Abstract
Natural killer (NK) cells play an important role in immunity against infection and tumors. Aging-related functional NK cell deficiency is well-documented in humans and mice. However, the mechanism for this is poorly understood. Using an adoptive transfer approach in mice, we found that NK cells from both young and aged mice responded vigorously to priming by pathogen-derived products after being co-transferred into young mice. In contrast, NK cells from young mice responded poorly to priming by pathogen-derived products after being transferred to aged mice. In addition to defects in NK cell priming, maturation of NK cells under steady state conditions is also impaired in aged mice resulting in decreased proportion of CD27- mature NK cells. We found that bone marrow from young and aged mice gave rise to CD27- mature NK cells similarly in young mixed bone marrow chimeric mice. Furthermore, by using a novel bone marrow transfer approach without irradiation, we found that after being transferred to aged mice, bone marrow from young mice gave rise to NK cells with maturation defects. Finally, we found that aging-related functional NK cell deficiency was completely reversed by injecting soluble IL-15/IL-15Rα complexes. In contrast, blockade of IL-10 signaling, which broadly augments inflammatory responses to pathogen-derived products, had little effect on aging-related defects in NK cell priming. These data demonstrate that the aged host environment is responsible for aging-related functional NK cell deficiency. In addition, our data suggest that IL-15 receptor agonists may be useful tools in treating aging-related functional NK cell deficiency.
Introduction
The peripheral blood of humans and animals contains a unique subgroup of large granular lymphocytes that are able to kill tumor cells expressing aberrant class I MHC molecules (1-3). These cells were later found to reside within a population of leukocytes that express the surface molecule NKp46/CD335 (4). Although not all NKp46+ are cytolytic, these cells are commonly referred to as NK cells. NK cells are a heterogeneous group of cells. In addition to cytolytic potential (5, 6), many surface molecules are also differentially expressed by subsets of NK cells in both mice (7-9) and humans (7, 10-15). For example, in mice, NK cells can be divided into CD11b+ and CD11b- subsets (16). The CD11b+ subset can be further divided into CD27- and CD27+ subsets (8, 9). Finally, the CD27-CD11b+ subset can be divided into KLRG1+ and KLRG1- subsets (17-19). However, all NK cells express the heterodimeric IL-15Rβγ IL-15 receptor (20). Variation in IL-15 signaling plays an important role in NK cell biology. In one extreme where no signal is received, NK cells die within a few hours (21). In another extreme, sustained high level signal leads to accumulation of large numbers of NK cells with impaired functions (22). In addition, when IL-15 signal is reduced from its normal physiological level, CD27- NK cells fail to develop (23, 24). Further reduction of the signal leads to the elimination of CD11b+ NK cells (23). Conversely, when IL-15 signal is increased from its normal physiological level, the proportion of KLRG1+ NK cells increases (18, 21, 22, 25).
Human NK cell deficiency can be divided into two categories, namely classical and functional NK cell deficiency. Classical NK cell deficiency is a rare disorder characterized by the absence of NK cells. Functional NK cell deficiency is a much more common and diverse disorder, in which NK cells are present, but their cytolytic function is severely impaired (26). The importance of cytolytic NK cells in human health is convincingly established by the fact that patients who have functional NK cell deficiency are highly susceptible to infections despite the presence of large numbers of NK cells (26). It is not clear if these patients are also susceptible to cancer development later in life because they die from herpes virus infections at a young age, but there is little doubt that cytolytic NK cells can efficiently kill many types of cancer cells in humans (27, 28). Furthermore, mice with NK cell deficiency have dramatically increased tumor metastasis and outgrowth (29).
Shortly after the discovery of NK cells, it was realized that cytolytic activity of NK cells is significantly lower in aged than in young mice (2, 30-32). Furthermore, unlike young mice, aged mice fail to increase NK cell activity after infection (30-33). It is well documented that much of the infection induced increase in cytolytic activity of NK cells is due to increased production of type I IFN in young mice (34-37). However, injection of type I IFN had little effect on NK cell cytolytic activity in aged mice (32, 38). Aging-related impairment in cytolytic function of human NK cells has been recognized for many years (2, 30-32, 39-42). In particular, despite the presence of large numbers of NK cells, cytolytic granules are poorly developed in NK cells from aged people in part because perforin expression is decreased dramatically with aging (41). Whereas a large majority of the NK cells in the peripheral blood of young people expressed KLRG1, which is up-regulated in cytolytic effector NK cells (43, 44), very few of the NK cells in the peripheral blood of aged people expressed this marker (42). In contrast, the total number of NK cells changed little with aging (41, 42). Thus, in both mice and humans, aging is associated with functional NK cell deficiency. However, the mechanism for this is unknown.
In this study, we employed adoptive cell transfers to address this question and found that NK cells from aged mice proliferated and up-regulated KLRG1 normally in response to pathogen-derived products when transferred to young mice, suggesting that NK cells in aged mice are not intrinsically defective. In contrast, NK cells from young mice failed to proliferate and up-regulate KLRG1 in response to pathogen-derived products when transferred to aged mice, suggesting that the host environment is responsible for aging-related NK cell deficiency. Finally, aging-related NK cell defects could not be reversed by increasing the production of inflammatory cytokines such as TNFα, IL-12 and IFNγ. However, soluble IL-15/IL-15Rα complexes effectively reversed the aging-related functional NK cell deficiency.
Materials and Methods
Mice
Male C57BL/6 mice at 2-24 months of age were obtained from the National Institute on Aging contract colony at Harlan Laboratories (Indianapolis, IN) or from the Jackson Laboratory (Bar Harbor, ME). Mice were individually identified by ear punches and maintained under specific pathogen-free conditions and provided food and water ad libitum. Aging status of the NK cell population of each individual mouse was monitored by periodic flow cytometric analysis of peripheral blood samples collected via a tail vain nick. Necropsy examinations were performed at the time of mouse sacrifice in order to exclude individuals with organ failure or neoplasms. Male C57BL/6 congenic mice (CD45.1+CD45.2-) were purchased from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 F1 congenic mice (CD45.1+CD45.2+) were produced by crossing male C57BL/6 congenic (CD45.1+CD45.2-) with female C57BL/6 (CD45.1-CD45.2+) mice. The University of Michigan Committee on Use and Care of Animals (UCUCA) approved all animal studies.
NK cell priming in vivo
NK cells were primed by intra-peritoneal injection of either polyinosinic:polycytidylic acid (poly I:C) (InvivoGen, San Diego, CA) at 100 ug/mouse or LPS (Sigma, St. Louis, MO) at 10 ug/mouse in 0.5 ml PBS. Some mice were primed by injecting both poly I:C (50 ug/mouse ) and LPS (10 ug/mouse). In other mice, IL-10 signaling was block by intra-peritoneal injection of anti-CD210 (1B1.3A) (IL-10R blocking antibody) at 0.5 mg/mouse (BioXcell, West Lebanon, NH) 1 day before priming by poly I:C and LPS. Control mice in these experiments were given rat IgG at 0.5 mg/mouse (Sigma, St. Louis, MO). When NK cells were primed with IL-15/IL-15Rα complex, each 1 ug of recombinant murine IL-15 (R&D Systems, Minneapolis, MN) was incubated with 6 ug of recombinant murine IL-15Rα-human-IgG1-Fc (R&D Systems, Minneapolis, MN) for 30 minutes in 200 ul of PBS at 37°C. The IL-15/IL-15Rα complex was injected I.P. at 100 ng of IL-15/mouse in 0.5 ml of PBS.
