Abstract
Background
Schizophrenia is associated with immune system dysfunction, including abnormal blood immune cell parameters. We performed a meta-analysis of these associations, considering the effect of clinical status and antipsychotic treatment following an acute exacerbation of psychosis.
Methods
We identified articles by searching Pub Med, PsychInfo, and ISI, and the reference lists of identified studies.
Results
Sixteen studies of blood lymphocytes met the inclusion criteria. There was insufficient data for a meta-analysis of the mononuclear phagocytic system. In cross-sectional studies, there was a significant increase in the CD4% and CD56% in acutely relapsed inpatients (AR). Absolute levels of total lymphocytes, CD3, and CD4, and the CD4/CD8 ratio were significantly increased, and the CD3% was significantly decreased in drug-native first-episode psychosis (FEP). In longitudinal studies, the CD4/CD8 ratio appeared to be state-related markers, as it decreased following antipsychotic treatment for acute exacerbations of psychosis. Absolute CD56 levels appeared to be a trait marker, as levels significantly increased following antipsychotic treatment for relapse.
Discussion
Blood lymphocyte abnormalities in drug-naïve FEP suggest an effect that may be independent of antipsychotic medications. While some parameters (CD4/CD8) may be state markers for acute exacerbations of psychosis, others (CD56) may be trait markers, however more longitudinal studies are needed. Although these findings could provide the basis for future hypothesis testing, a relatively small number of studies and subjects, lack of correlative data with clinical features, and inadequate consideration of potential confounding factors limit the results.
Keywords: Schizophrenia, Monocytes, Lymphocytes, Meta-analysis, First-episode psychosis
Introduction
Schizophrenia is a heterogeneous disorder with respect to symptomatology, disease course, and outcome (1). Nonetheless, the clinical course is often characterized by recurrent relapses, which are associated with adverse outcomes, including treatment-resistant symptoms, cognitive decline, and functional disability. Immune system abnormalities in schizophrenia have been one of the more enduring findings in the field, albeit with significant heterogeneity in results, including negative studies. Despite the inherent complexity of this area of research, several recent findings provide further support for an association between immune system abnormalities in the pathophysiology of some patients with schizophrenia. Immune system-related genes, including cytokines (2), cytokine pathways (3), and the major histocompatibility complex (4-6) are all associated with schizophrenia. Five randomized, double-blinded trials in relapsed patients found that adjunctive treatment with non-steroidal anti-inflammatory drugs (NSAIDs) significantly improved psychopathology (7-11), and that baseline blood cytokine levels may a predictor of treatment response (9,12). Another study found that some patients with FEP have potentially pathogenic autoantibodies to central nervous system (CNS) antigens in the absence of overt signs of encephalitis (13). In a meta-analysis in Biological Psychiatry, we found that serum interleukin (IL)-1β, IL-6, and transforming growth factor (TGF)-β appeared to be state markers for acute psychosis (levels significantly increased in patients with acute relapse of schizophrenia and first-episode drug-naïve psychosis (FEP), and then significantly decreased following treatment for relapse), whereas serum IL-12, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and soluble IL-2 receptor (sIL-2R) appeared to be trait markers for schizophrenia (levels significantly increased in acute psychosis, but did not significantly decrease following treatment for relapse (14).
Cytokines are key signaling molecules that coordinate both innate (e.g., granulocytes, monocytes/macrophages, and natural killer cells) and adaptive (e.g., B- and T-lymphocytes) arms of the immune system, and exert effects in the periphery and the brain. They are produced by both immune and non-immune cells, and exert their effects by binding specific cytokine receptors on a variety of target cells. In our previous work it was not possible to identify the specific cell source (s) of the observed cytokine abnormalities, as studies did not simultaneously measure cytokines and immune cells (14). Abnormal immune cells in the peripheral blood of patients with an acute exacerbation of schizophrenia, independent of antipsychotic medications, were first reported almost 50 years ago (15). While previous studies of immune cells in schizophrenia have been summarized (16), to date a systematic, quantitative review of these findings has not been performed. Such a quantitative review is an important “next step” to understand potential mechanisms whereby immune system dysfunction might contribute to the pathophysiology of schizophrenia. We present a meta-analysis of blood immune cell parameters (absolute levels and/or proportions), considering the effects of clinical status, antipsychotic treatment following an acute exacerbation of psychosis, and correlations with clinical features. The primary aim was to establish the characteristic immune profile that emerges in schizophrenia, and in doing so, to integrate these findings with data on cytokine abnormalities and further evaluate leading hypotheses for the immune-cytokine basis of schizophrenia.
