Abstract
Little agreement exists as to acute dopamine (DA) manipulation effects on intertemporal choice in humans. We previously found that catechol-O-methyltransferase (COMT) Val158Met genotype predicts individual differences in immediate reward selection bias among adults. Moreover, we and others have shown that the relationship between COMT genotype and immediate reward bias is inverted in adolescents. No previous pharmacology studies testing DA manipulation effects on intertemporal choice have accounted for COMT genotype, and many have included participants in the adolescent age range (18–21) as adults. Moreover, many studies have included female subjects without strict cycle phase control, although recent evidence demonstrates that cyclic estradiol elevations interact with COMT genotype to affect DA-dependent cognition. These factors may have interacted with DA manipulations in past studies, potentially occluding detection of effects. Therefore, we predicted that among healthy adult males (ages 22–40), frontal DA tone, as indexed by COMT genotype, would interact with acute changes in DA signaling to affect intertemporal choice. In a double-blind, placebo-controlled design, we decreased central DA via administration of an amino acid beverage deficient in the DA precursors, phenylalanine and tyrosine (P/T[−]), and tested effects on immediate reward bias in a delay-discounting (DD) task and working memory (WM) in an n-back task. We found no main effect of beverage on DD or WM performance, but did find significant beverage*genotype effects. These results suggest that the effect of DA manipulations on DD depends on individual differences in frontal DA tone, which may have impeded some past efforts to characterize DA’s role in immediate reward bias in humans.
Keywords: delay discounting, executive function, frontal, immediate reward bias, tyrosine
INTRODUCTION
Little agreement exists among numerous studies on the effects of acute dopamine (DA) signaling changes on impulsive decision-making in intertemporal choice, or delay discounting (DD) tasks. Some data indicate that elevating DA increases impulsive decision-making (A. Pine, T. Shiner, B. Seymour, & R. J. Dolan, 2010), while other data argue that elevating DA decreases (de Wit, Enggasser, & Richards, 2002; Kayser, Allen, Navarro-Cebrian, Mitchell, & Fields, 2012) or does not change (Acheson & de Wit, 2008; Hamidovic, Kang, & de Wit, 2008) impulsive decision-making. Acutely reducing striatal DA signaling produces no apparent effect on impulsive choice (A. Pine et al., 2010). To date, no studies have tested whether functional genetic polymorphisms in the DA system modulate the effect of acute DA manipulations on impulsive decision-making. However, a recent study of WM in females found that the cognitive effects of a cyclic elevation of estradiol depend on a genetic index of tonic frontal DA, a polymorphism in the gene encoding the catechol-O-methyltransferase (COMT) enzyme (Jacobs & D'Esposito, 2011), which is thought to reflect the stimulatory effect of estrogen on DA signaling (Becker, 1990; Kritzer & Kohama, 1998). Moreover, the effects of COMT inhibition on both WM and gambling task performance depend on COMT-genotype (Farrell, Tunbridge, Braeutigam, & Harrison, 2012). A valine (val)-to-methionine (met) substitution at codon 158 of COMT (Val158Met; rs4680) yields a 4-fold reduction in COMT enzymatic activity (Lachman et al., 1996), resulting in lower cortical DA in val/val homozygotes relative to the met/met genotype (Chen et al., 2004; Wu et al., 2012), as COMT metabolizes DA (Gogos et al., 1998; Kaenmaki et al., 2010; Tunbridge, Bannerman, Sharp, & Harrison, 2004; Yavich, Forsberg, Karayiorgou, Gogos, & Mannisto, 2007) and is the primary regulator of DA clearance in the prefrontal cortex (PFC) (Kaenmaki et al., 2010; Karoum, Chrapusta, & Egan, 1994).
Our previous work has found that COMT Val158Met genotype predicts individual differences in impulsive decision-making among adults. Specifically, adults with the COMT val/val genotype discount delayed rewards to a larger extent than met allele carriers (Boettiger et al., 2007; Smith & Boettiger, 2012), and have increased activity in the dorsal PFC compared to met allele carriers (Boettiger et al., 2007). Therefore, we hypothesized that frontal DA tone, as indexed by COMT genotype, would interact with acute changes in DA signaling in affecting impulsive choice. Rather than replicate previous methods of acutely manipulating DA, we chose to take a novel approach to acutely reducing DA signaling. Specifically, we transiently reduced DA by administering an amino acid beverage deficient in the amino acids required for DA synthesis, phenylalanine and tyrosine (P/T; (Karobath & Baldessarini, 1972). We then tested whether acutely lowering DA signaling via P/T depletion (Booij, Van der Does, & Riedel, 2003; Leyton et al., 2004; Moja, Lucini, Benedetti, & Lucca, 1996; Montgomery, McTavish, Cowen, & Grasby, 2003; B. Sheehan, P. Tharyan, S. McTavish, G. Campling, & P. Cowen, 1996) in healthy adult male volunteers changed impulsive decision-making in a delay-discounting task (Altamirano, Fields, D'Esposito, & Boettiger, 2011). To evaluate whether the effect of transient DA changes on impulsive choice might be mediated through effects on working memory function (WM), we also tested WM performance using an n-back task (Gray, Chabris, & Braver, 2003; Jacobs & D'Esposito, 2011). Critically, all participants were genotyped for the COMT Val158Met polymorphism to assess whether acute behavioral effects of acute DA depletion depended on this underlying trait measure of frontal DA.
METHODS
Participants
Healthy males (n = 15) were recruited from the University of North Carolina at Chapel Hill (UNC) and surrounding community. Participants were 22–40 years old, native English speakers, and had at least a high school education. Subjects were screened for psychoactive medication/drug use, past or current psychiatric or neurological disorders, diabetes, and phenylketonuria. Participants gave written, informed consent as approved by the UNC Office for Human Research Ethics. Participants were paid for participating; payment did not depend on performance.
General Procedure
We used acute P/T depletion to temporarily reduce DA levels in a double-blind, placebo-controlled, within-subjects, counterbalanced design. Subjects followed a low protein (< 20 g) diet for 24 h prior to each session and fasted from midnight until session onset between 7 and 9 A.M. On arrival, participants completed the Brief Profile of Mood States (POMS) (McNair, Lorr, & Droppleman, 1971) and provided a blood sample via finger prick immediately prior to consuming either the placebo or P/T depleting amino acid beverage. POMS scores were converted to t-scores for statistical analyses. After a waiting period of ~5 h to achieve low P/T levels (B. D. Sheehan, P. Tharyan, S. F. McTavish, G. M. Campling, & P. J. Cowen, 1996), participants provided a second blood sample and immediately completed the computerized cognitive tasks, followed by completing the POMS. During the wait period, participants had access to low protein snacks, and after the computerized tasks were completed, participants were offered a high protein snack. Participants completed testing in two sessions, separated by ≥72 h. Testing was performed blind to genotype.