NK cell adoptive transfer
Single cell suspensions were prepared from freshly harvested spleens by mechanical dissociation. NK cells were enriched by depleting erythrocytes, T cells, B cells and granulocytes using negative enrichment kits (Miltenyi Biotec, Auburn, CA). For CFSE labeling, cells were resuspended at a concentration of 107 cells/ml in PBS (Sigma Chemical Co, St. Loius, MO). CFSE (Invitrogen, San Diego, CA) was added to the single cell suspension at a final concentration of 2 μM and incubated for 10 minutes at 37°C. After incubation, the cells were washed once with complete RPMI 1640 (Sigma Chemical Co.) and once with PBS. The cells were then checked for viability and fluorescent labeling using a fluorescent microscope. Approximately one million viable cells were transferred intravenously into each congenic recipient mouse.
Bone marrow stem cell adoptive transfer
Bone marrow chimeras were established using a novel approach based on a recent discovery which allows the replacement of a significant proportion of endogenous hematopoietic stem cells (HSCs) by adoptively transferred congenic HSCs without the disruption caused by irradiation (45). Recipient CD45.1-CD45.2+ mice were injected i.p. with 0.5 mg of anti-CD117 (ACK2) (eBioscience, San Diego, CA) in 0.5 ml of PBS on day 0. On day 7, single cell suspensions were prepared from freshly harvested bone marrow from CD45.1+CD45.2+ donors. Lineage-positive cells were labeled with biotinylated anti-CD4, CD8, CD5, CD19, B220, NK1.1 and CD11b, washed, labeled with anti-biotin magnetic beads (Miltenyi Biotec) and depleted in MACS columns. Lineage-depleted bone marrow cells were checked for viability and transferred intravenously in 0.3 ml of PBS. Cell transfer was repeated on day 9 to achieve maximal chimerism. A ratio of one donor mouse per one recipient was used. Donor HSCs routinely give rise to granulocytes, monocytes and NK cells by 2 weeks and T cells by 4 to 6 weeks after being transferred into CD117-treated mice. The levels of granulocyte-, monocyte- and NK cell-chimerism can reach as high as ~15% by 3 months and maintained thereafter. The level of chimerism did not seem to increase with the number of donor cells injected. Rather, the timing of donor cell injection appeared to be critical. Mixed bone marrow chimeras were generated by co-transferring bone marrow cells from CD45.1+CD45.2+ and CD45.1-CD45.2+ mice to CD45.1+CD45.2- congenic mice that were irradiated with a single dose of 7 Gy. Approximately 5 million bone marrow cells from each donor type were transferred to each recipient ~2 hours after the irradiation.
In vivo NK cell cytolytic activity assay
Single cell suspensions were prepared from freshly harvested spleens of H2K/D double knockout (KO) and WT C57BL6 mice. H2K/D KO cells were labeled with 0.25 uM CFSE, whereas WT cells were labeled with 0.05 uM CFSE. Approximately 5 million H2K/D KO cells and 5 million WT cells were mixed and injected intravenously into naive or IL-15/IL-15Rα complex treated mice. The ratio of the number of H2K/D KO (CFSEhi) and WT (CFSElo) cells before injection were determined by flow cytometric analysis of a sample of the cell mixture taken before injection. The ratio of the number of H2K/D KO (CFSEhi) and WT (CFSElo) cells 4 hours after injection were determined by flow cytometric analysis of peripheral blood samples taken 4 hours after injection. Specific % killing of H2K/D KO cells was estimated by using the following formula: (1-R(in)/R(out))×100, where R(in) and R(out) are the ratio of the number of KO/WT of injected and recovered cells respectively.
Flow Cytometry
Single cell suspensions were prepared from freshly harvested spleens, femurs, lung and lymph nodes by mechanical dissociation as previously described (46). Total cell yields were determined by standard hemocytometric counting. Approximately 1 million cells were stained with fluorochrome-labeled antibodies in 2% FBS-PBS buffer after blocking with anti-CD16/CD32. Stained cells were washed, fixed, acquired using a BD LSR II flow cytometer (equiped with 4 lasers and controled by FACSDiva software), and analysed with FlowJo software. NK cells were identified by their expression of NK1.1, which strictly co-expresses with NKp36, in CD3-CD5-CD19- leukocytes (CD45+). For peripheral blood analysis, 20 micro-liters of blood were collected via a tail vain nick. After lysing red blood cells, the entire sample were stained and subjected to flow cytometric analysis. The number of NK cells per microliter was calculated from the total number of NK cells recorded in the sample.
Monoclonal antibodies were purchased from BD PharMingen San Diego, CA or Serotec Raleigh, NC or eBioscience San Diego, CA including eFluor 450-, APC-, APC-Cy7, AF700, PerCPCy5.5-, FITC-, PE-, PE-Cy7- and PE-Cy5-conjugated anti-CD3 (145-2C11), anti-CD5 (53-7.3), anti-CD11b/Mac-1 (M1/70), anti-CD45 (30-F11), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD19 (eBio1D3), anti-NK1.1 (PK136), anti-CD27 (LG.3A10), anti-KLRG1 (2E1), anti-Granzyme B (GB11), anti-Ly-6G and Ly-6C (Gr-1, clone RB6-8C5), anti-CD11c (HL3 and N418), anti-I-Ab (11-5.2), anti-F4/80 (A3-1), anti-CD16/CD32 (2.4G2). Anti-NKp46 goat IgG was purchased from R&D Systems.
Statistical analysis
Single factor analysis of variance (ANOVA) was used for intergroup comparisons with P < 0.05 considered to indicate significance.
Results
NK cells in aged mice fail to expand and up-regulate KLRG1 in response to pathogen-derived products
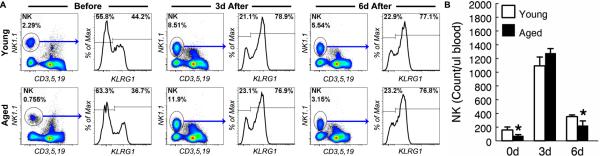
Under steady state conditions, cytolytic NK cells are vastly outnumbered by non-lytic NK cells in both humans and mice (5, 47). However, non-lytic NK cells can quickly give rise to a large number of lytic NK cells in response to pathogen-derived products in a process known as NK cell priming (5, 34, 48). To understand why aged mice fail to increase NK cell cytolytic activity in response to pathogen-derived products (30-33), we challenged young and aged mice with LPS and determined the number of NK cells in the blood at various time points after challenge. The blood was examined because it has been shown that cytolytic NK cells reside preferentially in peripheral blood, whereas non-lytic NK cells reside preferentially in secondary lymphoid organs (5, 47). We found that in young mice, the number of NK cells in the blood increased dramatically after priming with LPS reaching a peak leave 3 to 4 days after the challenge. In contrast, the number of NK cells in aged mice remained largely unchanged over a 6-day period after the challenge (Fig. 1a and 1b). In addition to population expansion, priming of NK cells also leads to up-regulation of KLRG1 expression, which is associated with cytolytic effecter cell development (43, 44). We found that the development of KLRG1+ NK cells after LPS challenge was severely impaired in aged mice (Fig. 1a and 1b). In addition, the baseline number of KLRG1+ NK cells was lower in aged mice than in young mice before the challenge. Similar aging-related defects were also demonstrable after mice were challenged with the TLR3 ligand, polyinosinic:polycytidylic acid (poly I:C) (Fig. 1c and 2d). Although poly I:C was able to induce KLRG1 up-regulation by day 6 in aged mice (Fig. 1c), population expansion was still impaired. These data suggested that impaired NK cell expansion and activation may be responsible for aging-related functional NK cell deficiency.
Figure 1. NK cells in aged mice fail to expand and up-regulate KLRG1 in response to pathogen-derived products.
