Abstract
Cognitive deficits in schizophrenia have been linked to disturbances in GABA neurons in the prefrontal cortex. Furthermore, cognitive deficits in schizophrenia appear well before the onset of psychosis and have been reported to be present during early childhood and even during the first year of life. Taken together, these data raise the following question: Does the disease process that produces abnormalities in prefrontal GABA neurons in schizophrenia begin prenatally and disrupt the ontogeny of cortical GABA neurons? Here, we address this question through a consideration of evidence that genetic and/or environmental insults that occur during gestation initiate a pathogenetic process that alters cortical GABA neuron ontogeny and produces the pattern of GABA neuron abnormalities, and consequently cognitive difficulties, seen in schizophrenia. First, we review available evidence from postmortem human brain tissue studies characterizing alterations in certain subpopulations of prefrontal GABA neuron that provide clues to a prenatal origin in schizophrenia. Second, we review recent discoveries of transcription factors, cytokine receptors, and other developmental regulators that govern the birth, migration, specification, maturation, and survival of different subpopulations of prefrontal GABA neurons. Third, we discuss recent studies demonstrating altered expression of these ontogenetic factors in the prefrontal cortex in schizophrenia. Fourth, we discuss the potential role of disturbances in the maternal-fetal environment such as maternal immune activation in the development of GABA neuron dysfunction. Finally, we propose critical questions that need to be answered in future research to further investigate the role of altered GABA neuron ontogeny in the pathogenesis of schizophrenia.
Keywords: parvalbumin, somatostatin, prefrontal cortex, interneuron, development
1. Introduction
Schizophrenia is a devastating psychiatric disorder that afflicts ~1% of all humans and is a leading cause of morbidity and early mortality (Insel and Scolnick, 2006). The features of the disorder with the strongest association to poor long-term outcomes include cognitive deficits (Green, 2006), such as impairments in working memory and cognitive control (Lesh et al., 2011). Disturbances in cognitive functioning are commonly seen in the illness (Keefe and Fenton, 2007) and certain cognitive deficits have been linked to dysfunction of subsets of inhibitory (GABA) neurons in the prefrontal cortex (PFC) (reviewed in (Lewis et al., 2012) and discussed in greater detail below). Furthermore, cognitive dysfunction in schizophrenia is only minimally responsive to antipsychotic medications (Keefe et al., 2007a; Keefe et al., 2007b). This lack of effective treatments for the cognitive features of schizophrenia indicates the need for greater insight into the pathogenetic processes that lead to disturbances in PFC GABA neurons.
Interestingly, cognitive disturbances are present before the onset of psychosis and well before the diagnosis of schizophrenia is typically made in late adolescence/early adulthood (Woodberry et al., 2008). These data suggest that the disease process affecting PFC GABA neurons is already active during development. In previous reviews, we discussed the potential role of postnatal developmental changes in PFC GABA neurons during adolescence in creating a sensitive period for environment insults, such as a cannabis exposure, that may lead to the emergence of PFC GABA neuron disturbances in schizophrenia (Hoftman and Lewis, 2011; Beneyto and Lewis, 2011). However, the initial onset of the disease process may begin at an even earlier stage of life. For example, individuals with schizophrenia exhibit delays in achieving developmental milestones in early childhood (Jones et al., 1994), even during the first year of life (Ridler et al., 2006; Sorensen et al., 2010; Clarke et al., 2011). Furthermore, in utero environmental insults, such as exposure to maternal infection, during the first and second trimester, which is the time period when cortical GABA neurons are born (Jakovcevski et al., 2011), are associated with an increased risk of schizophrenia in offspring (Brown and Derkits, 2010). In addition, murine models of maternal immune activation have been reported to disrupt the development of PFC GABA neurons (Meyer et al., 2008; Richetto et al., 2013).
Taken together, these data raise the following question: could the disease process that produces the cortical GABA neuron disturbances present in adults with schizophrenia begin much earlier than adolescence, perhaps even prenatally? Here, we address this question through a consideration of the evidence supporting the idea that genetic and/or environmental insults during gestation could initiate a pathogenetic process that alters the development (e.g. migration, phenotypic specification, maturation, and survival) of cortical GABA neurons, resulting in the pattern of GABAergic alterations, and consequently cognitive difficulties, seen in the disorder. First, we review the wealth of data accumulated over the past decade characterizing alterations in subsets of PFC GABA neuron that are relevant for cognitive dysfunction and may provide clues to a prenatal pathogenetic origin in schizophrenia. Second, we review recent discoveries of developmental regulators that govern the birth, migration, specification, maturation, and survival of PFC GABA neurons. Third, we discuss recent studies demonstrating altered expression of these ontogenetic factors in the PFC in schizophrenia. Fourth, we discuss the potential role of disturbances in maternal-fetal environment in the development of GABA neuron dysfunction in schizophrenia. Finally, we propose critical questions that need to be answered in future research on the role of altered GABA neuron ontogeny in the pathogenesis of schizophrenia.
2. Alterations in subpopulations of PFC GABA neurons contribute to cognitive dysfunction and provide clues to a prenatal pathogenetic origin in schizophrenia
The most consistently reported disease-related findings in the PFC in schizophrenia involve GABA neurons. For example, deficits in mRNA levels for the GABA synthesizing enzyme GAD67 have been replicated across multiple subject cohorts and do not appear to be attributable to antipsychotic medications (Akbarian et al., 1995; Guidotti et al., 2000; Volk et al., 2000; Vawter et al., 2002; Straub et al., 2007; Duncan et al., 2010; Curley et al., 2011). Interestingly, the majority of PFC GABA neurons appear to express normal levels of GAD67 mRNA in schizophrenia (Volk et al., 2000). Furthermore, approximately 50% of GABA neurons in primate PFC express the calcium-binding protein calretinin (Conde et al., 1994; Gabbott and Bacon, 1996). Calretinin mRNA levels have been reported to be unchanged in the PFC in schizophrenia (Hashimoto et al., 2003; Volk et al., 2012), suggesting that calretinin neurons are largely unaffected in the disorder.
