Abstract
The synthesis of a pilot scale library of 116 structurally diverse γ-lactams is reported. The library core structure emanates from a γ-lactam forming one-pot, four-component reaction of ammonium acetate, p-methoxythiophenol, p-methoxybenzaldehyde and maleic anhydride. Structural diversity then arises from amide coupling, thioaryl cleavage, N-functionalization and heterocycle forming reactions on this core structure. Computational analysis reveals that the library contains molecular properties and shape diversity suitable for drug lead and biological probe discovery.
Introduction
γ-Lactams are important structures for the synthesis of natural products and biological probes for drug discovery and development (Figure 1).1–9 The prevalence of γ-lactams in biologically significant molecules has resulted in the development of many syntheses of this substructure, and has led to the production of diverse libraries of small molecules for biological evaluation.10–18 It is likely that the use of this substructure in the prospective design of “lead-like” small molecules will be fruitful in the discovery of biological probes and drug leads.
Figure 1.
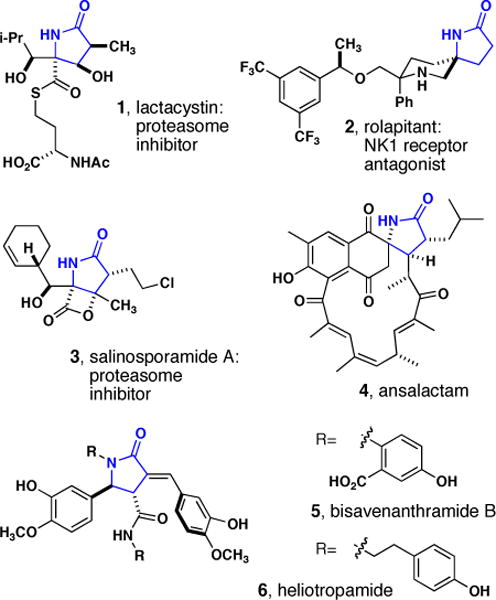
γ-Lactam natural products and lead compounds in drug discovery.
Multi component reactions (MCRs) are powerful transformations that involve the combination of three or more reagents in a one-pot procedure to rapidly generate molecular complexity with minimal effort.19 Our group reported a novel four-component reaction (4CR) for the synthesis of complex γ-lactams where in a single operation γ-lactams are synthesized in high yield and diastereoselectivity from the combination of an amine 7, an aldehyde 8, thiol 9 and maleic anhydride 10 (Scheme 1A).12 More recently, we reported a one-pot procedure for the multicomponent assembly of NH γ-lactams 13, from a 4CR with ammonium acetate 12, and subsequent N-functionalization of the amide nitrogen to generate structures 14 not immediately available for the original 4CR (Scheme 1B).13N-acylation was achieved using n-BuLi as a base followed by addition of an acylating agent, and N-arylation was accomplished with an arylboronic acid and stoichiometric amounts of copper(II) acetate (Scheme 1B). This study demonstrated that we could rapidly access complex γ-lactam structures not immediately available from the original 4CR. In our present study we demonstrate the utility of this methodology toward library development by preparing a pilot scale library of 116 structurally diverse γ-lactams for use in high-throughput screening experiments aimed at discovering drug leads and biological probes.
Scheme 1.
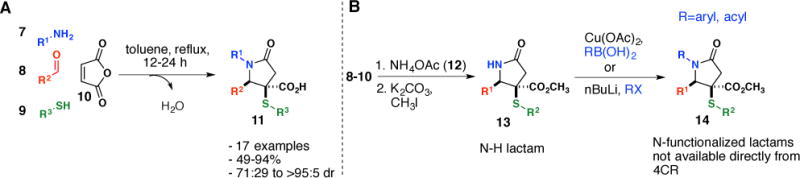
Assembly of (A)N-substituted and (B) N-H γ-lactams using a 4CR.
