between genders for early components, [N1 t(125)=−.041, p=.97, N2 t(125)=−1.18, p=.24].1
3. Results
3.1. Performance
Dichotic listening task performance data of schizophrenia patients, relatives of schizophrenia patients and control participants are presented in Table 2. Schizophrenia patients had reduced target detection (d′) and a greater tendency to respond (β) for the low-pitched pair of tones compared to the relatives of schizophrenia patients and controls, but the patients did not exhibit low performance for the high-tone pair. Schizophrenia patients were also slower in their responses to the low-pitched targets compared to control participants and the relative group. The relatives of schizophrenia patients and controls failed to differ on any behavioral index. Task performance data for patients with bipolar disorder, relatives of bipolar patients, and nonpsychiatric controls are presented in Table 3. Bipolar patients and relatives of bipolar patients failed to deviate from controls in dichotic listening task performance.
Table 2.
Dichotic Listening Task Performance for Schizophrenia Patients, First-Degree Relatives of Schizophrenia Patients, and Nonpsychiatric Control Groups.
| Task | Patients | Relatives | Controls | Test Value | p Value1 |
|---|---|---|---|---|---|
|
|
|
|
|||
| n=19 Mean (SD) |
n=37 Mean (SD) |
n=36 Mean (SD) |
|||
| Accuracy: Number of Correct Targets2 | |||||
| High Tones | 38.32 (3.42) | 39.54 (1.10) | 39.25 (1.66) | F(2,89)=2.5 | .09 |
| Low Tones | 32.78 (6.72)a,b | 38.28 (2.08) | 36.78 (5.05) | F(2,89)=8.8 | <.001 |
| Total | 71.06 (8.28)a,b | 77.81 (2.56) | 76.03 (5.83) | F(2,89)=9.3 | <.001 |
| Target Detection: d′ | |||||
| High Tones | 4.45 (.65) | 4.65 (.42) | 4.63 (.53) | F(2,89)=1.0 | n.s. |
| Low Tones | 3.73 (.79)a,b | 4.53 (.47) | 4.41 (.70) | F(2,89)=9.8 | <.001 |
| Total | 4.09 (.58)a,b | 4.59 (.33) | 4.52 (.53) | F(2,89)=6.9 | .002 |
| Response Threshold:β | |||||
| High Tones | .96 (.06)b | .98 (.02) | .98 (.03) | F(2,89)=2.9 | .06 |
| Low Tones | .87 (.10)a,b | .96 (.04) | .94 (.08) | F(2,89)=8.5 | <.001 |
| Total | .91 (.07)a,b | .97 (.02) | .96 (.05) | F(2,89)=9.3 | <.000 |
| Reaction Time for Targets: (msec) | |||||
| High Tones | 408 (139) | 369 (88) | 362 (69) | F(2,89)=1.6 | n.s. |
| Low Tones | 508 (121)a,b | 434 (92) | 440 (85) | F(2,89)=4.0 | .02 |
| Total | 460 (127)a,b | 399 (86) | 401 (73) | F(2,89)=3.1 | .05 |
Note. SD=Standard Deviation. A correction factor was used in computing d′ in cases of a perfect hit rate (1.0) or false-alarm rate (0.0) to allow for unbiased estimation of d′. A two-way ANOVA of d′ with group (patients, relatives, and controls) and trial type (high tones: 2400 Hz and 1600 Hz collapsed across attended side; low tones 1200 Hz and 800 Hz collapsed across attended side) revealed main effects for trial type, F(2,89) =20.16, p < .0001 and a group-by-trial type interaction, F(2,89)=4.84, p=.01. A similar two-way ANOVA of β yielded a main effect of trial type, F(2,89) =45.10, p < .0001 and a group-by-trial type interaction, F(2,89)=4.71, p < .05. Subjects responded faster to the high tone pairs of tones as compared to low tone pairs, F(1,89)=180.48, p<.0005.
Denotes significance level of Oneway ANOVA for specified set of trials.
Total number of targets presented at each tone was 40.
Schizophrenia Patients < Nonpsychiatric Controls,
Schizophrenia Patients < Relatives of Schizophrenia Patients.
Table 3.
Dichotic Listening Task Performance for Bipolar Patients, First-Degree Relatives of Bipolar Patients, and Nonpsychiatric Control Groups.
