Abstract
In children with idiopathic nephrotic syndrome rituximab can maintain short-term remission with withdrawal of prednisone and calcineurin-inhibitors. Long-term effects including number of repeated infusions to maintain remission are unknown.
We treated with rituximab 46 consecutive children with idiopathic nephrotic syndrome lasting for at least one year (6.3±4.1 years), who were maintained in remission with oral prednisone and calcineurin inhibitors. They received 1–5 rituximab courses during a median follow-up of three years (range 1–5). Oral agents were tapered after each infusion, and completely withdrawn within 45 days.
Rituximab was well tolerated. Six-month probabilities of remission were 48% after the first infusion and 37% after subsequent infusions. One- and two-year-remission probabilities were respectively 20% and 10%. Median time intervals between complete oral-agent withdrawal and relapse were 5.6 and 8.5 months respectively following the first and subsequent courses. Time to reconstitution of CD20 cells correlated with the duration of remission, but was not associated with variation in FcyR, CD20 or SMPDL-3B polymorphisms. Podocyte Src phosphorylation was normal.
Rituximab can be safely and repeatedly used as prednisone and calcineurin-inhibitor-sparing therapy in a considerable proportion of children with dependent forms of idiopathic nephrotic syndrome. Further research is needed to identify patients who will benefit most from rituximab therapy.
INTRODUCTION
Idiopathic Nephrotic Syndrome (INS) affects 2 to 3 new children per 100,000 children per year and is the most common kidney disease in the paediatric population after congenital abnormalities of the urinary tract and cystic disorders1. Although 90% of these children favourably respond to steroids, relapse rate is as high as 80% and a long-term combination of steroids and calcineurin-inhibitors is often required to maintain remission2, 3. Disease remission and prevention of kidney disease progression are the main long-term care objectives4. Given the toxicity of these agents, however, oral agent-free follow-up periods are attractive short-term goals2, 5, 6.
Rituximab (Mabthera)R is a monoclonal antibody directed against CD20, a 35kDa protein highly expressed on B lymphocytes from early to late B-cell stages7, 8. CD20 expression in most B-cell lymphomas supports its use for haematological malignancies9, 10. Studies in these settings showed that rituximab is safe in the mid-term, with most adverse events being limited to fever and chills at the first-infusion. Nephrologists started to use rituximab in children with INS following the observation of an anti-proteinuric effect in patients with focal and segmental glomerulosclerois associated with post-transplant lymphoproliferative disorders who were treated with rituximab11, 12. Although rituximab may not be effective in cortico-resistant forms of INS13, data from several small case series14–16 and a randomized controlled trial17 indicates that rituximab may successfully replace steroids and calcineurin-inhibitors in children with INS whose remission state is dependent on both these drugs. In these forms of INS, rituximab may be as effective as the combination of steroids and calcineurin-inhibitors in maintaining short-term remission, allowing withdrawal of the oral therapy for 9 months in 50% of cases16, 17
Despite these promising results, additional data are needed to inform clinical practice on how best to use rituximab in dependent forms of INS. For example, it is unknown whether individual characteristics and additional rituximab doses may favourably impact response duration and prolong disease remission18. INS tends to relapse and long-term data are necessary on factors affecting frequency and number of infusions, and patient tolerance or toxicity. Although the side effects and discomfort associated with administration can be minimized with pre-treatment and slowing the infusion speed, more severe complications such as fatal pulmonary fibrosis19 and progressive multifocal leukoencephalopathy20, 21 remain a concern.
Here we present long-term follow-up data from children with INS dependent on both prednisone and calcineurin-inhibitors for at least one year and in remission for at least six months. Consecutive children with these characteristics were treated with one or more rituximab infusions (up to 5) following the completion of a published clinical trial17, either because they had signs of toxicity from prednisone or calcineurin inhibitors, or to prevent toxicity following treatment for at least one year. We conducted clinical and vitro studies to describe: (1) the probability of maintaining disease remission for 6 and 12 months following rituximab infusion and prednisone and calcineurin-inhibitor withdrawal; (2) the risk of disease relapse in children who remained in remission following rituximab infusion and prednisone and calcineurin-inhibitor withdrawal; (3) the toxicity profile of repeated rituximab infusions; and (4) the relationship between variation in FcyR, CD20 and/or SMPDL-3B polymorphisms and response to rituximab.
RESULTS
Patient characteristics
Baseline characteristics of the study cohort (N=46) are summarized in Table 1. All children had INS dependent on oral prednisone and calcineurin inhibitors for at least one year (6.3±4.1 years), and repeatedly failed to withdraw oral agents (relapsing within one-two weeks of withdrawal). These children were maintained in disease remission with average oral doses of prednisone and calcineurin-inhibitors (Table 1). All had normal creatinine clearance, serum albumin, and cholesterol. Children were on average 10 years old and tended to be male (63%). Disease duration strongly correlated with age (rho=0.82; P<0.001). Signs of toxicity were present in 17 children and were significantly more likely in older children (Supplement Table 1). Use of angiotensin receptor blockers or angiotensin converting enzyme inhibitors was similar across age groups.
Table 1.
Baseline Characteristics by age above and below the sample median (9.4 years). Most data are given as mean±SD.