Young (4-6 mo.) and aged (20-22 mo.) were individually identified by ear punches and challenged with intra-peritoneal injection of 10 μg of LPS (A and B) or 100 μg of poly I:C (C and D). Twenty micro-liters of blood samples were analyzed before, 3 and 6 days after the challenge by flow cytometry. (A and C) NK cells were identified as NK1.1+ and CD3/CD5/CD19 triple-negative cells in gated CD45+ cells. Gated NK cells are shown to demonstrate the proportion of KLRG1+ cells. (B and D) The mean and standard deviation of the number of NK and KLRG1+ NK cells per microliter of blood are presented. Data are representative of 2 experiments (n = 4). * indicates statistical significance between young and aged mice with P <0.05.
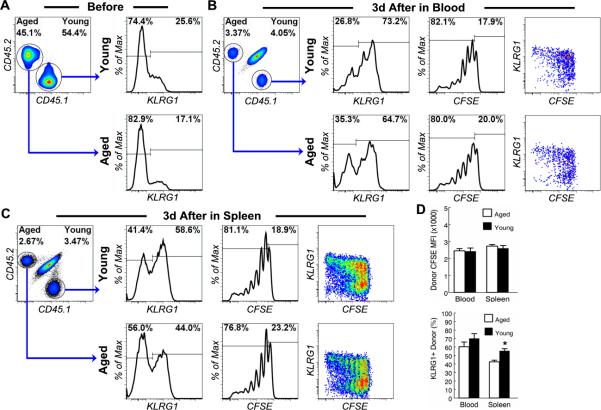
Figure 2. NK cells from aged mice proliferate and up-regulate KLRG1 normally in young mice in response to pathogen-derived products.
NK cells from the spleen of young (4-6 mo.) CD45.1+CD45.2- and aged (20-22 mo.) CD45.1-CD45.2+ C57BL6 mice were enriched by MACS negative selection, mixed, labeled with CFSE and transferred to young (3 mo.) CD45.1+CD45.2+ congenic hosts. The recipients were inject i.p. with 10 ug of LPS and 50 ug of poly IC 1 day after cell transfer. Donor NK cells were analyzed 3 day after LPS and poly IC injection. (A) Mixed donor NK cells were analyzed before transfer. Gated NK cells are shown to demonstrate the ratio of young and aged donor NK cells and the proportion of KLRG1+ cells in young and aged donor NK cells. (B and C) Donor NK cells from the blood (B) and spleen (C) of recipient mice were analyzed 3 days after LPS and poly IC injection. Gated NK cells are shown to demonstrate the ratio of young and aged donor NK cells, the proportion of KLRG1+ cells in young and aged donor NK cells and the fluorescent intensity of CFSE labeling of young and aged donor NK cells. (D) The mean and standard deviation of CFSE intensity of donor NK cells and the proportion of KLRG1+ donor NK cells are presented. Data are representative of 2 experiments (n = 4). * indicates statistical significance between young and aged donor cells with P <0.05.
NK cells from aged mice proliferate and up-regulate KLRG1 normally in young mice in response to pathogen-derived products
We previously reported that NK cells from aged mice do not have intrinsic defects in their ability to produce IFNγ in response to pathogen-derived products. Rather, the host environment was responsible for the aging-related defect in IFNγ production (49, 50). To determine if NK cells in aged mice are intrinsically defective in response to priming, we co-transferred young CD45.1+ and aged CD45.2+ CFSE-labeled NK cells to young recipients, which were then challenged with both LPS and poly I:C. It was found that transferred CFSE-labeled NK cells in blood and spleens from young and aged mice divided extensively and up-regulated KLRG1 3 days after priming in young hosts (Fig. 2b, 2c). Aged donor NK cells divided to the same degree as young donor NK cells as indicated by the level of the mean fluorescent intensity (MFI) of CFSE labeling of the donor NK cells (Fig. 2d). Although the proportion of KLRG1+ cells in aged donor NK cells was slightly lower than that in young donor NK cells 3 days after priming (Fig. 2d), it was unlikely due to an impaired response by aged donor NK cells but rather reflected the fact that the proportion of pre-transfer KLRG1+ cells in aged donor NK cells was lower than that in young donor NK cells (Fig. 2a). These data suggest that NK cells in aged mice are not intrinsically defective in their ability to proliferate and differentiate in response to priming.
NK cells from young mice fail to proliferate and up-regulate KLRG1 in aged mice in response to pathogen-derived products
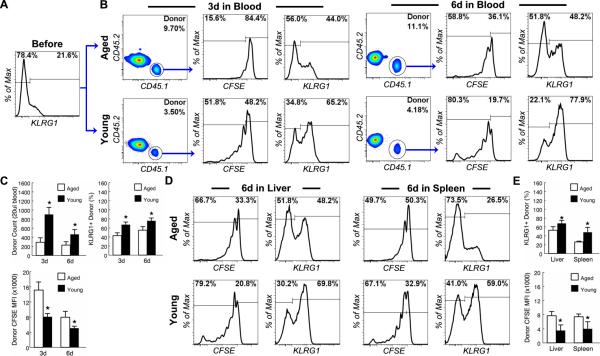
To determine if the aged host environment was responsible for the failure of NK cell expansion and activation after priming in aged mice, we transferred CFSE-labeled congenic CD45.1+ NK cells from young mice to either young or aged CD45.2+ recipients and analyzed the response of donor NK cells to priming by LPS and poly I:C. As expected, NK cells from young mice divided extensively and up-regulated KLRG1 3 days after priming in young hosts (Fig. 3b and 3c). Although donor NK cells continued to divide after day 3, as indicated by the decrease of the mean fluorescent intensity (MFI) of CFSE labeling of the donor NK cells, the absolute number of donor NK cells decreased rather than increased from day 3 to day 6 after priming in young mice, suggesting that many primed NK cells are short lived (Fig. 3b and 3c). In contrast, fewer young donor NK cells proliferated 3 days after priming in aged recipients (15.6% in old vs. 51.8% in young). Although the proportion of the dividing donor NK cells did increase by 6 days after priming in aged hosts, the MFI of CFSE labeling of donor NK cells was significantly higher in aged than in young hosts at both time points, indicating that donor NK cells divided less extensively in the aged environment (Fig. 3b and 3c). As a result, the expansion of donor NK cells was significantly lower in aged than in young recipient mice (Fig. 3c). Although the absolute number of donor NK cells in young recipient mice was significantly higher than in aged recipient mice (Fig. 3c), the percentage of donor NK cells among all NK cells (host and donor) in young recipients was much lower than in aged recipients (Fig. 3b) because host NK cells of the young recipients expanded more than those of the aged host recipients as demonstrated in Figure 1. In addition to impaired expansion, up-regulation of KLRG1 expression by young donor NK cells was also significantly impaired in aged as compared to young hosts (Fig. 3b and 3c). Impaired expansion of and KLRG1 up-regulation among donor NK cells in peripheral blood was not a result of altered tissue distribution because the same aging-related impairment was also observed in the recipient livers and spleens (Fig. 3d and 3e). These data suggested that the host environment was responsible for aging-related defects in NK cell priming by pathogen-derived products.
Figure 3. NK cells from young mice fail to proliferate and up-regulate KLRG1 in aged mice in response to pathogen-derived products.