We envisioned our library would be based on γ-lactam core structure 15, which emanates from a 4CR with ammonium acetate 12, maleic anhydride 10, p-methoxythiophenol 16 and p-methoxybenzaldehyde 17 (Figure 2A). Reaction of 15 with primary or secondary amines generated 15 diverse NH γ-lactam products 18 (Figure 2B). Next, a subset of these N-H γ-lactam were N-acylated using n-BuLi as a base followed by addition of an acylating reagent or N-arylated using arylboronic acids and copper(II) acetate to generate 69 γ-lactams 19 and 20 (Figure 2B). The thioaryl group of some N-H γ-lactams 18 was also cleaved and products were N-acylated to generate 21 new compounds 21 and 22 (Figure 2C). Finally, we generated 1,2,4-oxadiazoles 23 from the carboxylic acid handle of 15, and subsequent N-functionalization reactions generated 11 additional structures 24 and 25 (Figure 2D). Importantly, syntheses of library chemsets 18–22, 24 and 25 were conducted in parallel using a Heidolph reaction block, thus allowing for rapid and efficient generation of molecular complexity.
Figure 2.
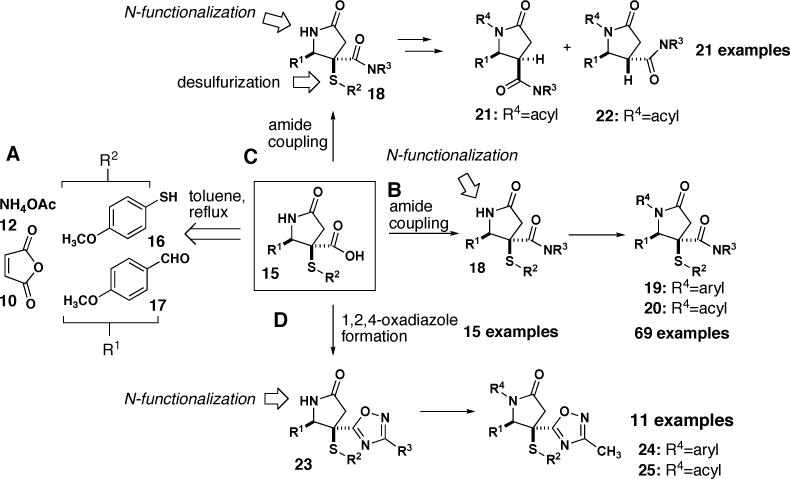
Summary of Library Strategy Based on the One-Pot, Four-Component Reaction (4CR).
Results and Discussion
Library Synthesis
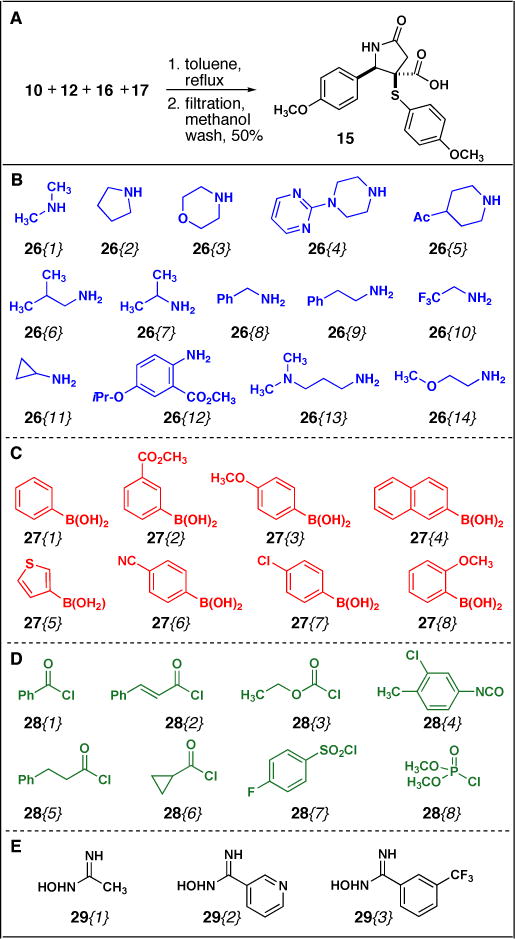
Multi-gram scale preparation of 15 was achieved from a 4CR with ammonium acetate 12, maleic anhydride 10, p-methoxythiophenol 16 and p-methoxybenzaldehyde 17 (Figure 3A). Filtration of the crude reaction mixture followed by washes with cold methanol provided 5 grams of 15, 50% yield, as a single diastereomer which was then used without additional purification (Scheme 2A). Library diversity based on 15 was then generated from amide-forming reactions with amines 26 (Figure 3B), N-functionalization reactions with arylboronic acids 27 and aclyating agents 28 (Figure 3B and C), and hetereocycle forming reactions with oximes 29 (Figure 3D).