| All | Age≤9.4 | Age>9.4 | P | |
|---|---|---|---|---|
| N=46 | N=23 | N=23 | ||
| Ages (yr) | 9.9±4.3 | 6.5±2.0 | 13.4±3.1 | <0.01 |
| Creatinine clearance ml/min | 98±13 | 96±10 | 100±15 | 0.29 |
| Total cholesterol mg/dL | 181±15 | 182±16 | 180±15 | 0.66 |
| Serum albumin gr/dL | 3.6± | 3.5±0.4 | 3.6±0.4 | 0.40 |
| Duration years mean±SD | 6.3±4.1 | 3.4±2.0 | 9.2±3.5 | <0.01 |
| 1 – 3 years (%) | 11 (24) | 11 (48) | 0 (0) | |
| 3 – 6 years (%) | 17 (37) | 12 (52) | 5 (22) | <0.01 |
| > 6 years (%) | 18 (39) | 0 (0) | 18 (78) | |
| Body Weight | 37.1±15.6 | 25.7±9.3 | 48.4±11.9 | <0.01 |
| Renal Histology n° | 16 | 5 | 11 | |
| Not performed | 30 | 18 | 12 | |
| FSGS | 2 | 0 | 2 | 0.999 |
| MCN | 10 | 4 | 6 | |
| IgM | 4 | 1 | 3 | |
| Prednisone (mg/kg/d) | 0.4±0.3 | 0.5±0.3 | 0.3±0.2 | 0.04 |
| CyA mg/Kg/d (n) | 2.5±1.2 (17) | 2.9±1.2 | 2.3±1.2 | 0.277 |
| FK mg/Kg/d (n) | 0.12±0.02 (19) | 0.11±0.005 | 0.12±0.01 | |
| Male | 29 (63%) | 16 (69%) | 13 (56%) | 0.359 |
| Toxicity | 17 (37%) | 4 (17%) | 13 (56%) | <0.01 |
| ACEI/ARB | 31 (67%) | 17 (74%) | 14 (61%) | 0.345 |
Doses of Tacrolimus (FK) are expressed in Cyclosporine A (CyA) equivalent doses; ACEI/ARB indicates ACE-inhibitors or angiotensin receptor blockers
Probability of remission
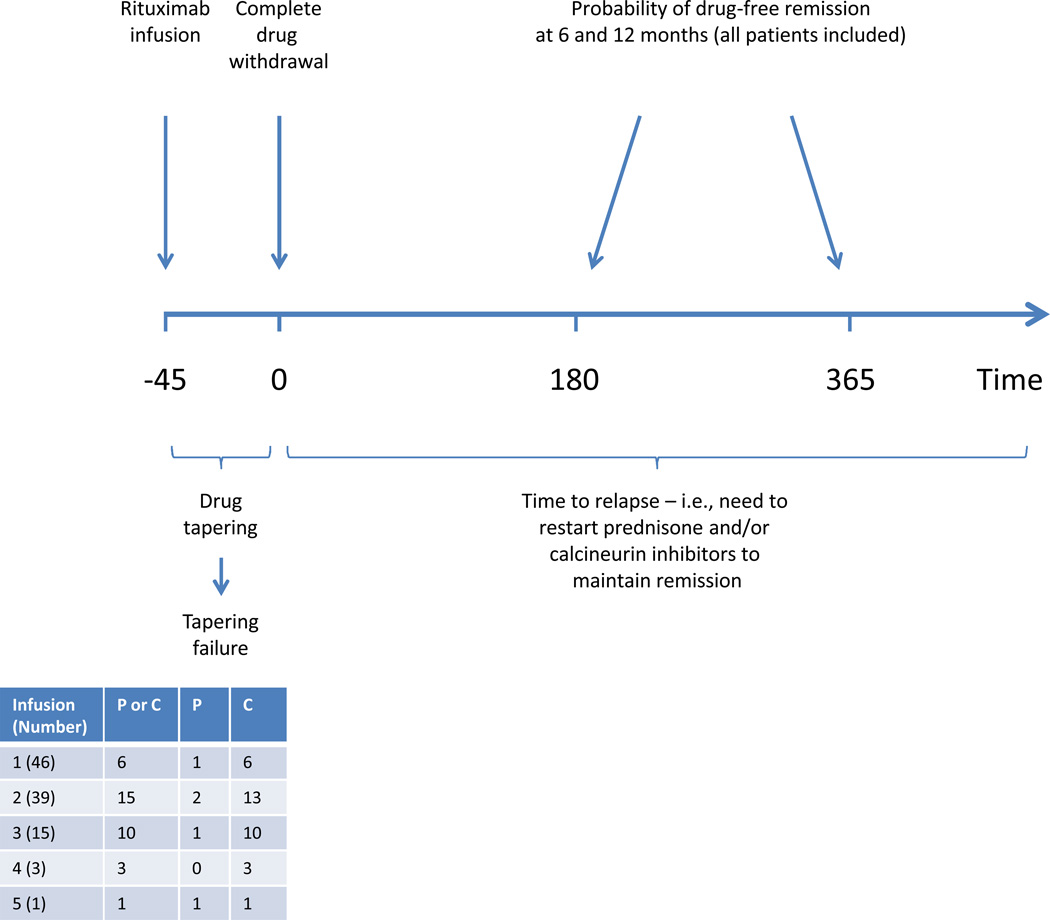
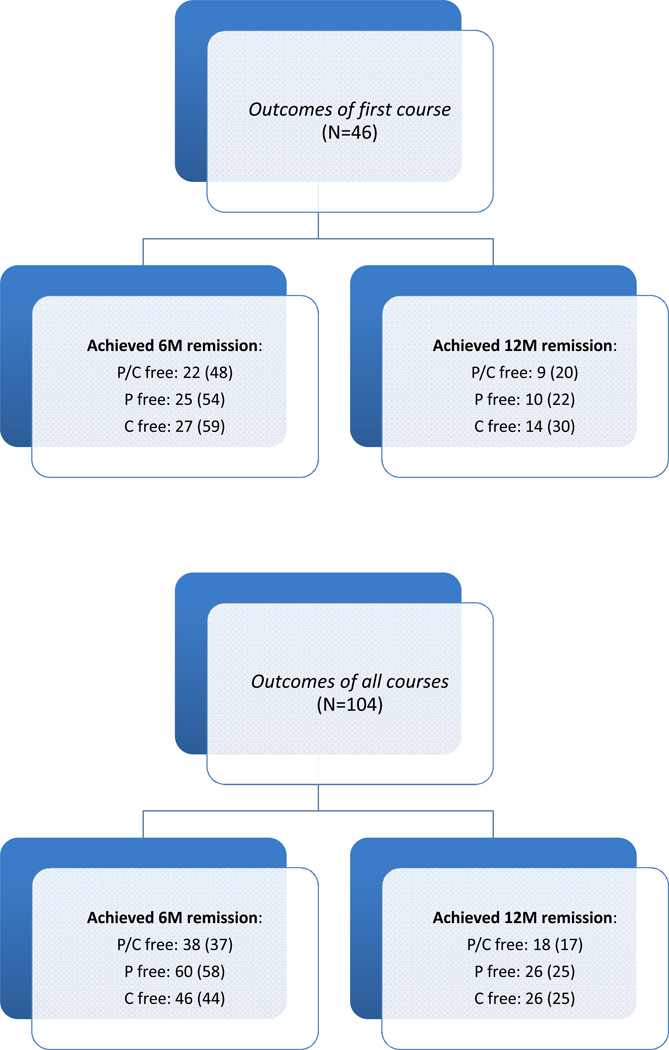
During an average follow-up of 3 years (range 1 to 5), the study participants received 1 to 5 courses of rituximab: all received 1; 39 received 2; 15 received 3; 3 received 4; and one received 5 courses (Figure 1). Children were considered in remission for at least six months following rituximab infusion if they (1) successfully completed prednisone and calcineurin-inhibitor tapering, and (2) remained in stable remission (see Methods) without oral agents from the day of complete drug withdrawal. We considered also 12-month remission, and single oral-agent withdrawal and withdrawal of both oral agents. Figure 2 shows the distribution of 6 and 12-month remissions after the initial treatment (top), and after any treatment (bottom), according to the oral agents that were withdrawn. At 6 months 22 children (48%) were in remission without oral agents; 25 (54%) were in prednisone-free remission (3 re-started calcineurin-inhibitors); and 27 (59%) were in calcineurin-inhibitor-free remission (5 re-started prednisone). Probabilities of 6-month remission following subsequent treatments were not statistically different. After one year from any treatment, approximately 20% of children were still in prednisone and calcineurin-inhibitor-free remission, and 25% of them were in single oral agent-free remission. All these children were still in remission at 18 months and half of them remained in remission for 24 months. Probabilities of single and dual oral agent-free remission at six months were significantly higher (P<0.001) in children older than 9.4 years than in those who were younger (68% vs. 32% for prednisone and calcineurin-inhibitor-free remission; 62% vs. 38% for prednisone-free remission; and 65% vs. 35% for calcineurin-inhibitor-free remission). In adjusted analyses (Table 2) age at diagnosis was the strongest predictor of drug-free remission for 6 and 12 months. Each year of age was associated with a 71 and 31% greater probability of prednisone and calcineurin-inhibitor-free remission for 6 and 12 months respectively. Results were similar in models of single oral agent-free remission for 6 and 12 months (Table 2). We found no difference between children who did and did not participate in the trial and between those who did and did not have signs of drug toxicity.
