Abstract
Foxp3+ regulatory T (Treg) cells modulate the functions of multiple immune cell types, and loss of Treg cells causes lethal, CD4+ T cell–dependent multi-organ autoimmune disease in both mice and humans. However, how different effector T cell subets contribute to the severe autoimmunity observed in the absence of Treg cells remains controversial. We found that although expanded populations of Th1, Th2, and Th17 cells can be detected in scurfy mice, Th1 cells predominate. Moreover, using a genetic approach we found that scurfy mice with deficiencies in type-1 immunity (sf×Ifngr1−/−, sf×Tbx21−/−, and sf×Ifngr1−/−/Tbx21−/−) have an extended lifespan that is associated with altered cytokine production and attenuated cutaneous and hepatic inflammation. By contrast, scurfy mice deficient in type-2 immune responses (sf×Stat6−/−) display a significantly reduced lifespan with increased hepatic inflammation, but decreased dermatitis. These data demonstrate that Th1 cells and their associated cytokines drive early immunopathology in Foxp3-deficient scurfy mice, highlighting the essential role of regulatory T cells in restraining Th1-mediated autoimmunity.
Keywords: T cells, cytokines, autoimmunity
Introduction
Foxp3-positive regulatory T (Treg) cells play a central role in enforcing peripheral tolerance[1]. In humans, mutations in FOXP3 result in immunodysregulation polyendocrinopathy enteropathy X-linked (IPEX) syndrome, a severe multi-organ autoimmune disease that presents with eczema, enteropathy, type I diabetes, thyroiditis, and other autoimmune symptoms[2]. Likewise, several different mouse models deficient in Foxp3-positive Treg cells have been developed, and all are associated with severe immunopathology and lethality at approximately 3–4 weeks of age[3–6]. Scurfy (sf) mice lack functional Treg cells due to a two–base pair insertion in Foxp3, which results in the premature termination of translation[7]. These mice rapidly develop lymphadenopathy, splenomegaly, anemia, and wasting, ultimately succumbing to disease within a month of birth[8;9]. Although disease in sf mice is caused by CD4+ T cells[10], there has only been a limited examination of how different functional subsets of CD4+ T cells contribute to immunopathology. The increased production of Th2-associated cytokines by CD4+ T cells from sf mice suggests that Th2 cells are major contributors to sf pathology[6;11;12]. However, using a genetic approach, we demonstrate that autoimmune pathology is substantially attenuated in sf mice lacking key components of type-1 immunity, whereas loss of type-2 immune responses exacerbates disease and significantly shortens lifespan. Thus, early lethality in sf mice is dependent on Th1 cells and their associated cytokines, whereas the observed type-2 immune responses in these animals partially counter this type-1 response and are therefore protective.
Results and Discussion
Increased lifespan of Tbx21- and Ifngr1-deficient sf mice
Although previous studies have suggested that autoimmunity in Foxp3-deficient mice may be largely driven by Th2 cell effector functions [6;12], the precise role of CD4+ T cell subsets in the pathogenesis of disease in sf mice is unknown. To examine this, we crossed sf mice to animals carrying targeted mutations in genes essential for the development and/or function of either Th1 (Tbx21, Ifngr1) or Th2 (Stat6) cells[8;9;13;14]. Surprisingly, the median life-span of sf×Tbx21−/− and sf×Ifngr1−/− mice was significantly increased from 28 days to 52 and 48 days, respectively (Figure 1A; p<0.001 compared with sf mice). Moreover, combined deficiency of Tbx21 and Ifngr1 further prolonged the median life-span of sf mice to 62 days (p<0.001 compared with sf mice). By contrast, the median lifespan of sf×Stat6−/− mice, which cannot respond to the major Th2-associated cytokines IL-4 and IL-13, was significantly decreased (21 days; p=0.002 compared with sf mice).
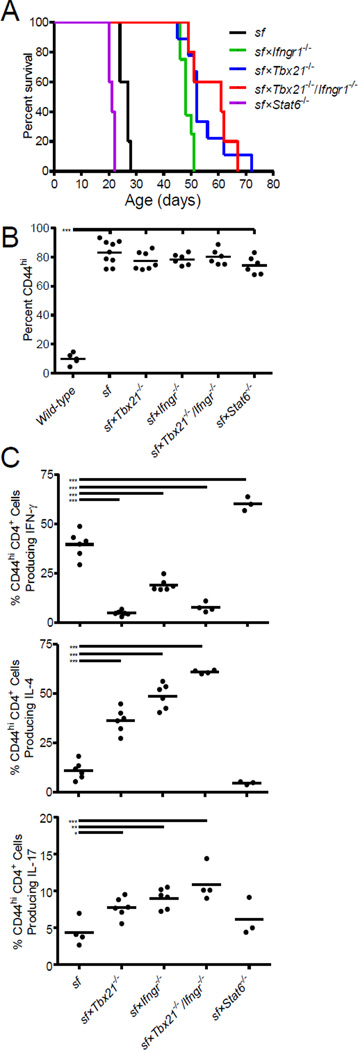
Figure 1. Increased survival of Th1 cell–deficient sf mice.
