Abstract
Objective
Survival of patients with pancreatic adenocarcinoma is limited and few prognostic factors are known. We conducted a two-stage genome-wide association study (GWAS) to identify germline variants associated with survival in patients with pancreatic adenocarcinoma.
Design
We analyzed overall survival in relation to single nucleotide polymorphisms (SNPs) among 1,005 patients from two large GWAS datasets, PanScan I and ChinaPC. Cox proportional hazards regression was used in an additive genetic model with adjustment for age, sex, clinical stage and the top four principal components of population stratification. The first stage included 642 cases of European ancestry (PanScan), from which the top SNPs (P≤10−5) were advanced to a joint analysis with 363 additional patients from China (ChinaPC).
Results
In the first stage of cases of European descent, the top-ranked loci were at chromosomes 11p15.4, 18p11.21, and 1p36.13, tagged by rs12362504 (P=1.63×10−7), rs981621 (P=1.65×10−7), and rs16861827 (P=3.75×10−7), respectively. One-hundred thirty-one SNPs with P ≤ 10−5 were advanced to a joint analysis with cases from the ChinaPC study. In the joint analysis, the top-ranked SNP was rs10500715 (minor allele frequency, 0.37; P=1.72×10−7) on chromosome 11p15.4, which is intronic to the SET binding factor 2 (SBF2) gene. The hazard ratio (95% CI) for death was 0.74 (0.66–0.84) in PanScan I, 0.79 (0.65–0.97) in ChinaPC, and 0.76 (0.68–0.84) in the joint analysis.
Conclusion
Germline genetic variation in the SBF2 locus was associated with overall survival in patients with pancreatic adenocarcinoma of European and Asian ancestry. This association should be investigated in additional large patient cohorts.
Keywords: Pancreatic cancer, GWAS, single nucleotide polymorphism, SET binding factor 2
INTRODUCTION
Pancreatic cancer is a major cause of cancer-related death across the globe, and five-year overall survival is approximately five percent.[1–2] Nevertheless, patient survival times are variable and only partially explained by traditional clinical and pathologic features.[3] Accumulating evidence indicates that germline genetic variability can provide important prognostic information for patients with cancer.[4–6] One mechanism by which germline genetic variability may impact patient survival is through modification of tumor-host interactions. A defining feature of pancreatic adenocarcinoma is the recruitment of host cells, including fibroblasts, immune cells, and endothelial cells, which surround the tumor in a dense stromal matrix.[7–9] This host-derived desmoplastic stroma actively engages with tumor cells and plays a critical role in promoting tumor development and progression.[10] Notably, laboratory studies suggest that treatments which modify the interaction of pancreatic cancer cells with its surrounding stroma can impact survival in genetically engineered mouse models of pancreatic adenocarcinoma.[8, 11–12]
Prior studies of germline variants and pancreatic cancer survival have primarily focused on the evaluation of candidate genes in pathways of suspected importance.[13–17] However, this approach relies upon our relatively incomplete understanding of tumor and host biology. In contrast, genome-wide approaches are available that allow a more comprehensive evaluation of germline genetic variants that is not reliant upon a priori hypotheses. Recently, genome-wide association studies (GWAS) have identified several genetic variants associated with the development of pancreatic adenocarcinoma in European and Chinese populations.[18–20] In a two-stage genome-wide study of survival, we used these data to evaluate the association of germline variants with overall survival in over 1,000 cases of pancreatic adenocarcinoma.
MATERIALS AND METHODS
PanScan Population
The Pancreatic Cancer Cohort Consortium (PanScan) genome wide association study (GWAS) has been described previously, in detail.[18, 21] In short, PanScan I included cases and controls from 11 prospective cohort studies from European populations in the United States and Europe, including Alpha-Tocopherol Beta-Carotene Cancer Prevention Study (ATBC), CLUE II, American Cancer Society Cancer Prevention Study-II (CPS II); European Prospective Investigation into Cancer and Nutrition Study (EPIC); Health Professional's Follow-up Study (HPFS); New York University Women's Health Study (NYU-WHS); Nurses' Health Study (NHS); Physicians' Health Study I (PHS I); Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO); Women’s Health Initiative (WHI); and Women’s Health Study (WHS). In each cohort, a defined population of subjects was followed prospectively with assessments of lifestyle factors and ascertainment of cancer diagnoses. Cases included subjects with incident primary pancreatic adenocarcinoma (ICD-O-3 code C250-C259 or C25.0-C25.3, C25.7-C25.9). All subjects with non-exocrine pancreatic tumors (C25.4, histology type, 8150, 8151, 8153, 8155, 8240, 8246) were excluded.
Each cohort study selected participants with blood or buccal cells collected prior to cancer diagnosis. Incident pancreatic cancer cases identified by self-report, report of next-of-kin, linkage with local or national cancer registries, or through national death indices were confirmed by subsequent medical record review, cancer registry report, and/or death registry report, without prior knowledge of genetic data. In the 11 participating cohorts, covariate data were collected though written questionnaires or in-person interviews. Data were requested from each cohort on participants’ age, gender, and race/ethnicity (European, Asian, African, other). Detailed descriptions of data collection methods have been published previously.[18, 21] Cohorts obtained consent from participants and approval from their Institutional Review Board (IRB). The Special Studies Institutional Review Board (SSIRB) of the National Cancer Institute approved the pooled PanScan study.