Figure 3.
Scheme 2.
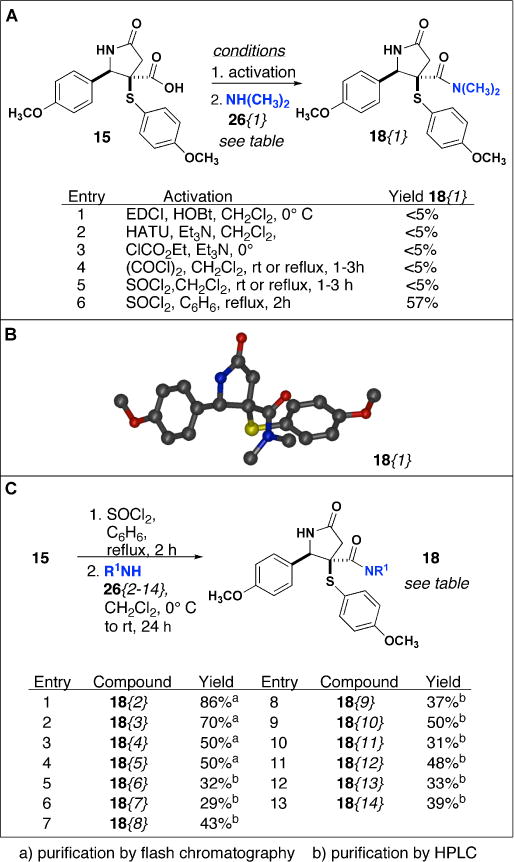
(A) Optimization of amide coupling conditions with 26{1}(B) crystal structure of 18{1}, and (C) synthesis of 18{2–14}.
Conditions for amide formation were optimized with 15 and N,N-dimethylamine 26{1} under a variety of reaction conditions to synthesize amide 18{1} (Scheme 2A). Initial attempts to form amide 18{1} were made using peptide coupling reagents. Treatment of 15 with EDCI and HOBt, or HATU in the presence of N,N-dimethylamine 26{1} were unsuccessful and starting material was isolated in all cases (Scheme 2A, entries 1–2). Similarly, amide synthesis via formation of the mixed anhydride by treatment of 15 with ethyl chloroformate followed by addition of 26{1} gave similar results (Scheme 2A, entry 3). We next attempted amide formation through conversion to the acid chloride. Treatment of 15 with oxalyl chloride or thionyl chloride in DCM under a variety of mild reaction conditions failed to produce any amide product 18{1} (Scheme 2A, entries 4–5). 13CNMR and 1H NMR spectra of the presumed acid chloride intermediate revealed acid chloride was not forming under these conditions and thus lead us to investigate harsher reaction conditions for acid chloride synthesis. We ultimately found refluxing 15 in benzene and thionyl chloride initially for 8 hours, and then optimized to 2 hours, provided the requisite acid chloride, as confirmed by 13CNMR and 1H NMR spectroscopy (data not shown). Subsequent addition of N,N-dimethylamine 26{1} in a second step gave the desired amide product 18{1} in 57% yield (Scheme 2A, entry 6), and crystallization of 18{1} confirmed the relative stereochemistry of 15 (Scheme 2B). Finally, using these optimized conditions, we were able to synthesize NH γ-lactams 18{2–14} in 29–86% yields (Scheme 2C).