| Task | Patients | Relatives | Controls | Test Value | p Value1 |
|---|---|---|---|---|---|
|
|
|
|
|||
| n=18 Mean (SD) |
n=25 Mean (SD) |
n=36 Mean (SD) |
|||
| Accuracy: Number of Correct Targets2 | |||||
| High Tones | 39.35 (1.17) | 39.76 (.44) | 39.25 (1.66) | F(2,75)=1.2 | n.s. |
| Low Tones | 36.65 (3.90) | 38.56 (1.80) | 36.78 (5.05) | F(2,75)=1.7 | .18 |
| Total | 76.00 (4.64) | 78.32 (2.06) | 76.03 (5.83) | F(2,75)=2.1 | .13 |
| Target Detection: d′ | |||||
| High Tones | 4.41 (.54)b | 4.84 (.18) | 4.63 (.53) | F(2,75)=4.0 | .02 |
| Low Tones | 4.12 (.82)b | 4.64 (.40) | 4.41 (.70) | F(2,75)=2.9 | .06 |
| Total | 4.27 (.61)b | 4.74 (.23) | 4.52 (.53) | F(2,75)=4.4 | .01 |
| Response Threshold:β | |||||
| High Tones | .99 (.04) | .98 (.004) | .98 (.03) | F(2,75)=0.6 | n.s. |
| Low Tones | .93 (.07) | .96 (.04) | .94 (.08) | F(2,75)=1.5 | n.s. |
| Total | .96 (.04) | .97 (.02) | .96 (.05) | F(2,75)=1.3 | n.s. |
| Reaction Time for Targets: (msec) | |||||
| High Tones | 343 (85) | 350 (72) | 362 (69) | F(2,75)=0.4 | n.s. |
| Low Tones | 442 (98) | 418 (79 | 440 (85) | F(2,75)=0.5 | n.s. |
| Total | 392 (89) | 384 (71) | 401 (73) | F(2,75)=0.3 | n.s. |
Note. SD=Standard Deviation. A correction factor was used in computing d′ in cases of a perfect hit rate (1.0) or false-alarm rate (0.0) to allow for unbiased estimation of d′. A two-way ANOVA of d′ with group (patients, relatives, and controls) and trial type (high tones: 2400 Hz and 1600 Hz collapsed across attended side; low tones 1200 Hz and 800 Hz collapsed across attended side) revealed main effects for trial type, F(2,75) =10.64, p < .005 but no group-by-trial type interaction. A similar two-way ANOVA of β yielded a main effect of trial type, F(2,75) =28.03, p < .0001 but no group-by-trial type interaction. Participants responded faster to the high tone pairs of tones as compared to low tone pairs, F(1,75)=206.32, p <.0005.
Denotes significance level of Oneway ANOVA for specified set of trials.
Total number of targets presented at each tone was 40.
Bipolar Patients < Relatives of Bipolar Patients.
3.2. Electrophysiological Responses
3.2.1. Sensory Registration and Early Auditory Attention (N1)
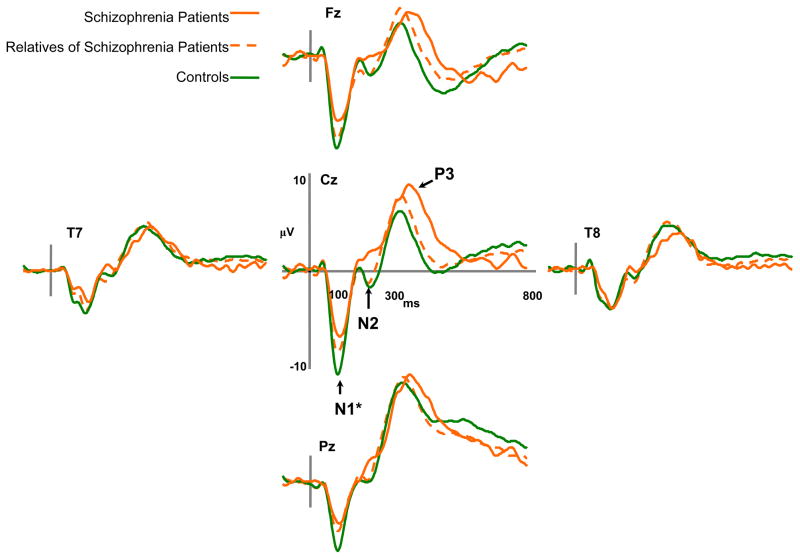
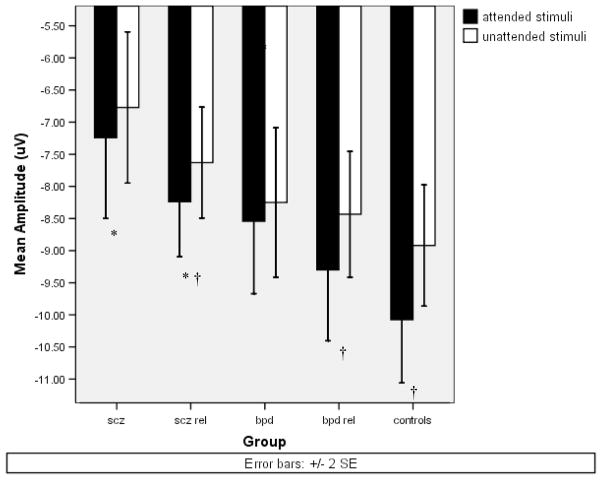