NK cells from the spleen of young (3 mo.) CD45.1+CD45.2- C57BL6 mice were enriched by MACS negative selection, labeled with CFSE and transferred to young (4-6 mo.) and aged (20-22 mo.) CD45.1-CD45.2+ congenic mice. The recipients were injected i.p. with 10 ug of LPS and 50 ug of poly IC 1 day after cell transfer. (A) Donor NK cells were analyzed before transfer. Gated NK cells are shown to demonstrate the proportion of KLRG1+ cells in donor NK cells. (B) Donor NK cells from the blood were analyzed 3 and 6 day after LPS and poly IC injection. Gated donor NK cells from young and aged hosts are shown to demonstrate the proportion of KLRG1+ cells and the fluorescent intensity of CFSE labeling. (C) The mean and standard deviation of the number of donor NK cells, CFSE intensity of the donor NK cells and the proportion of KLRG1+ donor NK cells are presented. Data are representative of 2 experiments (n = 4). * indicates statistical significance between young and aged donor cells with P <0.05. (D and E) Donor NK cells from the liver and spleen of recipient mice were analyzed 6 days after LPS and poly IC injection. (D) Gated donor NK cells are shown to demonstrate the proportion of KLRG1+ cells and the fluorescent intensity of CFSE labeling. (E) The mean and standard deviation of CFSE intensity of the donor NK cells and the proportion of KLRG1+ donor NK cells are presented. Data are representative of 2 experiments (n = 4). * indicates statistical significance between young and aged donor cells with P <0.05.
Blockade of IL-10 signaling failed to reverse aging-related defects in NK cell priming by pathogen-derive products
We previously reported that aging is associated with increased production of IL-10 by macrophages in response to pathogen-derived products in vivo (49, 50). In addition, blockade of IL-10 signaling by an anti-CD210 (IL-10 receptor) antibody dramatically increased the level of a wide range of cytokines and chemokines including dendritic cell-derived IL-12 in aged mice, which in turn reversed the aging-related defect in IFNγ production by NK cells (49, 50). To determine if increased IL-10 production was also responsible for aging-related defects in NK cell priming, we blocked IL-10 signaling by injecting anti-CD210 in aged mice, which were then challenged with LPS and poly I:C. As shown in Fig. 4, blockade of IL-10 signaling in aged mice had no significant effect on NK cell expansion and KLRG1 up-regulation. In addition, injection of recombinant IFNγ, IL-12 and IL-18 each individually or in combination failed to reverse aging-related defects in NK cell priming by LPS (data not shown). These data suggest that unlike aging-related defects in IFNγ production by NK cells, aging-related defects in NK cell priming is not a result of impaired production of the cytokines due to age-related increases in IL-10 production by macrophages.
Figure 4. Blockade of IL-10 signaling failed to reverse aging-related defects in NK cell priming by pathogen-derive products.
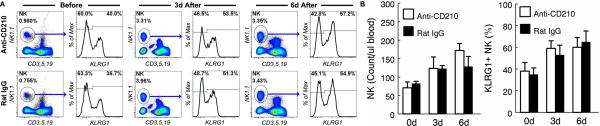
Aged (20-22 mo.) C57BL6 mice were injected i.p. with anti-CD210 (1B1.3A) (IL-10R blocking antibody) or control rat IgG. One day later, mice were challenged i.p. with 10 ug of LPS and 50 ug of poly IC. Twenty micro-liters of blood samples were analyzed before, 3 and 6 days after the challenge by flow cytometry. (A) Gated CD45+ cells are shown to demonstrate the proportion of NK cells. Gated NK cells are shown to demonstrate the proportion of KLRG1+ NK cells. (B) The mean and standard deviation of the number of NK cells per micro-liter of blood and the proportion of KLRG1+ cells are presented. Data are representative of 2 experiments (n = 3).
NK cell maturation is impaired in aged mice
NK cells in naive mice are phenotypically diverse and can be divided into CD27- and CD27+ subsets (8, 9, 16). CD27- NK cells, which are considered to be mature NK cells, are enriched in peripheral blood and non-lymphoid tissues such as the lung, whereas CD27+ NK cells, which are the immediate immature precursors of CD27- NK cells, are enriched in bone marrow and lymph nodes (ref. (9) and Fig. 5). The effect of aging on NK cell maturation in naive mice can be easily detected using flow cytometry (51). For example, aging is associated with a shift from CD27- to CD27+ in NK cells in peripheral blood, lymph node, bone marrow, lung and spleen (Fig. 5). To better define the levels of population shift, the following formula was used to calculate the percent shift in CD27-/+ NK population with aging: percent shift = (1-R(aged)/R(young)) × 100, where R = the number of CD27- NK/the number of CD27+ NK. As shown in Fig. 5b, in all the organs tested, an approximately 50% shift was found when mice were aged from 4 months to 20 months of age. These data suggest that NK cells in aged mice are intrinsically defective due to a maturation defect. However, studies have shown that CD27+ to CD27- NK cell maturation is regulated by IL-15-presentation (23, 24). In particular, the level of IL-15-presentation required for the acquisition of the CD27- phenotype is higher than the level required for the development of CD27+ NK cells (23). Thus, when IL-15-presentation by macrophages or DCs is reduced the maturation of NK cells appears to be arrested at the CD27+ stage (23, 24). Therefore, the host environment could also be responsible for aging-related maturation defect in NK cell development.
Figure 5. NK cell maturation is impaired in aged mice.
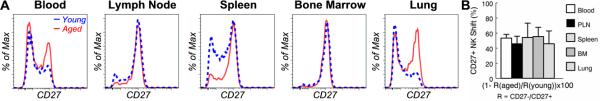
Single-cell suspensions were prepared from peripheral blood, lymph nodes, spleen, bone marrow and lung of young (4 months) (blue) and aged (20 months) (red) mice and were analyzed using flow cytometry. NK cells are identified as in Figure 1. (A) Gated NK cells are shown to demonstrate aging-related shift in the proportion of CD27+ and CD27- cells. Data are representative of 5 experiments with 1 young and 1 aged mouse in each experiment. (B) Percent CD27+/- population shift with aging was calculated (see text) for each experiment. The mean and standard deviation of the percent CD27+ NK cell shift from the 5 experiments are presented.
Bone marrow from aged mice give rise to CD27- NK cells normally in young hosts
To determine if the host environment is responsible for NK cell maturation, we first tested if the aging-related impairment in NK cell development is a result of a defect in bone marrow stem cells by co-transferring bone marrow cells from CD45.1+CD45.2+ young (~3 months) and CD45.1+CD45.2- aged (~20 months) donors to sub-lethally irradiated congenic CD45.2+CD45.1- young recipients. As shown in Figure 6, transferred bone marrow, which contain very few CD27-mature NK cells (Fig. 5), gave rise to mature CD27- NK cells as early as 3 weeks after transfer and continue to do so for at least 3 months (Fig. 6a). Importantly, the proportion of CD27- cells in NK cells that were derived from aged bone marrow was not significantly different from the proportion of CD27- cells in NK cells that were derived from young bone marrow (Fig. 6b). These data suggest that aged immature NK cells and bone marrow hematopoietic stem cells (HSC) are not intrinsically defective in their ability to give rise to mature CD27- NK cells.
Figure 6. Bone marrow from aged mice give rise to CD27- NK cells normally in young hosts.