Amino Acid Beverages
Amino acid mixtures (prepared by SHS International, Liverpool, UK consisted of (in g):
Placebo: L-alanine, 4.1; L-arginine, 3.7; L-cysteine, 2.0; L-glycine, 2.4; L-histidine, 2.4; L-isoleucine, 6; L-leucine, 10.1; L-lysine, 6.7; L-methionine, 2.3; L-phenylalanine, 4.3; L-Journal of Cognitive Neuroscience proline, 9,2; L-serine, 5.2; L-threonine, 4.9; L-tryptophan, 3.0; L-tyrosine, 5.2; and L-valine, 6.7. Total: 78.2g
P/T[−]: same as above, except phenylalanine and tyrosine were omitted. Total: 68.7g.
To reduce the risk of nausea and emesis, 20% reductions in the above quantities were administered to participants weighing <160 lbs (n = 2). Beverages were mixed with 8oz of cold tap water and a flavoring packet (Nutricia, Gaithersburg, MD) in a sterile cup.
Behavioral Inventories
Participants completed a battery of questionnaires prior to cognitive testing in session one. These data allowed us to determine whether the genotype groups differed in terms of socioeconomic status (SES) or behavioral trait measures that could influence impulsive choice. SES was quantified according to Hollingshead (Hollingshead, 1975). According to the method of Barrat, for current students, only parental SES information was included in the calculation, while both parental and personal SES information was included for non-students (W. Barratt, 2006). Other standard questionnaires included: the Alcohol Use Disorders Identification Test (Saunders, Aasland, Babor, de la Fuente, & Grant, 1993), the Barratt impulsivity scale (E. S. Barratt, 1994), the Drug Use Screening inventory (Tarter, 1990), the Family Tree Questionnaire (Mann, Sobell, Sobell, & Pavan, 1985), the Future Time Perspective Inventory (Wallace, 1956), Rotter’s Locus of Control Scale (Rotter, 1966), Spielberger’s State-Trait Anxiety Inventory (Spielberger, 1985), the Beck Depression Inventory (Beck & Steer, 1987), and the Anti-social Practices Scale of the Minnesota Multiphasic Personality Inventory 2 (Butcher, Graham, Williams, & Ben-Porath, 1990). Genotype groups did not differ on any of these measures (Table 1).
Table 1.
Behavioral comparison by COMT genotype.
| met carrier (n = 11) |
val/val (n = 4) |
t(13) | p | |
|---|---|---|---|---|
| General | ||||
| Age | 26.4 ± 1.5 | 26.3 ± 2.8 | 0.04 | ns |
| SES | 51.2 ± 3.2 | 46.4 ± 6.2 | 0.74 | ns |
| Personal and Familial Substance Use | ||||
| AUDIT | 4.7 ± 1.0 | 3.3 ± 0.8 | 0.82 | ns |
| DUSI | 0.13 ± 0.06 | 0.22 ± 0.08 | −0.70 | ns |
| FTQ | 0.20 ± 0.05 | 0.13 ± 0.13 | 0.74 | ns |
| Psychometric Measures | ||||
| BIS | 56.6 ± 1.6 | 59.3 ± 4.8 | −0.69 | ns |
| FTPI-Mean Extension | 11.2 ± 2.5 | 8.1 ± 3.1 | 0.67 | ns |
| FTP1-Max Extension | 42.6 ± 8.9 | 38.5 ± 14.2 | 0.24 | ns |
| ASP | 8.0 ± 1.2 | 8.0 ± 0.7 | 0.00 | ns |
| BDI | 3.6 ± 0.9 | 7.0 ± 2.5 | −1.65 | ns |
| STAI-Trait Score | 31.6 ± 2.5 | 39.5 ± 6.1 | −1.47 | ns |
| LOC | 11.7 ± 1.0 | 13.8 ± 1.8 | −1.00 | ns |
Values are reported as mean ± standard deviation. Reported p-values reflect the results of unpaired two-tailed comparison between groups. Exact p-values reported unless p < 0.001.
p-value represents results of χ2 test.
ASP, Anti-social Practices; AUDIT, Alcohol Use Disorders Identification Test; BDI, Beck Depression Inventory; BIS, Barratt Impulsivity Scale; DUSI, Drug Use Screening Inventory; FTQ, Family Tree Questionnaire; FTPI, Future Time Perspective Inventory; LOC, Locus of Control; SES, Socioeconomic Status; STAI, State-Trait Anxiety Inventory.
Amino Acid Plasma Analysis
Blood samples were analyzed to determine the total plasma P/T levels and the ratio of P/T to other large, neutral amino acids (P+T/ΣLNAA ratio) before and after consumption of the P/T[−] beverage. This ratio was calculated from the total serum concentrations of P and T divided by the sum of the other large neutral amino acids (tryptophan, valine, isoleucine, and leucine). This ratio is a measure of the availability of dopamine the brain (Montgomery et al., 2003). Using aseptic technique, we used a contact-activated lancet (BD Biosciences, San Jose, CA) to collect 150µL of blood from the finger. Samples were stored frozen at −20°C until analysis. A 50µL aliquot of serum sample was extracted with 150µL of pre-cooled (−20°C) methanol and vortexed for 30 seconds. After storing for 10 minutes at −20°C, samples were centrifuged at 10,000g for 10 min at 4°C. An aliquot of the 160µL supernatant was transferred to a glass sampling vial to vacuum dry at room temperature. The residue was derivatized using a two-step procedure. First, 80µL methoxyamine (15 mg/mL in pyridine) was added to the vial and kept at 30°C for 90 minutes. An amount of 10µL retention index compounds (the mixture of C10-C40, 50µg/mL), and 80µL BSTFA (1%TMCS) were added into the reaction vials. The samples were then subjected to 70°C for 120 min for the derivatization reaction. Each 1µL aliquot of the derivatized solution was injected in splitless mode into an Agilent 7890N gas chromatograph coupled with a Pegasus HT time-of-flight mass spectrometer (Leco Corp., St Joseph, MI). Separation was achieved on a DB-5 ms capillary column (30m × 250µm I.D., 0.25µm film thickness; Agilent Technologies, Folsom, CA), with He as the carrier gas at a constant flow rate of 1.0 ml/min. The temperature of injection, transfer interface, and ion source were set to 260°C, 260°C, and 210°C, respectively. The GC temperature programming was set to 2 min isothermal heating at 80°C, followed by 10°C/min oven temperature ramps to 220 °C, 5 °C/min to 240°C, and 25°C/min to 290 °C, and a final 8 min maintenance at 290°C. Electron impact ionization (70eV) at full scan mode (m/z 40–600) was used, with an acquisition rate of 20 spectra/second in the TOFMS setting. The data generated from the GC-TOFMS instrument were analyzed with ChromaTOF software (v4.22, Leco Corp., St Joseph, MI).
Genotyping
COMTval158met (rs4680) genotyping was performed on DNA extracted from saliva samples (DNA Genotek, Kanata, Ontario, Canada) using TaqMan technology (Applied Biosystems, Foster City, CA), as described previously (Boettiger et al., 2007). Genotyping was performed by the Duke Center for Human Genetics. Allele frequencies in this sample did not deviate from Hardy–Weinberg equilibrium (χ2 = 1.55, df = 1, p = 0.21). Met carriers included individuals homozygous and heterozygous for the met allele.