Next, treatment of NH amides 18{1–4} with arylboronic acids 27{1–8} and stoichiometric amounts of copper(II) acetate gave amides 19{1–4, 1–8} in 4.5–69% yield (Scheme 3). As we observed previously,13 ortho-substituted arylboronic acids were not very reactive, and in the case of o-methoxyphenylboronic acid 27{8} we observed slight reactivity with amide 18{2} to yield lactam 19{2, 8}. Then, reaction of NH amides 18{1–5} with n-BuLi at −78° C followed by addition of various acylating agents 28{1–8} provided lactams 20{1–5, 1–8} in 12–90% yield (Scheme 4). Acid chlorides tended to provide the best yields while sulfonylation and phosphonylation worked less well. In most cases, yields for arylation and acylation reactions were determined based on material recovered after purification by HPLC. In general, products were recovered in higher yield from flash chromatography than they were after purification by HPLC.
Scheme 3.
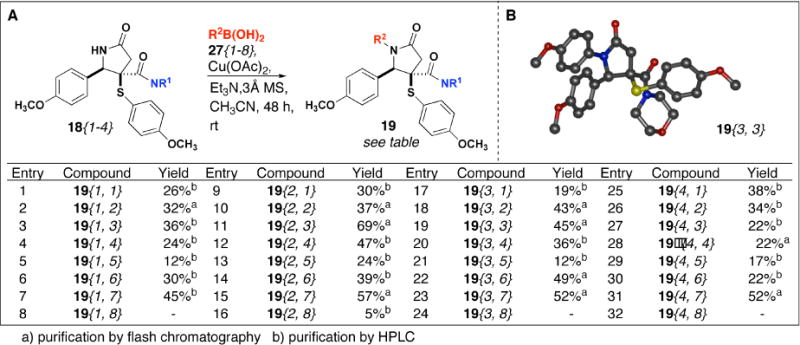
(A)N-arylation of N-H amides 18{1–4} and (B) crystal structure of 19{3,3}.
Scheme 4.
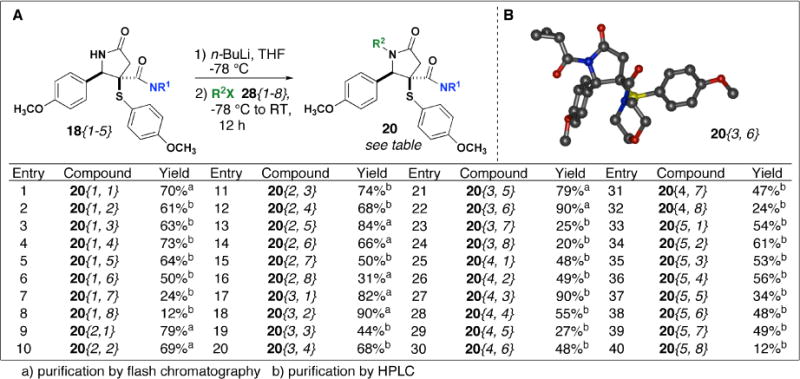
(A)N-acylation of N-H amides 18{1–5} and (B) crystal structure of 20{3,6}.
We next aimed to access N-functionalized compounds 21 and 22 through a concise synthetic route requiring only a single N-acylation reaction to provide both syn 21 and anti 22 products (Scheme 5A). We initially envisioned generating 21 and 22 beginning with a stereoselective desulfurization reaction to cleave the thioaryl group of NH γ-lactams 18{2–3} (Scheme 5A). Next, N-functionalization of 30 would provide syn21, which could then be epimerized to give anti products 22. Initial attempts to cleave the thioaryl group of 18{2–3} using free radical conditions with tristrimethylsilyl silane12 or tributyltinhydride20 were unsuccessful and in both cases a mixture of undesired products were observed (data not shown). Next, we envisioned a similarly concise and efficient route in which thioaryl group cleavage of 18{2–3} with Raney Nickel could be used to provide 31 as a 50:50 mixture of syn and anti diastereomers which could then be N-functionalized as a mixture to provide 21 and 22. Reaction of 18{2} with Raney Nickel12 worked well to a give a 50:50 mixture of syn and anti 31{2}, however, desulfurization of 18{3} provided 31{3} as 10:90 mixture of diastereomers (Scheme 5B). We obtained an X-ray crystal structure of the syn diastereomer 30{3} which allowed us to identify the syn and anti products resulting from N-acylation of 31 (Scheme 5C). 31{2–3} were acylated as a mixture of diastereomers using n-BuLi and products were isolated by flash chromatography or HPLC (Scheme 5D). Syn products 21{3, 1–7} were detectable in the 1HNMR spectra resulting from acylation of 31{3}, yet, we were only able to isolate anti products 22{3, 1–7} in 17–56% yield (Scheme 5D). Both syn 21{2, 1–8} and anti 22{2, 1–8} products resulting from acylation of 31{2} were isolable by flash chromatography or HPLC in 10–27% yield (Scheme 5D).