However, two other subsets of GABA neurons have been consistently reported to be altered in the PFC of subjects with schizophrenia. For example, a subpopulation of GABA neurons identified as abnormal in schizophrenia includes those that express the calcium-binding protein parvalbumin (PV), which includes ~25% of PFC GABA neurons in primate PFC (Conde et al., 1994; Gabbott and Bacon, 1996). Lower PV mRNA levels in schizophrenia have also been consistently reported in PFC gray matter by different research groups (Hashimoto et al., 2003; Mellios et al., 2009; Fung et al., 2010; Volk et al., 2012). Diminished PV neuron regulation of pyramidal neuron activity may have negative consequences for cognitive functioning in schizophrenia (Lewis et al., 2012). Fast-spiking PV neurons provide powerful perisomatic inhibitory regulation of pyramidal neuron output and enable synchronization of cortical neuron activity at gamma frequencies (30–80 Hz) (Sohal et al., 2009; Sohal, 2012). Gamma frequency oscillations are important for perceptual and PFC-related cognitive processes such as working memory (Howard et al., 2003), and individuals with schizophrenia show altered PFC gamma activity while performing tasks that require cognitive control (Cho et al., 2006; Minzenberg et al., 2010). Disrupting PV neuron function results in reduced gamma oscillatory power (Whittington et al., 1998; Gulyas et al., 2010). Thus, pathological processes affecting PV neurons may adversely affect the synchronization of cortical neural activity and contribute to cognitive dysfunction in schizophrenia.
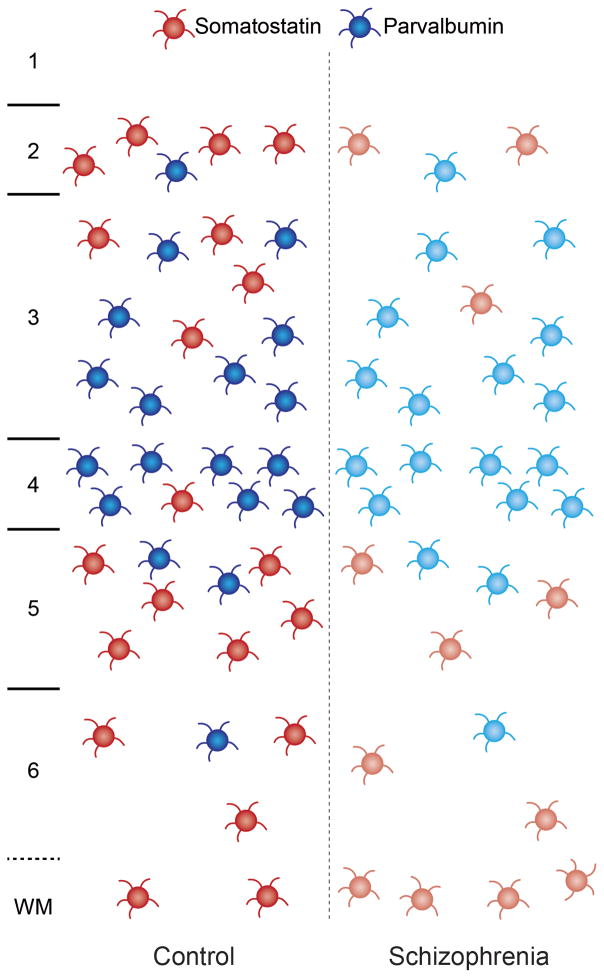
Interestingly, in situ hybridization grain counting studies have found that PFC GABA neurons underexpress PV mRNA, but that the number of neurons expressing detectable PV mRNA levels in the PFC gray matter appears unchanged (Hashimoto et al., 2003). Similarly, immunohistochemistry studies have also found a normal complement of PV neurons in the PFC in schizophrenia (Woo et al., 1997). Some studies have reported a lower density of PFC PV-immunoreactive neurons in the disorder (Beasley and Reynolds, 1997; Beasley et al., 2002), but these findings could reflect subthreshold levels of PV protein due to insufficient transcript levels which rendered the neurons undetectable under conditions suboptimal for immunohistochemistry (Stan and Lewis, 2012). Furthermore, 50% of PFC PV neurons lack detectable GAD67 mRNA in schizophrenia (Hashimoto et al., 2003). Thus, PV neurons may complete the process of tangential migration but fail to develop the normal GABAergic phenotype reflected in lower levels of PV and GAD67 mRNAs (Figure 1).
Figure 1. Schematic illustrating reported disturbances in PV and SST neurons in the PFC in schizophrenia.
In the PFC of healthy human subjects (left panel), PV neurons (blue) are predominantly found in layers deep 3 and 4, while SST neurons (red) are predominantly localized to cortical layers 2, superficial 3, 5, and 6 and are also found in the superficial white matter (Hashimoto et al., 2003; Morris et al., 2008). In schizophrenia subjects (right panel), reduced mRNA levels of GAD67 and PV have been reported in PV neurons without a change in the number of PV neurons per tissue area (Hashimoto et al., 2003), suggesting incomplete phenotypic specification and/or maturation of PV neurons (lighter shade of blue) in the disorder. In contrast, a lower density of gray matter neurons that express detectable levels of SST mRNA has been reported in schizophrenia (Morris et al., 2008), which could reflect fewer gray matter SST neurons and/or neurons that do not express detectable levels of SST mRNA (lighter shade of red). Interestingly, some (Yang et al., 2011), though not all (Morris et al., 2008), studies have found a higher density of SST neurons in cortical white matter in the disorder, suggesting that the migration of some SST neurons may be arrested, leading to fewer SST neurons reaching their final destination in gray matter.