Figure 1.
Outcome definitions: We studied probability of prednisone and calcineurin-inhibitor-free remission (top) for at least 6 and 12 months (primary outcomes; no children were excluded from these analyses). Remission (absence of proteinuria) could be attained with complete withdrawal of both prednisone (P) and calcineurin-inhibitors (C) or either of them. We studied also time to relapse (proteinuria or need to reintroduce prednisone or calcineurin-inhibitors) after successful tapering (bottom). This analysis included only children who completed drug withdrawal, as children who failed to complete drug withdrawal could not be considered at risk for relapse (secondary outcome).
Figure 2.
Distribution of 6 and 12-month remissions after the initial treatment (top), and after any treatment (bottom), according to the drugs that were successfully withdrawn: 6M and 12M remissions indicate remission for 6 and 12 months; P/C indicates prednisone and calcineurin-inhibitors-free remission; P indicates prednisone-free remission; and C indicates calcineurin-inhibitor-free remission. Absolute frequencies (%) are reported for each outcome.
Table 2.
Effect of age (per year) on the probability of remission for 6 and 12 months
| 6 months | 12 months | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Both P and C | 1.71 | 1.11 to 2.62 | 1.31 | 1.04 to 1.65 |
| Prednisone | 1.23 | 1.05 to 1.45 | 1.19 | 1.06 to 1.32 |
| CyA/FK506 | 1.45 | 1.07 to 1.96 | 1.39 | 1.09 to 1.79 |
Results of primary analysis are in bold (6-month remission free from both oral agents). For each of the 6 models the outcome is defined based on the duration of the remission (6 and 12 months) and the drug that was withdrawn. OR indicates odds ratio; 95% CI indicate 95% confidence intervals. All models take into account the correlation in the data due to repeated observations in the same subject (mixed models) and are adjusted for number of rituximab cycles (see methods). Disease duration, toxicity, sex and baseline body weight did not have any effect. ‘Both P and C’ indicates remission free from both prednisone and calcineurin-inhibitors; ‘Prednisone’ indicates remission free from prednisone, but not from calcineurin-inhibitors; ‘CyA/FK506’ indicates remission free from calcineurin-inhibitors but not from prednisone.
Risk of relapse
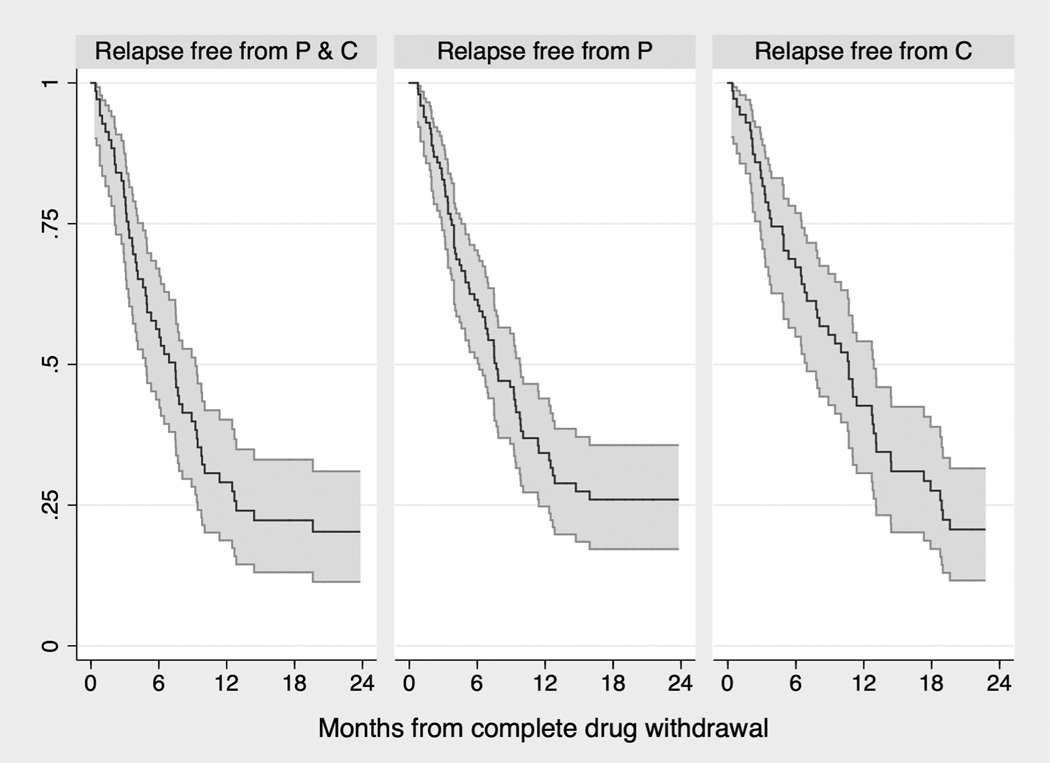
We studied the duration of the remission state as the time interval between complete oral-agent-withdrawal following the first and subsequent rituximab infusions and relapse of nephrotic syndrome or need to re-start oral agents or study end (see Methods). Tapering failures (N=35) were excluded from this analysis (Figure 1). Figure 3 summarizes relapse-free survival probabilities according to whether remission was free from both oral agents, free from prednisone only and free from calcineurin-inhibitors only. Median survival times were 5.6 to 7.3 months after the first treatment and 8.5 to 11.6 months after the second and subsequent treatments (Table 3). Each year of age at diagnosis predicted longer relapse-free survival (Supplement Table 2).
