To the Editor
Swallowed topical steroids, allergen elimination diets, or both are the primary treatment for patients with eosinophilic esophagitis (EoE).1 To date, 2 steroid preparations, fluticasone and budesonide, have been shown to offer therapeutic benefit for patients with EoE.2-4 Clinical experience suggests that some patients are unresponsive to either of these topical steroids. Whether this is related to inadequate dosing, improper technique of administration, low steroid concentrations on the epithelial surface, genetically determined steroid unresponsiveness, or poor adherence is uncertain, but additional therapeutic options are necessary.
Ciclesonide is a topical steroid of proved benefit in the treatment of allergic diseases, such as asthma, allergic rhinitis, and allergic conjunctivitis.5-7 It is a nonhalogenated parent compound that is converted by epithelial esterases to form the biologically potent desisobutyryl-ciclesonide (des-CIC). This conversion facilitates a high concentration of active metabolite at the mucosal surface.8 In vitro comparisons demonstrate that des-CIC exhibits a 100-fold greater glucocorticoid receptor binding than the parent compound ciclesonide. des-CIC possesses a number of mechanistic properties, one of which is limiting eosinophil migration.9 Measurements of systemic levels of biologically active steroid components are less in patients administered ciclesonide compared with those administered fluticasone or budesonide (inhaled corticosteroids).10 In vitro animal and human studies comparing ciclesonide with alternative inhaled corticosteroids in asthmatic patients provide evidence that equivalent or lower doses of ciclesonide result in similar clinical responses. These studies also indicate that there are fewer systemic steroid effects (osteopenia, adrenal suppression, and oral candidiasis).6,10 In combination, these features suggest that ciclesonide might be an attractive therapeutic option for the treatment of EoE. Therefore we hypothesized that ciclesonide would provide therapeutic efficacy in the treatment of children with EoE.
As an initial step, we investigated whether esophageal squamous epithelia expressed carboxylesterases known to esterify ciclesonide. As a part of an institutional review board–approved protocol, RT-PCR was performed on esophageal mucosal biopsy samples from children undergoing endoscopy at Children's Hospital Colorado who had normal esophageal mucosa, active EoE, or treated EoE. As seen in Fig 1, all esophageal mucosal samples expressed carboxylesterases 1 and 2, a finding that supports the use of ciclesonide in the treatment of EoE. We performed a chart review to identify patients who received ciclesonide as treatment for EoE in the Gastrointestinal Eosinophilic Diseases Program at Children's Hospital Colorado and National Jewish Health. The diagnosis of EoE had been made according to previously established published guidelines.1 Six patients in our program were treated with ciclesonide, but only the 4 who were treated for at least 2 months and had undergone pretreatment and posttreatment biopsies were reviewed. Clinical features, including symptoms, doses of medication, endoscopic appearances, and histopathologic findings, were recorded as shown in Table I. Patients had received instructions to swallow 2 actuations of the metered-dose inhaler (80 or 160 μg) twice a day without using a spacer, rinsing their mouth, or eating or drinking for 30 minutes afterward.
Fig 1.
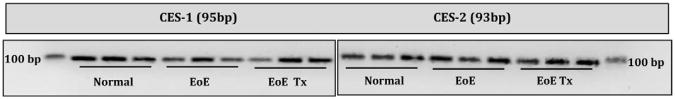
Esterase expression in esophageal tissues. Esophageal biopsy specimens were collected from healthy subjects, patients with active EoE, and patients with treated EoE. cDNA measurements were made (Applied Biosystems, Foster City, Calif), and quantitative PCR was completed (Absolute Blue QPCR Rox Mix; Thermo Scientific, Waltham, Mass) by using high-fidelity TaqMan probes (Applied Biosystems). Gene probe identification numbers Hs00275606 and Hs01077951 were for carboxylesterases 1 (95 bp) and 2 (93 bp), respectively. Products were visualized on a 2.5% agarose gel (Invitrogen, Carlsbad, Calif). EoE Tx, Treated EoE.
Table 1. Clinical summary of children receiving ciclesonide as their primary treatment for EoE.
| Patients | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Age (y) | 4 | 4 | 16 | 7 |
| Sex | Male | Male | Female | Male |
| Weight (kg) | 12.7 | 18 | 49 | 24.5 |
| Dose, total/d (μg) | 320 | 640 | 320 | 320 |
| Food allergy | Y | Y | Y | Y |
| Atopic history | Y | Y | Y | Y |
| Months of treatment | September-November | August-November | August-October | June-August |
| Dietary restriction | Y | Y | Y | Y |
| Pre/Post | Pre/Post | Pre/Post | Pre/Post | |
| Treatment: | ||||
| Symptoms | Slow growth/Weight gain | Reflux, dysphagia, pain/None | Dysphagia/None | Vomiting, dysphagia/None |
| Endoscopic findings: | Normal/Normal | Normal/Normal | Trachealization/Furrowing | Furrowing/Furrowing |
| Histology | ||||
| Proximal biopsy: | ||||
| Eosinophil/hpf (×40) | 58/3 | 105/0 | 46/4 | 75/0 |
| Basal zone hyperplasia | Y/Y | Y/N | Y/N | Y/Y |
| Rete peg elongation | N/N | Y/N | Y/N | Y/N |
| Spongiosis/edema | Y/N | Y/N | N/N | N/N |
| Degranulation | Y/N | N/N | Y/N | N/N |
| Microabscess | N/N | N/N | N/N | N/N |
| Distal biopsy: | ||||
| Eosinophil/hpf (×40) | 75/0 | 100/0 | 30/3 | 100/0 |
| Basal zone hyperplasia | Y/Y | Y/N | Y/N | Y/Y |
| Rete peg elongation | Y/N | Y/N | Y/N | Y/N |
| Spongiosis/edema | N/N | Y/N | N/N | N/N |
| Degranulation | N/N | N/N | Y/N | N/N |
| Microabscess | N/N | N/N | N/N | N/N |
hpf, High-power field; N, no; Pre, before ciclesonide; Post, after ciclesonide; Y, yes.