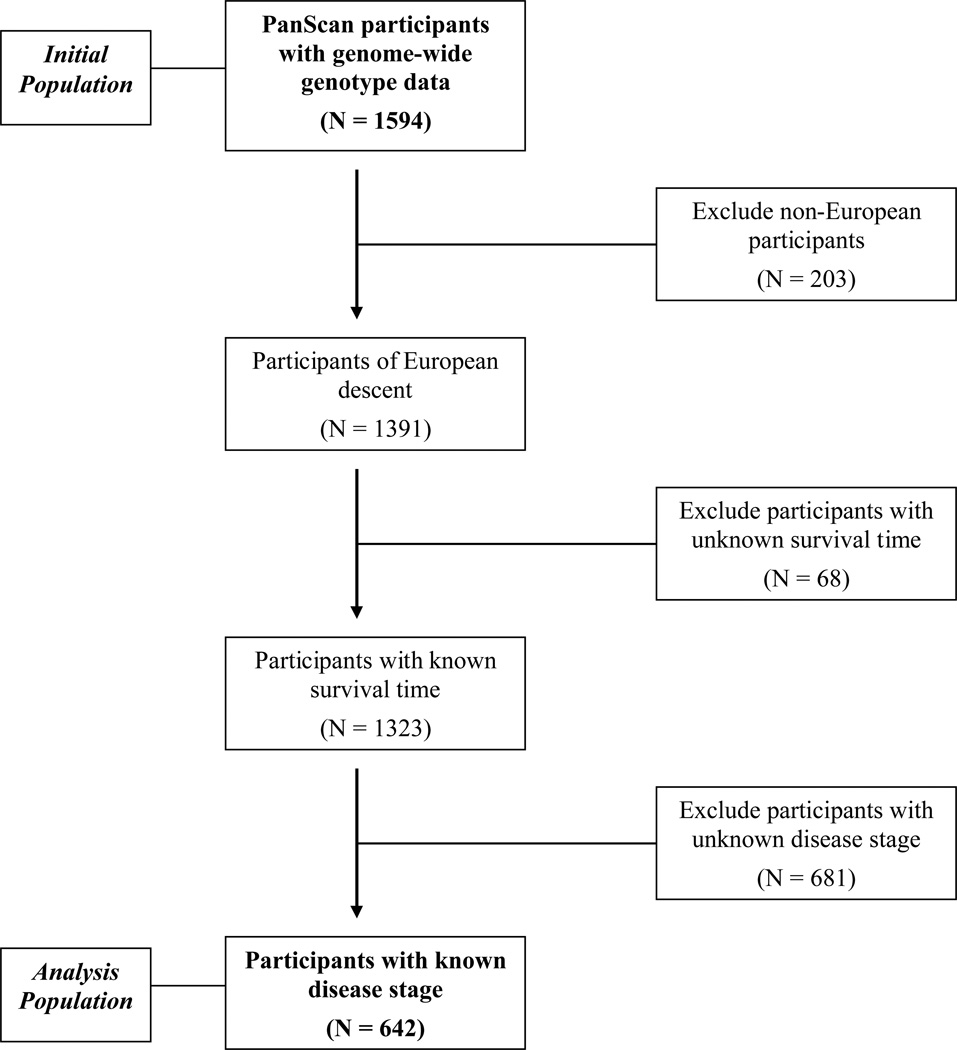
For the current survival analysis, each cohort also provided survival time and stage information for pancreatic cancer cases included in PanScan. Survival time was defined as the number of days between the date of diagnosis and the date of death or date of last known contact. Stage data were harmonized into three categories: (1) local disease amenable to surgical resection; (2) locally advanced disease with extra-pancreatic extension rendering it unresectable, but without distant metastases, (3) distant metastatic disease. For eight cohorts (CLUE II, EPIC, HPFS, NHS, NYU-WHS, PHS, PLCO and WHS), American Joint Committee on Cancer (AJCC)/International Union for Cancer Control (IUCC) TNM staging [22] was converted to the above categories, with AJCC/IUCC stages I and II indicating local disease, stage III indicating locally advanced disease, and stage IV indicating metastatic disease. Two cohorts (CPS and WHI) provided data using Surveillance Epidemiology End Results summary staging,[23] which classifies tumors as localized, regional, or distant. These stages were included as local, locally advanced and metastatic disease, respectively. Stage data were not available for one study (ATBC). Given the known strong association between stage and survival, subjects of European descent were included in the final analysis if they had available survival time and stage information, in addition to genotype data. From PanScan I, 1323 cases of European descent were available with genome-wide genotype data and survival time. Among these cases, 642 cases had stage information and were included in the analysis (Figure 1 and Supplementary Table 1). Median survival times were slightly shorter among the full population of patients with survival information versus the subset of patients with stage information (Supplemental Table 1). Overall, median survival time (MST) was 5.0 months for all cases and 5.9 months for the subset of cases with available stage data. Age and gender were similar in the full PanScan population of European descent (median age, 68 years; 48% male) and the population with survival and stage information (median age, 71 years; 37% male).
Figure 1.
Flow chart of PanScan case eligibility
ChinaPC Population
The ChinaPC case-control GWAS has been described previously in detail.[20] In short, a GWAS was performed among pancreatic cancer cases and controls collected in an ongoing molecular epidemiological study of pancreatic cancer. These case subjects were recruited from Cancer Hospital, Chinese Academy of Medical Sciences (Beijing), and Cancer Hospital, Fudan University (Shanghai), between 2000 and 2011. At recruitment, informed consent was obtained from each subject. The study was approved by the Institutional Review Board of the Chinese Academy of Medical Sciences Cancer Institute. All case subjects had pancreatic ductal adenocarcinoma confirmed histopathologically or cytologically by at least two pathologists according to the World Health Organization classification. Genomic DNA for GWAS analysis was isolated from peripheral blood lymphocytes at the time of diagnosis. Characteristics and clinical information including age, sex, race and tumor stage, were obtained from patients’ medical records. As for the PanScan subjects, survival time was defined as the number of days between the date of diagnosis and the date of death or date of last known contact. Stage data were harmonized into three categories: (1) local disease amenable to surgical resection; (2) locally advanced disease with extra-pancreatic extension rendering it unresectable, but without distant metastases, (3) distant metastatic disease. Those subjects with available genome-wide genotype data, survival time, and stage information were included in the analysis. From ChinaPC, 600 cases had genome-wide genotype data and survival times. After exclusion of 237 cases without stage information, 363 cases were available for analysis. Median survival times were 7.1 months in both those cases with available stage information and those cases without such information.
Genotype and Imputation Analysis
Genotypes of patients in PanScan and ChinaPC were generated using the HumanHap550 chip (Illumina, San Diego, CA) and Affymetrix GeneChip Human Mapping 6.0 set (Affymetrix, Santa Clara, CA), respectively. The procedures of genotyping and quality control in each genome-wide association study have been described previously. [18, 20] In brief, samples with <98% completion and SNP assays with call rates <90% were excluded. Only SNPs with minor allele frequency >0.01 and mapped on autosomal chromosomes were included for analysis. A principal component analysis (PCA) of DNA samples in this study was performed with EIGENSTRAT. Four principal components were effective for distinguishing significant population groups and were included as quantitative covariates to correct for genetic admixture. To increase the spectrum of variants tested in the current study of overall survival, we used MACH software to impute untyped markers using linkage disequilibrium (LD) and haplotype information from HapMap phase II CEU and CHB+JPT as the reference sets for PanScan and ChinaPC participants, respectively. After quality control of imputation data, 2,731,086 SNPs in PanScan and 2,307,550 SNPs in ChinaPC were available for analysis.
Cis-eQTL analysis
To examine gene expression differences by genotype at our top locus, we inspected a publically available eQTL database.[24] The database includes 405 children of British descent, organized into 206 sibships, including 297 sib pairs and 11 half-sib pairs. Global gene expression in lymphoblastoid cell lines was measured using Affymetrix HG-U133 Plus 2.0 chips. All 405 children and their parents were genotyped using the Illumina Sentrix Human-1 Genotyping BeadChip. The number of principal components used was chosen to maximize the number of cis-eQTLs with genome-wide significance. Association analysis was applied with the FASTASSOC option implemented in MERLIN.