Scheme 5.
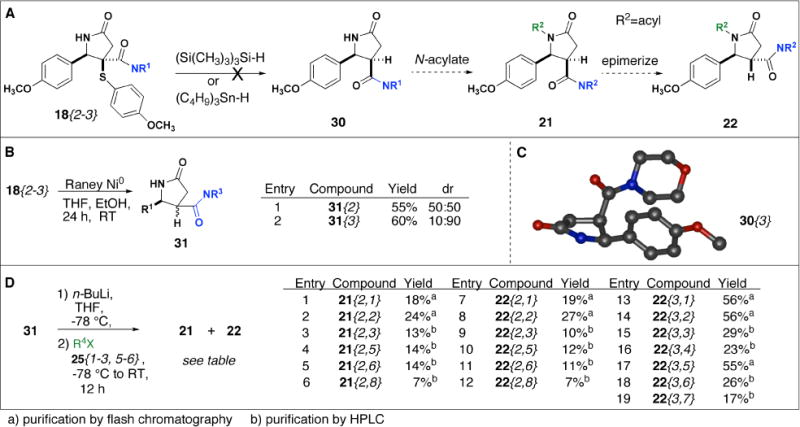
Desulfurization reactions
Finally, we synthesized 1,2,4-oxadiazoles from the carboxylic acid handle of 15 (Scheme 6). We initially attempted a single pot procedure in the microwave with 15, oxime 29{1}, trichloroacetonitrile and polystyrene bound triphenylphosphine,21 however, did not observe formation of product 23{1} (data not shown). In a two-step procedure, 15 was first converted to the acid chloride with thionyl chloride and treated with oximes 29{1–3}. Subsequent heating in refluxing toluene for one and a half to six hours provided the desired NH γ-lactams 23 in 15–40% yield over two steps (Scheme 6A).22 Next, N-functionalization of 23{1} provided the desired products 24 and 25 in 20 to 58% yield (Scheme 6B).
Scheme 6.
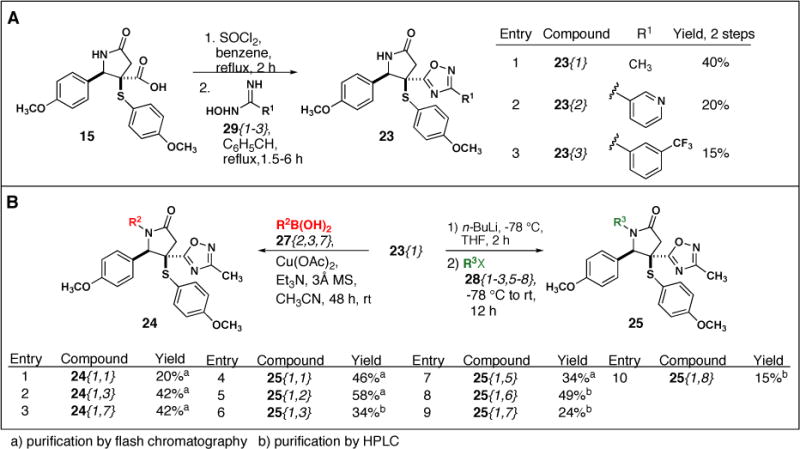
(A) Synthesis of 1,2,4-oxadiazoles 23 and (B)N-functionalization of 23{1}.