Indications for using ciclesonide were lack of clinical response to fluticasone, dietary restrictions, or parental concerns about systemic steroid exposure. During ciclesonide treatment, there were no changes in patients' dietary restrictions, maintenance medications, or use of oral systemic steroids. Patients were prescribed ciclesonide at varied times between the summer and fall seasons (Table I). Environmental allergen sensitization in patient 1, a 4-year-old boy with eczema, had not been assessed at the time of treatment. Patients 2, 3, and 4 were sensitized to spring, fall, and perennial allergens, as determined by means of skin testing, and patient 4 was receiving maintenance allergen immunotherapy.
All 4 patients experienced both a clinical and histologic benefit from the use of swallowed topical ciclesonide. Symptoms resolved in all patients, and eosinophil numbers decreased significantly in both proximal biopsy specimens (71 ± 25.5 vs 1.75 ± 2 eosinophils/high-power field; P = .007, before vs after treatment, respectively) and distal biopsy specimens (76.25 ± 33 vs 0.75 ± 1.5 eosinophils/high-power field; P = .009, before vs after treatment, respectively). In addition, when present on initial biopsy specimens, a number of histologic features, including basal cell hyperplasia, rete peg elongation, spongiosis, and edema, also decreased after ciclesonide treatment. No side effects were found, including thrush, acne, hirsutism, increased bruising, or mood swings, at 2 months after initiation of treatment.
Ciclesonide might have offered clinicopathologic benefit in children with EoE because of high local des-CIC concentrations. des-CIC exhibits local anti-inflammatory effects that include reduction in eosinophil migration, diminished lymphocyte cytokine production, decreased fibroblast eotaxin release, and reduced smooth muscle actin expression.9 Clinically, ciclesonide possesses potent actions in asthmatic patients, as evidence by reduced sputum eosinophils, reduction in exhaled nitric oxide levels, and improvement in FEV1.5,6 The formulation in a metered-dose inhaler delivers a lipophilic particle (1.1-2.1μm) that is subject to high first-pass metabolism by the hepatic CY3PA4 system, thus leading to low systemic bioavailability. Because of its low systemic bioavailability, ciclesonide can potentially be used at higher doses for EoE than other steroid preparations. All of these features make ciclesonide an attractive therapeutic compound for mucosal diseases, including EoE.
Other factors could have contributed to clinicopathologic improvements, including improved adherence to this prescribed drug, undocumented changes in diet, or change of season. In addition, and deserving of more study, is the fact that the oral bioavailability of oropharyngeal ciclesonide is low.7 Whether epithelial expression of esterases varies between esophageal and oral epithelia is currently not known.
Our results support our hypothesis that ciclesonide is another topical steroid that might offer benefit in treating children with EoE. Because this is a small case series report, we suggest caution with ciclesonide use. Nonetheless, our findings provide preliminary data to support further clinical and mechanistic studies of ciclesonide in the treatment of EoE.
Acknowledgments
We thank Sophie Fillon, PhD, Karim El Kasmi, MD, PhD, and Rachel Harris, BA, for their technical assistance.
Footnotes
Disclosure of potential conflict of interest: The authors declare that they have no relevant conflicts of interest.
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell JM, Blanchard C, Collins MH, Putnam PE, Kaul A, Aceves SS, et al. Glucocorticoid-regulated genes in eosinophilic esophagitis: a role for FKBP51. J Allergy Clin Immunol. 2010;125:879–88. e8. doi: 10.1016/j.jaci.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Straumman A, Conus S, Degan L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139:1526–37. e1. doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 4.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102:2271–9. doi: 10.1111/j.1572-0241.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 5.Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once-daily ciclesonide in children: efficacy and safety in asthma. J Pediatr. 2006;148:377–83. doi: 10.1016/j.jpeds.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Pearlman DS, Berger WE, Kerwin E, Laforce C, Kundu S, Banerji D. Once-daily ciclesonide improves lung function and is well tolerated by patients with mild-to-moderate asthma. J Allergy Clin Immunol. 2005;116:1206–12. doi: 10.1016/j.jaci.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Richter K, Kanniess F, Biberger C, Nave R, Magnussen H. Comparison of the oropharyngeal deposition of inhaled ciclesonide and fluticasone propionate in patients with asthma. J Clin Pharmacol. 2005;45:146–52. doi: 10.1177/0091270004271094. [DOI] [PubMed] [Google Scholar]
- 8.Mutch E, Nave R, Zech K, Williams FM. Esterases involved in the hydrolysis of ciclesonide in human tissues. Eur Respir J. 2003;22(suppl):S267–8. [Google Scholar]
- 9.Rohatagi S, Arya V, Zech K, Nave R, Hochhaus G, Jensen BK, Barrett JS. Population pharmacokinetics and pharmacodynamics of ciclesonide. J Clin Pharmacol. 2003;43:365–78. doi: 10.1177/0091270002250998. [DOI] [PubMed] [Google Scholar]
- 10.Stoeck M, Reidel R, Hochhaus G, Häfner D, Masso JM, Schmidt B, et al. In vitro and in vivo anti-inflammatory activity of new glucocorticoid ciclesonide. J Pharmacol Exp Ther. 2004;1:249–58. doi: 10.1124/jpet.103.059592. [DOI] [PubMed] [Google Scholar]