The neuropeptide somatostatin (SST), which is expressed by ~20% of GABA neurons, has also been shown to have lower mRNA levels in the PFC across several large cohorts of schizophrenia subjects (Morris et al., 2008; Mellios et al., 2009; Fung et al., 2010; Volk et al., 2012). In situ hybridization grain counting analysis found a lower density of gray matter neurons that express detectable levels of SST mRNA in the PFC, which could reflect fewer gray matter SST neurons and/or SST neurons that do not express adequate levels of SST mRNA to reach the threshold of detection (Morris et al., 2008). Interestingly, some (Yang et al., 2011), though not all (Morris et al., 2008), studies have found a higher density of SST neurons in cortical white matter in the disorder. One potential parsimonious explanation for this pattern of findings across studies involves a pathogenetic process in some subjects that arrests the migration of SST neurons in white matter early in development leading to fewer SST neurons reaching their final destination in gray matter (Figure 1) (Yang et al., 2011). Indeed, other studies have also reported a higher density of neurons in the interstitial white matter in schizophrenia (Anderson et al., 1996; Kirkpatrick et al., 2003; Eastwood and Harrison, 2005), including nitric oxide synthase-containing neurons in deeper white matter (Akbarian et al., 1993). Understanding the potential divergence of prenatal pathogenetic origins for PV (i.e. incomplete phenotypic specification) and SST (i.e. failure to complete tangential migration) neuron disturbances in schizophrenia requires knowledge of the developmental factors that regulate cortical GABA neuron ontogeny.
3. Ontogenetic transcription factors regulate different stages of prenatal development of cortical GABA neuron subpopulations
In the past decade, great advances have been made in understanding the prenatal ontogeny of cortical GABA neurons. In humans, calretinin neurons appear to derive from the subventricular zone of the dorsal pallium (Letinic et al., 2002; Zecevic et al., 2005; Fertuzinhos et al., 2009; Zecevic et al., 2011; Jakovcevski et al., 2011). In contrast, evidence from studies of holoprosencephaly suggests that PV and SST neurons in humans, as in mice, originate from the ganglionic eminence of the subpallium (Fertuzinhos et al., 2009). In mice and humans, the medial region of the ganglionic eminence is the primary site of origin for these neurons (Xu et al., 2004; Butt et al., 2005; Cobos et al., 2006; Zecevic et al., 2011). Thus, a developmental pathogenetic process focused in the medial ganglionic eminence may contribute to the selective disturbance in PV and SST neurons, while sparing calretinin neurons, in schizophrenia.
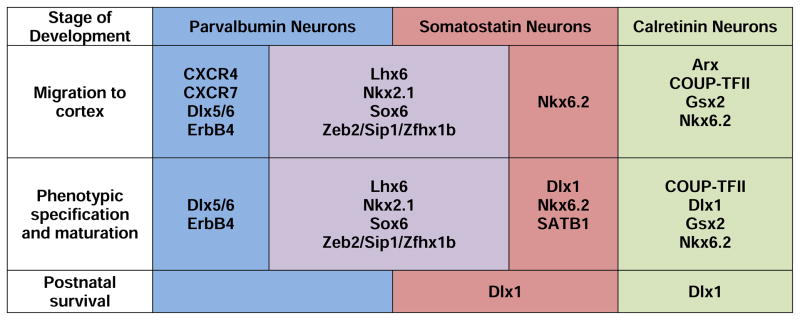
The migration, phenotypic specification, maturation, and survival of cortical PV and SST neurons depend upon adequate expression of cell-type specific ontogenetic transcription factors (Figure 2). For example, the transcription factor Lhx6 is expressed by cortical PV and SST progenitor cells (Liodis et al., 2007; Zhao et al., 2008; Neves et al., 2012) in the medial ganglionic eminence as early as 7 weeks gestation in humans (Jakovcevski et al., 2011). A complete loss of Lhx6 at this critical developmental stage leads to deficits in neurodevelopmental signaling molecules, delayed migration, and impeded differentiation into PV and SST neurons (Liodis et al., 2007; Zhao et al., 2008; Neves et al., 2012). Similarly, other transcription factors such as Nkx2.1, Sox6, MafB, Zeb2/Sip1/Zfhx1b, and Dlx5/6 are also expressed early in gestation in the medial ganglionic eminence and regulate the migration, specification, and maturation of PV and/or SST, but not calretinin, neurons (Figure 2) (Sussel et al., 1999; Cobos et al., 2006; Du et al., 2008; Xu et al., 2008; Azim et al., 2009; Batista-Brito et al., 2009; Wang et al., 2010; van, V et al., 2013; McKinsey et al., 2013).
Figure 2. Figure illustrating some of the major roles of developmental regulators including ontogenetic transcriptional regulators, cytokine receptors, and other factors in various stages of development of cortical PV, SST, and calretinin neurons.
This figure is based on published reports that largely utilized single, complete loss of gene function murine models. This list of factors is not exhaustive, and many of the factors may play additional roles beyond those listed here. Since many gene mutations are lethal postnatally, knowledge of the potential role of many factors in postnatal maturation and survival is not known. Full gene name and associated references: Arx: aristaless related homeobox (Colombo et al., 2007); COUP-TFII: chicken ovalbumin upstream promoter-transcription factor II (Kanatani et al., 2008; Reinchisi et al., 2012); CXCR4 and CXCR7: chemokine (C-X-C motif) receptors 4 and 7 (Wang et al., 2011; Sanchez-Alcaniz et al., 2011; Meechan et al., 2012); Dlx1: Distal-less homeobox 1 (Cobos et al., 2005); Dlx5/6: Distal-less homeobox 5/6 (Wang et al., 2010); ErbB4: receptor tyrosine-protein kinase erbB-4 (Flames et al., 2004; Fazzari et al., 2010; Ting et al., 2011); Gsx2: genomic screened homeobox 2 (Fogarty et al., 2007); Lhx6: LIM homeodomain factor 6 (Liodis et al., 2007; Zhao et al., 2008; Neves et al., 2012); Nkx2.1: NK2 homebox 1 (Sussel et al., 1999; Xu et al., 2004; Butt et al., 2008; Nobrega-Pereira et al., 2008); Nkx6.2: NK6 homeobox 2 (Fogarty et al., 2007); SATB1: Special AT-rich DNA Binding Protein 1 (Denaxa et al., 2012); Sox6: SRY (sex determining region Y)-box 6 (Azim et al., 2009; Batista-Brito et al., 2009); Zeb2/Sip1/Zfhx1b: zinc finger E-box binding homeobox 2 (van, V et al., 2013; McKinsey et al., 2013).