(A) Survival curves of the indicated gene-deficient sf mice. At least five mice from at least three litters of each geneotype were analyzed. (B) The proportion of activated CD44hi cells among splenic CD4+ T cells (CD4+ CD44hi) in each of the indicated gene-deficient sf mice, as determined using flow cytometry. At least five mice of each genotype were analyzed over at least three separate experiments. (C–E) The proportion of splenic CD4+CD44hi T cells producing IFN-γ (C), IL-4 (D), or IL-17 (E) following stimulation with PMA and ionomycin in the indicated gene-deficient sf mice, as determined using flow cytometry. At least three mice of each genotype were analyzed over at least three separate experiments. (B–E) Each symbol represents data from an individual mouse; the horizontal bar represents the mean; *, p<0.05; ***, p<0.001 determined using ANOVA, with a post hoc Dunnett’s test comparing the gene-deficient sf mice to wildtype mice (B) or sf mice (C–E).
Consistent with their lack of functional Treg cells, all of the sf mice examined showed a dramatic increase in the proportion of activated CD44hiCD4+ T cells (Figure 1B). However, as expected the frequency of IFN-γ-producing CD4+ T cells was significantly reduced in sf mice lacking Tbx21 and/or Ifngr1, but was significantly increased in sf×Stat6−/− animals (Figure 1C, top). Conversely, IL-4-producing Th2 cells were significantly expanded in the Th1-deficient sf mice, but largely absent in the sf×Stat6−/− animals (Figure 1C, middle). Moreover, consistent with the ability of T-bet and IFN-γ to inhibit Th17 differentiation, IL-17-producing cells were modestly elevated in the Tbx21- and Ifngr-deficient sf mice (p<0.05 compared with sf mice; Figure 1C, bottom). Importantly, in all of the sf mice examined, CD4+ T cells co-producing IFN-γ, IL-4, and/or IL-17 were rarely observed, demonstrating that loss of Treg cells does not lead to the emergence of poly-functional CD4+ T cells (data not shown). Overall, these data demonstrate that although autoimmune pathology in sf mice is associated with the expansion of multiple effector T cell subsets, early lethality in these animals can be prevented by the deletion of key genes involved in the development/function of type-1 immune responses despite corresponding increases in Th2 and Th17 cells. Furthermore, the increased Th1 cell expansion in the sf×Stat6−/− mice (Figure 1C) suggests that lack of IL-4/13-mediated counter-regulation of Th1 cell development may underlie the decreased life-span of these animals. Furthermore, our results in B6.sf×Stat6−/− mice contrast the finding that loss of Stat6 prolongs the life-span of Foxp3-deficient Balb/c mice[6], indicating that genetic background can influence the pathogensis of autoimmunity caused by loss of Treg cells.
Attenuated inflammation in Tbx21- and Ifngr1-deficient sf mice
By approximately 3 weeks of age, sf mice are severely runted and display extensive exfoliative dermatitis. Sf mice also present with lymphadenopathy, splenomegaly, inflammatory infiltration targeting the liver and lungs, and hematological abnormalities, including severe anemia[3;8]. Although substantially prolonging their lifespan, ablation of Tbx21 and Ifngr did not completely protect sf mice from disease, suggesting that the disease pathogenesis is multifaceted. However, whereas both Th1-associated (e.g., IFN-γ) and Th2-associated (e.g., IL-4, -5, and -13) cytokines are overexpressed in sf mice [11], the contributions of the different effector T cell lineages to each aspect of sf pathology has not been carefully examined. Therefore, to determine how Th1 and Th2 effector functions influence disease development in sf mice, we performed a detailed phenotypic analysis of sf mice lacking functional type-1 or type-2 immunity at 21–24 days of age.