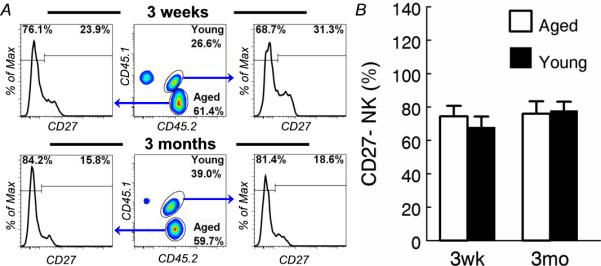
Bone marrow cells from young (~3 months) CD45.1+CD45.2+ and aged (~20 months) CD45.1-CD45.2+ C57BL6 mice were mixed and transferred to irradiated young (~3 months) CD45.1+CD45.2-congenic recipients. Blood samples from the recipients were analyzed 3 weeks and 3 months after bone marrow transfer. (A) NK cells are identified as in Figure 1 (not shown). Young and aged donor NK cells are identified by their expression of CD45.1 and CD45.2 in gated NK cells. Gated young and aged donor NK cells are shown to demonstrate the proportion of CD27+ and CD27- cells. (B) The mean and standard deviation of the percentage of CD27- NK cells are presented. Data are representative of 2 experiments (n = 3).
Bone marrow stem cells from young mice fail to reverse the aging-related defect in NK cell maturation
To determine if the host environment is responsible for aging-related defect in NK cell maturation, we transferred lineage-depleted bone marrow cells from young mice to aged mice. Bone marrow transfer is traditionally done only in irradiated recipients. However, for our purposes, whole body irradiation had to be avoided because it disrupts the host environment we are investigating. Previous studies show that only ~0.1% of the hematopoietic stem cell (HSC) niche is accessible via the blood under steady state conditions (52). Consistent with these data, our attempts to engraft HSCs in non-irradiated recipients failed as judged by the fact that no donor-derived leukocytes were generated regardless of how many stem cells were transferred (data not shown). Recently, it was reported that the HSC niche space could be made transiently accessible after injecting an anti-CD117 monoclonal antibody. The space remained accessible for a few days after the antibody was cleared from the system, providing a brief time window for engrafting donor HSCs (45). Using this approach, we were able to consistently engraft HSCs from CD45.1+CD45.2+ F1 B6 mice into CD45.1-CD45.2+ B6 mice. As shown in Fig. 7, donor-derived NK cells were found in the blood 3 weeks but not 1 week after transfer of lineage-depleted BM cells from young CD45.1+CD45.2+ mice to anti-CD117-treated aged CD45.1-CD45.2+ C57BL6 mice (Fig. 7a). As shown, HSCs from young mice did not reverse the aging-related defect in NK cell maturation. Instead, young HSCs gave rise to NK cell populations that resembled aged host NK cells with more CD27+ cells than CD27- cells (Fig. 7a). The maturation defect of the donor derived NK cells remained unchanged over a 3 month period and was observed not only in peripheral blood but also in the spleen and bone marrow (Fig. 7b). To better define the levels of phenotypic correction by young HSCs, the following formula was used to calculate the percent correction of aging-related CD27-/+ NK population shift by young donor HSCs: percent shift = (1-R(donor)/R(host)) × 100, where R = the number of CD27- NK/the number of CD27+ NK. As shown in Fig. 7c, no consistent correction by young HSCs was observed. These data indicated that the host environment rather than bone marrow stem cell defects are responsible for impaired, aging-related NK cell maturation.
Figure 7. Bone marrow stem cells from young mice fail to reverse the aging-related defect in NK cell maturation.
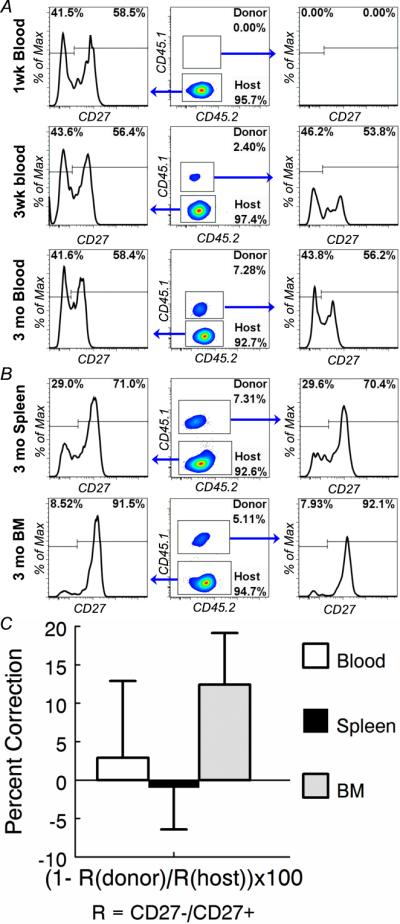
Lineage-depleted BM cells from young (2 months) CD45.1+CD45.2+ mice were transferred into anti-CD117-treated aged (20 months) CD45.1-CD45.2+ recipients. (A) Blood samples from the recipients were analyzed 1 week, 3 weeks and 3 months after bone marrow transfer. (B) Splenocytes from the recipients were analyzed 3 months after bone marrow transfer. NK cells are identified as in Figure 1. Donor-derived and host NK cells are identified by their expression of CD45.1 and CD45.2 in gated NK cells. Gated donor-derived and host NK cells are shown to demonstrate the proportion of CD27+ and CD27- cells. (C) Percent correction of aging-related CD27+ NK cell population shift by donor young HSCs was calculated (see text) for each host. The mean and standard deviation of the percent correction by donor young HSCs are presented. Data are representative of 3 experiments (n = 2)
NK cells in aged mice expand and up-regulate KLRG1 normally in response to IL-15/IL-15Rα stimulation
Based on current understanding of NK cell maturation, it is likely that the aging-related defect in CD27+ to CD27- NK cell transition (Fig. 5 to 7) is a result of impaired IL-15 presentation (23, 24). Recently, it has also become clear that NK cell priming is mediated by macrophages and DCs in an IL-15-dependent manner (5, 6, 34, 48, 53-58). Interaction between IL-15-presenting macrophages/DCs and NK cells is responsible not only for NK cell proliferation but also for up-regulation of granzyme B and perforin in non-lytic NK cells during NK cell priming (5, 6, 53, 59). Interestingly, NK cell priming by IL-15 signaling appears to be independent of cellular context because NK cells can be primed effectively by soluble IL-15/IL-15Rα complex (22, 60). If NK cells from aged mice are not intrinsically defective, they should be primed by IL-15/IL-15Rα complex. To test this, we injected recombinant IL-15/IL-15Rα complex in young and aged mice. In contrast to pathogen-derived products (Fig. 1), IL-15/IL-15Rα complex expanded NK cells to the same degree in young and aged mice (Fig. 8a and 8b). In addition, the complex also up-regulated KLRG1 expression by NK cells similarly in young and aged mice (Fig. 8a). These data demonstrated that aging-related defects in NK cell maturation/priming can be reversed by IL-15 receptor agonists.
Figure 8. NK cells in aged mice expand and up-regulated KLRG1 normally in response to IL-15/IL-15Rα.
Young (4-6 mo.) and aged (20-22 mo.) C57BL6 mice were injected i.p. with 100 ng of IL-15 conjugated with 600 ng of IL-15Rα human Ig. (A) NK cells in the blood were analyzed before, 3 and 6 days after IL-15/IL-15Rα injection. Gated CD45+ cells are shown to demonstrate the percentage of NK cells. Gated NK cells are shown to demonstrate the proportion of KLRG1+ cells. (B) The mean and standard deviation of the number of NK cells per micro-liter of blood are presented. Data are representative of 2 experiments (n = 3). * indicates statistical significance between young and aged donor cells with P <0.05.