Statistical Analysis
For each of the selected SNPs, we performed Cox proportional hazards regression under a log-additive genetic model with adjustment for covariates that might influence patient survival, including age (continuous), sex (male or female), stage of disease (local, locally advanced and metastatic as ordinal categories) and the top four principal components of population stratification in both PanScan I and ChinaPC studies.[18, 20] The overall survival time was defined as the time from pancreatic cancer diagnosis to either death or the last known date alive. Patients known to be alive were censored at the time of last contact. The top SNPs with P≤10−5 found in PanScan were advanced to a combined analysis in patients independently recruited from the ChinaPC study. To summarize results for the two datasets, we performed a meta-analysis to obtain the summary HR and 95% CI using METAL software (http://www.sph.umich.edu/csg/abecasis/metal). Haploview software was used to determine pair-wise linkage disequilibrium structure across the studied genomic regions. Kaplan-Meier survival estimates were plotted and P-values were assessed using the log-rank test. Survival analyses were performed with SAS software. All statistical tests were two-sided.
RESULTS
Patient Characteristics
The characteristics of the 642 pancreatic cancer cases from the PanScan cohort and the 363 cases from the ChinaPC study are shown in Table 1. Median follow-up time for cases still alive was 64 months in PanScan and 17 months in ChinaPC. In PanScan, 609 (94.9%) patients had died, while 334 (92.0%) had died in the ChinaPC study. In the combined analysis, we included 1,005 patients with pancreatic cancer, 19.6% with localized disease, 32.5% with locally advanced disease, and 47.9% with metastatic disease. As expected, stage was strongly associated with survival in both studies (P<0.0001); median survival time (MST) was 13.5, 9.7 and 3.8 months for patients with local, locally advanced and metastatic disease, respectively, in the combined study. The MST for patients in PanScan and ChinaPC studies were 5.9 months and 7.1 months, respectively.
Table 1.
PanScan and ChinaPC patient characteristics and overall survival
| PanScan (N=642) |
ChinaPC (N=363) |
Pooled Analysis (N=1005) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No (%) | MST* | P† | No (%) | MST* | P† | No (%) | MST* | P† | |
| Vital Status | |||||||||
| Dead | 609 (94.9) | 5.9 | 334 (92.0) | 7.1 | 943 (93.8) | 6.1 | |||
| Alive | 33 (5.1) | 29 (8.0) | 62 (6.2) | ||||||
| Sex | 0.4522 | 0.4207 | 0.9193 | ||||||
| Male | 239 (37.2) | 5.1 | 218 (60.1) | 6.9 | 457 (45.5) | 6.0 | |||
| Female | 403 (62.8) | 6.3 | 145 (39.9) | 7.0 | 548 (54.5) | 6.4 | |||
| Age§ | 0.0104 | 0.2627 | 0.0024 | ||||||
| ≤68 years | 253 (39.4) | 7.7 | 264 (72.7) | 6.5 | 517 (51.4) | 7.0 | |||
| >68 years | 389 (60.6) | 5.1 | 99 (27.3) | 5.6 | 488 (48.6) | 5.1 | |||
| Stage | <0.0001 | <0.0001 | <0.0001 | ||||||
| Local disease | 119 (18.5) | 17.6 | 78 (21.5) | 9.3 | 197 (19.6) | 13.5 | |||
| Locally advanced disease | 170 (26.5) | 10.6 | 157 (43.2) | 8.4 | 327 (32.5) | 9.7 | |||
| Metastatic disease | 353 (55.0) | 3.2 | 128 (35.3) | 4.6 | 481 (47.9) | 3.8 | |||
MST = median survival time, months
P values were calculated using the log-rank test
Median age was 68 years among all cases
Germline Variants Associated with Incident Pancreatic Cancer or Patient Survival in Previous GWAS
Four susceptibility loci, 13q22.1, 1q32.1, 5p15.33 and 9q34, have been associated with pancreatic cancer risk in two pancreatic cancer GWAS of European ancestry (PanScan I and II).[18–19] To determine if the top SNPs at these loci might also be associated with patient survival, we investigated the associations between these SNPs and survival time in the PanScan cohort. We also evaluated rs167020 on chromosome 7q36, which was associated with incident pancreatic cancer in the prospective cohorts participating in PanScan I.[18] None of the top SNPs at these loci were significantly associated with survival (P>0.05) (Table 2).
Table 2.
Hazard ratios and median survival times by genetic variants previously associated with incident pancreatic cancer in PanScan
| SNP, Chromosome, Gene | No. (%) | MST* | HR (95% CI)† | P† |
|---|---|---|---|---|
| rs9543325, Chr13, None | 1.09 (0.96–1.24) | 0.1769 | ||
| TT | 207 (32.2) | 6.1 | ||
| TC | 331 (51.6) | 6.4 | ||
| CC | 104 (16.2) | 5.1 | ||
| rs3790844, Chr1, NR5A2 | 1.04 (0.90–1.21) | 0.5699 | ||
| AA | 414 (64.5) | 5.4 | ||
| AG | 203 (31.6) | 6.6 | ||
| GG | 25 (3.9) | 6.9 | ||
| rs401681, Chr5, CLPTM1L-TERT | 1.06 (0.95–1.19) | 0.2916 | ||
| CC | 189 (29.4) | 6.5 | ||
| CT | 316 (49.2) | 5.2 | ||
| TT | 137 (21.4) | 7.7 | ||
| rs505922, Chr9, ABO | 1.05 (0.93–1.18) | 0.4590 | ||
| TT | 224 (34.9) | 5.1 | ||
| TC | 312 (48.6) | 5.3 | ||
| CC | 106 (16.5) | 8.0 | ||
| rs167020, Chr7, SHH | 0.99 (0.87–1.12) | 0.8724 | ||
| GG | 314 (48.9) | 5.7 | ||
| GA | 264 (41.1) | 6.1 | ||
| AA | 64 (10.0) | 5.0 | ||
MST = median survival time, months
HR (95% CI) = hazard ratio (95% confidence interval). HR and P-value calculated using multivariable-adjusted Cox regression under a log-additive genetic model, adjusting for age, sex, stage of disease, and the top four principal components of population stratification
We then investigated a genetic locus on chromosome 6, which was associated with survival in patients with advanced pancreatic adenocarcinoma participating in a randomized clinical trial of gemcitabine plus placebo versus gemcitabine plus bevacizumab (CALGB 80303).[25] The top SNP from the analysis in CALGB 80303 (rs763780) was also associated with survival time in the PanScan cohort (P = 0.0008). However, the risk allele identified in CALGB 80303 was protective in the PanScan cohort. Comparing the survival time of patients with the TC genotype versus the TT genotype (referent) resulted in a HR of 3.3 (95% CI, 2.1–5.1) in CALGB 80303,[26] while it resulted in a HR of 0.64 (95% CI, 0.50–0.83) in PanScan. We noted similar results when including only subjects with metastatic disease in the PanScan cohort (data not shown). In CALGB 80303, results were similar after stratification by treatment arm.[25] Although further investigation is required, differences in patient populations between a large, randomized phase III trial and participants from pospective cohorts may have contributed to the discordant results.