Figure 3.
Time to relapse in patients who completed prednisone and/or calcineurin-inhibitor tapering and withdrawal
The three Kaplan-Meier survival curves (with 95% confidence intervals) estimate the time to relapse according to whether remission (absence of proteinuria) was maintained without prednisone and calcineurin-inhibitors (‘P and C’; left plot); avoiding prednisone (‘P’, with or without use of calcineurin-inhibitors; mid plot); or avoiding calcineurin-inhibitors (‘C’, with or without use of prednisone; right plot).
Table 3.
Median relapse-free survival time (in months) in patients who completed prednisone and/or calcineurin-inhibitor tapering and withdrawal
| First course | Second and subsequent courses | |||
|---|---|---|---|---|
| Median | Interquartile range | Median | Interquartile range | |
| Both P and C | 5.6 | 4.3 to 8.1 | 8.5 | 6.5 to 11.7 |
| Prednisone | 6.5 | 4.8 to 9.6 | 10.9 | 8.1 to 15.6 |
| CyA/FK506 | 7.3 | 5.6 to 10.2 | 11.6 | 8.9 to 15.8 |
Median times are the times at which half of the sample is still event free. Each of the three estimates is from a lognormal model where the outcome is defined based on drug that was successfully tapered and withdrawn (models are described in Table 3). ‘Both P and C’ indicates remission free from both prednisone and calcineurin-inhibitors; Prednisone indicates remission free from prednisone, but not from calcineurin-inhibitors; CyA/FK506 indicates remission free from calcineurin-inhibitors but not from prednisone.
Other follow-up data
CD20 count: CD20 remained undetectable for 195 days (SD 90) after the first treatment and for 219 days (SD 142) after the second and subsequent treatments (P=0.444). Adjusting for age, CD20 reappearance was on average 185 days (95% CI from 104 to 267) in children who achieved prednisone and calcineurin-inhibitor-free remission for 6 months vs. those who did not (109 days; 45 to 172; P=0.01); 152 days (76 to 227) in children who achieved prednisone-free remission for 6 months vs. those who did not (96 days; 27 to 165; P=0.06); and 161 days (77 to 246) in children who achieved calcineurin-inhibitor-free remission for 6 months vs. those who did not (109 days; 45 to 173; P=0.01).
Adverse events: Five patients required rituximab infusion in intensive care unit for initial bronchospasm, which improved after slowing the infusion rate. No further medication was necessary. Three patients presented fever with migrating skin rash and acute arthritis appearing 2–4 weeks from infusion that rapidly resolved with non-steroidal anti-inflammatory medications. Two patients had neutropenia associated with transient viral infection. One patient (with known vescico-ureteral reflux) developed acute pyelonephritis with neutropenia and negative markers for viral infection.
Cost data: see supplemental results.
Laboratory data
FCGR2B and FCGR3A polymorphisms: FCGR2B and FCGR3A polymorphisms affect rituximab binding to NK cells and drive its antibody-dependent cellular toxicity22. The comparison of FCGR2A rs1801274 SNP allelic and genotype frequencies between patients presenting different sensitivity to rituximab (prednisone and calcineurin-inhibitors-free remission for 12 months) versus 320 controls showed a trend to a higher frequency of the C allele coding for Arg (P=0.059) in those patients who successfully stopped both oral agents for 12 months (Supplement Table 3). All other comparisons were not statistically significant (not shown).
CD20 (MS4A1) and SMPDL3b (SMPDL3B) variations: the search for variants/mutations in MS4A1 (exon 4) gene coding for CD20 epitopes 170ANPS173 or 182YCYSI186 and in SMPDL3b (epitope 156ELWKPW161) was negative.
Modulation of Podocyte shape and biochemistry: see supplemental results.
DISCUSSION
In this follow-up study we enrolled 46 children with INS dependent on both steroids and calcineurin-inhibitors. These children had been in remission for at least one year and failed any attempts to withdraw either oral agent. Participants received one to 5 rituximab treatments (104 in total) during a median follow-up of 3 years (range 1 to 5). We found that: (1) the probability of prednisone and calcineurin-inhibitor-free remission for 6 months was achieved in approximately half of the cases after rituximab treatment, with greater response rates for single oral-agent-free remission and considerable proportions of children still in remission at one (20%) and two (10%) years; (2) median time to relapse in children who completed prednisone and calcineurin-inhibitors was between 5.6 and 7.3 months following the initial rituximab infusion and between 8.5 and 11.6 months following the second and subsequent infusions; (3) rituximab adverse effects were mild and limited; finally (4) we found that successful response to rituximab was associated with older age at diagnosis and longer time to reconstitution of circulating CD20. We did not find any association between probability of remission and variation in FcyR, CD20 and/or SMPDL-3B polymorphisms.