Cytokine receptors and a diverse array of other molecules also play a crucial role in the ontogeny of cortical PV neurons (Figure 2). For example, the cytokine receptors CXCR4 and CXCR7 are heavily expressed in the medial ganglionic eminence and are required for successful tangential migration of PV neurons (Wang et al., 2011; Sanchez-Alcaniz et al., 2011; Meechan et al., 2012). Furthermore, ErbB4 is a receptor tyrosine kinase for the trophic factor neuregulin 1 that is involved in the migration of, and development of excitatory inputs to, PV neurons (Flames et al., 2004; Fazzari et al., 2010; Ting et al., 2011). In addition, homozygous deletion of urokinase plasminogen activator receptor (Powell et al., 2003), a key factor in hepatocyte growth factor activation, or fibroblast growth factor receptor 1 (Muller et al., 2008), results in disturbances in cortical PV (and SST neurons for fibroblast growth factor receptor 1) but not calretinin neurons.
Furthermore, other transcription factors and developmental regulators are involved in the maturation and survival of different classes of cortical GABA neurons (Figure 2). For example, Dlx1 was one of the earliest reported factors that regulates cortical GABA neuron development (Anderson et al., 1997). More recent evidence suggests that Dlx1 is not required for tangential migration, but is required for maintenance of GAD67 expression postnatally (Cobos et al., 2005). Furthermore, Dlx1 is expressed by SST neurons but not PV neurons postnatally, and accordingly homozygous deletion of Dlx1 results in a failure of SST but not PV neurons to survive the preadolescent period in mice (Cobos et al., 2005). In addition, nuclear matrix and genome organizer Special AT-rich DNA Binding Protein 1 (SATB1) begins to be expressed by most cortical PV and SST, but not by calretinin neurons (Denaxa et al., 2012), after tangential migration has completed. Homozygous knockout of SATB1 produces large reductions in mRNA and protein levels of SST (but not PV) without a reduction in cell density indicating a role in terminal differentiation and maturation of SST neurons (Denaxa et al., 2012). In contrast, the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) is initially expressed by PV neurons postnatally after migration is complete and is required for the development of the PV phenotype (Lucas et al., 2010).
In summary, normal expression of a broad range of molecules is required for successful early development of cortical GABA neurons (Figure 2). These findings raise the following question which we address next: Could altered prenatal expression of these ontogenetic regulators initiate a disease process that disrupts the normal developmental trajectory of cortical GABA neurons and produces the phenotype of GABA abnormalities seen in schizophrenia?
4. Lhx6 and other candidate neurodevelopmental regulators may be involved in PFC PV and SST neuron dysfunction in schizophrenia
While it is not feasible to directly study developmental processes in the prenatal brains of individuals who will eventually develop schizophrenia, many ontogenetic factors continue to be robustly expressed in a cell-type specific manner in PV and SST neurons in adult cortex (Cobos et al., 2006; Georgiev et al., 2012). Thus, knowledge of whether certain key candidate developmental regulators are abnormally expressed in adult schizophrenia brain may provide clues to earlier pathogenetic processes that lead to alterations selective for PV and/or SST neurons. For example, we recently found deficits in Lhx6 mRNA levels in the PFC in schizophrenia (Volk et al., 2012). Most Lhx6-containing neurons had lower Lhx6 mRNA levels, and Lhx6 mRNA deficits were found in the same cortical layers that contain most PV neurons (layers 3–4) (Hashimoto et al., 2003) and SST neurons (layers 2, 5, 6) (Morris et al., 2008), suggesting that PV and SST neurons expressed lower Lhx6 mRNA levels. However, Lhx6 mRNA levels did not differ in layer 1 where calretinin neurons are more common than PV or SST neurons. Lhx6 mRNA levels were also not related to substance abuse, psychotropic medications, or smoking in schizophrenia, suggesting that Lhx6 mRNA deficits reflect the disease process of schizophrenia and not factors that commonly accompany the illness.
Given the role of Lhx6 in the tangential migration, cell type specification, and maturation of cortical PV and SST neurons, the disease-related consequences of Lhx6 deficits may depend upon the developmental stage at which Lhx6 deficits first appear. For example, deficits in Lhx6 that occur at the earliest gestational periods in the medial ganglionic eminence may impair the migration of cortical GABA neurons. The findings of lower densities of Lhx6- and SST-containing neurons in the gray matter (Morris et al., 2008), and a higher density of SST-containing neurons in the white matter (Yang et al., 2011), in the PFC in schizophrenia are consistent with arrested migration of some SST neurons. Alternatively, lower levels of Lhx6 present after migration is complete might interfere with continued maturation of the GABAergic phenotype. In this case, the lower density of PFC Lhx6-containing neurons could also mean that some of these neurons successfully migrated but have undetectable levels of Lhx6 mRNA and an altered phenotype. Consistent with this interpretation, the density of PFC PV mRNA-containing neurons is not altered in schizophrenia, but many of these neurons have lower levels of PV and GAD67 mRNAs (Hashimoto et al., 2003).
However, since the timing of the emergence of Lhx6 deficits in the disorder cannot be directly assessed, we cannot rule out that deficits in Lhx6 first appear in late adolescence or early adulthood after cellular maturation has been completed. Consequently, other upstream etiological factors must also be considered. Interestingly, another ontogenetic transcription factor Nkx2.1 regulates Lhx6 levels prenatally and a loss of Nkx2.1 profoundly disrupts the development of PV and SST neurons but not calretinin neurons (Sussel et al., 1999; Du et al., 2008; Xu et al., 2008), suggesting that a loss of Nkx2.1 could be upstream to Lhx6 deficits in schizophrenia. However, while Nkx2.1 is strongly expressed prenatally in the medial ganglionic eminence, Nkx2.1 becomes undetectable in adult human brain (unpublished data) and thus cannot be directly studied in postmortem tissue from individuals with schizophrenia.