Schizophrenia patients, relatives of schizophrenia patients, and control participants differed in their overall N1 peak amplitude [F(2,89)=6.29, p<.005]. Bonferroni post hoc tests revealed that across conditions both schizophrenia patients [M=−7.01, SD=2.50][Mean difference=2.49, p<.005] and relatives of schizophrenia patients [M=−7.93, SD=2.54][Mean difference=1.56, p<.05] exhibited reduced N1 amplitude in comparison to control participants [M=−9.50, SD=2.82]. The schizophrenia patients and relatives had similar N1 amplitudes. Figure 1a depicts ERPs to target stimuli for schizophrenia patients, biological relatives of schizophrenia patients, and control participants. The omnibus ANOVA of N1 also revealed a main effect of attention [F(1,89)=23.81, p<.0001]. Figure 2 depicts N1 mean amplitudes for participant groups as a function of attention. Controls [t(35)=−5.22, p<.0001] and relatives [t(36)=−3.02, p=.005] exhibited significantly greater N1 amplitudes for tones in the attended ear than the unattended ear, but schizophrenia patients failed to show significant augmentation in N1 peak amplitude in the attended condition [t(18)=−1.15, p=.27]. Nevertheless, the interaction of group and attention for N1 failed to reach significance [F(2,89)=2.04, p=.14]. N1 amplitude also showed main effects of pitch [F(1,89)=22.45, p<.0001], probability [F(1,89)=8.41, p=.005], and an interaction of pitch and probability [F(1,89)=25.29, p<.0001]. As expected, overall amplitude was larger to rare tones [M=−8.56, SD=2.92] compared to frequent tones [M=−8.15, SD=2.81]. N1 amplitude was generally larger in response to the low pitched tone set (800Hz and 1200Hz) [M=−8.75, SD=2.93] compared to higher pitched tone set (1600Hz and 2400Hz) [M=−7.96, SD=2.85].
Figure 1.
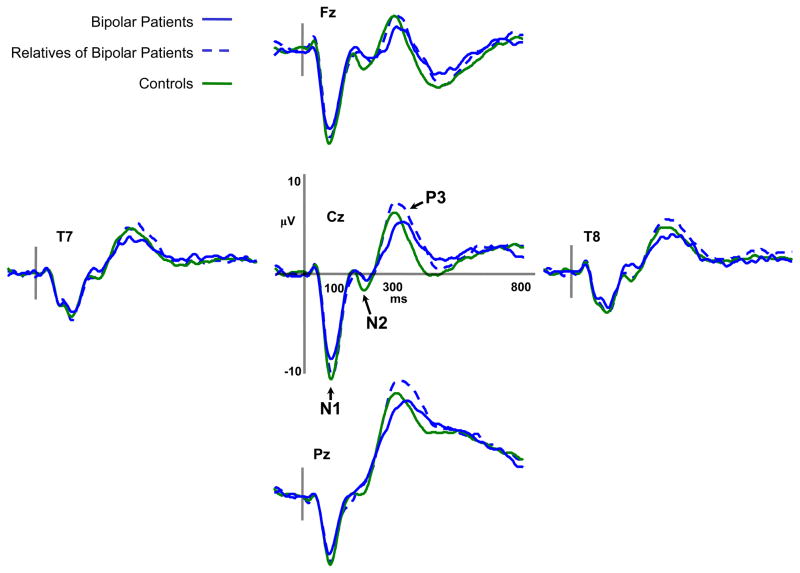
a) Average event-related potentials for schizophrenia patients, first-degree biological relatives of schizophrenia patients, and nonpsychiatric control participants for target trials during the dichotic listening task. b) Average event-related potentials for bipolar patients, first-degree biological relatives of bipolar patients, and nonpsychiatric control participants for target trials during the dichotic listening task.
Figure 2.
Means of N1 peak amplitude at site CZ for attended and unattended stimuli of schizophrenia patients, first-degree biological relatives of schizophrenia patients, bipolar patients, first-degree biological relatives of bipolar patients, and nonpsychiatric control participants.
*=LSD post hoc significantly different from control participants.
†= Paired t-test attended stimuli significantly different from unattended stimuli (p<.005).
