Abstract
Developmental processes are governed by diverse regulatory mechanisms including a suite of signaling pathways employing reversible phosphorylation. With the advent of large-scale phosphoproteomics it is now possible to identify thousands of phosphorylation sites from tissues at distinct developmental stages. We describe here the identification of over 6,000 non-redundant phosphorylation sites from neonatal murine brain. When compared to nearly three times the number of phosphorylation sites identified from three-week-old murine brain, remarkably one-third of the neonatal sites were unique. This fraction only dropped to one-quarter when allowing the site to stray plus or minus 15 residues. This provides evidence for considerable change in the profiles of developmentally-regulated phosphoproteomes. Using quantitative mass spectrometry we characterized a novel phosphorylation site (Ser265) identified uniquely in the neonatal brain on Doublecortin (Dcx), a protein essential for proper mammalian brain development. While the relative levels of Dcx and phospho-Ser265 Dcx between embryonic and neonatal brain were similar, their levels fell precipitously by postnatal day 21, as did phospho-Ser297, a site required for proper neuronal migration. Both sites lie near the microtubule-binding domain and may provide functionally similar regulation via different kinases.
Keywords: Phosphoproteomics, Brain Development, Quantitative Mass Spectrometry, Doublecortin (Dcx), phosphorylation
Cell fates during development are fashioned by a host of signal transduction pathways that utilize reversible phosphorylation. Several specific phosphoregulatory mechanisms have been characterized biochemically and have been shown genetically to play profound roles in mammalian brain development [1–3]. Toward uncovering novel phosphoregulatory mechanisms and toward a more comprehensive understanding of their dynamics across brain development we conducted a phosphoproteomic analysis of neonatal murine brain. Postnatal day zero (P0) mice have already undergone significant brain development including the formation of the cerebral cortical plate [4, 5]. However, brain structures including the cerebellum continue to develop after birth [6]. We present here a phosphoproteomic database and analysis of P0 murine brain and compare our finding to a recently published phosphoproteomic analysis of postnatal day 21 (P21) murine brain [7], a stage when mice are considered to be early adults. Furthermore, we use quantitative mass spectrometry to monitor a novel phosphorylation site (Ser265) on Doublecortin (Dcx) a protein which is critical to proper brain development [8] and whose phosphorylation at a neighboring site (Ser297) has been shown to regulate neuronal migration [2].
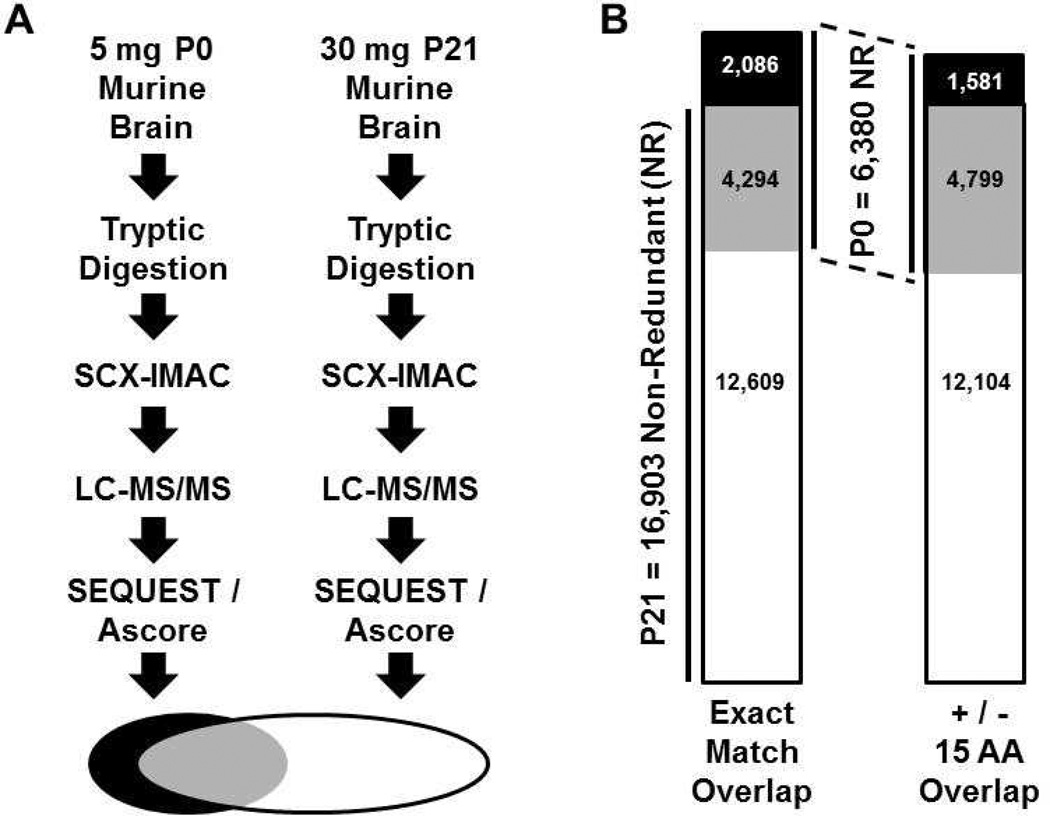