In addition, mRNA levels for Dlx1 have recently been reported to be reduced in orbital frontal cortex gray matter in schizophrenia (Joshi et al., 2012). Since Dlx1 plays a critical role in the postnatal maturation and survival of SST neurons (Cobos et al., 2005), reduced levels of Dlx1 may potentially contribute to deficits in SST mRNA levels and in the number of neurons that express detectable levels of SST mRNA in cortical gray matter in schizophrenia (Morris et al., 2008).
5. Cortical GABA neuron development is disrupted by disturbances in the maternal-fetal environment such as maternal immune activation
In utero environmental exposures may also play a role in the etiopathogenesis of cortical GABA neuron dysfunction in schizophrenia. For example, maternal exposure to parasitic (Brown et al., 2005) or viral (Brown et al., 2004a) infections during the first and second trimesters, the time period when cortical PV and SST neurons are born (Jakovcevski et al., 2011), and the induced immune response of elevated serum cytokine levels (Brown et al., 2004b) are associated with an increased risk of schizophrenia in offspring. Furthermore, the population attributable risk for schizophrenia due to maternal infection has been recently estimated to be ~30% (Brown and Derkits, 2010).
As described earlier, the cytokine receptors CXCR4 and CXCR7 are expressed prenatally by cortical PV neurons in the medial ganglionic eminence, and disrupted expression of these receptors or their ligand (CXCL12) adversely affects the development of cortical PV neurons (Wang et al., 2011; Sanchez-Alcaniz et al., 2011; Meechan et al., 2012). These data suggest that altered cytokine levels in fetal brain in response to maternal immune activation may disrupt the development of CXCR4/7-expressing PV neurons. Consistent with this hypothesis, animal models using the viral mimic poly I:C, a synthetic double stranded RNA that binds to and activates toll-like receptor 3, have shown that maternal immune activation alters levels of multiple cytokines and CXCR4/7 in fetal brain (Meyer et al., 2006; Oskvig et al., 2012). Furthermore, maternal immune activation induces long-lasting epigenetic changes in the promoter regions of genes which could also contribute to perturbations of gene expression postnatally (Tang et al., 2013). In addition, postmortem brain tissue studies have reported higher mRNA levels for markers of immune activation in schizophrenia (Arion et al., 2007; Saetre et al., 2007; Fillman et al., 2012) which may in part reflect an early immune challenge that may have acted earlier during brain development (Arion et al., 2007) though additional proof-of-principle testing is needed. Interestingly, the effects of poly I:C-induced maternal immune activation on offspring include lower PV immunoreactivity and deficits in GAD67 mRNA and protein levels in the PFC (Meyer et al., 2008; Richetto et al., 2013) and impaired spatial working memory (Meyer et al., 2008). Taken together, these data suggest that in some schizophrenia subjects, maternal immune activation disrupts cortical PV neuron ontogeny and leads to disturbances in PFC PV neurons (Hashimoto et al., 2003; Curley et al., 2011; Volk et al., 2012). Furthermore, the shared site of origin and developmental regulation of PV and SST neurons suggests that maternal immune activation may also have similar deleterious effects on SST neuron development.
6. Unanswered Questions and Topics for Future Research
In summary, evidence from postmortem brain tissue studies from schizophrenia subjects suggests that abnormalities in PFC GABA neurons are consistent with a prenatal pathogenetic origin linked to the medial ganglionic eminence. The critical role that ontogenetic transcription factors and other developmental regulators play in the birth, migration, specification, maturation, and survival of cortical PV and SST neurons suggests that altered expression of these factors in schizophrenia could contribute to GABA-related disturbances in the PFC. Consistent with this hypothesis, recent postmortem studies have found deficits in the cortical expression of some ontogenetic transcription factors such as Lhx6 and Dlx1 in schizophrenia. Finally, evidence from animal models suggests that perturbations of maternal-fetal environment such as maternal immune activation alter levels of cytokines, cytokine receptors, and developmental regulators in fetal brain that also disrupt the development of cortical PV and SST neurons. These findings suggest the following important areas for future study.
6.1 Postmortem studies of GABA-related markers
As described above, some in situ hybridization studies of postmortem brain tissue have reported evidence consistent with arrested tangential migration of SST neurons (Yang et al., 2011) and incomplete phenotypic specification of PV neurons (Hashimoto et al., 2003) in schizophrenia. However, cellular levels of analysis have also yielded some inconsistent results in regards to neuron density across subject cohorts such as higher (Yang et al., 2011) and lower (Morris et al., 2008) densities of SST neurons in the superficial white matter and no change (Woo et al., 1997; Hashimoto et al., 2003) or lower (Beasley and Reynolds, 1997; Beasley et al., 2002) densities of PV neurons in the gray matter in schizophrenia. One possible explanation for the discrepancy among these findings is that the pathogenetic mechanisms underlying disturbances in SST and PV neurons differ among individuals with schizophrenia. That is, distinct disease processes, such as those involving abnormal expression of certain developmental regulators (section 6.3), may interfere with different stages of GABA neuron ontogeny (i.e. birth, migration, specification, maturation, and/or survival) in different schizophrenia subjects. However, these distinct and individualized pathogenetic processes (i.e. impaired neuronal migration, incomplete phenotypic specification, failure to complete maturation, excessive apoptosis) may all produce the commonly observed molecular phenotype of deficits in SST and PV mRNA levels in gray matter homogenates that has been replicated across large schizophrenia cohorts (Mellios et al., 2009; Fung et al., 2010; Volk et al., 2012). Future studies involving detailed cellular level analysis of PV and SST neurons in large cohorts of schizophrenia subjects and also measures of key candidate developmental regulators may permit the identification of subgroups of subjects with distinctive disease processes that yield the commonly reported finding of deficits in gray matter SST and PV mRNA levels (section 6.3).