Genetic Variants Associated with Patient Survival in Genome-wide Genotyping
The manhattan plot for the GWAS of pancreatic cancer survival in PanScan is shown in Supplementary Figure 1. We identified three independent regions most associated with survival on chromosomes 11p15.4 (four SNPs), 18p11.21 (12 SNPs) and 1p36.13 (one SNP) , which were tagged by rs12362504 (HR, 1.40; 95% CI, 1.23–1.58; P=1.63×10−7), rs981621 (HR, 1.39; 95% CI, 1.23–1.57; P=1.65×10−7) and rs16861827 (HR, 1.70; 95% CI, 1.39–2.09; P=3.75×10−7), respectively (Table 3 and Supplemental Table 2). These SNPs were clustered in SBF2 on Chr11p15.4, C18orf1 on Chr18p11.21, and IGSF21 on Chr1p36.13. LD plots of the SNPs on chromosomes 11p15.4 and 18p11.21 are shown in Supplementary Figure 2. In 200 kb regions flanking rs16861827, there were 56 nominally significant SNPs with P-values ranging from 4.96×10−5 to 0.048.
Table 3.
Hazard ratios and median survival times by genotype for significant tagSNPs (P<5×10−7) in PanScan survival GWAS.
| SNP, Chromosome, Gene | No. (%) | MST* | HR (95% CI)† | P† |
|---|---|---|---|---|
| rs12362504, Chr11p15.4, SBF2 | 1.40 (1.23–1.58) | 1.63×10−7 | ||
| TT | 319 (49.7) | 6.9 | ||
| TC | 266 (41.4) | 4.6 | ||
| CC | 57 (8.9) | 5.1 | ||
| rs981621, Chr18p11.21, C18orf1 | 1.39 (1.23–1.57) | 1.65×10−7 | ||
| AA | 266 (41.4) | 8.1 | ||
| AG | 298 (46.4) | 5.0 | ||
| GG | 78 (12.2) | 3.7 | ||
| rs16861827, Chr1p36.13, IGSF21 | 1.70 (1.39–2.09) | 3.75×10−7 | ||
| CC | 513 (79.9) | 6.5 | ||
| CT | 125 (19.5) | 3.8 | ||
| TT | 4 (0.6) | 2.5 | ||
MST = median survival time, months
HR (95% CI) = hazard ratio (95% confidence interval). HR and P-value calculated using multivariable-adjusted Cox regression under a log-additive genetic model, adjusting for age, sex, stage of disease, and the top four principal components of population stratification
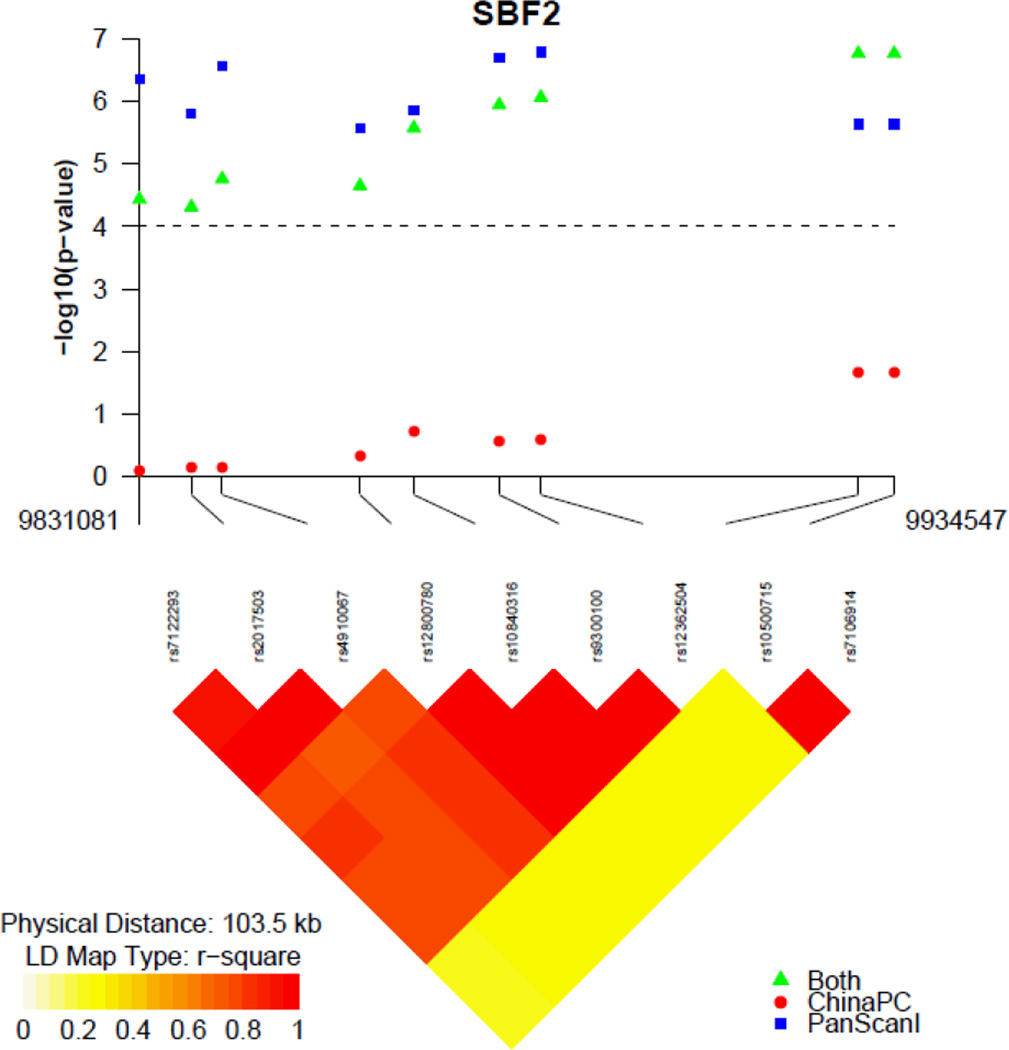
We selected the SNPs with P ≤ 10−5 from PanScan (131 SNPs) to evaluate in a joint analysis with cases from the ChinaPC study (Supplementary Table 2). In the joint analysis, the top two SNPs, rs10500715 and rs7106914, were identified on chromosome 11p15.4. These two SNPs were also located in the SBF2 gene, 43,520 and 48,429 base pairs from the top SNP identified in the PanScan analysis, respectively. These two SNPs were in perfect LD with each other in both populations; we selected rs10500715 as the tag SNP in this region for further analysis. In PanScan and ChinaPC participants, rs10500715 was associated with a HR for death in an additive model of 0.74 (95% CI, 0.66–0.84) and 0.79 (95% CI, 0.65–0.97), respectively. In the meta-analysis of the two studies, we observed a HR of 0.76 (95% CI, 0.68–0.84) with a P-value of 1.72×10−7 (Table 4). The P-value for heterogeneity was 0.30 across the two studies. The association of rs10500715 with patient survival was similar by disease stage, with a HR of 0.80 (95% CI, 0.64–1.01) in patients with localized disease, HR of 0.78 (95% CI, 0.65–0.93) in those with locally advanced disease, and HR of 0.81 (95% CI, 0.70–0.92) in those with metastatic disease. rs10500715 genotype was not statistically significantly associated with clinical stage in a joint analysis (P=0.10).