The tandem strong cation exchange chromatography (SCX)-immobilized metal affinity chromatography (IMAC) phosphopeptide enrichment schema [9] has been used with high success in multiple laboratories and was used here. Dissected P0 brain tissue from Swiss-Webster mice was lysed and sonicated in a solution containing 8M urea, reduced and alkylated with iodoacetamide, diluted to 2M urea, digested with trypsin, and peptides were desalted using solid phase extraction columns as described [9]. SCX was performed on 8mg of dried peptides as described [9] except that 16 three-minute fractions were collected. The IMAC procedure including solvents, buffers and resin were as described [9] except that we divided the desalted peptides from each fraction in half and each was subjected to a different column length of IMAC resin (10 µl or 30 µl of resin was packed in a gel loader tip column after incubation with peptides) as described [10]. After incubations in batch, the resins were packed in column formats. The peptides were again passed across the columns, and then the columns were washed and peptides were eluted from the IMAC resin as described [9]. Peptides were desalted using Stage tips [9, 11] and subjected to LC-MS/MS using a linear ion trap-orbitrap hybrid mass spectrometer set up as described [12]. A total of 32 MS runs were conducted and used to search a concatenated target-decoy [13] mouse IPI database (version 3.6) using SEQUEST (Thermo Electron, Version 27, Revision 12). A total of 12,692 phosphopeptides were identified from P0 brain with a false discovery rate (FDR) of 0.45% (Supplementary Table 1). Of these, 6,380 sites were non-redundant. We compared the P0 brain dataset to the published P21brain dataset of 45,435 phosphopeptides (16,903 non-redundant) and a FDR of 0.15% (Supplementary Table 2)[7]. Despite nearly exact workflows (Fig. 1A) and a P21 dataset with nearly three times the number of non-redundant phosphorylation sites, the P0 dataset had 2,086 unique sites that were not identified in the P21 dataset (Fig. 1B and Supplementary Table 3). This represents 33% of the P0 dataset. Of the 2,086 sites unique to the P0 dataset 392 (19%) were identified by three or more spectral counts (Supplementary Table 4). Of the 12,609 sites unique to the larger P21 dataset 4,120 (33%) were identified by three or more spectral counts (Supplementary Table 4). Taken together these data suggest that a large-scale alteration in the phosphoproteomic profile is occurring over the first three postnatal weeks. A potential indication of whether the phosphorylation sites from the P0 brain are functionally different from those in the P21 brain would be if the P0 sites were not located in close proximity to the P21 sites. We therefore determined the number of P0 sites that were positioned plus or minus 15 amino acids from any given site in the P21 dataset. While this increased the overlap between the datasets, we found that 25% of the P0 database was still unique (Supplementary Table 3). Given that both datasets were generated from searches using the same reference proteome and the same parameters hosted on the same server, and given both were generated after subjecting the spectra to the same phosphorylation site assessment program (Ascore[14]), the datasets are highly comparable and the exact match overlap is not likely to contain a high number of sites that differ simply due to methods or site assignment differences. Nevertheless, the plus or minus 15 residue proximity analysis would eliminate assignment ambiguity mismatches while giving a rough estimate of differences in regional phosphorylation or phosphotopology.