6.2 Animal models that disrupt cortical GABA neuron ontogeny
While knowledge of the role of ontogenetic transcription factors, cytokine receptors, and other developmental regulators in the birth, migration, specification, maturation and survival of cortical GABA neurons has increased exponentially over the past decade, applying this knowledge to hypotheses of the disease process of schizophrenia requires addressing additional questions. First, are the effects of a loss of gene function on cortical GABA neuron development dose-dependent? For example, most studies of factors that regulate GABA neuron development utilize animal models with a complete loss of gene function. However, schizophrenia is not a genetic disorder of homozygous null mutations. Indeed, evidence from postmortem human brain tissue studies have reported partial reductions in the levels of ontogenetic transcription factors such as Lhx6 (Volk et al., 2012) and Dlx1 (Joshi et al., 2012). Consequently, animal models that employ a partial loss of gene function such as heterozygous null mutation mice are needed to determine whether smaller losses of ontogenetic transcription factors are sufficient to produce disturbances in cortical PV and SST neurons similar to those seen in schizophrenia.
Second, what is the combined effect of smaller deficits in multiple ontogenetic transcription factors on cortical GABA neuron development? For example, a reduction in expression of both Dlx1 and Lhx6 in the same individuals may be predicted to have an additive effect that could impact multiple stages of migration, phenotypic specification, maturation and even survival of PFC PV and SST neurons in schizophrenia. However, at present time, mRNA levels of Dlx1 and Lhx6 have not been studied by quantitative PCR or in situ hybridization in the same cohort of schizophrenia subjects, and studies of the ontogeny of cortical GABA neurons have not included partial manipulations of more than one transcription factor. Still, reductions in Dlx1 and Lhx6, while not affecting each other directly (Cobos et al., 2005; Zhao et al., 2008), have multiple downstream effects on other transcription factors (Zhao et al., 2008; McKinsey et al., 2013), which could create a deleterious cascade of transcriptional regulator deficiencies with diverse disrupting effects on cortical GABA neuron development. Animal models that employ smaller deficits in multiple transcription factors found to be deficient in schizophrenia would represent an important advance in modeling potential pathogenetic processes in the disorder.
Third, are the relevant developmental processes conserved across species? The vast majority of studies of cortical GABA neuron ontogeny employ mouse models. However, significant differences exist in the composition and phenotypic properties of cortical GABA neuron subpopulations between rodent and primate brain (Conde et al., 1994; Gabbott and Bacon, 1996; Povysheva et al., 2008). Furthermore, while the vast majority of cortical GABA neurons are derived from the ganglionic eminence in mouse, a substantial number of cortical GABA neurons, likely calretinin, have been reported to derive from the subventricular zone of the pallium in humans (Letinic et al., 2002; Zecevic et al., 2005; Fertuzinhos et al., 2009; Zecevic et al., 2011; Jakovcevski et al., 2011). Thus, additional studies in embryonic human brain are needed to determine the applicability of mouse-related findings to human brain.
6.3 Altered expression of ontogenetic transcription factors in schizophrenia
While Lhx6 and Dlx1 have recently been studied in schizophrenia (Volk et al., 2012; Joshi et al., 2012), further investigation into the expression levels of key candidate developmental regulators in schizophrenia may provide additional insight into potential mechanisms that could disrupt cortical GABA neuron development at different stages of the disorder. In particular, knowledge of the status of developmental regulators in the same schizophrenia subjects for whom we have knowledge of the nature of disturbances in PFC PV and SST neurons (section 6.1) may provide insight into individualized pathogenetic processes. For example, Sip1 is expressed early in the medial ganglionic eminence and is necessary for tangential migration of cortical PV and SST neurons (van, V et al., 2013; McKinsey et al., 2013). Thus, schizophrenia subjects with deficits in Sip1 would be predicted to have fewer PFC PV and SST neurons. In addition, PGC1α is critical for mature levels of PV mRNA expression, but not migration of PV neurons (Lucas et al., 2010). PGC1α also continues to be expressed in adult brain. Thus, inadequate levels of PGC1α during development in schizophrenia would be predicted to not affect the migration of PV neurons but instead to interfere with the development of the GABAergic phenotype of PV neurons, as has been reported in schizophrenia (Hashimoto et al., 2003). In contrast, SATB1 plays a critical role in the terminal differentiation and maturation, but not migration, of cortical SST neurons (Denaxa et al., 2012). Thus, schizophrenia subjects with deficits in SATB1 would be predicted to have normal numbers of SST-containing neurons but less SST per neuron. Finally, since calretinin mRNA levels appear to be largely unaffected, or even slightly higher (Volk et al., 2012), in schizophrenia, one may predict that developmental regulators that are not specific to PV and SST neurons and that are also involved in the development of calretinin neurons (Figure 2) (e.g., Arx (Colombo et al., 2007; Colasante et al., 2008), COUP-TF II (Kanatani et al., 2008; Reinchisi et al., 2012), Gsx2 (Fogarty et al., 2007), Nkx6.2 (Fogarty et al., 2007)) will not be altered in schizophrenia. Thus, while basic neuroscience studies continue to elucidate the role of important neurodevelopment regulators in the ontogeny of GABA neurons, additional postmortem schizophrenia brain tissue studies are needed to characterize and correlate any deficits (or potential compensatory upregulation) of these markers to alterations in SST and PV neurons in the same schizophrenia subjects.
6.4 Applying models of maternal immune activation to schizophrenia
What stage of cortical GABA neuron development is most susceptible to maternal immune activation? One study recently reported that maternal immune activation at a late gestational stage in mice (gestation day 17) leads to deficits in PFC GAD67 mRNA levels (Richetto et al., 2013) similar to that seen in schizophrenia (Curley et al., 2011). However, are certain stages of cortical GABA neuron development such as birth or migration more susceptible to maternal immune activation? Identifying the most susceptible gestational period for maternal immune activation may inform preventative strategies involving maternal prenatal care to reduce infection rates and potentially reduce risks for developing schizophrenia in at-risk offspring. Furthermore, additional studies are needed to determine the extent to which maternal immune activation can reproduce the pattern of abnormalities in PFC PV and SST neurons reported in schizophrenia (section 2). Finally, the extent to which maternal immune activation alone is sufficient to replicate disease-related pathology in PFC GABA neurons in schizophrenia is unclear; some evidence suggests that maternal immune activation interacts with other insults, such as peripubertal stress (Giovanoli et al., 2013) or preexisting genetic abnormalities, to produce more severe disease pathology. For example, the combination of Lhx6 deficits and maternal immune activation may interact to severely disrupt PV and SST neuron development. In fetal brain, loss of Lhx6 induces deficits in CXCR4/7 (Zhao et al., 2008), and maternal immune activation lowers Lhx6 levels (Oskvig et al., 2012). Consequently, disturbances in PFC PV and SST neurons in schizophrenia may reflect the long-lasting consequences of an interaction in prenatal insults that are fetal (i.e. deficits in developmental regulators such as Lhx6) and/or maternal (i.e. immune activation) in origin.