Computational Analysis of Molecular Properties and Shape Diversity
Computational analysis of molecular properties was calculated for this collection of γ-lactams (Figure 4) (see Supporting Information) and average property values are displayed in Table 1. Analysis of molecular properties indicates some compounds may have desirable qualities for drug lead discovery, while others could function more suitably as biological probes (Figure 4 and 5).23–25 The average molecular weights of 18, 21 and 22 fall within the acceptable range (molecular weight less than 500 daltons) for drug-like compounds,23 while higher average molecular weight structures 19, 20, 24 and 25 that also lack hydrogen bond donors may be suitable as biological probes for disrupting protein-protein interactions.24 Analysis of library molecular shape diversity using the method of Sauer and Schwarz was also performed. 25 This method calculates and plots principal moments of inertia for each library member and characterizes molecular shape as rod- (e.g. acetylene), sphere- (e.g. adamantane) or disc-like (e.g. benzene). Greater shape diversity of a library correlates with increased likelihood of the library containing bioactive molecules. Due to the propensity for molecules to bind to a biological target in many possible conformations, principle moments of inertia were calculated and plotted for all conformers ≤3 kcal/mol in energy from the minimum energy conformer. The shape diversity of our library indicates an increase in the odds of a compound binding to a biological target.
Figure 4.
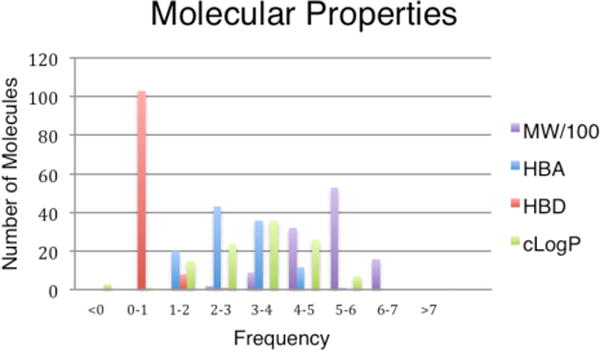
Molecular properties of library
Table 1.
Average molecular property values
| chemset | |||||
|---|---|---|---|---|---|
| property | 18 (n=18) |
19 (n=29) |
20 (n=40) |
21 + 22 (n=15) |
24 + 25 (n=10) |
| MW | 437 | 552 | 572 | 395 | 518 |
| HBD | 2 | 0 | 0 | 0 | 0 |
| HBA | 3 | 3 | 4 | 3 | 4 |
| cLogP | 3 | 4 | 3 | 2 | 5 |
Figure 5.
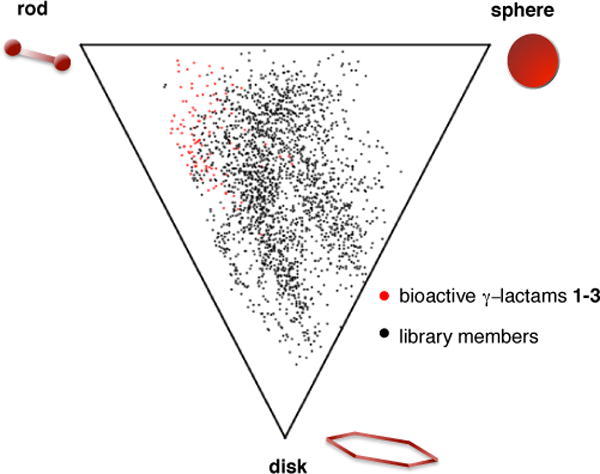
Scatter plot with principle moments of inertia (PMI) ratios plotted to compare molecular shape diversity of γ-lactam library. Ratios were calculated for all conformers ≤3 kcal/mol from the minimum energy conformer. Bioactive γ-lactams 1–3 are in red and library members are in black.