We carried out similar analyses to test whether N1 amplitude reduction was specific to schizophrenia by examining bipolar patients and relatives of bipolar patients. Figure 1b depicts ERPs to target stimuli for bipolar disorder patients, biological relatives of bipolar patients, and control participants. Unlike the schizophrenia patient comparison there failed to be a group effect for N1 [F(2,75)=1.14, p=.32]. Nevertheless, there was a main effect of attention [F(1,75)=18.53, p<.0001] with N1 peak amplitude being greater for tones presented to the attended ear [M=−9.49, SD=2.80] than the unattended ear [M=−8.62, SD=2.60]. Like schizophrenia patients, bipolar patients failed to show significant augmentation of the N1 in the attended condition [t(16)=−.61, n.s.] though relatives of bipolar patients exhibited significantly greater N1 amplitude to attended than unattended tones [t(24)=3.11, p=.005]. Similar to the schizophrenia group comparison the interaction of group and attention was not significant [F(2,75)=1.89, p=.16]. The omnibus ANOVA revealed larger N1 amplitudes to rare tones [M=−9.46, SD=2.65] compared to frequent tones [M=−8.65, SD=2.70] [F(1,75)=33.19, p<.0001] and a trend toward a group-by-pitch interaction [F(2,75)=2.83, p=.06]. The bipolar patient group had similar N1 amplitudes for low and high-tone sets [t(16)=−.96, ns], while the relatives of bipolar patients [t(24)=1.95, p=.062] and controls [t(35)=2.51, p<.02] tended to exhibit larger N1 amplitudes to the low-tone set compared to the high-tone set. 2
3.2.2. Mid-latency Auditory Stimulus Classification (N2)
To examine electrophysiological processes associated with stimulus classification we carried out repeated measures ANOVAs of N2 peak amplitude. Although analysis of schizophrenia patients, relatives of schizophrenia patients, and controls failed to reveal a main effect of group [F(2,89)=.023, p=n.s.] on N2 amplitude, there was a significant interaction between group and probability [F(2,89)=3.42, p=.037]. Overall, N2 amplitude was larger for the rare tones [M=−3.23, SD=3.27] compared to the frequent tones [M=.48, SD=1.95] [F(1, 89)=103.4, p<.0001]. The schizophrenia patients showed the least augmentation of N2 amplitude from frequent [M=−.56, SD=1.98] to rare tones [M=−2.45, SD=3.04] [t(18)=−2.881, p<.01], followed by the relative group [frequent: M=.48, SD=1.95; rare: M=−3.23, SD=3.27] [t(36)=−6.97, p<.0001]. The control group exhibited the largest difference in N2 amplitude in relation to the probability of tones [frequent: M=.46, SD=2.81; rare: M=−3.45, SD=4.24] [t(35)=−9.84, p<.0001]. See Figure 1a to view the N2 component for infrequent target stimuli. N2 amplitude was also increased to tones in the attended ear [M=−1.74, SD=3.29] compared to the unattended ear [M=−1.16, SD=2.39] [F(1,89)=5.38, p<.05]. Unlike the N1 component, N2 peak amplitude was larger for the higher tones (1600Hz and 2400Hz) [M=−1.75, SD=2.92] than the lower tones (800Hz and 1200Hz) [M=−1.15, SD=2.70] [F(1,89)=9.83, p<.005].
To explore the specificity of N2 amplitude anomalies to schizophrenia a repeated measures ANOVA was carried out on N2 amplitude in bipolar disorder patients, relatives of bipolar patients, and controls. The groups exhibited similar N2 amplitudes [F(2,75)=.082, ns] and there were no interactions involving group (see Figure 1b). N2 amplitude was greater for tones presented to the attended ear [F(1,75)=8.79, p<.005], and were of higher pitch [F(1,75)=20.88, p<.0001] and rare [F(1,75)=174.64, p<.0001].
3.2.3. Target Detection and Context Updating (P3)
To evaluate late electrophysiological processes related to target detection a repeated measures ANOVA was computed with P3 peak amplitude to infrequent tones as the dependent variable. Group (schizophrenia patients, relatives of schizophrenia patients, controls) and gender were the between subjects factors, and attention (attended ear infrequent, ignored ear infrequent) and electrode site (FZ, CZ, PZ) were the within subjects factors. The analysis failed to reveal a group main effect [F(2,89)=.73, n.s.] or any interaction involving group. There was a trend towards a main effect for gender [F(2,89)=3.32, p=.07] and a trend towards an interaction of gender and group [F(2,89)=2.75, p=.07]. In the control group women exhibited significantly greater P3 amplitude [t(32)=3.09, p=.004], but in the relative group [t(34)=−.91, p=.37] and schizophrenia patients [t(17)=−1.07, p=.30] the difference between genders was not significant. As expected, there was a significant increase in P3 amplitude for the attended ear [F(1, 89)=96.48, p<.0005], and a main effect of site [F(2,178=128.09, p<.0005] with the greatest P3 amplitude at PZ. A similar ANOVA testing for left hemisphere P3 amplitude reductions (Salisbury et al., 1994) using electrode sites (T7, T8) neither revealed a main effect of group [F(1,53)=.305, n.s.] nor an interaction of group and site [F(1,53)=.631, n.s.].
An analysis of P3 amplitude in bipolar disorder patients and their relatives also failed to show a group main effect [F(2, 75=.22, n.s.] but did reveal a trend towards a gender by group interaction [F(2, 75=2.35, p=.10], though no other interactions with group were significant. Women with bipolar disorder exhibited significantly greater P3 amplitude than men with the disorder [t(16)=2.68, p=.02], but in the relative group there was no difference between genders [t(23)=−.863, p=.40]. There were expected increases in amplitude for the attended ear [F(1, 75)=63.53, p <.0001] and from frontal to parietal midline sites [F(2, 150=97.97, p <.0001]. Thus, analyses of P3 amplitude revealed no overall abnormalities of late processing for either of the patient groups or the groups of relatives. Female nonpsychiatric control participants and women with bipolar disorder exhibited greater P3 amplitude than males within their diagnostic group. For all groups, P3 amplitude was maximal at site PZ. Please see the supplemental materials for analyses of P3 latency.