Table 4.
Hazard ratios and median survival times by rs10500715 T>G genotypes in the PanScan and ChinaPC studies
| rs10500715 |
||||
|---|---|---|---|---|
| No. (%) | MST* | HR (95% CI)† | P† | |
| PanScan Study | 0.74 (0.66–0.84) | 2.33×10−6 | ||
| TT | 204 (31.8) | 4.1 | ||
| GT | 313 (48.8) | 6.6 | ||
| GG | 125 (19.4) | 7.5 | ||
| ChinaPC Study | 0.79 (0.65–0.97) | 0.0216 | ||
| TT | 235 (64.7) | 6.1 | ||
| GT | 113 (31.1) | 7.9 | ||
| GG | 15 (4.2) | 5.4 | ||
| Meta-Analysis | 0.76 (0.68–0.84) | 1.72×10−7 | ||
| TT | 439 (43.7) | 5.7 | ||
| GT | 426 (42.4) | 7.1 | ||
| GG | 140 (13.9) | 7.3 | ||
MST = median survival time, months
HR (95% CI) = hazard ratio (95% confidence interval). HR and P-value calculated using multivariable-adjusted Cox regression under a log-additive genetic model, adjusting for age, sex, stage of disease, and the top four principal components of population stratification
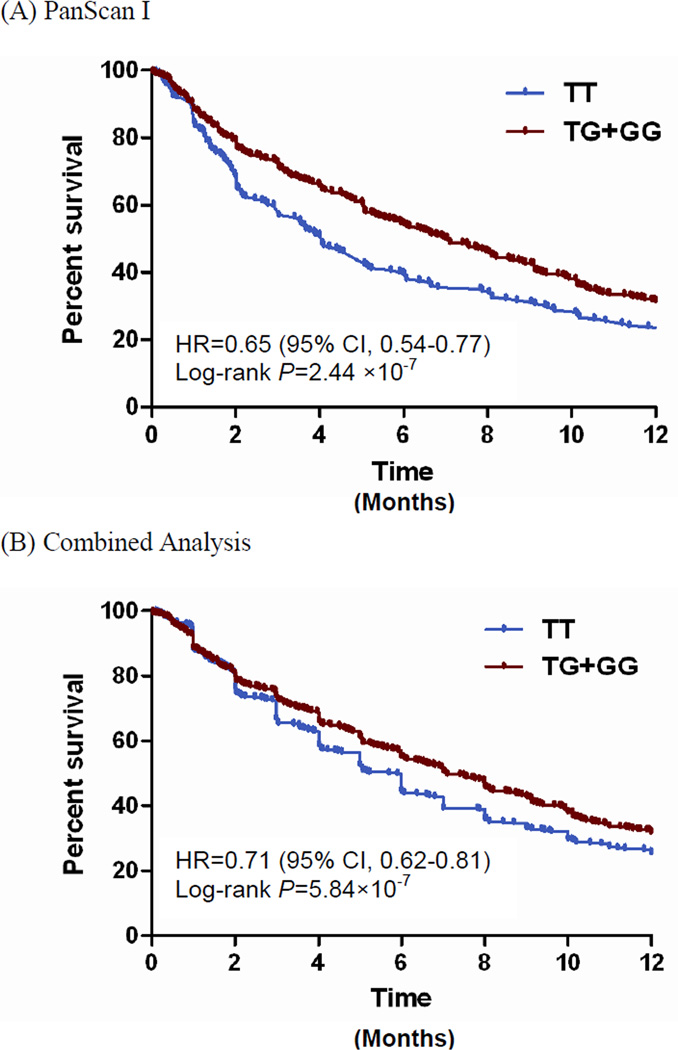
In a dominant model for rs10500715, the median overall survival was 4.1 months for cases with the TT genotype and 7.0 months for those with TG or GG genotype in PanScan (Figure 2). Multiple additional SNPs at the SBF2 gene locus were of marginal statistical significance in the PanScan cohort and combined analysis (Figure 3); seven SNPs in high LD (r2=0.76–1.00) in PanScan were associated with overall survival with P-values of 8.64×10−7 to 0.0002. We examined whether the top SNPs in SBF2 map to reported expression quantitative trait loci (eQTLs) for nearby genes using data from a published eQTL dataset.[24] However, we did not find that the SNPs were associated with known eQTLs in this dataset.
Figure 2.
Kaplan-Meier curves by rs10500715 T>G genotypes using a dominant model in the PanScan and Combined Analyses
Abbreviations: CI, confidence interval; HR, hazard ratio
Figure 3.
Association with survival and linkage disequilibrium of single nucleotide polymorphisms at the SBF2 gene locus
Association results are shown in the top panel for the joint analysis (green triangles), PanScan I (blue squares) and ChinaPC (red circle). The linkage disequilibrium plot was based on genotypes in the PanScan I cohort. Locations are from NCBI Genome Build 36.