The implications of our findings are four fold. First, rituximab can be used to maintain remission for 6 to 12 months without prednisone and calcineurin inhibitors in approximately 50% of children with INS. Since previous toxicity does not affect response to treatment, rituximab may represent an attractive alternative therapy to spare steroids and calcineurin-inhibitors in the long-term in these children. Second, relapse free survival ranged from 6 months to one year and this time interval may guide decision making to personalize long-term treatment based on repeated rituximab infusions. Since relapse free intervals are longer following repeated infusions, additional treatments of frequent relapses may be justified provided that tapering of oral agents is successful. Third, age at diagnosis was strongly associated with better outcomes. Although age is not a modifiable factor, it carries important prognostic information to plan long-term care, guide additional diagnostic testing and inform future research including studies on mechanisms of sensitivity to rituximab therapy. Fourth, our study suggests that rituximab, as other immunosuppressive agents, may successfully control but does not cure the disease.
We did not observe severe side effects. Risks related to discomfort during rituximab infusion can be minimized with pre-treatment and slowing the infusion rate. We observed only few episodes of acute arthritis and no rituximab-associated lung injury, which is very rare and may not be related to rituximab considering that the unique patient with nephrotic syndrome who developed pulmonary fibrosis had respiratory symptoms before the first infusion19. The same seems true for other long-term complications including leukoencephalopathy20 considering that JC reactivation is not restricted to patients treated with riruximab21, and has never been reported in case of isolated nephrotic syndrome. Considering the potential cost saving of one rituximab infusion as compared to six-month treatment with tacrolimus (see in Supplements a dedicated section on costs) and the low risks associated with the use of rituximab, including repeated infusions, equipoise may exist as to whether rituximab dependency may be less toxic than combined steroid and calcineurin-inhibitor dependency.
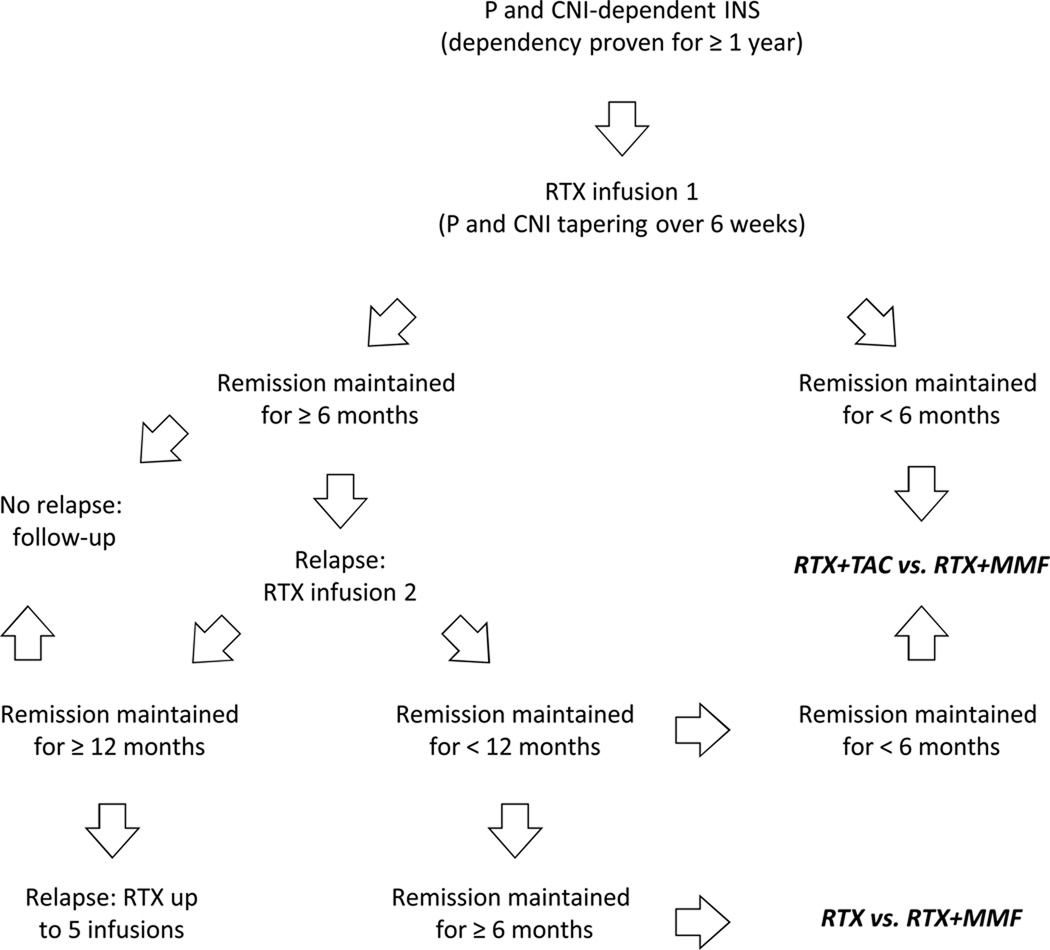
Failure to identify molecular predictors of sensitivity to rituximab and the tendency of INS to maintain some form of treatment-dependency (i.e., either on oral drugs or rituximab) opens the question of how best and in whom rituximab can be used today. Our study suggests that rituximab may be used as a steroid and calcineurin-inhibitor sparing agent in children in whom oral agents have been successful for at least one year and in whom, however, any attempts to taper them has failed. Depending on the duration of oral-agent-free remission following rituximab infusion, different strategies can be considered (Figure 4). Our data indicate that attaining an oral-agent-free remission for at least six months following the first rituximab supports sensitivity to rituximab therapy and potentially better response to subsequent infusions. However, longer remission times are the goal of the second and subsequent infusions. In case of long-lasting remission (>12 months) relapses may be treated with additional rituximab pulses, although safety data on repeated rituximab infusions beyond five courses are not available. If remission time is shorter, new strategies may be considered including approaches currently being tested in clinical trials (Figure 4) (EudraCT 2010-020184-20).
Figure 4.
Proposed strategies to maintain remission in prednisone and calcineurin inhibitor-dependent idiopathic nephrotic syndrome.
P indicates prednisone; CNI indicates calcineurin inhibitors; RTX indicates rituximab; TAC indicates tacrolimus; MMF indicates mycophenolate mofetil; bold indicate comparisons currently being tested in clinical trials (EudraCT number 2010-020184-20).