Delay Discounting Task
The delay discounting paradigm was based on a previously described task (Altamirano et al., 2011; Mitchell, Fields, D'Esposito, & Boettiger, 2005; Smith & Boettiger, 2012). Subjects were given task instructions and a short practice session to become familiar with the task. Participants viewed stimuli on a color LCD screen and used a manual keypad for response selection; the task was completed within a mock scanner. In the task, subjects made a series of choices between smaller, sooner (“Now”) and larger, later (“Later”) hypothetical monetary rewards and were instructed to choose as if they would actually receive their choices. Later amounts were $2, $5, $10, $20, or $100 at 1 of 5 future delays: 1 week, 2 weeks, 1 month, 3 months, or 6 months. The Now option was always available “TODAY” and was 70, 85, 90, or 95% of the Later amount. Left/right position of the Now option was equifrequent and pseudorandomized. Participants were shown a cue in each trial indicating how to select between the subsequently displayed options. Instruction cues included: WANT (W), DON'T WANT (DW), SOONER, and LARGER, with the latter two conditions considered together as “CONTROL” (CON). In W trials, subjects indicated their preferred option. Our primary dependent measure was the proportion of Now choices relative to all W condition choices made, the impulsive choice ratio (ICR). A secondary dependent measure was the area under the curve (AUC), calculated from the ICR plotted as a function of delay time. In DW trials, subjects were asked to make the same choice, but to press the button corresponding to the opposite choice. On CON trials, subjects made objective choices based on instruction cue. The task consisted of 8 blocks of 42 trials each. The order of trial types was the same for all subjects; however, the delayed amount, delay time, and discount were pseudorandomly ordered. Trial type frequencies were weighted with ratios of 1/2 for the W condition and 1/6 for each of the other conditions. Inter-trial intervals varied between 2–4 sec, with order fixed across subjects. Subjects saw each of the 120 possible choices 2–3 times, although not necessarily in the same trial type context.
Working Memory Task
The n-back task was a modified version of a published task (Jacobs & D'Esposito, 2011). Subjects were given task instructions and a short practice session prior to testing. Participants viewed stimuli on a colored LCD screen and used a keypad for manual response selection. Briefly, participants completed 16 blocks of 20 trials each, at three working memory loads (eight 0-back blocks, four 2-back blocks, and four 3-back blocks) ordered in one of two pseudorandom sequences counterbalanced by session across participants. Subjects were instructed on the condition (0-, 2-, or 3-back) at the start of each block. A block consisted of serially presented white consonant letters (duration: 1 sec each), with a 1 sec delay between letters. Participants were instructed to press one button for targets (“1” key on keypad) and another button for non-targets (“2” key). For 0-back trials, the target letter was the letter “X,” while in 2- and 3-back trials, target letters matched the letter seen 2 or 3 previously in the stream, respectively. Lures were letters seen previously in the stream but at ±1 position from the target position (e.g. lures in the 3-back condition were 2- or 4-back letter matches). Accuracy and reaction time measures were collected for each trial. One subject was excluded from the working memory analysis because of computer equipment failure.
Statistical analysis
For single factor statistical comparisons within and between groups, we used paired and unpaired two-tailed t-tests, respectively. For multi-factorial comparisons, we used repeated measures ANOVA. To ensure the validity of parametric statistical tests, we used an arcsine-root transformation of ICR data prior to statistical comparisons. When necessary, a Greenhouse-Geisser non-sphericity correction was applied. All statistical tests were conducted using Excel or SPSS (SPSS Inc., Chicago, IL). Values reported as mean ± SEM, unless otherwise stated. Effect sizes for ANOVA are reported as η2, while effect sizes for t-tests are reported as Cohen’s d.
RESULTS
Dopamine precursor manipulation
P/T depletion produced significant reductions in both the concentrations of P/T and the P/T/ΣLNAA ratio. A repeated measures ANOVA revealed a significant two-way interaction between beverage (P/T[−], placebo) and time point (baseline, +5 h), resulting in significant reductions in phenylalanine levels (F(1,14)=82.0; p<0.001, η2=0.34), tyrosine levels (F(1,14) = 44.0; p<0.001, η2=0.34), and the P/T/ΣLNAA ratio (F(1,14)=28.5; p<.001, η2=0.14), 5 h post-P/T depletion relative to placebo. Simple effects analyses showed a significant decrease in plasma phenylalanine levels (t(14)=14.98, p<.001, d=−2.12) and tyrosine levels (t(14)=4.85, p<.001, d=− 1.11) in the P/T depletion session, averaging 45.7±3.3 % and 50.7±2.9%, respectively. There was also a significant decrease in the P/T/ΣLNAA ratio (t(14)=13.72, p<0.001, d=−3.39) in the P/T depletion session, averaging 76%.
Lowering dopamine does not affect blood pressure or mood state
Although P/T depletion has been reported to decrease systolic and diastolic blood pressure (Moja et al., 1996), a repeated measures ANOVA found no significant effect of P/T depletion on either systolic blood pressure (time × beverage interaction, F(1,14)=0.89; p=0.36, η2=0.02) or diastolic blood pressure (time × beverage interaction, F(1,14)=1.68; p=0.22, η2=0.02). The discrepancy may be due to differences in amino acid beverage formulation between our and Moja and colleagues’ study.
Confirming a recent meta-analysis of P/T depletion effect in healthy controls (Ruhe, Mason, & Schene, 2007), we found that P/T depletion did not significantly affect mood state. A comparison of POMS scores before consuming the beverage and after the 5h wait period found no significant effect of P/T depletion on mood (time × beverage interaction, F(1,14)=1.49; p=0.24, η2=0.01).