DISCUSSION
In this genome-wide interrogation of germline genetic variants associated with pancreatic cancer survival, we used a two-stage analytical approach that took advantage of two large GWAS of pancreatic cancer in two independent populations. In both the PanScan population that included cases of European descent and in a combined analysis with cases from China, SNPs in the SET binding factor 2 (SBF2) gene were associated with survival time among patients with pancreatic adenocarcinoma. With a P-value <5×10−7, this association is likely to be replicated in follow-up studies,[26] although it did not reach P<5×10−8, which is often cited as a threshold for genome-wide significance in GWAS of incident disease. We also identified two additional genetic loci associated with pancreatic cancer survival in the PanScan cohort, which were not significant at P<5×10−7 in the combined analysis. Several loci associated with incident pancreatic cancer in two prior PanScan studies were not associated with patient survival.
The SNPs most highly associated with pancreatic cancer survival in the PanScan population (rs12362504) and in the joint analysis (rs10500715) are located intronic to the SBF2 gene and are in moderate linkage disequlibrium (r2=0.33) in the CEU population. SBF2 spans > 500 kb and 40 exons on chromosome 11p15.4 and is highly conserved across eukaryotes.[27, 28] This gene encodes for a protein in the myotubularin family of lipid phosphatases and is also known as myotubularin-related protein-13 (MTMR13). Similar to the known tumor suppressor gene phosphatase and tensin homolog (PTEN),[29] MTMR proteins function as phosphoinositide- 3-phosphatases and antagonize the activity of specific classes of phosphatidylinositol 3-kinases.[30] Although it contains an inactive phosphatase domain, SBF2 enhances the catalytic activity of and may alter the cellular localization of MTMR2, a phosphatase-competent member of the MTMR family.[31]
Notably, mutations in either SBF2 or MTMR2 lead to the development of Charcot-Marie-Tooth disease type 4B (CMT4B), an autosomal recessive disorder characterized by severe demyelinating peripheral neuropathy.[32–33] The known mutations in human SBF2 lead to a shortened or truncated protein,[33–34] and mice genetically engineered for loss of murine SBF2 develop peripheral neuropathy similar to that seen in humans with CMT4B.[35–36] The pathogenic mechanisms appear related to altered membrane trafficking of 3-phosphoinositides within nerve-supporting Schwann cells, due to malfunctioning of the SBF2-MTMR2 complex.[37] Importantly, in other cell types, SBF2 and MTMR2 appear to influence the sorting and degradation of cell surface receptors, such as the epidermal growth factor receptor (EGFR), with resultant alterations in downstream signaling.[30] For the SNPs identified in SBF2 in the current study, we did not identify alterations in expression of SBF2 or nearby genes using a publically available eQTL database. Further investigation will be necessary to determine the causative one or more SNPs marked by the single nucleotide changes identified in the current study, and the functional impact of such changes.
Recently, two genome wide association studies have implicated SBF2 as a susceptibility locus for circulating lipoproteins in European populations[28] and human stature in European and Chinese populations.[38] The most strongly associated SNPs from these studies were rs7938647 with circulating HDL and rs10734652 with stature, also located within introns of SBF2. The most strongly associated SNP in our combined analysis, rs10500715, is in moderate linkage disequilibrium with rs7938647 and rs10734652, with r2 values of 0.40 and 0.56, respectively in the PanScan cohort. Interestingly, pancreatic cancer incidence or mortality has been associated with height, obesity and metabolic derangements related to insulin resistance.[39–42]
In the PanScan population, variants at chromosomes 18p11.21 and 1p36.13 were also associated with survival. However, these associations were no longer significant in the combined analysis with cases from the ChinaPC study. Furthermore, the genes at these loci, chromosome 18 open reading frame 1 (C18orf1) and immunoglobin superfamily member 21 (IGSF21), respectively, encode for proteins with unclear function. Additional studies of these variants in populations of European and Asian ancestry will be necessary to determine whether these loci are truly associated with survival of patients with pancreatic adenocarcinoma.
The current study has a number of important strengths. In the PanScan population, large numbers of cohort participants provided germline DNA at a baseline time point and were then followed prospectively for development of disease. Pancreatic cancer diagnoses were subsequently determined from notification near the time of diagnosis or review of cancer and death registries. Therefore, the full spectrum of cases were ascertained, in terms of disease aggressiveness and stage of disease, rather than only those patients well enough to be captured in case-controls studies. This is of particular importance to studies of rapidly fatal diseases, such as pancreatic cancer, in which a better-prognosis population can result when subjects donate biologic samples after diagnosis. Furthermore, our study included a large number of pancreatic cancer cases with genome-wide SNP data, and these data originated from two well-established GWAS with strict quality control procedures.[18–20] We also pursued a two-stage design, with an initial analysis in PanScan participants and a subsequent combined analysis with ChinaPC participants, in an attempt to reduce the likelihood of false positive results.
Our study also has limitations. Among our participants, treatment programs likely varied, and we could not control for differences in treatment as the PanScan cohorts generally did not collect this information. Nevertheless, chemotherapy and radiation have had only a modest impact on patient survival,[3] and it is highly unlikely that treatment programs varied systematically by germline genotype. As described, we also performed combined analyses with a second group of pancreatic cancer cases, drawn from a case-control study in China, to reduce the chance of false positive results. However, linkage disequilibrium differs between Europeans and Asians, which can lead to false negative results in analyses that combine subjects of different race/ethnicity. Specifically, some loci may impact survival only in a particular race/ethnicity or the index signal may be best tagged by different polymorphisms in subjects of different race/ethnicity; phenomena demonstrated in studies of cancer risk.[19–20, 43–44] The difference in study design (nested prospective cohort study versus hospital-based case-control study) can also lead to false negative results in combined analyses due to recruitment of patients with dissimilar disease characteristics. However, all patients were known to have pancreatic adenocarcinoma, and all had available information on disease stage. Our top SNPs had significance levels of P<5×10−7; further studies are necessary to replicate these findings in additional large patient cohorts. As is inherent in the GWAS design, we have identified loci associated with pancreatic cancer survival, but further work is necessary to investigate the biologic mechanisms by which polymorphisms at these loci impact survival. Although we examined published eQTL datasets, we did not identify known gene expression changes related to the most significant SNPs in SBF2. Finally, we utilized overall mortality data in our analyses, as opposed to pancreatic cancer-specific mortality. However, pancreatic cancer is a highly lethal malignancy with cure rate less than five percent, such that overall mortality is a good surrogate for cancer-specific mortality in patients with this disease.
In summary, we performed a genome-wide analysis of germline genetic variants and survival of patients with pancreatic adenocarcinoma. Our large study implicates the SBF2 locus on chromosome 11p15.4 as a genetic region associated with overall survival among these patients. These results further implicate altered membrane trafficking of 3-phosphoinositides in pancreatic cancer growth and progression. Additional large datasets are needed to evaluate germline genetic variants and survival in patients with this highly lethal malignancy.
Supplementary Material
Significance of this study.
What is already known about the subject?