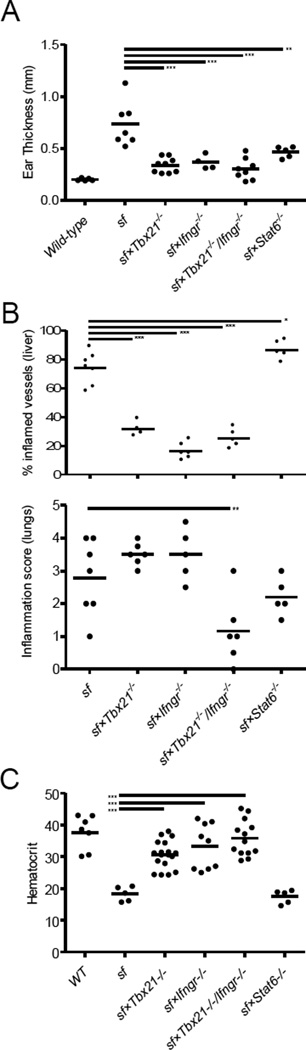
All of the sf mice examined were runted and displayed similar degrees of splenomegaly and lymphadenopathy (Supplemental Figure 1). Moreover, sf mice deficient in either type-1 or type-2 immunity displayed dramatically attenuated dermatitis, evidenced by decreased inflammatory infiltration and thickening of the ears (Figure 2A and Supplemental Figure 2). However, hepatic inflammation was significantly attenuated in all Th1-deficient sf mice, whereas consistent with the elevated frequency of IFN-γ-producing Th1 cells observed in sf×Stat6−/− mice, these animals presented with pervasive perivascular and parenchymal hepatic inflammation and extensive hepatocyte necrosis that was more widespread than in wild-type sf animals (Figure 2B, top and Supplemental Figure 2). Interestingly, the extensive bronchial and avelolar inflammation characteristic of sf mice was significantly attenuated only in the sf×Ifngr1−/−/Tbx21−/− animals (Figure 2B, bottom and Supplemental Figure 2). Thus, both type-1 and type-1 immune responses contribute to the development of the autoimmune pathology in sf mice in an organ-specific manner, and distinct mechanisms appear to underlie the inflammation at the different tissue sites, with hepatic inflammation being predominantly IFN-γ and T-bet-dependent, whereas dermatitis requires both intact Th1 and Th2 responses. Moreover, the development of pulmonary inflammation in sf×Tbx21−/− and sf×Ifngr1−/− mice indicates that multiple Th1-associated inflammatory pathways contribute to immune pathology of the lungs in sf mice. This is consistent with the ability of cells, such as CD8+ T cells and NK cells, to produce IFN-γ in the absence of Tbx21[15], and of Th1 cells to cause IFN-γ-independent immunopathology[16]. The attenuated tissue inflammation in sf mice lacking Tbx21 and/or Ifngr1 is similar to what was observed in IL-2-deficient sf animals[17], and IL-2 was recently shown to directly influence the development of Th1 effector cells[18], providing a potential link between these findings.
Figure 2. Attenuated inflammation in Th1 cell–deficient sf mice.
(A) Ear thickness in each of the indicated gene-deficient sf mice measured on either 21-day-old (Stat6−/− mice) or 24-day-old (all other strains). (B) The percent of inflamed blood vessels in the liver was quantified in H&E-stained tissue sections; the proportion of inflamed vessels from two tissue levels is shown. (C) Lung inflammation was quantified based on the number of inflammatory foci present in two tissue levels. (D) The hematocrit values in each of the indicated gene-deficient sf mice measured on either day 21 (Stat6−/− mice) or day 24 (all other strains). Each symbol represents data from an individual mouse. At least four mice of each genotype were analyzed over at least three separate experiments; the horizontal bar represents the mean; *, p<0.05; **, p<0.01; ***, p<0.001 determined using ANOVA, with a post hoc Dunnett’s test comparing the gene-deficient sf mice to sf mice (A–C).