Conclusion
The diversity-oriented synthesis of a “pilot scale” library of complex γ-lactams has been achieved. Large scale preparation of library core structure 15 and use of a reaction block for parallel synthesis allowed for library production with minimal effort. Computational analysis indicates molecular properties suitable for drug lead and biological probe discovery.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (CAREER award to JTS) and the National Institutes of Health (NIGMS/P41GM089153). JCF acknowledges support from the NSF (CHE-0840444) for the purchase of the Bruker SMART DUO diffractometer used for X-ray crystallography. The authors thank Patrick Porubsky (University of Kansas) for conducting mass-directed purification of all final library members at the Center for Methodology and Library Development (KU-CMLD), which is funded by the NIH (NIGMS/P50GM069663). Phillip P. Painter (Tantillo research group, UC Davis) is acknowledged for calculating the molecular properties and principle moments of inertia othe library using OpenEye software (www.eyesopen.com).
Footnotes
Detailed experimental procedures, full characterization data, and purification details for all new compounds, representative library members and .cif files for compounds 18{1}, 19{3,3}, 20{3,6}, and 30{3} are reported. This material is available free of charge via the Internet at http://pubs.acs.org. In addition, all library members have been submitted to the National Small Molecule Repository where they will be made available for high-throughput screening.
References
- 1.Manam RR, Teisan S, White DJ, Nicholson B, Grodberg J, Neuteboom STC, Lam KS, Mosca DA, Lloyd GK, Potts BCM. Lajollamycin, a nitro-tetraene spiro-β-lactone-γ-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J Nat Prod. 2005;68:240–243. doi: 10.1021/np049725x. [DOI] [PubMed] [Google Scholar]
- 2.Mori T, Takahashi K, Kashiwabara M, Uemura D, Katayama C, Iwadare S, Shizuri Y, Mitomo R, Nakano F, Matsuzaki A. Structure of oxazolomycin, a novel β-lactone antibiotic. Tetrahedron Lett. 1985;26:1073–6. [Google Scholar]
- 3.Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed. 2003;42:355–357. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]
- 4.Fenteany G, Standaert RF, Lane WS, Choi S, Corey EJ, Schreiber SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science (Washington, D C) 1995;268:726–31. doi: 10.1126/science.7732382. [DOI] [PubMed] [Google Scholar]
- 5.Guntern A, Ioset JR, Queiroz EF, Sandor P, Foggin CM, Hostettmann K. Heliotropamide, a Novel Oxopyrrolidine-3-carboxamide from Heliotropium ovalifolium. J Nat Prod. 2003;66:1550–1553. doi: 10.1021/np0302495. [DOI] [PubMed] [Google Scholar]
- 6.Okazaki Y, Ishizuka A, Ishihara A, Nishioka T, Iwamura H. New Dimeric Compounds of Avenanthramide Phytoalexin in Oats. J Org Chem. 2007;72:3830–3839. doi: 10.1021/jo0701740. [DOI] [PubMed] [Google Scholar]
- 7.Wilson MC, Nam S-J, Gulder TAM, Kauffman CA, Jensen PR, Fenical W, Moore BS. Structure and biosynthesis of the marine streptomycete ansamycin ansalactam A and its distinctive branched chain polyketide extender unit. J Am Chem Soc. 2011;133:1971–1977. doi: 10.1021/ja109226s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omura S, Fujimoto T, Otoguro K, Matsuzaki K, Moriguchi R, Tanaka H, Sasaki Y. Lactacystin, a novel microbial metabolite, induces neuritogenesis of neuroblastoma cells. J Antibiot. 1991;44:113–16. doi: 10.7164/antibiotics.44.113. [DOI] [PubMed] [Google Scholar]