Priming of NK cells by IL-15/IL-15Rα complex reverses aging-related functional NK cell deficiency
To determine if IL-15/IL-15Rα complex can also successfully prime NK cell cytolytic activity in aged mice, we first enumerated the number of granzyme B expressing NK cells in naive and IL-15/IL-15Rα complex-injected young and aged mice. It was found that granzyme B is strictly co-expressed with KLRG1 by NK cells in peripheral blood of both young and aged mice, supporting the use of KLRG1 as a marker of cytolytic NK cells (Fig. 9a and 9b). Furthermore, the number of granzyme B and KLRG1 double positive NK cells was significantly higher in naive young mice than naive aged mice (Fig. 9c). Injection of IL-15/IL-15Rα complex dramatically increased the number of these double positive NK cells in both young and aged mice (Fig. 9c). Finally, we measured NK cell cytolytic functional activity in vivo in these mice, NK cell-susceptible target cells from the spleens of H2K/D double knockout (KO) mice were injected intravenously into naive and IL-15/IL-15Rα complex-primed young and aged mice along with control NK cell-resistant cells from the spleens of syngeneic wild type (WT) mice. Specific percent target killing of H2K/D KO cells was calculated by using the following formula: (1-R(in)/R(out))×100, where R(in) and R(out) are the ratio of the number of KO/WT of injected and recovered cells respectively. Consistent with previous reports (5, 34, 48), we found that without priming, NK cell cytolytic activity in young mice was quite low (Fig. 9c). However, unprimed naive young cells showed higher baseline cytotoxicity than naïve aged NK cells (Fig 9c). The cytolytic activity in both young and aged mice increased dramatically 3 days after IL-15 injection (Fig. 9c). Thus, the functional assay precisely paralleled the phenotypic analysis (Fig. 9c). These data demonstrated that aging-related functional NK cell deficiency can be reversed by administration of IL-15 receptor agonists
Figure 9. Priming of NK cells by IL-15/IL-15Rα reverses aging-related functional NK cell deficiency.
Young (4-6 mo.) and aged (20-22 mo.) C57BL6 mice were primed with IL-15/IL-15Rα as in Fig. 8. Naive and 3d-primed mice were injected intravenously with CFSE labeled cells from the spleen of syngenic wild type (low CFSE labeled control target cells) and H2K/D double knockout (high CFSE labeled NK-susceptible target cells) mice. Peripheral blood samples were analyzed 4 hrs after target cell injection. NK cells are identified as in Figure 1. (A and B) Gated NK cells in naive (A) and primed (B) mice are shown to demonstrate that granzyme B and KLRG1 were co-expressed by NK cells. Gated target cells are shown to demonstrate the ratio of control and NK-susceptible target cells. (C) The mean and standard deviation of the number of granzyme B and KLRG1 double positive NK cells per micro-liter of blood and % specific target killing (see text of formula) are presented. Data are representative of 2 experiments (n = 3). * indicates statistical significance between young and aged donor cells with P <0.05.
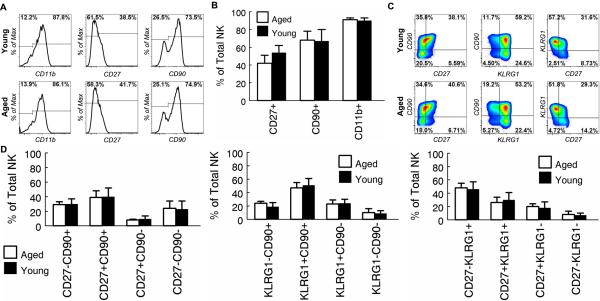
Recent transcriptome-wide analysis showed that priming affects a large number of genes in NK cells (44). Up-regulation of KLRG1, CD11b and CD90 protein was also detected by flow cytometry (44). In addition, we have found that although CD27 is down-regulated during NK cell maturation, it is transiently up-regulated after priming (data not shown). To further characterized the effect of IL-15/IL-15Ra on NK cells in aged mice, we compared the expression levels of these molecules by NK cells in young and aged mice after injection of IL-15/IL-15Rα complex. We first used a single marker, which divides NK cells into two subpopulations (Fig. 10a). It was found that the proportion of NK cell subpopulations defined by CD11b, CD27 or CD90 in aged mice was not significantly different from that in young mice 3 days after injection of IL-15/IL-15Rα complex (Fig. 10a and 10b). The proportion of NK cell subpopulations defined by KLRG1 in aged mice was also not significantly different from that in young mice 3 days after injection of IL-15/IL-15Rα complex (Fig. 8 and 9). We next used a combination of two markers, which divides NK cells into 4 subpopulations (Fig. 10c). For these experiments, we focused on CD27, CD90 and KLRG1. CD11b was not included because only a small minority of NK cells are CD11b- (Fig. 10a). Again, the proportion of the subpopulations defined by these markers in aged mice was not significantly different from that in young mice 3 days after injection of IL-15/IL-15Rα complex (Fig. 10c and 10d). The proportion of subpopulations that were defined by using a combination of 3 markers (CD27, CD90 and KLRG1), which divides NK cells into 8 subpopulations, in aged mice was also not significantly different from that in young mice 3 days after injection of IL-15/IL-15Rα complex (data not shown). Together, these data suggest that NK cells in aged mice are activated similarly as those in young mice in response to IL-15 receptor agonists.
Figure 10. NK cells in young and aged mice display a similar phenotype after priming with IL-15/IL-15Rα.
Young (4-6 mo.) and aged (20-22 mo.) C57BL6 mice were primed with IL-15/IL-15Rα as in Fig. 8. Peripheral blood NK cells were analyzed 3 days after priming. (A) Gated NK cells are shown to demonstrate the percentage of CD11b+, CD27+ and CD90+ cells. (B) The mean and standard deviation of the percentage of CD11b+, CD27+ and CD90+ NK cells are presented. Data are representative of 2 experiments (n = 5 in experiment 1 and n = 3 in experiment 2). (C) Gated NK cells are shown to demonstrate the percentage of NK cell subsets defined by the 2 markers shown in the figure. (D) The mean and standard deviation of the percentage of NK cell subsets that are defined in (C) are presented. Data are representative of 2 experiments (n = 5 in experiment 1 and n = 3 in experiment 2).
Discussion
Although aging-related functional NK cell deficiency is well-documented (2, 30-37, 39-42), the mechanism is poorly understood. By transferring NK cells from young to aged mice or vise versa, we demonstrated that the aged host environment rather than an intrinsic NK cell defect is responsible for aging-related defect in NK cell priming by pathogen-derived products. It has long been known that steady state NK cells in aged animals and humans are phenotypically different from those in their younger counterparts. However, the mechanism for these observations has not been reported. By transferring bone marrow stem cells from young to aged mice or vise versa, we demonstrated that the host environment rather than stem cells are responsible for aging-related defect in NK cell maturation under steady state conditions. Furthermore, we showed that aging-related functional NK cells deficiency can be completely reversed by injecting soluble IL-15/IL-15Ra complexes. In contrast, blockade of IL-10 signaling, which broadly augments inflammatory responses to pathogen-derived products, has little effect on aging-related defect in NK cell priming. Our data thus demonstrate that the aged host environment is primarily responsible for aging-related functional NK cell deficiency.
Although the exact nature of the host factors responsible for aging-related functional NK cell deficiency remain to be defined, our data suggest that insufficient access to IL-15-presentation by NK cells and their precursors is responsible for aging-related functional NK cell deficiency. Both NK cell maturation (21, 61-63) and priming (54-58) are dependent on IL-15-presentation and both are impaired in aging mice. It was surprising that blockade of IL-10, which broadly enhances inflammatory responses, had no effect on NK cell priming after LPS and poly I:C challenge of aged mice, whereas soluble recombinant IL-15/IL-15Rα alone completely reversed the aging-related functional NK cell deficiency. These data suggest that IL-15-presentation plays a dominant role in NK cell priming. Without sufficient IL-15 stimulation, other inflammatory cytokines such as IL-12 and IFNγ appear to contribute little to NK cell priming.