Figure 1.
Phosphoproteomic workflow and comparison of identified P0 brain phosphorylation sites with published P21 brain phosphorylation sites. (A) Phosphoproteomic workflows for P0 and P21 brain tissue were similar. (B) 6,380 non-redundant phosphorylation sites were identified from P0 brain and 16,903 non-redundant phosphorylation sites were identified from P21 brain. The overlap between datasets was calculated by exact phosphorylation site matches (4,294 sites overlapping) or by a proximity-based overlap (4,799 sites overlapping). Proximity overlap was counted when a phosphorylation site in one dataset was plus or minus 15 amino acids from a phosphorylation site in the second dataset.
Frequently, regions of proteins are targeted by multiple kinases to achieve similar or synergistic functional effects. For example, the pro-apoptotic protein Bad has a cluster of phosphorylation sites targeted by several kinases that synergistically participate in its negative regulation [15, 16]. Similarly, the Collapsin Response Mediator Protein (CRMP) family and the Doublecortin (Dcx) family have clusters of phosphorylation sites targeted by multiple kinases in regions regulating their binding to tubulin (Fig. 2A) [2, 8, 17, 18]. Interestingly, our P0 phospho-dataset identified a novel phosphorylation site in Dcx (Ser265) adjacent to the tubulin binding domain (Fig. 2A) that was not identified in the P21 dataset. Given the importance of Ser297 phosphorylation in this region in regulating neuronal migration [2] we took a quantitative mass spectrometry approach to directly validate Ser265 phosphorylation and to compare its relative abundance across development. Dcx was immunopreciptitated from murine brain at three stages, embryonic day 16.5 (E16.5), P0 and P21. A tenth of the immunoprecipate was subjected to SDS-PAGE and immunoblotting to determine the levels of both Dcx and phospho-Ser297 Dcx (Fig. 2B). The remaining portion was subjected to SDS-PAGE and staining with Coomassie blue. The immunoblots and the Coomassie staining showed that E16.5 and P0 samples had roughly proportional levels of Dcx and phospho-Ser297 Dcx per mg of protein extract, whereas by P21 the relative levels of Dcx and phospho-Ser297 had fallen precipitously. To examine the relative levels of Dcx phospho-Ser265 and unphosphorylated Ser265 in these immune complexes, synthetic “tryptic” peptides encompassing Ser265 and containing stable isotope mass tags [19] were added at fixed amounts to the dried peptides extracted from in-gel tryptic digests of the Dcx-containing regions of the SDS-PAGE gel (Fig. 2B). The peptides were then subjected to LC-MS/MS analysis in a linear ion trap-orbitrap tandem mass spectrometer and mass spectra were used for peptide identification and quantification (Fig. 3 and Supplementary Figs. 1 and 2).
Figure 2.
(A) Location of a novel phosphorylation site (Ser265) on Dcx found in P0 brain. The position of Ser265 relative to known and conserved phosphorylation sites in Dcx, as well as in the homologous Dclk1 and Dclk2, is indicated. The boxed regions of the protein schematic (at top) are Dcx microtubule binding domains with comprised amino acids indicated. For the alignment, Mm = Mus musculus; Hs = Homo sapiens; “*” = conservation identity; “.” and “:” indicate increasing degrees of conservation similarity. Known phosphorylation sites are boxed black with white text. Boxed in gray are putative phosphorylation sites based on conservation with known sites. (B) Developmental characterization of immunoprecipitated Dcx from E16.5, P0 and P21 murine brain. One mg of protein extract from each stage was subjected to anti-Dcx immunoprecipitation. Immune complexes were separated by SDS-PAGE with 1/10 of each sample being subjected to immunoblotting with the indicated antibodies (upper panels) and the remainder being subjected to Coomassie staining (lower panel) with the indicated gel regions subjected to MS analysis. Molecular weights are indicated in kDa.