Time from rituximab-induced CD20-depletion to CD20-reconstitution may reflect the degree of immunosuppression and mirror sensitivity to rituximab. CD20-depletion involves mechanisms including apoptosis and complement or antibody dependent cell cytotoxicity mediated by Fcy receptos in NK cells22. We were unable to support an association between functionally relevant polymorphisms (including FCGR3A rs396991 and FCGR2A rs1801274 SNPs encoding the NK cell FcyRIII protein) and rituximab activity that have been identified in patients with follicular lymphoma23–26. Similarly, our data do not support the effect of other potential modifiers of rituximab activity including mutations in exon 4 of MS4A1 coding for CD20 binding sites (extracellular loops Rp15-C9 and Rp5-L)27 and in SMPDL3B coding for sphingomyelin-phosphodiesterase-acid-like-3b18, 28, 29, an additional target of rituximab in human podocytes. Finally, our experimental data did not reveal any difference in the degree of actin cytoskeleton remodelling and suPAR-dependent signalling observed in normal human podocytes cultured in the presence of sera from patients with different clinical response to rituximab. In contrast to what we observed in recurrent FSGS after transplantation18, rituximab administration in vitro did not prevent actin cytoskeleton remodelling observed after exposure to patient sera. This apparent discrepancy may be due to the more benign course in this study population, in whom circulating suPAR levels were similar to age-matched normal controls. These negative findings suggest that in this patient population, systemic rather than direct effects on the podocyte may mediate the response to rituximab.
Although our study is the first longitudinal cohort study of rituximab in INS it has limitations: it is not a clinical trial, the population enrolled is of limited size and data were collected in a single tertiary referral center. Further data from international collaborations are necessary to confirm our results. To minimize bias we followed patients prospectively using the infrastructure of a clinical trial including verification of eligibility criteria for study entry, standardization of interventional drug delivery, outcome assessment and safety monitoring17. These elements make our study difficult to compare with current literature data, which are based on uncontrolled case series or retrospective studies of heterogeneous populations14–16, including patients who were dependent or resistant to a single drug (in general steroids), and cases with variable sensitivity to an association of steroids and calcineurin-inhibitors. In addition, no studies on pharmacogenomics of rituximab have been done so far. Additional data from more homogeneous patient populations are needed.
In summary, rituximab therapy can be safely and repeatedly used to attain prednisone- and calcineurin-inhibitor-free remission in a considerable proportion of children with INS dependent on both these agents. Remission can be achieved for 6 months in half of these children, with longer remission times following repeated treatments, greater response rates for single oral agent-free remission and better outcomes in older children. However, in a similar proportion of cases rituximab is ineffective or may allow limited steroid or calcineurin-inhibitor sparing. Further research is needed to understand the mechanism of action of rituximab in INS and to identify patients who will benefit most from rituximab therapy. Although rituximab represents a potential steroid- and calcineurin inhibitor-sparing agent in several children with dependent forms of INS, rituximab alone does not seem to cure this disease. Our study suggests that remission of nephrotic syndrome tends to remain dependent on some form of treatment, and highlights how far behind we are in the management of this disease.
METHODS
Design Overview
Half of the children who participated in a previous clinical trial17(13 children originally assigned to the intervention group and 14 originally assigned to the control group who received rituximab at the end of the trial) gave consent for long-term follow-up. Children who were followed outside the coordinating center were excluded (n=27). Nineteen additional children, who were consecutively referred for INS after the completion of the trial, were eligible for the present study17. To be enrolled children had to be 16 year-old or younger with an estimated creatinine clearance >60 ml/min per 1.73 m2. They had to have a history of INS responsive to, and dependent on, both prednisone and calcineurin-inhibitors for at least one year with remission (proteinuria <4 mg/m2 per hour) maintained with both oral agents. At least three attempts to withdraw oral agents had to be unsuccessful with disease relapse within one-two weeks of oral-agent withdrawal. Criteria for diagnosis of INS were the same as in the published trial17. Briefly, INS was diagnosed in the presence of nephrotic range proteinuria (>40 mg/m2 per hour or >1 g/d per m2, or protein to creatinine ratio [P/C] >4 in a single urine specimen) or between 5 and 40 mg/m2 per hour associated with hypoalbuminemia or dyslipidemia. According to our protocol no renal biopsy was done because of clear drug-sensitivity. Criteria for exclusion were infantile onset (<1 year), previous history of macro-hematuria, hepatitis B, hepatitis C, or HIV infection, positivity of any marker of autoimmunity (ANA, nDNA, ANCA), low C3 levels, and need of diuretics, albumin, or anticoagulant therapy. Steroid dependency was defined as responsiveness to full doses of prednisone (2 mg/kg) and calcineurin-inhibitors (5 mg/kg) with two consecutive relapses occurring during prednisone tapering or within 2 weeks of prednisone withdrawal. Toxicity was defined based on previously published criteria17 (Supplement Table 1). Our study has been approved by the local Ethical Committee of the Institute Giannina Gaslini and was registered in the European Registry EudraCT (EudraCT registration numbers 2007-007796-16; 2008-004486-26).
Healthy controls: Three hundred and twenty healthy controls were genotyped as controls for FCGR2B and FCGR3A polymorphism studies. All the controls were Caucasian of Italian origin.
Laboratory investigations: see supplemental material.
Initial rituximab infusion and treatment of relapses: All participants received an intervention schedule based on rituximab infusion and tapering of prednisone and calcineurin-inhibitors. Tapering was done following the scheme below that allows drug withdrawal within 45 days. Rituximab (Mabthera; 375 mg/m2 IV) was given intravenously once (in the absence of clinical signs of toxicity from steroids and/or cyclosporine) or twice (after 2 weeks from the first infusion in the presence of toxicity). The medication was diluted in normal saline (1 mg/ml) and administered at increasing speeds (0.5 to 1.5 ml/min) over approximately 6 hours. The infusion was preceded by 2.5 to 5 mg of intravenous chlorfenamine maleate, 2 mg/kg methyl prednisolone in normal saline, and 8 mg/kg of oral paracetamol. Starting at 15 days, prednisone was tapered off by 0.33 mg/kg per week if proteinuria was <1 g/d. After 1 week, calcineurin-inhibitors were also decreased by 50% and withdrawn after 4 additional days. In case of relapse of proteinuria, prednisone 1 mg/kg was started and continued until stable remission. After one week a single dose of rituximab (375 mg/m2 IV) was repeated and prednisone tapered in the same way. In case of persistence of proteinuria, or of a new recurrence within 5 months, cyclosporine was added at the starting dose of 4 mg/Kg for 6 months followed by reduction to 3 mg/Kg. Rituximab infusion was repeated in case of relapse (proteinuria >40 mg/m2 per hour or >1 g/d per m2, or protein to creatinine ratio [P/C] >4 in a single urine specimen or between 5 and 40 mg/m2 per hour associated with hypoalbuminemia or dyslipidemia). Angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers were used at the discretion of the investigators, but kept constant during the study.