4. Discussion
Utilizing a dichotic listening task, we found evidence of deficient early auditory processing (N1) in schizophrenia outpatients and first-degree biological relatives of schizophrenia patients, but no such anomaly in bipolar outpatients and first-degree biological relatives of bipolar patients. Both schizophrenia and bipolar patients failed to modulate early processing (N1) by selective attention while the relatives of both patient groups exhibited attentional effects. Schizophrenia patients also had diminished electrophysiological components (N2) reflective of poor auditory stimulus classification. Bipolar outpatients and both groups of relatives failed to exhibit significant N2 decrement. Thus, N1 abnormalities may be an expression of genetic liability specific to schizophrenia when contrasted with another severe mental disorder under genetic influence. Failure to augment the auditory N1 amplitude with selective attention appears to be associated with the clinical conditions of schizophrenia and bipolar disorder, but not genetic liability.
Although our finding of reduced auditory N1 amplitude in schizophrenia patients is consistent with previous studies (Ahveninen et al., 2006; O’Donnell et al., 2004; Wood et al., 2006), this is the first published report of reduced auditory N1 amplitude evident in biological relatives of schizophrenia patients but absent in bipolar disorder patients and their biological relatives. Neural populations responsible for scalp-recorded N1 have been investigated using high spatial-resolution methods such as intracranial recordings and magnetoencephalography (For review, see Naatanen & Picton, 1987). Using data from intracranial electrode arrays recorded during dichotic auditory paradigms researchers have identified neural generators of the scalp-recorded N1 as potentially residing in the superior temporal gyrus (Neelon et al., 2006). Reduced volume of the left posterior superior temporal gyrus has been described in several studies of patients with schizophrenia (McCarley et al., 2002; Onitsuka et al., 2004; Shenton, Dickey, Frumin, & McCarley, 2001), in contrast to studies of patients with affective disorders (Hirayasu et al., 2000; Hirayasu et al., 1998). In light of N1 amplitude reduction being evident in relatives of schizophrenia, one investigation found gray matter reductions of the left lateral temporal regions specific to the genetic risk for schizophrenia while genetic risk for bipolar disorder was associated with gray matter reductions of the anterior cingulate and ventral striatum (McDonald et al., 2004). Thus, auditory N1 decrement may be the functional expression of reduced superior temporal gyrus volume associated with schizophrenia. Although N1 peak amplitude is typically maximum at the vertex (e.g., site CZ) (Baribeau, Laurent, & Decary, 1993; Clementz & Blumenfeld, 2001; Karoumi et al., 2000) neural sources have been estimated as residing in the Sylvian fissure (Kayser & Tenke, 2006). Electrical fields from left and right hemispheres likely combine to form a midline maximum for the component and may in part mask the functional expression of lateralized structural abnormalities in N1 amplitude.3 Magnetic recordings and lesion studies point to the N1 as generated by a broad region of the supratemporal plane extending beyond primary auditory cortex and related to transient detection of stimuli and the initial readout of information from “sensory analyzers” (Naatanen & Picton, 1987).
Control participants and both groups of relatives exhibited modulation of the N1 in relation to directed attention, while schizophrenia and bipolar patients did not, suggesting that both patient groups possess a ‘top-down’ deficit in attentional control of sensory detection. Generally, N1 amplitude is increased to attended versus unattended stimuli (Hillyard et al., 1973; Sabri, Liebenthal, Waldron, Medler, & Binder, 2006; Woldorff et al., 1993). Studies of stimulus sequence effects on neural responses to auditory stimuli have provided evidence that N1 abnormalities in schizophrenia reflect difficulty with control and maintenance of selective auditory processing (Baribeau-Braun, Picton, & Gosselin, 1983) or ‘insufficient representation of stimulus significance and context’ (Gilmore, Clementz, & Buckley, 2005). Although the exact balance of exogenous and endogenous influences on the auditory N1 is unknown, selective control of early processing appears reduced in individuals with schizophrenia and bipolar disorder.
The N2 component is thought to be a measure of stimulus categorization and has been found to be disrupted in schizophrenia patients (Potts, Hirayasu, O’Donnell, Shenton, & McCarley, 1998). Schizophrenia patients have been shown to exhibit similar responses to target and standard tones thus failing to modulate N2 amplitude in relation to the category of a stimulus (Gilmore et al., 2005). Given that the N2 failed to be associated with performance and relatives demonstrated no abnormalities in the component or behavioral deficits, evidence suggests that the diminished N2 may reflect neural dysfunction contributing to poor identification of auditory stimuli in schizophrenia but unrelated to genetic vulnerability for the disorder.