6.5 Summary
In summary, a cross-species translational approach is required to investigate the potential interaction between genetic and gestational environmental insults that could initiate a disease process that disrupts cortical GABA neuron ontogeny and produces the pattern of GABA neuron abnormalities, and consequently cognitive difficulties, seen in schizophrenia. Such studies may provide a biological basis for preventative strategies that target the prenatal period, such as maternal prenatal care to reduce infection rates, and potentially reduce risks for developing schizophrenia in at-risk offspring.
Highlights.
Cortical GABA neuron abnormalities in schizophrenia may have a prenatal origin.
Transcription regulators and other developmental factors govern GABA neuron ontogeny.
Deficits in ontogenetic transcription factors have been reported in schizophrenia.
Maternal immune activation may be involved in GABA neuron deficits in schizophrenia.
Acknowledgments
Dr. Lewis currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and serves as a consultant in the areas of target identification and validation and new compound development to Bristol-Myers Squibb and Concert Pharmaceuticals.
Grant Support: Supported by NIH grants MH-084016 (Dr. Volk) and MH-043784 and MH-084053 (Dr. Lewis) and the Hamilton Family Award for Basic Neuroscience Research in Psychiatry (Dr. Volk).
Footnotes
Disclosures:
Dr. Volk has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Bunney WE, Jr, Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry. 1993;50:169–177. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Anderson S, Volk DW, Lewis DA. Increased density of microtubule-associated protein 2-immunoreactive neurons in the prefrontal white matter of schizophrenic subjects. Schizophr Res. 1996;19:111–119. doi: 10.1016/0920-9964(96)88521-5. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JLR. Interneuron migration from basal forebrain to neocortex: Dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Arion D, Unger T, Lewis DA, Levitt P, Mirnics K. Molecular evidence for increased expression of genes related to immune and chaperone function in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2007;62:711–721. doi: 10.1016/j.biopsych.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jabaudon D, Fame RM, Macklis JD. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12:1238–1247. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63:466–481. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Lewis DA. Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry. Int J Dev Neurosci. 2011;29:295–304. doi: 10.1016/j.ijdevneu.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004a;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004b;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MC, Tanskanen A, Huttunen M, Leon DA, Murray RM, Jones PB, Cannon M. Increased risk of schizophrenia from additive interaction between infant motor developmental delay and obstetric complications: evidence from a population-based longitudinal study. Am J Psychiatry. 2011;168:1295–1302. doi: 10.1176/appi.ajp.2011.11010011. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Cobos I, Long JE, Thwin MT, Rubenstein JL. Cellular patterns of transcription factor expression in developing cortical interneurons. Cereb Cortex. 2006;16(Suppl 1):i82–i88. doi: 10.1093/cercor/bhk003. [DOI] [PubMed] [Google Scholar]
- Colasante G, Collombat P, Raimondi V, Bonanomi D, Ferrai C, Maira M, Yoshikawa K, Mansouri A, Valtorta F, Rubenstein JL, Broccoli V. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J Neurosci. 2008;28:10674–10686. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, Collombat P, Colasante G, Bianchi M, Long J, Mansouri A, Rubenstein JL, Broccoli V. Inactivation of Arx, the murine ortholog of the X-linked lissencephaly with ambiguous genitalia gene, leads to severe disorganization of the ventral telencephalon with impaired neuronal migration and differentiation. J Neurosci. 2007;27:4786–4798. doi: 10.1523/JNEUROSCI.0417-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaxa M, Kalaitzidou M, Garefalaki A, Achimastou A, Lasrado R, Maes T, Pachnis V. Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Rep. 2012;2:1351–1362. doi: 10.1016/j.celrep.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon WC. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatry Res. 2010;44:673–681. doi: 10.1016/j.jpsychires.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neuron density in the dorsolateral prefrontal cortex and parahippocampal gyrus in schizophrenia. Schizophr Res. 2005;79:181–188. doi: 10.1016/j.schres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex. 2009;19:2196–2207. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Flames N, Long JE, Garratt AN, Fischer TM, Gassmann M, Birchmeier C, Lai C, Rubenstein JL, Marin O. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Gabbott PLA, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a, b, c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–636. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Georgiev D, Gonzalez-Burgos G, Kikuchi M, Minabe Y, Lewis DA, Hashimoto T. Selective Expression of KCNS3 Potassium Channel alpha-Subunit in Parvalbumin-Containing GABA Neurons in the Human Prefrontal Cortex. PLoS One. 2012;7:e43904. doi: 10.1371/journal.pone.0043904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Schedlowski M, Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(Suppl 9):3–8. [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull. 2011;37:493–503. doi: 10.1093/schbul/sbr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Mayer N, Zecevic N. Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb Cortex. 2011;21:1771–1782. doi: 10.1093/cercor/bhq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Joshi D, Fung SJ, Rothwell A, Weickert CS. Higher gamma-aminobutyric Acid neuron density in the white matter of orbital frontal cortex in schizophrenia. Biol Psychiatry. 2012;72:725–733. doi: 10.1016/j.biopsych.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Kanatani S, Yozu M, Tabata H, Nakajima K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci. 2008;28:13582–13591. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Davis CE, Hsiao JK, Lieberman JA. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007a;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33:912–920. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Sweeney JA, Gu H, Hamer RM, Perkins DO, McEvoy JP, Lieberman JA. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007b;164:1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias NC, Conley RR, Roberts RC. Interstitial cells of the white matter in the dorsolateral prefrontal cortex in deficit and nondeficit schizophrenia. J Nerv Ment Dis. 2003;191:563–567. doi: 10.1097/01.nmd.0000087181.61164.e1. [DOI] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharm. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EK, Markwardt SJ, Gupta S, Meador-Woodruff JH, Lin JD, Overstreet-Wadiche L, Cowell RM. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1alpha. J Neurosci. 2010;30:7227–7235. doi: 10.1523/JNEUROSCI.0698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey GL, Lindtner S, Trzcinski B, Visel A, Pennacchio LA, Huylebroeck D, Higashi Y, Rubenstein JL. Dlx1&2-dependent expression of Zfhx1b (Sip1, Zeb2) regulates the fate switch between cortical and striatal interneurons. Neuron. 2013;77:83–98. doi: 10.1016/j.neuron.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Cxcr4 regulation of interneuron migration is disrupted in 22q11.2 deletion syndrome. Proc Natl Acad Sci U S A. 2012;109:18601–18606. doi: 10.1073/pnas.1211507109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharm. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller SK, Fagel DM, Stevens HE, Rabenstein RL, Maragnoli ME, Ohkubo Y, Picciotto MR, Schwartz ML, Vaccarino FM. Deficiency in inhibitory cortical interneurons associates with hyperactivity in fibroblast growth factor receptor 1 mutant mice. Biol Psychiatry. 2008;63:953–962. doi: 10.1016/j.biopsych.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Neves G, Shah MM, Liodis P, Achimastou A, Denaxa M, Roalfe G, Sesay A, Walker MC, Pachnis V. The LIM Homeodomain Protein Lhx6 Regulates Maturation of Interneurons and Network Excitability in the Mammalian Cortex. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs159. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega-Pereira S, Kessaris N, Du T, Kimura S, Anderson SA, Marin O. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–745. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26:623–634. doi: 10.1016/j.bbi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Rotaru DC, Gonzalez-Burgos G, Lewis DA, Krimer LS. Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J Neurophysiol. 2008;100:2348–2360. doi: 10.1152/jn.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinchisi G, Ijichi K, Glidden N, Jakovcevski I, Zecevic N. COUP-TFII expressing interneurons in human fetal forebrain. Cereb Cortex. 2012;22:2820–2830. doi: 10.1093/cercor/bhr359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetto J, Calabrese F, Riva MA, Meyer U. Prenatal Immune Activation Induces Maturation-Dependent Alterations in the Prefrontal GABAergic Transcriptome. Schizophr Bull. 2013 doi: 10.1093/schbul/sbs195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridler K, Veijola JM, Tanskanen P, Miettunen J, Chitnis X, Suckling J, Murray GK, Haapea M, Jones PB, Isohanni MK, Bullmore ET. Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:15651–15656. doi: 10.1073/pnas.0602639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetre P, Emilsson L, Axelsson E, Kreuger J, Lindholm E, Jazin E. Inflammation-related genes up-regulated in schizophrenia brains. BMC Psychiatry. 2007;7:46. doi: 10.1186/1471-244X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alcaniz JA, Haege S, Mueller W, Pla R, Mackay F, Schulz S, Lopez-Bendito G, Stumm R, Marin O. Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron. 2011;69:77–90. doi: 10.1016/j.neuron.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Sohal VS. Insights into cortical oscillations arising from optogenetic studies. Biol Psychiatry. 2012;71:1039–1045. doi: 10.1016/j.biopsych.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen HJ, Mortensen EL, Schiffman J, Reinisch JM, Maeda J, Mednick SA. Early developmental milestones and risk of schizophrenia: a 45-year follow-up of the Copenhagen Perinatal Cohort. Schizophr Res. 2010;118:41–47. doi: 10.1016/j.schres.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, Vakkalanka RK, Kolachana BS, Kleinman JE, Weinberger DR. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Tang B, Jia H, Kast RJ, Thomas EA. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav Immun. 2013;30:168–175. doi: 10.1016/j.bbi.2013.01.086. [DOI] [PubMed] [Google Scholar]
- Ting AK, Chen Y, Wen L, Yin DM, Shen C, Tao Y, Liu X, Xiong WC, Mei L. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31:15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van dB V, Stappers E, Vandesande B, Dimidschstein J, Kroes R, Francis A, Conidi A, Lesage F, Dries R, Cazzola S, Berx G, Kessaris N, Vanderhaeghen P, van Ijcken W, Grosveld FG, Goossens S, Haigh JJ, Fishell G, Goffinet A, Aerts S, Huylebroeck D, Seuntjens E. Directed migration of cortical interneurons depends on the cell-autonomous action of Sip1. Neuron. 2013;77:70–82. doi: 10.1016/j.neuron.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, Freed WJ. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: A preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, Sampson A, Hashimoto T, Lewis DA. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. doi: 10.1176/appi.ajp.2012.12030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dye CA, Sohal V, Long JE, Estrada RC, Roztocil T, Lufkin T, Deisseroth K, Baraban SC, Rubenstein JL. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J Neurosci. 2010;30:5334–5345. doi: 10.1523/JNEUROSCI.5963-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li G, Stanco A, Long JE, Crawford D, Potter GB, Pleasure SJ, Behrens T, Rubenstein JL. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. 2011;69:61–76. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Faulkner HJ, Jefferys JG, Chettiar K. Morphine disrupts long-range synchrony of gamma oscillations in hippocampal slices. Proc Natl Acad Sci U S A. 1998;95:5807–5811. doi: 10.1073/pnas.95.10.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo T-U, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La CE, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fung SJ, Rothwell A, Tianmei S, Weickert CS. Increased interstitial white matter neuron density in the dorsolateral prefrontal cortex of people with schizophrenia. Biol Psychiatry. 2011;69:63–70. doi: 10.1016/j.biopsych.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Hu F, Jakovcevski I. Interneurons in the developing human neocortex. Dev Neurobiol. 2011;71:18–33. doi: 10.1002/dneu.20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Flandin P, Long JE, Cuesta MD, Westphal H, Rubenstein JL. Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. J Comp Neurol. 2008;510:79–99. doi: 10.1002/cne.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]