Study hypothesis, follow-up data and outcomes
Hypothesis: We hypothesized that age (main exposure) and other patient characteristics, including gender, toxicity status and disease duration, may affect the effects rituximab. Sensitivity to rituximab linked to variation in FcyR, CD20 and/or SMPDL-3B may play addictive effects30, 31.
Follow-up data: Children were seen every three months by a nephrologist, and as many times as necessary in case of complications or relapses. Study coordinators maintained ongoing contact with children and families to monitor clinical status and report potential adverse events. Routinary clinical exams (including WBC count) were done montly in the first 3 months than every three months. Blood pressure was measured at regular intervals (2 times per week) by the family paediatrician who contacted the referent nephrologist for data reporting and therapy update. Urine protein excretion was measured weekly on 24-hour urine collection, or more often in case of dipstick positivity. Determination of proteinuria at baseline and after 3 months was performed at a central laboratory. Dipstick for proteinuria determination was evaluated daily. In case of dipstick positivity, the presence of proteinuria was confirmed with 24-hour urine collection. Kidney function, plasma proteins, and cholesterol were obtained monthly. Counts of white blood cells and lymphocyte populations were monitored monthly.
Outcomes: The primary efficacy measure was the probability of maintaining remission (absence of proteinuria) for at least 6 months after successful tapering and complete withdrawal of prednisone and calcineurin-inhibitors following the first rituximab infusion. Time was measured from the date of complete prednisone and calcineurin-inhibitor withdrawal (Figure 1). Remission for 12 months was also studied. We also studied single-oral-agent-free remission for 6 and 12 months. Secondary outcome was the relapse free survival (time to relapse) from complete drug withdrawal (tapering failures were excluded). Relapse was diagnosed in the presence of proteinuria (see criteria for INS above) or re-start prednisone or calcineurin-inhibitors to maintain remission. A data safety monitoring board reviewed the safety data of the present study. Protocol and consent documents were approved by the ethics committee of Giannina Gaslini Hospital.
Statistical analysis
We compared quantitative and qualitative variables with t-test and chi-square method.
Probability of remission: We used mixed logistic models to study the relationship between age and the probability of remission for 6 and 12 months. Patient identifier was treated as random factor to account the correlation of repeated measures in the same subject (i.e., each subject could receive several rituximab infusions and fail several times during follow-up). In the analysis of the first treatment mixed modeling reduced to ordinary logistic regression.
Risk of relapse: We used parametric models for repeated survival times to study the relationship between age and the risk of disease relapse in children who completed tapering and oral-agent withdrawal following rituximab infusion. Relapse was defined as the restart of either or both steroids and calcineurin inhibitors to maintain remission. As to the remission analysis, rituximab could be repeated several times and the same child could return at risk following each rituximab infusion if oral-agent tapering was successful. We censored observations at the study end date (September 25, 2012) if children were still in remission, or when they left the study. We studied how the hazard function changed over time by plotting non-parametric (smoothed) estimates of this function. We also estimated the crude hazard rates for time intervals 0–1, 1–3, 3–6, 6–12, and >12 months. We selected a reasonable set of parametric survival models from the three-parameter gamma family by comparing observed rates within time intervals with model predictions and found the lognormal model provided the best fit. We compared parametric survival models using formal tests (including testing the parameters of gamma nested models) and graphical assessment of the goodness of fit. We accounted for the correlation in the data due to recurrences and repeated treatments in the same patient using robust variance.
Modeling strategy: For both analyses, the initial model included age (exposure), sex, body weight, previous toxicity and disease duration. Number of infusion was categorized as 1 and 2 or more (outcomes in 2 and 3 or more were similar). Previous randomization group in participants who were part of the clinical trial was tested as effect modifier. We considered overall model fit and graphical tests based on residuals. Variations of the exposure regression coefficient were monitored while removing manually non-significant variables. Analyses were performed using STATA (www.stata.com).
Supplementary Material
Acknowledgment
Grant support: The Giannina Gaslini Institute provided financial and logistic support to the study. This work was also supported by the Italian Ministry of Health ’Ricerca Corrente’ and from contributions derived from ‘Cinque per mille dell’IRPEF’. We also acknowledge contributions from the Renal Child Foundation, Fondazione Mara Wilma e Bianca Querci (project ”Ruolo dello stress reticolare nella progressione del danno renale e tumorale”), Fondazione La Nuova Speranza (‘Progetto integrato per la definizione dei meccanismi implicati nella glomerulo sclerosi focale’). The Alberta Kidney Disease Network – www.AKDN.info/research, provided analytical support. The funding sources had no role in any stage of the design and conduct of the study, in the collection, management, analysis, and interpretation of data in the study, or in the preparation, review, or approval of the manuscript.
Footnotes
Contributions: Drs. Ghiggeri and Ravani designed the study, take responsibility for the integrity of the data and the accuracy of the data analysis, and drafted the paper. Chiara Siciliano, Alessandro Ponticelli and Alberto Magnasco coordinated follow-up data collection and ensured the quality of data entry. All authors provided critical inputs for important intellectual content and final approval. Drs. Ghiggeri obtained funding and had the final responsibility to submit the manuscript for publication. Data safety and monitoring board members included Antonella Trivelli MD, Giovanni Candiano PhD, and Giorgio Piaggio MD. Dr. Sandra Merscher-Gomez and Dr Alessia Fornoni performed and analyzed the in vitro experiments.
Potential conflicts of interests: none to declare.