Progressive IL-17-associated pathology in Tbx21- and Ifngr1-deficient scurfy mice
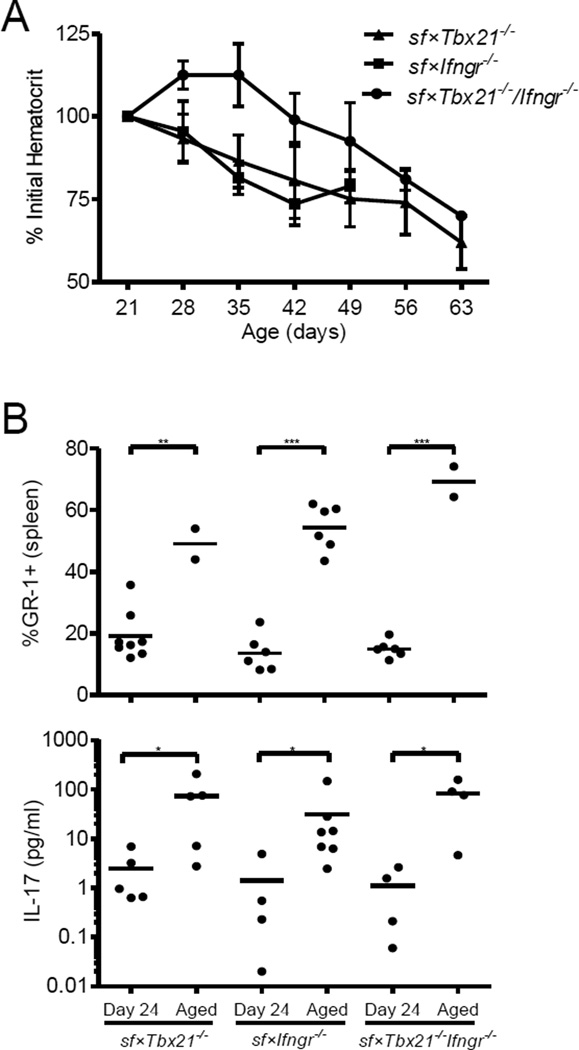
The development of early-onset anemia was at least partially rescued in the sf mice lacking Tbx21 and/or Ifngr1, but not in the sf×Stat6−/− animals (Figure 2C). However, all of these animals developed progressive anemia and severe cutaneous and pulmonary inflammation with age, and this was associated with a dramatic increase in the abundance of splenic GR-1++ neutrophils (Figure 3A, B and data not shown). By contrast, hepatic inflammation did not increase significantly in these aged (>42 days old) mice (data not shown), underscoring the importance of type-1 immunity in this aspect of autoimmune pathology. IL-17 can contribute to development of neutrophilia, anemia, and cutaneous/pulmonary inflammation[19–21], and indeed we found that the serum IL-17 level was significantly increased in the aged Tbx21/Ifngr1–deficient sf mice (Figure 3B, bottom). Thus, in the absence of functional type-1 immunity, a slowly progressing, potentially IL-17-associated, systemic autoimmune pathology develops in sf mice, resulting in their death by approximately 2 months of age.
Figure 3. Th1 cell–deficient sf mice develop a progressive, IL-17-associated disease.
(A) Hematocrit values in the indicated Th1 cell–deficient sf mouse strains were measured weekly beginning at 21 days of age and are displayed as the mean percent of the initial hematocrit measurement ± SD (n=4–8). (B) Frequency CD4−CD8−B220−GR-1+ neutrophils among total splenocytes in the young (24 days old) and aged (> 42 days old) Th1 cell–deficient sf mice, as determined using flow cytometry. Each symbol represents data from an individual mouse from two separate experiments; the horizontal bar represents the mean. (C) Serum concentration of IL-17 in young (24 days old) and aged (>42 days old) Th1 cell–deficient sf mice, as determined using a Bio-plex Magnetic Cytokine Assay (Biorad). Each symbol represents data from an individual mouse from two separate experiments; the horizontal bar represents the mean; *, p<0.05; **, p<0.01; ***, p<0.001 as determined using a Mann-Whitney U test comparing the 24-day-old mice to the aged mice of the same genotype.
Concluding Remarks
Our results demonstrate that type-1 and type-2 immune responses play distinct roles in the morbidities observed in sf mice. In particular, loss of type-1 immune function results in reduced organ-specific inflammation in the skin, lungs and liver, delayed development of anemia and a significantly prolonged lifespan, whereas loss of Stat6-mediated Th2 cell function attenuated cutaneous inflammation, but enhanced Th1 cell expansion and reduced the lifespan. Thus, although loss of Treg cells results in the expansion of Th1, Th2 and Th17 cell populations, dysregulated Th1 responses appear to underlie much of the early pathology observed in sf mice. Moreover, these data demonstrate that inflammation in different tissues of sf mice is driven by distinct disease processes. However, as Th1-deficient sf mice age, they develop IL-17-associated morbidities, including anemia, neutrophilia, and cutaneous and pulmonary inflammation[19–21]. Together, our data further demonstrate the importance of Treg cells in restraining multiple CD4+ effector T cell populations and highlight their particular importance in inhibiting destructive autoreactive Th1 cells.