-
►
Five-year overall survival of patients with pancreatic cancer is approximately five percent.
-
►
Germline genetic variability can provide important prognostic information for patients with cancer.
-
►
Genome-wide association studies (GWAS) have identified several genetic variants associated with the development of pancreatic adenocarcinoma in European and Chinese populations, but few studies have examined variants related to survival
What are the new findings?
-
►
Previously identified genetic loci associated with the development of pancreatic adenocarcinoma were not associated with survival among those with the disease
-
►
In the first stage of the GWAS of patient survival, three regions, 11p15.4, 18p11.21, and 1p36.13, were the top-ranked loci among patients of European ancestry
-
►
In the joint analysis of > 1000 pancreatic cancer cases, variants at the SBF2 gene on chromosome 11p15.4 defined the top genetic locus associated with overall survival among patients of European and Chinese descent.
-
►
rs10500715 in SBF2 was associated with a HR for death in an additive model of 0.76 (95% CI, 0.68–0.84; P-value, 1.72×10−7) which was similar in European and Chinese populations and by disease stage.
How might it impact on clinical practice in the foreseeable future?
-
►
In patients with pancreatic adenocarcinoma, several germline variants were associated with overall survival. If confirmed in further replication and functional studies, these variants may add important information to define patient prognosis, with the potential to impact treatment decisions and clinical trial design.
-
►
Our results highlight a potential role for SBF2 in pancreatic tumorigenesis and further implicate altered membrane trafficking of 3-phosphoinositides in pancreatic cancer growth and progression.
Acknowledgments
Funding / Support:
The NYU Women’s Health Study is supported by research grants R01CA034588, R01CA098661, center grant P30CA016087 from the NCI and the center grant ES000260 from the National Institute of Environmental Health Sciences.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118–32119, 32122, 42107-26, 42129-32, and 44221. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators is available.
PHS was supported by grants CA 97193, CA 34944, CA 40360, HL 26490, and HL 34595 from the National Institutes of Health (Bethesda, MD).
The NHS, HPFS and WHS at Harvard were supported by the National Cancer Institute, National Institutes of Health (Grants No. P01 CA87969, P01 CA55075, P50 CA127003, R01 CA124908). Brian Wolpin, MD, MPH was supported by NCI K07 CA140790, an American Society of Clinical Oncology Career Development Award, Howard Hughes Medical Institute Early Career Physician-Scientist Award, and the Lustgarten Foundation for Pancreatic Cancer Research.
The Shanghai Men’s Health Study was supported by the National Cancer Institute extramural research grant [R01 CA82729]. The Shanghai Women’s Health Study was supported by the National Cancer Institute extramural research grant [R37 CA70867] and, partially for biological sample collection, by the Intramural Research Program of National Cancer Institute (Division of Cancer Epidemiology and Genetics). We are in debt to the contributions of Drs. Yu-Tang Gao and Yong-Bing Xiang in these two cohort studies. The studies would not be possible without the continuing support and devotion from the study participants and staff of the SMHS and SWHS.
PLCO was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS. The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the Screening Center investigators and staff of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Susan Yurgalevitch and staff at Westat, Inc for their assistance with survival data abstraction, and Craig Williams, Michael Furr and staff at Information Management Services, Inc. Most importantly, we acknowledge the study participants for their contributions to making this study possible.
The ATBC Study was supported by U.S. Public Health Service contracts N01-CN-45165, N01-RC-45035, N01-RC-37004, and HHSN261201000006C from the National Cancer Institute, Department of Health and Human Services, and by funding from the Intramural Research Program of the National Cancer Institute.
For the EPIC cohorts, all coauthors coordinated the initial recruitment and management of the studies. All authors contributed to the final paper. The authors thank all of the participants who took part in this research and the funders and support and technical staff who made this study possible. The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue contre le Cancer, Société 3M, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Medicale (France); Deutsche Krebshilfe, Deutsches Krebsforschungszentrum and Federal Ministry of Education and Research (Germany); Ministry of Health and Social Solidarity, Stavros Niarchos Foundation and Hellenic Health Foundation (Greece); Italian Association for Research on Cancer (AIRC) and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Health Research Fund (FIS), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Scientific Council and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK, Medical Research Council (United Kingdom).
CLUE II was supported by National Institute of Aging grant (5U01AG018033) and National Cancer Institute grants (CA105069, CA73790). The authors express their appreciation to the participants of the CLUE II cohort, and thank the staff at the George W. Comstock Center for Public Health Research and Prevention for their dedication and contributions to the study: Judy Hoffman-Bolton, Clara Krumpe, Kitty Spoonire and Betty Miner. Cancer incidence data have been provided by the Maryland Cancer Registry, Center for Cancer Surveillance and Control, Department of Health and Mental Hygiene, 201 W. Preston Street, Room 400, Baltimore, MD 21201, www.fha.state.md.us/cancer/registry/, 410-767-4055. We acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention (CDC) for the funds that helped support the availability of the cancer registry data.