Effects of lowering dopamine on delay discounting behavior interact with COMT genotype
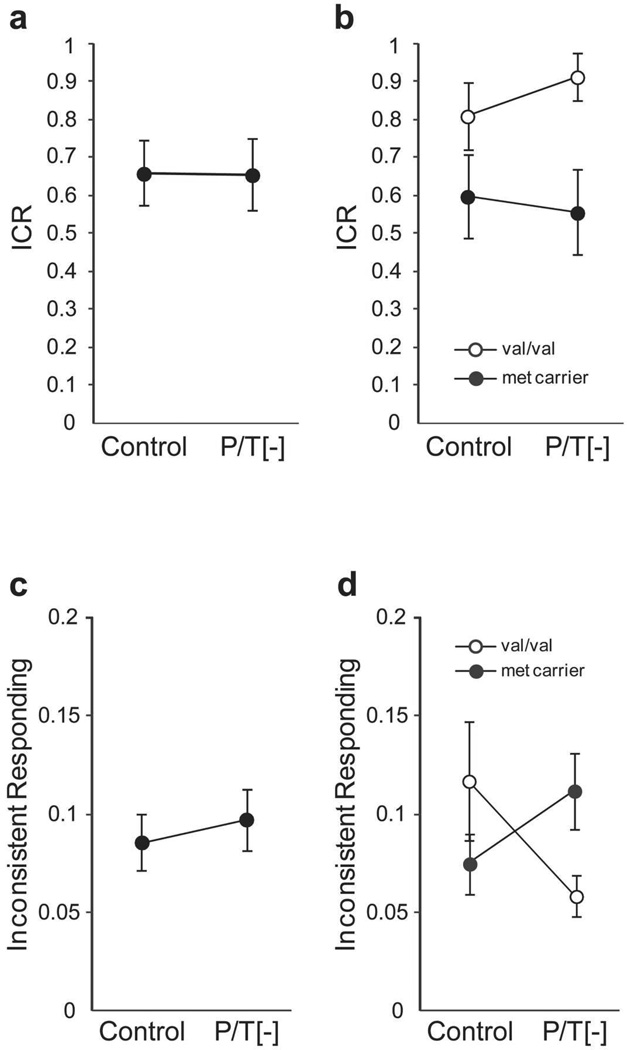
Our analysis of the ICR data found no significant effect of P/T depletion on the ratio of impulsive choices (t(14)=0.25, p=0.80; Fig. 1a). When the participants were separated by COMT genotype, however, we observed a significant beverage by genotype interaction (F(1,13)=4.96; p=0.044, η2=0.27; Fig. 1b). There was no significant difference in ICR when comparing the P/T[−] and placebo sessions within COMT genotype group, but there were large but non-significant increases in ICR among val/val participants (t(3)=−1.62, p=0.21, d=0.61) and non-significant decreases in ICR among met carriers (t(10)=1.44, p=0.18, d=−0.15) after P/T depletion. Considering a secondary dependent measure of immediate reward bias, the area under the ICR by delay curve (AUC), we likewise found a significant beverage by genotype interaction effect on AUC (F(1,13)=6.05, p=0.029, η2 = 0.31). As for the ICR measure, after P/T depletion, AUC increased for val/val participants (t(3)=−1.89, p=0.16, d=0.57) and decreased for met carriers (t(10)=1.81, p=0.10, d=−0.16), although neither change reached statistical significance. The plasma P/T/ΣLNAA data also indicate that greater biochemical depletion after P/T depletion predicted larger changes in ICR after P/T depletion (compared with placebo) (r=0.33, p=0.17), but this effect did not reach statistical significance. The interacting effects of COMT genotype and P/T depletion on immediate reward bias does not appear to be due to differences between genotype groups in terms of age, SES, personal or familial substance use, or any of several psychometric measures (Table 1).
Figure 1.
Effect of P/T depletion on ICR and within-session response inconsistency is COMT genotype dependent. a) Comparison of average ICR in the control and P/T depletion conditions for the sample as a whole. b) Comparison of ICR across beverage conditions when participants are separated by COMT genotype (beverage × group interaction, F(1,13) = 4.96; p = 0.044). c) Comparison of inconsistency of impulsive choices in the control and P/T depletion conditions for the sample as a whole. d) Comparison of choice consistency when participants are separated by COMT genotype (beverage × group interaction, F(1,13) = 6.1; p = 0.029). P/T depletion reduced inconsistent responding among the val/val participants (t(3) = 3.45, p = 0.04). ICR, impulsive choice ratio; P/T[−], phenylalanine/tyrosine-depletion session. met, methionine; val, valine.
We also examined the effect of P/T depletion on the consistency of response selection by determining the inferred ICR (iICR) from the DW trials as a function of delay time, and calculating the average of the absolute difference between the ICR and iICR values at each delay time. This value represents a gross measure of motor impulsiveness, with larger values indicating less controlled response selection. In considering the whole sample, we found no significant effect of P/T depletion on response consistency (t(14)=−.75, p=0.47; Fig. 1c). Separating participants by COMT genotype, however, revealed a significant beverage by genotype interaction (F(1,13)=6.1; p=0.029, η2=0.32; Fig. 1d). This finding reflects a large, significant increase in response consistency among val/val participants following P/T depletion (t(3)=3.45, p=0.041, d=1.03), and a moderate, but non-significant, decrease in response consistency among met carriers following P/T depletion (t(10)=−1.82, p=0.10, d=−0.61). Greater biochemical depletion in the P/T [−] condition did not predict larger changes in response consistency (r=0, p=0.99). This result indicates that the trend toward higher ICR among val/val participants following P/T depletion is not likely due to an increase in motor impulsiveness.
Accuracy in the CON trials did not differ significantly between the P/T[−] (94.2±1.9%) and placebo session (94.2±1.2%; t(14)=−0.04, p=0.97, d=−0.01). We did however observe a trend toward a beverage by genotype interaction (F(1,13)=3.6; p=0.080, η2=0.21), with val/val participants performing more accurately on the P/T[−] beverage (98.3±0.7%) compared to the control beverage (93.4±3.3%), and met carriers performing slightly less accurately on the P/T[−] beverage (92.8±2.5%) compared to the control beverage (94.4±1.2%).
Dopamine depletion effects on working memory depend on COMT genotype
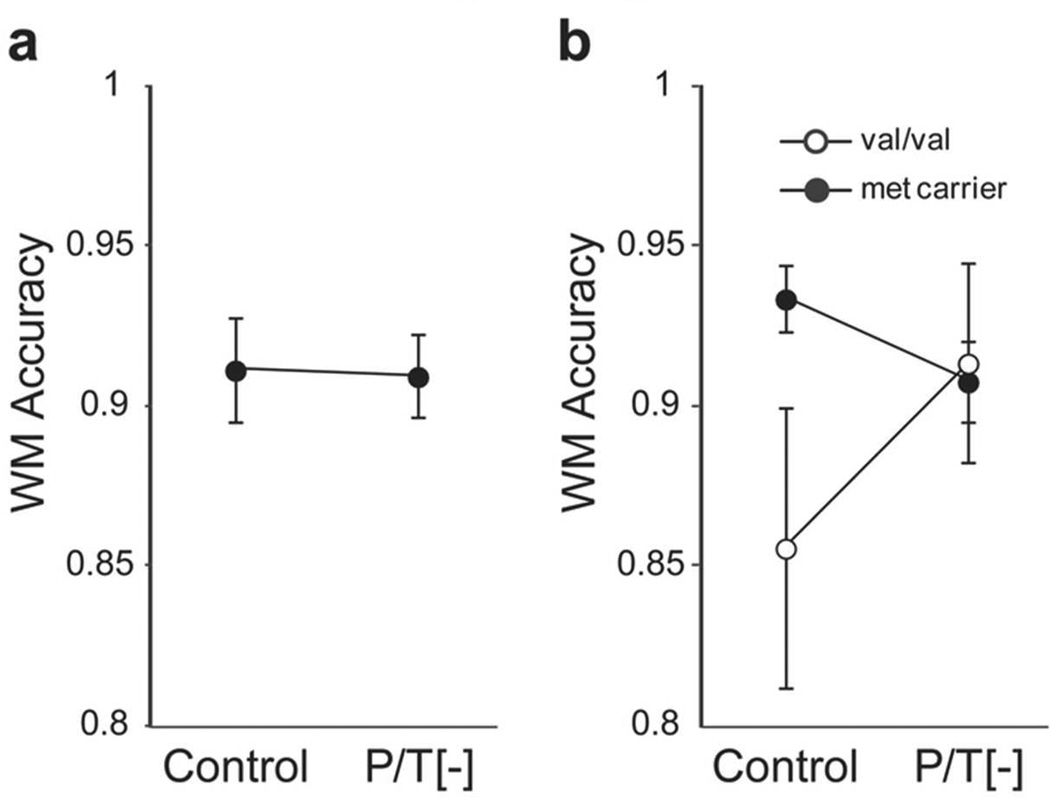
Given the well-established role of DA in modulating WM performance, we also tested whether our P/T depletion manipulation influenced WM performance on an n-back task. The results of a mixed measures ANOVA (beverage × load) found a main effect of load (F(2,26)=32.6; p<0.001, η2=0.57), whereby accuracy decreased with increasing load. This analysis failed to detect either a main effect of beverage (F(1,26)=1.3; p=0.27, η2=0) or a significant interaction between beverage and load (F(2,26)=0.35; p=0.71, η2=0). Given our a priori hypothesis that depleting dopamine should impair WM performance, we compared average accuracy in the 2- and 3-back conditions between beverage conditions. Considering the sample as a whole, we found no significant main effect of P/T depletion on WM accuracy (F(1,12)=0.002; p=0.97; Fig. 2a). However, separating participants by COMT genotype revealed a nearly significant beverage by genotype interaction (F(1,24)=4.57; p=0.054, η2 = 0.25; Fig. 2b). This result reflects a significant reduction in WM accuracy in the met carriers following P/T depletion (t(9)=−3.3, p=0.009, d=−1.17), coupled with no significant effect on accuracy among val/val participants (t(3)=0.70, p=0.53, d=0.41). The magnitude of biochemical depletion in the P/T[−] session did not predict changes in WM performance (r=−0.13, p=0.63). We observed no significant effect of P/T depletion on accuracy in 0-back trials (F(1,12)=0.56; p=0.47, η2=0), even when participants were separated by COMT genotype (beverage × genotype interaction, F(1,12)=0.06; p=0.82, η2=0).