The biological activity of IL-15 is mediated by the heterodimeric IL-15 receptor, IL-15Rβγ (64). In IL-15 producing cells, IL-15 and its high affinity IL-15 receptor, IL-15Rα, are pre-assembled before being presented on the cell surface or released from the cell (65). Because bioactive IL-15 is presented on cell surface (24, 65-67), access to IL-15-presentation by NK cells depends not only on the production and consumption of IL-15 but also on successful recruitment of NK cells by IL-15 presenting cells, which in turn depends on the production and consumption of NK cell-recruiting factors by IL-15-presenting cells. Unfortunately, as reported by others (36), the level of IL-15 presentation was too low to be reliably quantified with currently available reagents (our unpublished data). Therefore, it remains unclear if aging alters levels of IL-15-presention by the various subsets of mononuclear phagocytes. This question can be addressed once aged IL-15 reporter mice become available (36). Currently, the factors mediating NK cell recruitment by IL-15-presenting cells are poorly understood and the effect of aging is completely unknown. However, other aging-related defects in macrophages have been documented (49, 50, 68-70). In addition to decreased production, increased competition for IL-15 and NK cell-recruiting factors may be responsible for insufficient access to IL-15 by NK cells and their precursors (14). In young mice, NK cells represent the largest cell population that expresses the heterodimeric IL-15Rβγ IL-15 receptor. In contrast, in aged mice, memory phenotype CD8 T cells become the dominant population expressing this receptor (our unpublished data). The potential role of competing memory phenotype CD8 T cells in contributing to the aging-related NK cell deficiency is currently under investigation.
Studies of cytolytic activities of human NK cells rely heavily on the radioactive in vitro chromium-release assay (71). With this assay, a low level of “spontaneous” cytolytic activity can be detected in peripheral blood of normal donors (1). Such in vitro NK cell activity is known to be amplified by IL-2, being many folds higher in the presence of IL-2 (72). Data regarding whether or not aging impairs spontaneous NK cell function are sometimes contradictory (73). The low level of spontaneous cytolytic activity combined with high sensitivity to in vitro manipulation may explain conflicting results. Although IL-2 is commonly used to prime NK cells, the vast majority of NK cells express the heterodimeric IL-15Rβγ IL-15 receptor, which is also known as intermediate affinity IL-2 receptor (64), but not the high affinity IL-2 receptor, IL-2Rαβγ (64, 74). These data suggest that human NK cell priming by IL-2 in vitro is actually being mediated via the IL-15 receptor. Furthermore, in contrast to IL-2, which is produced transiently by recently activated T cells in secondary lymphoid tissues, IL-15 is produced constitutively in a wide variety of tissues (75). Thus, in vivo, the heterodimeric IL-15Rβγ IL-15 receptor is activated primarily by IL-15 not IL-2. Although it is not yet clear if the host environment is responsible for aging-related functional NK cell deficiency in humans, studies have shown that NK cells from aged humans can be successfully primed by IL-2 in vitro (41, 76). In addition, recent studies using humanized mouse models suggest that IL-15-presentation plays a similar role in human NK cell development and maturation as in mouse NK cell development and maturation. Thus, current data appear to be consistent with the hypothesis that insufficient access to IL-15-presentation by NK cells is likely responsible for aging-related functional NK cell deficiency in humans.
In summary, our study significantly advances our understanding of the effect of aging on NK cells function by providing novel evidence that the host environment is responsible for aging-related functional NK cell deficiency. In addition, our data suggest that IL-15 receptor agonists may be useful tools in treating aging-related functional NK cell deficiency, which may contribute to the impaired viral and tumor immunity.
Acknowledgments
This work was supported by NIH-NIAID grant A143460 and in part by the Department of Veterans Affairs.
Abbreviations used in this paper
- KLRG1
killer cell lectin-like receptor G1
- DC
dendritic cell
- poly I:C
polyinosinic:polycytidylic acid
- MFI
mean fluorescent intensity
- HSC
hematopoietic stem cell
References
- 1.Pross HF, Jondal M. Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes. Clin Exp Immunol. 1975;21:226–235. [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh K, Suzuki R, Umezu Y, Hanaumi K, Kumagai K. Studies of murine large granular lymphocytes. II. Tissue, strain, and age distributions of LGL and LAL. J Immunol. 1982;129:395–405. [PubMed] [Google Scholar]
- 3.Kumagai K, Itoh K, Suzuki R, Hinuma S, Saitoh F. Studies of murine large granular lymphocytes. I. Identification as effector cells in NK and K cytotoxicities. J Immunol. 1982;129:388–394. [PubMed] [Google Scholar]
- 4.Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, Jaeger S, Andre P, Gauthier L, Daniel L, Chemin K, Morel Y, Dalod M, Imbert J, Pierres M, Moretta A, Romagne F, Vivier E. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, Moretta A, Munz C. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 6.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Vossen MT, Matmati M, Hertoghs KM, Baars PA, Gent MR, Leclercq G, Hamann J, Kuijpers TW, van Lier RA. CD27 defines phenotypically and functionally different human NK cell subsets. J Immunol. 2008;180:3739–3745. doi: 10.4049/jimmunol.180.6.3739. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 9.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK, Liu S, McClory S, Marcucci G, Trotta R, Caligiuri MA. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115:274–281. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom-Tullberg M, Michaelsson J, Rottenberg ME, Guzman CA, Ljunggren HG, Malmberg KJ. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood. 2010;116:3853–3864. doi: 10.1182/blood-2010-04-281675. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juelke K, Killig M, Luetke-Eversloh M, Parente E, Gruen J, Morandi B, Ferlazzo G, Thiel A, Schmitt-Knosalla I, Romagnani C. CD62L expression identifies a unique subset of polyfunctional CD56dim NK cells. Blood. 2010;116:1299–1307. doi: 10.1182/blood-2009-11-253286. [DOI] [PubMed] [Google Scholar]
- 14.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 15.Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ. Evidence for NK cell subsets based on chemokine receptor expression. J Immunol. 2006;177:7833–7840. doi: 10.4049/jimmunol.177.11.7833. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 17.Soderquest K, Powell N, Luci C, van Rooijen N, Hidalgo A, Geissmann F, Walzer T, Lord GM, Martin-Fontecha A. Monocytes control natural killer cell differentiation to effector phenotypes. Blood. 2011;117:4511–4518. doi: 10.1182/blood-2010-10-312264. [DOI] [PubMed] [Google Scholar]
- 18.Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Hofmann M, Wang Q, Teng L, Chlewicki LK, Pircher H, Mariuzza RA. Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing self recognition. Immunity. 2009;31:35–46. doi: 10.1016/j.immuni.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 21.Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ, Tabarias H, Degli-Esposti MA, Dewson G, Willis SN, Motoyama N, Huang DC, Nutt SL, Tarlinton DM, Strasser A. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc Natl Acad Sci U S A. 2010;107:21647–21652. doi: 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee GA, Liou YH, Wang SW, Ko KL, Jiang ST, Liao NS. Different NK cell developmental events require different levels of IL-15 trans-presentation. J Immunol. 2011;187:1212–1221. doi: 10.4049/jimmunol.1100331. [DOI] [PubMed] [Google Scholar]