Though amplitude reductions of the auditory P3 is one of the most replicated findings in schizophrenia research (Ford, 1999) our analyses indicated no abnormalities or asymmetry related to genetic liability for schizophrenia. Investigators that failed to detect a P3 reduction but found early processing deficits in schizophrenia patients have speculated that medication effects, clinical severity, and poor task performance may affect P3 amplitude. Others have suggested that intact P3 amplitudes in the context of diminished early auditory processing in schizophrenia reflects a compensatory function for the early abnormalities (Kayser et al., 2001). As P3 amplitude was associated with target detection and schizophrenia participants exhibited target detection impairment to only low tones, a task that more greatly discriminates groups on performance may result in significant P3 amplitude reductions in schizophrenia patients. In addition, studies show a significant number of unaffected relatives of schizophrenia patients to have P3 amplitudes similar to control participants and thus the electrophysiological response elicited by auditory oddball paradigms has been construed as a variable indicator of genetic liability for the disorder (Winterer et al., 2003).
There are several caveats to the present study. Because the investigation was not a twin study we were unable to directly test for genetic contributions to neural responses. Also, the sample of relatives of bipolar patients was smaller than that of the control subjects. Although the effect sizes were small for differences in neural responses between relatives of bipolar patients and controls, findings need to be replicated in a larger sample of relatives of bipolar patients. Additionally, to fully establish specificity of the observed auditory processing abnormalities to liability to schizophrenia more disorders must be studied. To conclude, in a task requiring attention to be directed to select auditory stimuli, schizophrenia patients and relatives of schizophrenia patients demonstrated reductions in an early neural response (i.e. N1) suggestive of deficient sensory registration while bipolar patients and relatives of bipolar patients did not exhibit such an abnormality. Both patient groups failed to significantly augment N1 amplitude with attention and schizophrenia patients did not augment N2 amplitudes to stimulus frequency suggesting a disorder specific deficit in stimulus classification. Given evidence for early neural response anomalies in schizophrenia patients and their relatives reduced N1 amplitudes may mark genetic liability for schizophrenia and possibly serve as an endophenotype for the disorder.
Supplementary Material
Acknowledgments
We gratefully acknowledge John J. Stanwyck, Sarah M. Sass, and Robb Hunter for assistance with data collection.
Footnotes
Though the gender ratios of the patient groups are relatively similar, the relatives of schizophrenia patients group had a majority of females. Repeated measures ANOVAs (2×2×2) on peak N1 amplitude with gender and group as between subject factors and pitch (high-tone set, low-tone set), attention (attend, unattended), and probability (rare, frequent) as within subjects factors of group comparisons yielded no significant main effect of gender nor significant interaction between group and gender. The following F values are for the main effect of gender as well as the gender by group interactions: For bipolar patients, schizophrenia patients and controls [F(1,67)=.65, p=.42], interaction [F(2,67)=.93, p=.40], relatives of bipolar patients, relatives of schizophrenia patients and controls [F(1,94)=.02, p=.89], interaction [F(2,94)=1.61, p=.21], schizophrenia patients, their biological relatives, and controls [F(1,88)=.04, p=.85], interaction [F(2,88)=.88, p=.42], and the bipolar patients, their biological relatives, and controls [F(1,73)=.95, p=.33], interaction [F(2,73)=1.29, p=.28]. Similar repeated measures ANOVAs (2×2×2) on peak N2 amplitude with gender and group as between subject factors and pitch (high-tone set, low-tone set), attention (attend, unattended), and probability (rare, frequent) as within subjects factors indicated no significant main effect of gender or significant interaction between group and gender. The following F values are for the main effect of gender as well as the gender by group interactions: For the bipolar patients, schizophrenia patients and controls group [F(1,67)=.15, p=.70], interaction [F(2,67)=.75, p=.48], the relatives of bipolar patients, relatives of schizophrenia patients and controls group [F(1,94)=1.03, p=.31], interaction [F(2,94)=.74, p=.48], schizophrenia patients, their biological relatives, and controls group [F(1,88)=.54, p=.47], interaction [F(2,88)=.70, p=.50], and the bipolar patients, their biological relatives, and controls group [F(1,73)=.81, p=.45], interaction [F(2,73)=.81, p=.45].