Figure 2.
Effect of P/T depletion on working memory performance is COMT dependent. a) Comparison of working memory accuracy for the 2-back and 3-back trials in the control and P/T depletion conditions for the sample as a whole. b) When participants are separated by COMT genotype, there is a significant beverage × group interaction (F(1,24) = 4.57; p = .054). met, methionine; P/T[−], phenylalanine/tyrosine-depleted beverage; val, valine; WM, working memory.
Dopamine depletion effects on reaction time
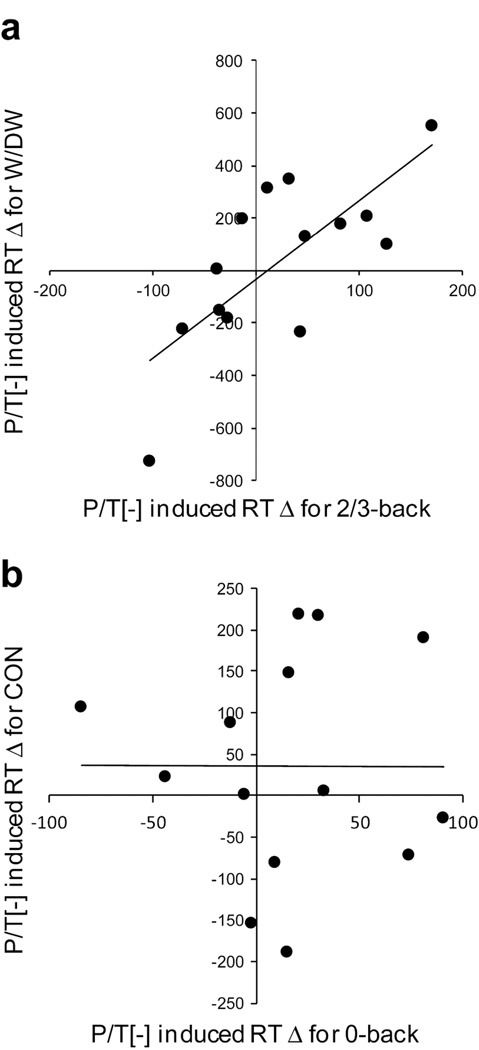
While profoundly depleting dopamine should induce Parkinsonian motor impairment, we did not observe significant effects of P/T depletion on reaction time (RT) in either the delay discounting task or the n-back task (Table 2). When participants were grouped by COMT genotype, we observed no beverage by genotype interaction for either the delay discounting or n-back tasks (maximum F=0.6). We did, however, find a significant positive correlation between the effect of P/T depletion on average RT in the cognitively demanding (2- and 3-back) n-back trials and the cognitively demanding (W and DW) delay discounting task trials (r=0.74, p=0.003; Fig. 3a). In other words, the degree to which P/T depletion slowed a participant’s RT in the 2- and 3-back trials predicted the degree to which their RT slowed in the W and DW trials. This relationship was specific to more cognitively demanding trials, as we found no significant relationship between the effect of P/T depletion on average RT in the 0-back trials of the n-back task and the CON trials of the delay discounting task (r=−0.01, p=98; Fig. 3b). The magnitude of change in the PT:LNAA ratio in the P/T[−] session was significantly correlated with P/T depletion-induced slowing in the W/DW trials (r=0.53, p=0.042), and was nearly significantly correlated with depletion-induced RT slowing in the 2/3-back trials (r=0.52, p=0.058). The RT slowing effect of P/T depletion in the subjective choice trials of the delay discounting task did not correlate with P/T depletion effects on ICR (r=0.07, p=0.79). In contrast, the RT slowing in the WM trials of the n-back task was positively correlated with improved WM accuracy (r=0.59, p=0.042).
Table 2.
The effect of P/T depletion on RT for the impulsive decision making and working memory tasks
| Control | P/T[−] | |||
|---|---|---|---|---|
| Delay discounting | t(14) | p value | ||
| CON | 1209 ± 87 | 1233 ± 72 | −0.68 | ns |
| W | 1662 ± 135 | 1694 ± 117 | −0.40 | ns |
| DW | 1795 ± 139 | 1805 ± 127 | −0.10 | ns |
| n-back | t(13) | p value | ||
| 0-back | 458 ± 13 | 473 ± 16 | −1.24 | ns |
| 2-back | 675 ± 23 | 699 ± 30 | −1.08 | ns |
| 3-back | 689 ± 35 | 712 ± 41 | −0.89 | ns |
Values are reported as mean ± standard deviation. Reported p-values reflect the results of paired two-tailed comparisons for the effect of the beverage. Exact p-values reported unless p < 0.001. CON, control trials; DW, don’t want trials; P/T[−], phenylalanine/tyrosine-depletion; W, want trials.
Figure 3.
There is a relationship between the effect of P/T depletion on delay discounting task ICR RT and WM n-back task RT. a) There is significant positive correlation between the effect of P/T depletion on the RT in 2- and 3-back trials in the n-back task and RT during the W and DW trials of the delay discounting task (r = 0.74, p = 0.003). b) There is no significant relationship between the effect of P/T depletion on RT in 0-back trials and RT in the CON trials of the delay discounting task (r = −0.01, p = .98). CON, control trials; DW, don’t want trials; ICR, impulsive choice ratio; P/T[−], phenylalanine/tyrosine-depletion; RT, reaction time; W, want trials; WM, working memory.