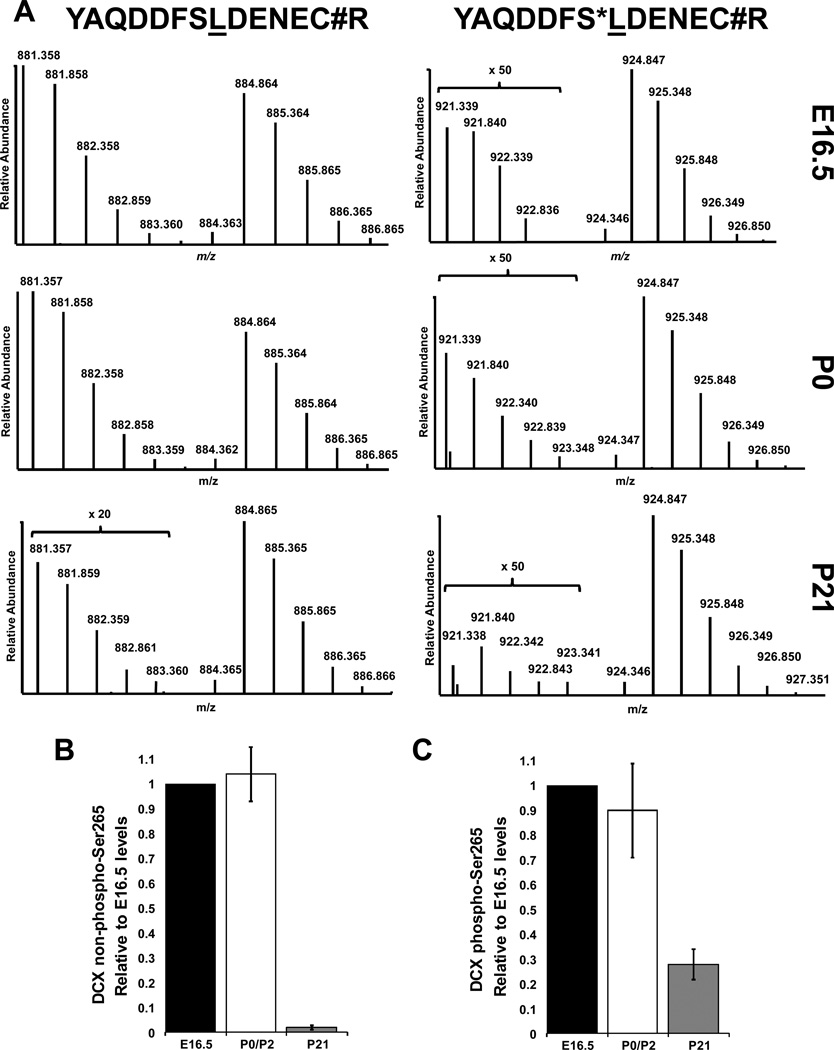
Figure 3.
The Coomassie-stained Dcx regions from Fig. 2B as well as a separate biological replicate (not shown) were subjected to in-gel reduction, alkylation with iodoacetamide, and tryptic digestion. Extracted peptides were combined with a fixed amount of unphosphorylated Ser265-containing, and phospho-Ser265-containing reference peptides of the indicated sequences. Reference peptides contained a leucine residue (underlined) labeled with one 15N and six 13C atoms. (A) The relative abundances of the native Dcx peptides were thereby quantified from each developmental stage (unphosphorylated samples are at left and phosphorylated samples are at right). “S*” indicates phosphorylated serine. “C#” indicates carbamiodomethylated cysteine introduced in-gel for the native sample or during peptide synthesis for the reference peptides. Relative levels of the unphosphorylated (B) and phosphorylated (C) peptides harboring Ser265 from each stage are indicated.