- 24.Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, Malynn BA, Ma A. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- 26.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 27.Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol. 2007;7:329–339. doi: 10.1038/nri2073. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa Y, Smyth MJ. Innate immune recognition and suppression of tumors. Adv Cancer Res. 2006;95:293–322. doi: 10.1016/S0065-230X(06)95008-8. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albright JW, Albright JF. Age-associated impairment of murine natural killer activity. Proc Natl Acad Sci U S A. 1983;80:6371–6375. doi: 10.1073/pnas.80.20.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weindruch R, Devens BH, Raff HV, Walford RL. Influence of dietary restriction and aging on natural killer cell activity in mice. J Immunol. 1983;130:993–996. [PubMed] [Google Scholar]
- 32.Provinciali M, Muzzioli M, Fabris N. Timing of appearance and disappearance of IFN and IL-2 induced natural immunity during ontogenetic development and aging. Exp Gerontol. 1989;24:227–236. doi: 10.1016/0531-5565(89)90014-4. [DOI] [PubMed] [Google Scholar]
- 33.Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age-related changes in natural killer cells during primary influenza infection in mice. Mechanisms of Ageing and Development. 2008;129:223. doi: 10.1016/j.mad.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Gidlund M, Orn A, Wigzell H, Senik A, Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978;273:759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- 35.McCartney S, Vermi W, Gilfillan S, Cella M, Murphy TL, Schreiber RD, Murphy KM, Colonna M. Distinct and complementary functions of MDA5 and TLR3 in poly(I:C)-mediated activation of mouse NK cells. J Exp Med. 2009;206:2967–2976. doi: 10.1084/jem.20091181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colpitts SL, Stoklasek TA, Plumlee CR, Obar JJ, Guo C, Lefrancois L. Cutting edge: the role of IFN-alpha receptor and MyD88 signaling in induction of IL-15 expression in vivo. J Immunol. 2012;188:2483–2487. doi: 10.4049/jimmunol.1103609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Cella M, Gilfillan S, Colonna M. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J Immunol. 2010;184:2751–2755. doi: 10.4049/jimmunol.0903201. [DOI] [PubMed] [Google Scholar]
- 38.Plett PA, Gardner EM, Murasko DM. Age-related changes in interferon-alpha/beta receptor expression, binding, and induction of apoptosis in natural killer cells from C57BL/6 mice. Mech Ageing Dev. 2000;118:129–144. doi: 10.1016/s0047-6374(00)00164-0. [DOI] [PubMed] [Google Scholar]
- 39.Solana R, Mariani E. NK and NK/T cells in human senescence. Vaccine. 2000;18:1613–1620. doi: 10.1016/s0264-410x(99)00495-8. [DOI] [PubMed] [Google Scholar]
- 40.Mocchegiani E, Malavolta M. NK and NKT cell functions in immunosenescence. Aging Cell. 2004;3:177–184. doi: 10.1111/j.1474-9728.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- 41.Rukavina D, Laskarin G, Rubesa G, Strbo N, Bedenicki I, Manestar D, Glavas M, Christmas SE, Podack ER. Age-related decline of perforin expression in human cytotoxic T lymphocytes and natural killer cells. Blood. 1998;92:2410–2420. [PubMed] [Google Scholar]
- 42.Hayhoe RP, Henson SM, Akbar AN, Palmer DB. Variation of human natural killer cell phenotypes with age: identification of a unique KLRG1-negative subset. Hum Immunol. 2010;71:676–681. doi: 10.1016/j.humimm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med. 2011;208:357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bezman NA, Kim CC, Sun JC, Min-Oo G, Hendricks DW, Kamimura Y, Best JA, Goldrath AW, Lanier LL. Molecular definition of the identity and activation of natural killer cells. Nat Immunol. 2012;13:1000–1009. doi: 10.1038/ni.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiu BC, Shang XZ, Stolberg VR, Komuniecki E, Chensue SW. Population analysis of CD4+ T cell chemokine receptor transcript expression during in vivo type-1 (mycobacterial) and type-2 (schistosomal) immune responses. J Leukoc Biol. 2002;72:363–372. [PubMed] [Google Scholar]
- 47.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romagnani C, Juelke K, Falco M, Morandi B, D'Agostino A, Costa R, Ratto G, Forte G, Carrega P, Lui G, Conte R, Strowig T, Moretta A, Munz C, Thiel A, Moretta L, Ferlazzo G. CD56brightCD16- killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–4955. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 49.Chiu B-C, Stolberg VR, Chensue SW. Mononuclear Phagocyte-Derived IL-10 Suppresses the Innate IL-12/IFN-{gamma} Axis in Lung-Challenged Aged Mice. J Immunol. 2008;181:3156–3166. doi: 10.4049/jimmunol.181.5.3156. [DOI] [PubMed] [Google Scholar]
- 50.Chiu B-C, Stolberg VR, Freeman CM, Chensue SW. Mononuclear Phagocyte-Derived Interleukin-10 Suppresses the Innate Pulmonary Granuloma Cytokine Response in Aged Mice. Am J Pathol. 2007;171:829–837. doi: 10.2353/ajpath.2007.061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J Exp Med. 207:2369–2381. doi: 10.1084/jem.20100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunne J, Lynch S, O'Farrelly C, Todryk S, Hegarty JE, Feighery C, Doherty DG. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J Immunol. 2001;167:3129–3138. doi: 10.4049/jimmunol.167.6.3129. [DOI] [PubMed] [Google Scholar]
- 54.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, Diefenbach A. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic Cells Prime Natural Killer Cells by trans-Presenting Interleukin 15. Immunity. 2007;26:503. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beuneu H, Deguine J, Breart B, Mandelboim O, Di Santo JP, Bousso P. Dynamic behavior of NK cells during activation in lymph nodes. Blood. 2009;114:3227–3234. doi: 10.1182/blood-2009-06-228759. [DOI] [PubMed] [Google Scholar]
- 57.Coombes JL, Han SJ, van Rooijen N, Raulet DH, Robey EA. Infection-induced regulation of natural killer cells by macrophages and collagen at the lymph node subcapsular sinus. Cell Rep. 2012;2:124–135. doi: 10.1016/j.celrep.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garcia Z, Lemaitre F, van Rooijen N, Albert ML, Levy Y, Schwartz O, Bousso P. Subcapsular sinus macrophages promote NK cell accumulation and activation in response to lymph-borne viral particles. Blood. 2012;120:4744–4750. doi: 10.1182/blood-2012-02-408179. [DOI] [PubMed] [Google Scholar]
- 59.Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaelsson J, Malmberg KJ, Klingstrom J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 62.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–4893. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 64.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 68.Kelly J, Ali Khan A, Yin J, Ferguson TA, Apte RS. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cannizzo ES, Clement CC, Morozova K, Valdor R, Kaushik S, Almeida LN, Follo C, Sahu R, Cuervo AM, Macian F, Santambrogio L. Age-related oxidative stress compromises endosomal proteostasis. Cell Rep. 2012;2:136–149. doi: 10.1016/j.celrep.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, Chowers I, Edwards PA, Baldan A, Parks JS, Ory DS, Apte RS. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab. 2013;17:549–561. doi: 10.1016/j.cmet.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaritskaya L, Shurin MR, Sayers TJ, Malyguine AM. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev Vaccines. 2010;9:601–616. doi: 10.1586/erv.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Domzig W, Stadler BM, Herberman RB. Interleukin 2 dependence of human natural killer (NK) cell activity. J Immunol. 1983;130:1970–1973. [PubMed] [Google Scholar]
- 73.Mahbub S, Brubaker AL, Kovacs EJ. Aging of the Innate Immune System: An Update. Curr Immunol Rev. 2011;7:104–115. doi: 10.2174/157339511794474181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY. IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med. 2013;210:1179–1187. doi: 10.1084/jem.20122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 76.Kutza J, Murasko DM. Effects of aging on natural killer cell activity and activation by interleukin-2 and IFN-alpha. Cell Immunol. 1994;155:195–204. doi: 10.1006/cimm.1994.1112. [DOI] [PubMed] [Google Scholar]