The Cancer Prevention Study II Nutrition Cohort is supported by the American Cancer Society. The authors thank all of the men and women in the Cancer Prevention Study II Nutrition Cohort for their many years of dedicated participation in the study.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosures: Study authors have no conflicts to report
Author Contributions:
Study concept and design: Chen Wu, Peter Kraft, Charles Fuchs, Dongxin Lin, Brian Wolpin
Acquisition of data: All coauthors
Analysis and interpretation of data: Chen Wu, Peter Kraft, Charles Fuchs, Dongxin Lin, Brian Wolpin
Drafting of the manuscript: Chen Wu, Peter Kraft, Charles Fuchs, Dongxin Lin, Brian Wolpin
Critical revision of the manuscript for important intellectual content: All coauthors
Statistical analysis: Chen Wu, Emily Steplowski, Poorva Mudgal, Mousheng Xu, Peter Kraft
Obtained funding: Peter Kraft, Rachael Stolzenberg-Solomon, Particia Hartge, Charles Fuchs, Dongxin Lin, Brian Wolpin
Administrative, technical, or material support: Chen Wu, Peter Kraft, Rachael Stolzenberg-Solomon, Michelle Brotzman, Geoffry Tobias, Charles Fuchs, Dongxin Lin, Brian Wolpin
Study supervision: Peter Kraft, Charles Fuchs, Dongxin Lin, Brian Wolpin
License Language: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Gut editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Lyon, France: International Agency for Research on Cancer; 2010. [accessed on 30/03/2012]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 3.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 4.Azzato EM, Tyrer J, Fasching PA, et al. Association between a germline OCA2 polymorphism at chromosome 15q13.1 and estrogen receptor-negative breast cancer survival. J Natl Cancer Inst. 2010;102:650–662. doi: 10.1093/jnci/djq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Xu B, Yuan P, et al. Genome-wide interrogation identifies YAP1 variants associated with survival of small-cell lung cancer patients. Cancer Res. 2010;70:9721–9729. doi: 10.1158/0008-5472.CAN-10-1493. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Ye Y, Rosell R, et al. Genome-wide association study of survival in non-small cell lung cancer patients receiving platinum-based chemotherapy. J Natl Cancer Inst. 2011;103:817–825. doi: 10.1093/jnci/djr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson P, Hanahan D. Cancer. Breaching the cancer fortress. Science. 2009;324:1400–1401. doi: 10.1126/science.1175940. [DOI] [PubMed] [Google Scholar]
- 8.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19:429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 11.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asomaning K, Reid AE, Zhou W, et al. MDM2 promoter polymorphism and pancreatic cancer risk and prognosis. Clin Cancer Res. 2008;14:4010–4015. doi: 10.1158/1078-0432.CCR-07-4187. [DOI] [PubMed] [Google Scholar]
- 14.Couch FJ, Wang X, Bamlet WR, et al. Association of mitotic regulation pathway polymorphisms with pancreatic cancer risk and outcome. Cancer Epidemiol Biomarkers Prev. 2010;19:251–257. doi: 10.1158/1055-9965.EPI-09-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong X, Javle M, Hess KR, et al. Insulin-like growth factor axis gene polymorphisms and clinical outcomes in pancreatic cancer. Gastroenterology. 2010;139:464–473. doi: 10.1053/j.gastro.2010.04.042. 473 e461–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D, Liu H, Jiao L, et al. Significant effect of homologous recombination DNA repair gene polymorphisms on pancreatic cancer survival. Cancer Res. 2006;66:3323–3330. doi: 10.1158/0008-5472.CAN-05-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okazaki T, Javle M, Tanaka M, et al. Single nucleotide polymorphisms of gemcitabine metabolic genes and pancreatic cancer survival and drug toxicity. Clin Cancer Res. 2010;16:320–329. doi: 10.1158/1078-0432.CCR-09-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Miao X, Huang L, et al. Genome-wide association study identifies five loci associated with susceptibility to pancreatic cancer in Chinese populations. Nat Genet. 2012;44:62–66. doi: 10.1038/ng.1020. [DOI] [PubMed] [Google Scholar]
- 21.Wolpin BM, Kraft P, Gross M, et al. Pancreatic cancer risk and ABO blood group alleles: results from the pancreatic cancer cohort consortium. Cancer Res. 2010;70:1015–1023. doi: 10.1158/0008-5472.CAN-09-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Handbook. New York: Springer; 2002. [Google Scholar]
- 23.Young JLJ, Roffers SD, Ries LAG, et al. SEER Summary Staging Manual - 2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. NIH Pub. No. 01-4969. [Google Scholar]
- 24.Dixon AL, Liang L, Moffatt MF, et al. A genome-wide association study of global gene expression. Nat Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 25.Innocenti F, Owzar K, Cox NL, et al. A genome-wide association study of overall survival in pancreatic cancer patients treated with gemcitabine in CALGB 80303. Clin Cancer Res. 2012;18:577–584. doi: 10.1158/1078-0432.CCR-11-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panagiotou OA, Ioannidis JP. What should the genome-wide significance threshold be? Empirical replication of borderline genetic associations. Int J Epidemiol. 2012;41:273–286. doi: 10.1093/ije/dyr178. [DOI] [PubMed] [Google Scholar]
- 27.Kirfel J, Senderek J, Moser M, et al. Cloning, expression and characterization of the murine orthologue of SBF2, the gene mutated in Charcot-Marie-Tooth disease type 4B2. Gene Expr Patterns. 2006;6:978–984. doi: 10.1016/j.modgep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Chasman DI, Pare G, Mora S, et al. Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet. 2009;5:e1000730. doi: 10.1371/journal.pgen.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying H, Elpek KG, Vinjamoori A, et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov. 2011;1:158–169. doi: 10.1158/2159-8290.CD-11-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berger P, Tersar K, Ballmer-Hofer K, et al. The CMT4B disease-causing proteins MTMR2 and MTMR13/SBF2 regulate AKT signalling. J Cell Mol Med. 2011;15:307–315. doi: 10.1111/j.1582-4934.2009.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger P, Berger I, Schaffitzel C, et al. Multi-level regulation of myotubularin-related protein-2 phosphatase activity by myotubularin-related protein-13/set-binding factor-2. Hum Mol Genet. 2006;15:569–579. doi: 10.1093/hmg/ddi473. [DOI] [PubMed] [Google Scholar]
- 32.Bolino A, Muglia M, Conforti FL, et al. Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet. 2000;25:17–19. doi: 10.1038/75542. [DOI] [PubMed] [Google Scholar]
- 33.Senderek J, Bergmann C, Weber S, et al. Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy type 4B2/11p15. Hum Mol Genet. 2003;12:349–356. doi: 10.1093/hmg/ddg030. [DOI] [PubMed] [Google Scholar]
- 34.Azzedine H, Bolino A, Taieb T, et al. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet. 2003;72:1141–1153. doi: 10.1086/375034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tersar K, Boentert M, Berger P, et al. Mtmr13/Sbf2-deficient mice: an animal model for CMT4B2. Hum Mol Genet. 2007;16:2991–3001. doi: 10.1093/hmg/ddm257. [DOI] [PubMed] [Google Scholar]
- 36.Robinson FL, Niesman IR, Beiswenger KK, et al. Loss of the inactive myotubularin-related phosphatase Mtmr13 leads to a Charcot-Marie-Tooth 4B2-like peripheral neuropathy in mice. Proc Natl Acad Sci U S A. 2008;105:4916–4921. doi: 10.1073/pnas.0800742105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson FL, Dixon JE. Myotubularin phosphatases: policing 3-phosphoinositides. Trends Cell Biol. 2006;16:403–412. doi: 10.1016/j.tcb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Lei SF, Tan LJ, Liu XG, et al. Genome-wide association study identifies two novel loci containing FLNB and SBF2 genes underlying stature variation. Hum Mol Genet. 2009;18:1661–1669. doi: 10.1093/hmg/ddn405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 41.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 42.Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286:921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 43.Chung CC, Chanock SJ. Current status of genome-wide association studies in cancer. Hum Genet. 2011;130:59–78. doi: 10.1007/s00439-011-1030-9. [DOI] [PubMed] [Google Scholar]
- 44.Haiman CA, Chen GK, Blot WJ, et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet. 2011;7:e1001387. doi: 10.1371/journal.pgen.1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.