DISCUSSION
Temporarily lowering DA in adult male humans led to COMT-genotype dependent effects on immediate reward bias, within-session response selection consistency, and working memory performance. Across tasks, we also observed COMT-genotype independent RT slowing that was limited to the more cognitively demanding conditions.
Implications for the role of dopamine in immediate reward bias
These results provide the first demonstration in humans of a COMT genotype-dependent effect of manipulating DA on intertemporal choice behavior, suggesting that individual differences in frontal DA tone may have impeded many of the past efforts to characterize the influence of DA on the discounting of delayed rewards in human subjects. P/T depletion substantially increased immediate reward bias among val/val participants, while producing minimal change among met allele carriers. Studies in animal models strongly implicate DA in regulating immediate reward bias, generally finding that elevating DA reduces immediate reward bias (Doya, 2008). In contrast, in humans, the evidence as to whether acute DA changes can affect Now bias is mixed (Acheson & de Wit, 2008; de Wit et al., 2002; Dwoskin, Rauhut, King-Pospisil, & Bardo, 2006; Hamidovic et al., 2008; Kayser et al., 2012; Alex Pine, Tamara Shiner, Ben Seymour, & Raymond J. Dolan, 2010), except in impulsive populations, where elevating DA reduces Now bias (Pietras, Cherek, Lane, Tcheremissine, & Steinberg, 2003). Four factors may contribute to this discrepancy in the literature. First, 158Val/Val is the ancestral COMT genotype, with the 158Met arising first in humans (Palmatier, Kang, & Kidd, 1999). As a consequence, all animal studies have been limited to 158Val/Val individuals, while the human studies have likely tested mixed COMT genotype samples. Second, excepting (Kayser et al., 2012), all DA manipulations used to date in humans act predominately on striatal, not cortical, DA. If frontal and striatal DA play different roles in modulating immediate reward bias, as proposed for other DA-dependent functions (Bilder, Volavka, Lachman, & Grace, 2004; Cohen, Braver, & Brown, 2002; Cools, Miyakawa, Sheridan, & D'Esposito, 2010; Crofts et al., 2001; Meyer-Lindenberg et al., 2005) elevating striatal DA could have distinct effects on immediate reward bias. Third, no pharmacological studies to date of immediate reward bias have accounted for genetic differences (such as COMT genotype) that could moderate the effects of DA manipulations. Finally, excepting (Hamidovic et al., 2008), previous studies have not accounted for cycle-dependent variance in frontal DA function (Jacobs & D'Esposito, 2011), which would be expected to modify the discounting behavior of female subjects. Finally, previous studies have tested mixed age populations from ages 18 to 30+, which adds another source of substantial variability (Smith & Boettiger, 2012). Given the small sample sizes in previous pharmacological studies, genetic, cycle, and age factors likely introduced variability that appeared random and may have occluded the detection of true pharmacological effects. We also note that COMT activity varies not only as a function of genotype, but also as a function of sex, with a globally reduced COMT activity in females relative to males (Chen et al., 2004); therefore, different sex ratios across genotypes may have further complicated findings in previous studies. Moreover, two studies have reported stronger impulsivity-related associations with Val alleles in females than in males (Lang, Bajbouj, Sander, & Gallinat, 2007; Qian et al., 2003). Future studies of DA regulation of impulsive choice specifically in females are warranted, but cyclic variation in DA signaling and interaction of DA manipulations with cycle via the infundibular DA system will necessarily complicate such studies.
Implications for the role of dopamine in working memory
The reduction in WM performance after P/T depletion in met carriers is consistent with a wide body of literature implicating frontal DA in WM performance (Cools & D'Esposito, 2011; Goldman-Rakic, Muly, & Williams, 2000; Sawaguchi & Goldman-Rakic, 1991; Williams & Castner, 2006). Moreover, these data are consistent with previous studies showing that P/T depletion impairs performance on tasks dependent on WM (Gijsman et al., 2002; Harmer, McTavish, Clark, Goodwin, & Cowen, 2001; Harrison et al., 2004; Nagano-Saito et al., 2008). Our finding that P/T depletion also increased response consistency in the DD task for val/val participants, while it decreased response consistency among met carriers suggests that P/T depletion had consistent genotype-dependent effects on executive control across tasks. The dissociation of immediate reward selection bias from executive control effects, however, indicate that immediate reward bias cannot be fully attributed to impaired executive function. However, our data imply that the involvement of DA in WM performance is not simply a reflection of frontal DA tone. DA depletion did not affect val/val subjects’ WM performance, despite the fact that their average placebo condition accuracy levels were >85%, leaving considerable room for degradation in performance. It’s important to consider that our P/T depletion does not selectively deplete frontal DA, and that effects of striatal DA depletion on task performance may occlude effects of depleting frontal DA. Indeed a recent study showing that COMT inhibition, which presumably elevates frontal DA selectively, has COMT-genotype dependent effects on WM performance found that elevating frontal DA worsened the performance of met/met individuals and improved the performance of val/val individuals (Farrell et al., 2012). Moreover, DA depletion effects on cognition likely reflect not only changes within DA-sensitive brain regions, but also changes in connectivity between DA-sensitive structures (Nagano-Saito et al., 2008). However, our finding that DA depletion did not globally slow RTs, but instead tended to slow RTs under cognitively demanding conditions, suggests the possibility that P/T depletion, at this dose, exerts relatively greater effects on cortical DA levels than on striatal DA signaling (at least the dorsal striatum). We also note that COMT is only indexing a presynaptic measure of frontal DA, while postsynaptic DA signaling factors may also be playing a role in modulating WM function (Meyer-Lindenberg et al., 2007).