In a direct test of diagnostic specificity, schizophrenia patients, bipolar patients and controls demonstrated a differences in N1 peak amplitude [F(2,74)=6.30, p<.01]]. Bonferroni post hoc tests revealed schizophrenia patients differed from control participants [p =.002, Cohen’s d=.93,] while bipolar patients did not [p =.31, Cohen’s d=.44]. Similarly, the relative groups were significantly different [F(2,101)=3.71, p<.03]. Bonferroni post hoc tests indicated that relatives of schizophrenia patients differed from control participants [p =.02, Cohen’s d=.56] while relatives of bipolar patients did not [p =.75, Cohen’s d=.24]. Demographic variables failed to show significant effects on N1 amplitude as covariates across groups (e.g, years of education [F(1,117)=.22, p=.64]) although there was a trend effect for IQ [F(1,117)=3.34, p=.07]
To test for laterality effects on N1 amplitude we conducted a repeated measures ANOVA with the between subjects factors of group (schizophrenia patients, relatives of schizophrenia patients, and controls) and the within subjects factor of side (electrode sites T7 and T8). The analysis failed to yield group differences in N1 peak amplitude [F(2,89)=.389, p=.68]. Further paired t-tests revealed no differences in overall N1 peak amplitude for schizophrenia patients (sites T7 and T8) [t(18)=.651, p=.523; left hemisphere site, M=−2.94, SD=1.23, right hemisphere site, M=−3.11, SD=1.13]. Similarly, analyses of laterality revealed no significant differences in overall N1 peak amplitude for relatives of schizophrenia patients (sites T7 and T8) [t(36)=1.72, p=.09; left hemisphere site, M=−2.94, SD=1.23, right hemisphere site, M=−3.11, SD=1.13].
Previous Presentation: Preliminary data from this study were presented at the Society for Research in Psychopathology, Toronto, Canada, October, 2003.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahveninen J, Jaaskelainen IP, Osipova D, Huttunen MO, Ilmoniemi RJ, Kaprio J, Lonnqvist J, Manninen M, Pakarinen S, Therman S, Naatanen R, Cannon TD. Inherited Auditory-Cortical Dysfunction in Twin Pairs Discordant for Schizophrenia. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.04.015. [DOI] [PubMed] [Google Scholar]
- American-Psychiatric-Association. Diagnostic and statistical manual of mental disorders. 4. Washington, D.C: American Psychiatric Association; 1994. [Google Scholar]
- Andreasen NC. Technical Report. University of Iowa; 1983a. The scale for the assessment of negative symptoms (SANS) [Google Scholar]
- Andreasen NC. Technical Report. University of Iowa; 1983b. The scale for the assessment of positive symptoms (SAPS) [Google Scholar]
- Anokhin AP, Vedeniapin AB, Heath AC, Korzyukov O, Boutros NN. Genetic and environmental influences on sensory gating of mid-latency auditory evoked responses: A twin study. Schizophr Res. 2006 doi: 10.1016/j.schres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7(4):405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Baribeau-Braun J, Picton TW, Gosselin JY. Schizophrenia: a neurophysiological evaluation of abnormal information processing. Science. 1983;219(4586):874–876. doi: 10.1126/science.6823555. [DOI] [PubMed] [Google Scholar]
- Baribeau J, Laurent JP, Decary A. Tardive dyskinesia and associated cognitive disorders: a convergent neuropsychological and neurophysiological approach. Brain Cogn. 1993;23(1):40–55. doi: 10.1006/brcg.1993.1043. [DOI] [PubMed] [Google Scholar]
- Bramon E, McDonald C, Croft RJ, Landau S, Filbey F, Gruzelier JH, Sham PC, Frangou S, Murray RM. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27(4):960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Brooker BH, Cyr JJ. Tables for clinicians to use to convert WAIS-R short forms. Journal of Clinical Psychology. 1986;42:982–986. [Google Scholar]
- Brown KJ, Gonsalvez CJ, Harris AW, Williams LM, Gordon E. Target and non-target ERP disturbances in first episode vs. chronic schizophrenia. Clin Neurophysiol. 2002;113(11):1754–1763. doi: 10.1016/s1388-2457(02)00290-0. [DOI] [PubMed] [Google Scholar]
- Bruder G, Kayser J, Tenke C, Amador X, Friedman M, Sharif Z, Gorman J. Left temporal lobe dysfunction in schizophrenia: event-related potential and behavioral evidence from phonetic and tonal dichotic listening tasks. Arch Gen Psychiatry. 1999;56(3):267–276. doi: 10.1001/archpsyc.56.3.267. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Modified nomenclature for the “10%” electrode system. J Clin Neurophysiol. 1988;5(2):183–186. [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001;139(4):377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–682. [PubMed] [Google Scholar]
- Frangou S, Sharma T, Alarcon G, Sigmudsson T, Takei N, Binnie C, Murray RM. The Maudsley Family Study, II: Endogenous event-related potentials in familial schizophrenia. Schizophr Res. 1997;23(1):45–53. doi: 10.1016/S0920-9964(96)00089-8. [DOI] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94(2):587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Clementz BA, Buckley PF. Stimulus sequence affects schizophrenia-normal differences in event processing during an auditory oddball task. Brain Res Cogn Brain Res. 2005;24(2):215–227. doi: 10.1016/j.cogbrainres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, Macdonald AW., 3rd Regionally Specific Cortical Thinning and Gray Matter Abnormalities in the Healthy Relatives of Schizophrenia Patients. Cereb Cortex. 2006 doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Balke M, Hajsamou S, Ruhrmann S, Schultze-Lutter F, Daumann J, Heekeren K. Orienting of attention in unmedicated patients with schizophrenia, prodromal subjects and healthy relatives. Schizophr Res. 2007;97:35–42. doi: 10.1016/j.schres.