- 9.Duffy RA, Morgan C, Naylor R, Higgins GA, Varty GB, Lachowicz JE, Parker EM. Rolapitant (SCH 619734): A potent, selective and orally active neurokinin NK1 receptor antagonist with centrally-mediated antiemetic effects in ferrets. Pharmacol Biochem Behav. 2012;102:95–100. doi: 10.1016/j.pbb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Fenical W, Jensen PR, Palladino MA, Lam KS, Lloyd GK, Potts BC. Discovery and development of the anticancer agent salinosporamide A (NPI-0052) Bioorg Med Chem. 2009;17:2175–2180. doi: 10.1016/j.bmc.2008.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masse CE, Morgan AJ, Adams J, Panek JS. Syntheses and biological evaluation of (+)-lactacystin and analogs. Eur J Org Chem. 2000:2513–2528. [Google Scholar]
- 12.Wei J, Shaw JT. Diastereoselective Synthesis of γ-Lactams by a One-Pot, Four-Component Reaction. Org Lett. 2007;9:4077–4080. doi: 10.1021/ol701911u. [DOI] [PubMed] [Google Scholar]
- 13.Tan DQ, Martin KS, Fettinger JC, Shaw JT. Ammonia synthons for the multicomponent assembly of complex γ-lactams. Proc Natl Acad Sci USA. 2011;108:6781–6786. S6781/1–S6781/91. doi: 10.1073/pnas.1015261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuk TL, Assink BK, Bates RC, Jr, Erdman DT, Fedij V, Jennings SM, Lassig JA, Smith RJ, Smith TL. An Efficient and Cost-Effective Synthesis of Pagoclone. Org Process Res Dev. 2003;7:851–855. [Google Scholar]
- 15.Ng PY, Tang Y, Knosp WM, Stadler HS, Shaw JT. Synthesis of diverse lactam carboxamides leading to the discovery of a new transcription-factor inhibitor. Angew Chem Int Ed. 2007;46:5352–5355. doi: 10.1002/anie.200700762. [DOI] [PubMed] [Google Scholar]
- 16.Tan DQ, Atherton AL, Smith AJ, Soldi C, Hurley KA, Fettinger JC, Shaw JT. Synthesis of a γ-Lactam Library via Formal Cycloaddition of Imines and Substituted Succinic Anhydrides. ACS Comb Sci. 2012;14:218–223. doi: 10.1021/co2001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raup DEA, Cardinal-David B, Holte D, Scheidt KA. Cooperative catalysis by carbenes and Lewis acids in a highly stereoselective route to γ-lactams. Nat Chem. 2010;2:766–771. doi: 10.1038/nchem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, DiRocco DA, Rovis T. N-Heterocyclic Carbene and Bronsted Acid Cooperative Catalysis: Asymmetric Synthesis of trans-γ-Lactams. J Am Chem Soc. 2011;133:12466–12469. doi: 10.1021/ja205714g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biggs-Houck JE, Younai A, Shaw JT. Recent advances in multicomponent reactions for diversity-oriented synthesis. Curr Opin Chem Biol. 2010;14:371–382. doi: 10.1016/j.cbpa.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Kim MB, Shaw JT. Synthesis of Antimicrobial Natural Products Targeting FtsZ: (+)-Totarol and Related Totarane Diterpenes. Org Lett. 2010;12:3324–3327. doi: 10.1021/ol100929z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Miller RL, Sauer DR, Djuric SW. Rapid and Efficient Synthesis of 1,2,4-Oxadiazoles Utilizing Polymer-Supported Reagents under Microwave Heating. Org Lett. 2005;7:925–928. doi: 10.1021/ol050007r. [DOI] [PubMed] [Google Scholar]
- 22.Zablocki J, Kalla R, Perry T, Palle V, Varkhedkar V, Xiao D, Piscopio A, Maa T, Gimbel A, Hao J, Chu N, Leung K, Zeng D. The discovery of a selective, high affinity A2B adenosine receptor antagonist for the potential treatment of asthma. Bioorg Med Chem Lett. 2005;15:609–612. doi: 10.1016/j.bmcl.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Arkin MR, Wells JA. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discovery. 2004;3:301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 25.Sauer WHB, Schwarz MK. Molecular Shape Diversity of Combinatorial Libraries: A Prerequisite for Broad Bioactivity. J Chem Inf Comput Sci. 2003;43:987–1003. doi: 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