Potential neural mechanisms and methodological limitations
There are a few limitations to the method we used to manipulate DA, acute P/T depletion. Although studies in rats (McTavish, Cowen, & Sharp, 1999) and humans (Leyton et al., 2004) have shown that P/T depletion reduces DA release in the striatum by at least 30%, it is possible that low DA levels in the brainstem may reduce DRD2 receptor-mediated somatodendritic autoinhibition, thereby increasing the firing of DA neurons and potentially increasing the release of DA in other projection regions. However, PET studies in humans have demonstrated that the degree to which procedure reduces DA receptor occupancy in the striatum predicts the level of executive function impairment (Mehta, Gumaste, Montgomery, McTavish, & Grasby, 2005); effects on extrastriatal targets have not yet been adequately investigated. The caveat remains that the effects of P/T depletion on dopaminergic neurotransmission in humans are not yet fully understood and warrant further study. Beyond changes in DA within target regions, P/T depletion has been shown to reduce frontostriatal connectivity (Nagano-Saito et al., 2008). This latter effect is particularly noteworthy here based on evidence that increasing frontostriatal connectivity predicts decreased immediate reward bias (Kayser et al., 2012). While studies in both rodents and humans suggest that P/T depletion does not affect norepinephrine (NE) dependent processes (Leyton et al., 2004; McTavish, Callado, Cowen, & Sharp, 1999; McTavish et al., 2001; B. Sheehan et al., 1996), one study found that P/T depletion reduced levels of a NE metabolite (Palmour, Ervin, Baker, & Young, 1998). However, acute P/T depletion does not affect NE-regulated melatonin levels, while it does alter DA-controlled prolactin levels (Harmer et al., 2001; B. Sheehan et al., 1996). Therefore, while P/T depletion effects on NE levels cannot be ruled out completely, a variety of evidence suggests that any such effects are relatively minimal. Another limitation of P/T depletion is that it is subtler in terms of degree of depletion, relative to profound global forebrain DA depletions, which have been used in animals. Given the profound dependence of motor function on DA, the subtlety of the effects of P/T depletion on cognition are actually advantageous in that it allows detection of behavioral effects without confounding motor impairment. Finally, one may question the scientific value of investigating such a global, neuroanatomically nonspecific manipulation. Although global P/T depletion presumably affects numerous neural functions, we examined its effects on those engaged by particular behavioral tasks designed to test specific hypotheses about the role of frontal DA in intertemporal choice. It is noteworthy that this global DA manipulation’s effects on executive functions were highly dependent on COMT genotype within adult males, and our findings imply that underlying individual differences should be taken into account in predicting or evaluating the effects of global DA manipulations on behavior. This issue is of biological and clinical relevance, because many drugs, both prescribed and illicit, act globally in the brain.
Our small sample size, particularly within the COMT val/val genotype group, is another significant limitation of this study. While recruiting without regard for COMT genotype allowed the advantage of testing blind to genotype, it also limited our statistical power. Future studies in which participants are recruited specifically by COMT genotype could avoid this pitfall. Another issue is that epistatic relationships between the COMT SNP may underlie unexplained variance in the P/T depletion effects. Our sample size did not allow for consideration of other genetic variants that have been reported to interact with COMT genotype in influencing immediate reward bias (Paloyelis, Asherson, Mehta, Faraone, & Kuntsi, 2010) or working memory (Stelzel, Basten, Montag, Reuter, & Fiebach, 2009; Tan et al., 2012). Moreover, other COMT SNPs have been identified as components of COMT haplotypes that influence behavior more predictably than do isolated SNPs (Pap et al., 2012; Tunbridge, 2010). Future studies adequately powered to consider these likely interacting factors may help further illuminate trait based interactions with temporary state changes in DA signaling.
Despite these caveats, we propose that our results demonstrate the interacting roles of acute state and tonic trait levels of DA signaling in modulating executive function in humans. We have shown the first demonstration that a genetic marker of tonic cortical DA is critical for predicting effects of global DA manipulations on behaviors that engage frontal structures. Our findings provide novel empirical support for a model of frontal DA function, in which the effects of acute manipulations on cognitive function depend upon pre-existing DA tone.
ACKNOWLEDGEMENTS
This work was supported by Award numbers KL2RR025746, UL1RR025747, and P60AA011605 and an IBM Junior Faculty Award (CAB), and by F32AA019838 and T32AA007573 (MKK), and by DK056350. We thank M. Beck, C. Green, C. Smith, and E. Steel and for their assistance on this project. The authors declare no competing financial interests. MKK and CAB designed research, MKK performed research, MKK and CAB analyzed data and wrote the paper.
REFERENCES
- Acheson A, de Wit H. Bupropion improves attention but does not affect impulsive behavior in healthy young adults. Exp Clin Psychopharmacol. 2008;16(2):113–123. doi: 10.1037/1064-1297.16.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamirano LJ, Fields HL, D'Esposito M, Boettiger CA. Interaction between Family History of Alcoholism and Locus of Control in the Opioid Regulation of Impulsive Responding under the Influence of Alcohol. Alcohol Clin Exp Res. 2011;35(11):1905–1914. doi: 10.1111/j.1530-0277.2011.01535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES. Violence and mental disorder: Developments in risk assessment. Chicago: University of Chicago Press; 1994. Impulsiveness and aggression; pp. 61–79. [Google Scholar]
- Barratt W. The Barratt Simplified Measure of Social Status (BSMSS) measuring SES. Unpublished manuscript, Indiana State University. 2006 [Google Scholar]
- Beck AT, Steer RA. Manual for the Revised Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118(2):169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, et al. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27(52):14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations: review. Mol Psychiatry. 2003;8(12):951–973. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- Butcher J, Graham J, Williams C, Ben-Porath Y. Development and Use of the MMPI-2 Content Scales. Minneapolis: University of Minnesota Press; 1990. p. 196. [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol. 2002;12(2):223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D'Esposito M. Enhanced frontal function in Parkinson's disease. Brain. 2010;133(Pt 1):225–233. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11(11):1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27(5):813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Doya K. Modulators of decision making. Nat Neurosci. 2008;11(4):410–416. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12(3–4):178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell SM, Tunbridge EM, Braeutigam S, Harrison PJ. COMT Val(158)Met genotype determines the direction of cognitive effects produced by catechol-O-methyltransferase inhibition. Biol Psychiatry. 2012;71(6):538–544. doi: 10.1016/j.biopsych.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsman HJ, Scarna A, Harmer CJ, McTavish SB, Odontiadis J, Cowen PJ, et al. A dose-finding study on the effects of branch chain amino acids on surrogate markers of brain dopamine function. Psychopharmacology (Berl) 2002;160(2):192–197. doi: 10.1007/s00213-001-0970-5. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95(17):9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31(2–3):295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6(3):316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J Clin Psychopharmacol. 2008;28(1):45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, McTavish SF, Clark L, Goodwin GM, Cowen PJ. Tyrosine depletion attenuates dopamine function in healthy volunteers. Psychopharmacology (Berl) 2001;154(1):105–111. doi: 10.1007/s002130000613. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Olver JS, Norman TR, Burrows GD, Wesnes KA, Nathan PJ. Selective effects of acute serotonin and catecholamine depletion on memory in healthy women. J Psychopharmacol. 2004;18(1):32–40. doi: 10.1177/0269881104040225. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. New Haven: Yale University; 1975. [Google Scholar]