REFERENCES
- 1.Braden GL, Mulhern JG, O'Shea MH, Nash SV, et al. Changing incidence of glomerular diseases in adults. Am J Kidney Dis. 2000;35:878–883. doi: 10.1016/s0272-6386(00)70258-7. [DOI] [PubMed] [Google Scholar]
- 2.Trompeter RS, Lloyd BW, Hicks J, White RH, et al. Long-term outcome for children with minimal-change nephrotic syndrome. Lancet. 1985;1:368–370. doi: 10.1016/s0140-6736(85)91387-x. [DOI] [PubMed] [Google Scholar]
- 3.Ponticelli C, Rizzoni G, Edefonti A, Altieri P, et al. A randomized trial of cyclosporine in steroid-resistant idiopathic nephrotic syndrome. Kidney Int. 1993;43:1377–1384. doi: 10.1038/ki.1993.194. [DOI] [PubMed] [Google Scholar]
- 4.Ghiggeri GM, Catarsi P, Scolari F, Caridi G, et al. Cyclosporine in patients with steroid-resistant nephrotic syndrome: an open-label, nonrandomized, retrospective study. Clin Ther. 2004;26:1411–1418. doi: 10.1016/j.clinthera.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Kyrieleis HA, Lowik MM, Pronk I, Cruysberg HR, et al. Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol. 2009;4:1593–1600. doi: 10.2215/CJN.05691108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghiggeri GM, Altieri P, Oleggini R, Valenti F, et al. Cyclosporine enhances the synthesis of selected extracellular matrix proteins by renal cells "in culture". Different cell responses and phenotype characterization. Transplantation. 1994;57:1382–1388. doi: 10.1097/00007890-199405150-00017. [DOI] [PubMed] [Google Scholar]
- 7.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678–1685. [PubMed] [Google Scholar]
- 8.Deans JP, Li H, Polyak MJ. CD20-mediated apoptosis: signalling through lipid rafts. Immunology. 2002;107:176–182. doi: 10.1046/j.1365-2567.2002.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leget GA, Czuczman MS. Use of rituximab, the new FDA-approved antibody. Curr Opin Oncol. 1998;10:548–551. doi: 10.1097/00001622-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Leonard JP. Monoclonal antibody therapy for B-cell non-Hodgkin's lymphoma. N Engl J Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 11.Nozu K, Iijima K, Fujisawa M, Nakagawa A, et al. Rituximab treatment for posttransplant lymphoproliferative disorder (PTLD) induces complete remission of recurrent nephrotic syndrome. Pediatr Nephrol. 2005;20:1660–1663. doi: 10.1007/s00467-005-2013-7. [DOI] [PubMed] [Google Scholar]
- 12.Pescovitz MD, Book BK, Sidner RA. Resolution of recurrent focal segmental glomerulosclerosis proteinuria after rituximab treatment. N Engl J Med. 2006;354:1961–1963. doi: 10.1056/NEJMc055495. [DOI] [PubMed] [Google Scholar]
- 13.Magnasco A, Ravani P, Edefonti A, Murer L, et al. Rituximab in children with resistant idiopathic nephrotic syndrome. J Am Soc Nephrol. 2012;23:1117–1124. doi: 10.1681/ASN.2011080775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guigonis V, Dallocchio A, Baudouin V, Dehennault M, et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol. 2008;23:1269–1279. doi: 10.1007/s00467-008-0814-1. [DOI] [PubMed] [Google Scholar]
- 15.Gulati A, Sinha A, Jordan SC, Hari P, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol. 2010;5:2207–2212. doi: 10.2215/CJN.03470410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemper MJ, Gellermann J, Habbig S, Krmar RT, et al. Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant. 2012;27:1910–1915. doi: 10.1093/ndt/gfr548. [DOI] [PubMed] [Google Scholar]
- 17.Ravani P, Magnasco A, Edefonti A, Murer L, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:1308–1315. doi: 10.2215/CJN.09421010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 3:85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaumais MC, Garnier A, Chalard F, Peuchmaur M, et al. Fatal pulmonary fibrosis after rituximab administration. Pediatr Nephrol. 2009;24:1753–1755. doi: 10.1007/s00467-009-1195-9. [DOI] [PubMed] [Google Scholar]
- 20.Carson KR, Focosi D, Major EO, Petrini M, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. Lancet Oncol. 2009;10:816–824. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu C, Kimura S, Yoshida Y, Nezu A, et al. Acute leucoencephalopathy during cyclosporin A therapy in a patient with nephrotic syndrome. Pediatr Nephrol. 1994;8:483–485. doi: 10.1007/BF00856540. [DOI] [PubMed] [Google Scholar]
- 22.Koene HR, Kleijer M, Algra J, Roos D, et al. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90:1109–1114. [PubMed] [Google Scholar]
- 23.Rezvani AR, Maloney DG. Rituximab resistance. Best Pract Res Clin Haematol. 24:203–216. doi: 10.1016/j.beha.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olejniczak SH, Hernandez-Ilizaliturri FJ, Clements JL, Czuczman MS. Acquired resistance to rituximab is associated with chemotherapy resistance resulting from decreased Bax and Bak expression. Clin Cancer Res. 2008;14:1550–1560. doi: 10.1158/1078-0432.CCR-07-1255. [DOI] [PubMed] [Google Scholar]
- 25.Terui Y, Mishima Y, Sugimura N, Kojima K, et al. Identification of CD20 C-terminal deletion mutations associated with loss of CD20 expression in non-Hodgkin's lymphoma. Clin Cancer Res. 2009;15:2523–2530. doi: 10.1158/1078-0432.CCR-08-1403. [DOI] [PubMed] [Google Scholar]
- 26.Cartron G, Dacheux L, Salles G, Solal-Celigny P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 27.Perosa F, Favoino E, Vicenti C, Guarnera A, et al. Two structurally different rituximab-specific CD20 mimotope peptides reveal that rituximab recognizes two different CD20-associated epitopes. J Immunol. 2009;182:416–423. doi: 10.4049/jimmunol.182.1.416. [DOI] [PubMed] [Google Scholar]
- 28.Bezombes C, Grazide S, Garret C, Fabre C, et al. Rituximab antiproliferative effect in B-lymphoma cells is associated with acid-sphingomyelinase activation in raft microdomains. Blood. 2004;104:1166–1173. doi: 10.1182/blood-2004-01-0277. [DOI] [PubMed] [Google Scholar]
- 29.Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746:284–294. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Binder M, Otto F, Mertelsmann R, Veelken H, et al. The epitope recognized by rituximab. Blood. 2006;108:1975–1978. doi: 10.1182/blood-2006-04-014639. [DOI] [PubMed] [Google Scholar]
- 31.Perosa F, Favoino E, Caragnano MA, Dammacco F. Generation of biologically active linear and cyclic peptides has revealed a unique fine specificity of rituximab and its possible cross-reactivity with acid sphingomyelinase-like phosphodiesterase 3b precursor. Blood. 2006;107:1070–1077. doi: 10.1182/blood-2005-04-1769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.