Methods
Mice
Mice were housed and bred at the Benaroya Research Institute, and all experiments were performed in accordance with the guidelines of the Benaroya Research Institute Animal Care and Use Committee. C57BL/6J, B6.129S7-Ifngr1tm1Agt/J (Ifngr1−/−), B6.129S2(C)-Stat6tm1Gru/J (Stat6−/−), and B6.Cg-Foxp3sf/J (sf) mice were from the Jackson Laboratory, and C57BL/6 Tbx21−/− mice were provided by A. Weinmann. As the sf mutation is X-linked, only male mice were analyzed in this study. Complete blood counts were obtained using a HemaVet 950S (Drew Scientific, Dallas, TX). Mice were euthanized at hematocrits less than 20%, upon development of severe cutaneous inflammation, or at a body condition score less than 2.
Cell purification and analysis
Cells from skin-draining lymph nodes and spleens were isolated and processed for flow cytometry using standard techniques. Cytokine production was determined following stimulation with 50 ng/ml PMA and 1 µg/ml ionomycin for 5 hours in the presence of 10 µg/ml monensin.
Histology
H&E-stained sections of ear, skin, liver, and lung tissue were obtained using standard techniques. Hepatic inflammation was quantified by counting the proportion of inflamed blood vessels in two tissue levels. Pulmonary inflammation was quantified based on the number of inflammatory foci present in two tissue levels: 0, no foci; 1, 1–2 foci; 2, 3–4 foci; 3, 4–5 foci; 4, 5–6 foci; 5, >6 foci.
Statistical Analysis
The survival of the mice was compared using the logrank test; all other comparisons were made using an ANOVA, with a post hoc Dunnett’s test comparing the gene-deficient sf mice to wildtype sf mice or wildtype mice as indicated, or a Mann-Whitney U test. p values less than 0.05 were considered significant.
Supplementary Material
Acknowledgements
We would like to thank K. Arumuganathan for help with flow cytometry and cell sorting, M. Warren for administrative assistance, and members of the Campbell lab for helpful discussions and comments on the manuscript. This work was supported by grants from the NIH to DJC (AR055695, DK072295, and AI067750).
Footnotes
Authorship
Contribution: T.J.S and N.R.P performed experiments; T.J.S. analyzed results and made the figures; T.J.S. and D.J.C designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat.Rev.Immunol. 2011;11:119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat.Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey VL, Wilkinson JE, Russell LB. X-linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am.J.Pathol. 1991;138:1379–1387. [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat.Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 5.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J.Exp.Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martin MG, Chatila TA. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J.Allergy Clin.Immunol. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat.Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 8.Lyon MF, Peters J, Glenister PH, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott-Aldrich syndrome. Proc.Natl.Acad.Sci.U.S.A. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc.Natl.Acad.Sci.U.S.A. 1991;88:5528–5532. doi: 10.1073/pnas.88.13.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair PJ, Bultman SJ, Haas JC, Rouse BT, Wilkinson JE, Godfrey VL. CD4+CD8- T cells are the effector cells in disease pathogenesis in the scurfy (sf) mouse. J.Immunol. 1994;153:3764–3774. [PubMed] [Google Scholar]
- 11.Kanangat S, Blair P, Reddy R, Daheshia M, Godfrey V, Rouse BT, Wilkinson E. Disease in the scurfy (sf) mouse is associated with overexpression of cytokine genes. Eur.J.Immunol. 1996;26:161–165. doi: 10.1002/eji.1830260125. [DOI] [PubMed] [Google Scholar]
- 12.Lahl K, Mayer CT, Bopp T, Huehn J, Loddenkemper C, Eberl G, Wirnsberger G, Dornmair K, Geffers R, Schmitt E, Buer J, Sparwasser T. Nonfunctional regulatory T cells and defective control of Th2 cytokine production in natural scurfy mutant mice. J.Immunol. 2009;183:5662–5672. doi: 10.4049/jimmunol.0803762. [DOI] [PubMed] [Google Scholar]
- 13.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 15.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J.Exp.Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma R, Sharma PR, Kim YC, Leitinger N, Lee JK, Fu SM, Ju ST. IL-2-controlled expression of multiple T cell trafficking genes and Th2 cytokines in the regulatory T cell-deficient scurfy mice: implication to multiorgan inflammation and control of skin and lung inflammation. J.Immunol. 2011;186:1268–1278. doi: 10.4049/jimmunol.1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haak S, Croxford AL, Kreymborg K, Heppner FL, Pouly S, Becher B, Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J.Clin.Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durrant DM, Gaffen SL, Riesenfeld EP, Irvin CG, Metzger DW. Development of allergen-induced airway inflammation in the absence of T-bet regulation is dependent on IL-17. J.Immunol. 2009;183:5293–5300. doi: 10.4049/jimmunol.0803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarzenberger P, La RV, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J.Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.