- Jacobs E, D'Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. J Neurosci. 2011;31(14):5286–5293. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaenmaki M, Tammimaki A, Myohanen T, Pakarinen K, Amberg C, Karayiorgou M, et al. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem. 2010;114(6):1745–1755. doi: 10.1111/j.1471-4159.2010.06889.x. [DOI] [PubMed] [Google Scholar]
- Karobath M, Baldessarini RJ. Formation of catechol compounds from phenylalanine and tyrosine with isolated nerve endings. Nat New Biol. 1972;236(68):206–208. doi: 10.1038/newbio236206a0. [DOI] [PubMed] [Google Scholar]
- Karoum F, Chrapusta SJ, Egan MF. 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem. 1994;63(3):972–979. doi: 10.1046/j.1471-4159.1994.63030972.x. [DOI] [PubMed] [Google Scholar]
- Kayser AS, Allen DC, Navarro-Cebrian A, Mitchell JM, Fields HL. Dopamine, corticostriatal connectivity, and intertemporal choice. J Neurosci. 2012;32(27):9402–9409. doi: 10.1523/JNEUROSCI.1180-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1998;395(1):1–17. [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Lang UE, Bajbouj M, Sander T, Gallinat J. Gender-dependent association of the functional catechol-O-methyltransferase Val158Met genotype with sensation seeking personality trait. Neuropsychopharmacology. 2007;32(9):1950–1955. doi: 10.1038/sj.npp.1301335. [DOI] [PubMed] [Google Scholar]
- Leyton M, Dagher A, Boileau I, Casey K, Baker GB, Diksic M, et al. Decreasing amphetamine-induced dopamine release by acute phenylalanine/tyrosine depletion: A PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2004;29(2):427–432. doi: 10.1038/sj.npp.1300328. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15(1–2):61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- McTavish SF, Callado L, Cowen PJ, Sharp T. Comparison of the effects of alpha-methyl-p-tyrosine and a tyrosine-free amino acid load on extracellular noradrenaline in the rat hippocampus in vivo. J Psychopharmacol. 1999;13(4):379–384. doi: 10.1177/026988119901300408. [DOI] [PubMed] [Google Scholar]
- McTavish SF, Cowen PJ, Sharp T. Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacology (Berl) 1999;141(2):182–188. doi: 10.1007/s002130050823. [DOI] [PubMed] [Google Scholar]
- McTavish SF, McPherson MH, Harmer CJ, Clark L, Sharp T, Goodwin GM, et al. Antidopaminergic effects of dietary tyrosine depletion in healthy subjects and patients with manic illness. Br J Psychiatry. 2001;179:356–360. doi: 10.1192/bjp.179.4.356. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Gumaste D, Montgomery AJ, McTavish SF, Grasby PM. The effects of acute tyrosine and phenylalanine depletion on spatial working memory and planning in healthy volunteers are predicted by changes in striatal dopamine levels. Psychopharmacology (Berl) 2005;180(4):654–663. doi: 10.1007/s00213-004-2128-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8(5):594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Straub RE, Lipska BK, Verchinski BA, Goldberg T, Callicott JH, et al. Genetic evidence implicating DARPP-32 in human frontostriatal structure, function, and cognition. J Clin Invest. 2007;117(3):672–682. doi: 10.1172/JCI30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA. Impulsive responding in alcoholics. Alcohol Clin Exp Res. 2005;29(12):2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Moja EA, Lucini V, Benedetti F, Lucca A. Decrease in plasma phenylalanine and tyrosine after phenylalanine-tyrosine free amino acid solutions in man. Life Sci. 1996;58(26):2389–2395. doi: 10.1016/0024-3205(96)00242-1. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, McTavish SF, Cowen PJ, Grasby PM. Reduction of brain dopamine concentration with dietary tyrosine plus phenylalanine depletion: an [11C]raclopride PET study. Am J Psychiatry. 2003;160(10):1887–1889. doi: 10.1176/appi.ajp.160.10.1887. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28(14):3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol-O-methyltransferase alleles. Biol Psychiatry. 1999;46(4):557–567. doi: 10.1016/s0006-3223(99)00098-0. [DOI] [PubMed] [Google Scholar]
- Palmour RM, Ervin FR, Baker GB, Young SN. An amino acid mixture deficient in phenylalanine and tyrosine reduces cerebrospinal fluid catecholamine metabolites and alcohol consumption in vervet monkeys. Psychopharmacology (Berl) 1998;136(1):1–7. doi: 10.1007/s002130050532. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35(12):2414–2426. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap D, Gonda X, Molnar E, Lazary J, Benko A, Downey D, et al. Genetic variants in the catechol-o-methyltransferase gene are associated with impulsivity and executive function: relevance for major depression. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(8):928–940. doi: 10.1002/ajmg.b.32098. [DOI] [PubMed] [Google Scholar]
- Pietras CJ, Cherek DR, Lane SD, Tcheremissine OV, Steinberg JL. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology (Berl) 2003;170(4):390–398. doi: 10.1007/s00213-003-1547-2. [DOI] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, Time, and Impulsivity in Humans. J. Neurosci. 2010;30(26):8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. J Neurosci. 2010;30(26):8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Q, Wang Y, Zhou R, Li J, Wang B, Glatt S, et al. Family-based and case-control association studies of catechol-O-methyltransferase in attention deficit hyperactivity disorder suggest genetic sexual dimorphism. Am J Med Genet B Neuropsychiatr Genet. 2003;118B(1):103–109. doi: 10.1002/ajmg.b.10064. [DOI] [PubMed] [Google Scholar]
- Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80(1):1–28. [PubMed] [Google Scholar]
- Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12(4):331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251(4996):947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- Sheehan B, Tharyan P, McTavish S, Campling G, Cowen P. Use of a dietary manipulation to deplete plasma tyrosine and phenylalanine in healthy subjects. Journal of Psychopharm. 1996;10(3):231–234. doi: 10.1177/026988119601000309. [DOI] [PubMed] [Google Scholar]
- Sheehan BD, Tharyan P, McTavish SF, Campling GM, Cowen PJ. Use of a dietary manipulation to deplete plasma tyrosine and phenylalanine in healthy subjects. J Psychopharmacol. 1996;10(3):231–234. doi: 10.1177/026988119601000309. [DOI] [PubMed] [Google Scholar]
- Smith CT, Boettiger CA. Age modulates the effect of COMT genotype on delay discounting behavior. Psychopharmacology (Berl) 2012;222(4):609–617. doi: 10.1007/s00213-012-2653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. Assessment of state and trait anxiety: conceptual and methodological issues. South Psychol. 1985;2:6–16. [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Effects of dopamine-related gene–gene interactions on working memory component processes. European Journal of Neuroscience. 2009;29(5):1056–1063. doi: 10.1111/j.1460-9568.2009.06647.x. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen AG, Kolachana B, Apud JA, Mattay VS, Callicott JH, et al. Effective connectivity of AKT1-mediated dopaminergic working memory networks and pharmacogenetics of anti-dopaminergic treatment. Brain. 2012;135(5):1436–1445. doi: 10.1093/brain/aws068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE. Evaluation and treatment of adolescent substance abuse: a decision tree method. Am J Drug Alcohol Abuse. 1990;16(1–2):1–46. doi: 10.3109/00952999009001570. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM. The catechol-O-methyltransferase gene: its regulation and polymorphisms. Int Rev Neurobiol. 2010;95:7–27. doi: 10.1016/B978-0-12-381326-8.00002-8. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24(23):5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. Future time perspective in schizophrenia. J Abnorm Psychol. 1956;52(2):240–245. doi: 10.1037/h0039899. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139(1):263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Wu K, O’Keeffe D, Politis M, O’Keeffe GC, Robbins TW, Bose SK, et al. The catechol-O-methyltransferase Val158Met polymorphism modulates fronto-cortical dopamine turnover in early Parkinson’s disease: a PET study. Brain. 2012;135(8):2449–2457. doi: 10.1093/brain/aws157. [DOI] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. 2007;27(38):10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]