Data from biological replicates were averaged and normalized to E16.5 levels. Unphosphorylated Ser265- and phospho-Ser265 Dcx-containing peptides were observed at similar levels in E16.5 and P0 samples. However, the P21 samples showed unphosphorylated- and phospho-Ser265-containing peptides to be 2.4% and 28% of E16.5 levels, respectively. Interestingly, while the P0 phospho-Ser265 levels were indeed more than forty times higher than those at P21, possibly explaining the lack of its detection in the P21 phosphoproteomic analysis, the relative stoichiometry of Dcx phospho-Ser265 was 14 times higher at P21. As amino acid analysis of the reference peptides provided a means for absolute quantification [19], the stoichiometry of Dcx Ser265 phosphorylation at E16.5, P0 and P21 was calculated to be 0.5%, 0.5% and 7.6% respectively. It is important to note this reflects the average of the entire tissue average and doesn’t give cell-specific stoichiometries which could be highly variability. Although this descriptive analysis does not permit conclusions regarding functionality, it uncovers the existence of a novel Dcx kinase that may provide additional regulation of tubulin binding. Ser265 lies within an acidic stretch of amino acids (262–270, DDFSLDENE) making it a possible target of casein kinase 2 (CK2), particularly as Ser265 has a CK2-preferred glutamate at the +3 position [20]. However, it should be noted that the non-exhaustive Scansite motif prediction program [21] predicts only two kinases as potential Ser265 kinases (CK2 and ATM). However, the prediction is tempered given the preference for an acidic residue at the +1 position for CK2 substrates and glutamine at the +1 position for ATM substrates. Still, if only considering these two kinases, CK2 may be preferred over ATM given six out of the top 500 Scansite predicted mammalian Swiss-Prot CK2 substrates contain a leucine at +1 compared to zero for ATM. CK2 has hundreds of known substrates and targeted disruption of CK2 activity in mice leads to embryonic lethality [22, 23]. However, conditional loss of CK2 activity in embryonic neural stem cells disrupts their proliferation and differentiation and leads to mice with neuronal development defects [24], which is certainly due to the sum of its pleiotropic effects. Thus, targeted experiments will be required to determine if CK2 is indeed the Dcx Ser265 kinase, and what the functional consequences are of Ser265 phosphorylation.
This comparative study highlights the dynamic and rich nature of developmentally-regulated phosphoproteomes, which are certainly highly correlated to developmental regulation of gene expression and/or the regulation of kinase and phosphatase activity. Ultimately, systems-level, comparative, quantitative, and stoichiometric phosphoproteomic profiling of developmental tissues will not only uncover additional novel sites and regions of phosphoregulation on specific proteins, but it will facilitate a more fluid description of the temporally-orchestrated molecular underpinnings of development.
Supplementary Material
Acknowledgments
The authors were supported by the following grants: NIH 5T32CA09657 (TG, XL, BAB); NIH HG00041 (TG, XL, BAB); NSF IOS 1021795 (EML, BAB); Vermont Genetics Network NIH INBRE--NCRR, 5P20RR16462-11 and NIGMS, 8 P20 GM103449-11 (AMS, EML, JJV, BAB); 2 P20 RR016435-06 NIH COBRE NCRR (AMS, EML, BAB). The authors thank Steven Gygi for support, server access, and sharing data prior to publication, and to Gygi lab members Willi Haas, Scott Gerber, Judit Villén and Sean Beausoleil for their contributions to method development. The authors also thank Jason Reynolds and Jeffrey Knott at CST for AQUA peptide synthesis and quantification. Julie Nardone at CST is thanked for integrating the datasets with PhosphoSitePlus curation. Studies were performed under IACUC protocol 07-091 at the University of Vermont (NIH Animal Welfare Insurance file A3301-01).