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Hall MH, Rijsdijk F, Picchioni M, Schulze K, Ettinger U, Toulopoulou T, Bramon E, Murray RM, Sham P. Substantial shared genetic influences on schizophrenia and event-related potentials. Am J Psychiatry. 2007;164(5):804–812. doi: 10.1176/ajp.2007.164.5.804. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182(108):177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57(7):692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley RW. Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry. 1998;155(10):1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- Karoumi B, Laurent A, Rosenfeld F, Rochet T, Brunon AM, Dalery J, d’Amato T, Saoud M. Alteration of event related potentials in siblings discordant for schizophrenia. Schizophr Res. 2000;41(2):325–334. doi: 10.1016/s0920-9964(99)00062-6. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stuart BK, Amador XF, Gorman JM. Event-related brain potentials (ERPs) in schizophrenia for tonal and phonetic oddball tasks. Biol Psychiatry. 2001;49(10):832–847. doi: 10.1016/s0006-3223(00)01090-8. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin Neurophysiol. 2006;117(2):348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Lukoff D, Nuechterlein KH, Ventura J. Manual for the Expanded Brief Psychiatric Rating Scale. Schizophr Bull. 1986;(12):594–602. [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biological Psychiatry. 2000;47(5):434–449. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Salisbury DF, Hirayasu Y, Yurgelun-Todd DA, Tohen M, Zarate C, Kikinis R, Jolesz FA, Shenton ME. Association between smaller left posterior superior temporal gyrus volume on magnetic resonance imaging and smaller left temporal P300 amplitude in first-episode schizophrenia. Arch Gen Psychiatry. 2002;59(4):321–331. doi: 10.1001/archpsyc.59.4.321. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E, Murray RM. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61(10):974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48(8):764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- Muir WJ, St Clair DM, Blackwood DH. Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med. 1991;21(4):867–879. doi: 10.1017/s003329170002986x. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Neelon MF, Williams J, Garell PC. The effects of auditory attention measured from human electrocorticograms. Clin Neurophysiol. 2006;117(3):504–521. doi: 10.1016/j.clinph.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int J Psychophysiol. 2004;53(1):45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Onitsuka T, Shenton ME, Salisbury DF, Dickey CC, Kasai K, Toner SK, Frumin M, Kikinis R, Jolesz FA, McCarley RW. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. 2004;161(9):1603–1611. doi: 10.1176/appi.ajp.161.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GF, Hirayasu Y, O’Donnell BF, Shenton ME, McCarley RW. High-density recording and topographic analysis of the auditory oddball event-related potential in patients with schizophrenia. Biol Psychiatry. 1998;44(10):982–989. doi: 10.1016/s0006-3223(98)00223-6. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17(4):555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Sabri M, Liebenthal E, Waldron EJ, Medler DA, Binder JR. Attentional modulation in the detection of irrelevant deviance: a simultaneous ERP/fMRI study. J Cogn Neurosci. 2006;18(5):689–700. doi: 10.1162/jocn.2006.18.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, O’Donnell BF, McCarley RW, Nestor PG, Faux SF, Smith RS. Parametric manipulations of auditory stimuli differentially affect P3 amplitude in schizophrenics and controls. Psychophysiology. 1994;31(1):29–36. doi: 10.1111/j.1469-8986.1994.tb01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber H, Stolz-Born G, Kornhuber HH, Born J. Event-related potential correlates of impaired selective attention in children at high risk for schizophrenia. Biol Psychiatry. 1992;32(8):634–651. doi: 10.1016/0006-3223(92)90294-a. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophr Res. 1999;37(1):65–79. doi: 10.1016/s0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim SR, McGuire KA, Stanwyck JJ. Neural anomalies during sustained attention in first-degree biological relatives of schizophrenia patients. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Swets JA, Green DM. Signal detection theory and psychophysics. New York: John Wiley and Sons; 1966. [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33(1):69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in first-episode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006;59(8):762–772. doi: 10.1016/j.biopsych.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Waldo MC. Co-distribution of sensory gating and impaired niacin flush response in the parents of schizophrenics. Schizophrenia Research. 1999;40(1):49–53. doi: 10.1016/s0920-9964(99)00031-6. [DOI] [PubMed] [Google Scholar]
- Winterer G, Egan MF, Raedler T, Sanchez C, Jones DW, Coppola R, Weinberger DR. P300 and genetic risk for schizophrenia. Arch Gen Psychiatry. 2003;60(11):1158–1167. doi: 10.1001/archpsyc.60.11.1158. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, Bloom FE. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc Natl Acad Sci U S A. 1993;90(18):8722–8726. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SM, Potts GF, Hall JF, Ulanday JB, Netsiri C. Event-related potentials to auditory and visual selective attention in schizophrenia. Int J Psychophysiol. 2006;60(1):67–75. doi: 10.1016/j.ijpsycho.2005.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.