Abbreviations
- Dcx
Doublecortin
- Dclk
Doublecortin-like kinase
Footnotes
All MS data from this study are freely available in the PRIDE database (http://www.ebi.ac.uk/pride) under the accession number 21326–21328 and can be viewed using the freely available PRIDE Inspector program (http://code.google.com/p/pride-toolsuite/wiki/PRIDEInspector).
For purposes of manuscript review, a reviewer’s account has been created and the following information was provided by PRIDE regarding access of the data:
Accession numbers: 21326–21328
-
-Username: review39869
-
-Password: #DtpW4gC
-
-PRIDE Inspector WebStart URL: http://tinyurl.com/7sxp74h
The authors have declared no conflict of interest.
References
- 1.Howell BW, Herrick TM, Hildebrand JD, Zhang Y, Cooper JA. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr Biol. 2000;10:877–885. doi: 10.1016/s0960-9822(00)00608-4. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka T, Serneo FF, Tseng HC, Kulkarni AB, et al. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron. 2004;41:215–227. doi: 10.1016/s0896-6273(03)00852-3. [DOI] [PubMed] [Google Scholar]
- 3.Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114:469–482. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- 4.Super H, Soriano E, Uylings HB. The functions of the preplate in development and evolution of the neocortex and hippocampus. Brain Res Brain Res Rev. 1998;27:40–64. doi: 10.1016/s0165-0173(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 5.Caviness VS, Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- 6.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 7.Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkmans TF, van Hooijdonk LW, Fitzsimons CP, Vreugdenhil E. The doublecortin gene family and disorders of neuronal structure. Cent Nerv Syst Agents Med Chem. 2010;10:32–46. doi: 10.2174/187152410790780118. [DOI] [PubMed] [Google Scholar]
- 9.Villen J, Gygi SP. The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat Protoc. 2008;3:1630–1638. doi: 10.1038/nprot.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goswami T, Ballif B. In: Sample Preparation in Biological Mass Spectrometry. Ivanov A, Lazaarev A, editors. Springer; 2011. pp. 627–655. [Google Scholar]
- 11.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 12.Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res. 2008;7:311–318. doi: 10.1021/pr0701254. [DOI] [PubMed] [Google Scholar]
- 13.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 14.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 15.Shimamura A, Ballif BA, Richards SA, Blenis J. Rsk1 mediates a MEK-MAP kinase cell survival signal. Curr Biol. 2000;10:127–135. doi: 10.1016/s0960-9822(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 16.Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–S70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 17.Buel GR, Rush J, Ballif BA. Fyn promotes phosphorylation of collapsin response mediator protein 1 at tyrosine 504, a novel, isoform-specific regulatory site. J Cell Biochem. 2010;111:20–28. doi: 10.1002/jcb.22659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A. 2003;100:6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvi M, Sarno S, Cesaro L, Nakamura H, Pinna LA. Extraordinary pleiotropy of protein kinase CK2 revealed by weblogo phosphoproteome analysis. Biochim Biophys Acta. 2009;1793:847–859. doi: 10.1016/j.bbamcr.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic acids research. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchou T, Vernet M, Blond O, Jensen HH, et al. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol. 2003;23:908–915. doi: 10.1128/MCB.23.3.908-915.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seldin DC, Lou DY, Toselli P, Landesman-Bollag E, Dominguez I. Gene targeting of CK2 catalytic subunits. Mol Cell Biochem. 2008;316:141–147. doi: 10.1007/s11010-008-9811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huillard E, Ziercher L, Blond O, Wong M, et al. Disruption of CK2beta in embryonic neural stem cells compromises proliferation and oligodendrogenesis in the mouse telencephalon. Mol Cell Biol. 30:2737–2749. doi: 10.1128/MCB.01566-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.