Methods and Materials
Study selection
Studies of blood monocytes and lymphocytes (including lymphocyte subsets) schizophrenia were systematically searched using Medline (PubMed), PsycInfo (via Ovid), and ISI (Science and Social Science Citation Index) in February 2012. The primary search strategy was “(lymphocytes or T-lymphocytes or B-lymphocytes or monocytes or macrophages) and (schizophrenia or psychotic disorders)”. Limiting results to human studies in English identified 419 articles from Pub Med, 254 for PsycInfo, and 700 for ISI, and the resulting matches were screened. From these sources, as well as a manual review of reference lists, we identified 50 potential studies for inclusion, which are described in the Supplement (16-65). The majority of initial matches were excluded because they 1) were review articles, 2) did not present data on monocyte and/or lymphocyte parameters (absolute levels or proportions), or 3) were genetic studies of peripheral blood mononuclear cells.
The inclusion criteria were: la) cross-sectional studies of blood monocytes or lymphocytes (including lymphocyte subsets) in patients with schizophrenia or related psychotic disorder (including schizophreniform disorder, brief psychotic disorder, psychotic disorder not otherwise specified, delusional disorder, and schizoaffective disorder) and healthy controls, or lb) studies assessing blood monocytes or lymphocytes (including lymphocyte subsets) in patients with an acute exacerbation of psychosis (defined as either AR or FEP) at baseline and again following a period of antipsychotic treatment for relapse, 2) clinical status of patients clearly defined as either: acutely relapsed inpatients (AR), first-episode psychosis (FEP), or stable medicated outpatients (SO), and 3) studies published in English. For studies that included patients with different clinical statuses (e.g., both AR and FEP), if stratified data were not presented in the manuscript, we attempted to contact study authors. The exclusion criteria were: 1) studies without a control group (except for studies with serial measurements of monocytes or lymphocytes in patients with an acute exacerbation of psychosis), 2) studies that did not present mean and standard deviations (SDs) for monocyte and/or lymphocyte parameters (after attempting to contact the study authors), 3) significant overlap in study population, 4) genetic studies related to peripheral blood mononuclear cells, 5) studies in which >20% of subjects were taking clozapine, and 6) studies of granulocytes and/or eosinophils. We excluded granulocytes and eosinophils as the majority of studies were on patients treated with clozapine, which can adversely affect these parameters. Furthermore, findings from our meta-analysis on cytokines also suggest that clozapine may have different effects on immune cell parameters (8).
After independent searches, review of study methods by three authors (BJM, BG, and DS) and attempts to contact the authors, 16 studies met the inclusion criteria. There was universal agreement on the included studies. There was insufficient data for metaanalysis of the blood mononuclear phagocyte system. Cross-sectional studies of blood lymphocytes included 10 studies of AR, 6 studies of FEP, and 2 studies of SO (these numbers exceed 16 because some studies included subjects with multiple clinical statuses). Additionally, 7 longitudinal studies assessed blood lymphocyte parameters in patients with an acute exacerbation of psychosis at baseline and again after antipsychotic treatment. Six of these seven studies also had data on controls, and so baseline data were also included among the cross-sectional studies of AR and/or FEP. Thirty-four studies were excluded due to: clinical status not available (n=13), means and/or SDs not available (n=10), stratified data not available by clinical status (n=4), no control group (n=3), CSF studies (n=2), significant study population overlap (n=1), and samples obtained from chronic inpatients (n=1). A flow chart summarizing the study selection process is presented in Figure S1 (see Supplement).
Data extraction and Meta-analysis
Data were extracted (sample size, mean, and standard deviation for schizophrenia and controls), for blood lymphocyte and/or lymphocyte subset parameters assessed in each study. One author (BJM) extracted all data, which was independently verified two other authors (BG, DS). The cell surface markers used to phenotype different immune cell parameters are described in Table 1. We then calculated effect size estimates (Hedges' g) for lymphocyte and/or lymphocyte subsets in each study, and these data are included in the Supplement. Random effects pooled effect size estimates and 95% confidence intervals were calculated using the method of DerSimonian and Laird. The random effects model is more conservative that the fixed effects model, as it yields a lower Type I error rate and wider confidence intervals, and its use was supported by significant heterogeneity between studies (65). Immune cell parameters assessed in only one study were not included. Separate meta-analyses were performed for blood lymphocyte parameters for cross-sectional studies by each clinical status (AR, FEP, and SO), as well as for longitudinal studies of these parameters between baseline and endpoint following antipsychotic treatment for an acute exacerbation of psychosis (AR or FEP). P-values were considered statistically significant at the α=0.05 level. The statistical analyses were performed in Stata 10.0 (StataCorp LP, College Station, TX).
Table 1. Cell Surface Markers Used for Phenotyping Immune Parameters.
| Marker | Immune Parameter |
|---|---|
|
|
|
| CD3 | T-lymphocytes |
| CD4 | T-helper lymphocytes |
| CD5 | B1a subset of B-lymphocytes |
| CD8 | T-suppressor/cytotoxic lymphocytes |
| CD4/CD8 | T-helper/suppressor lymphocyte ratio |
| CD19 | B-lymphocytes |
| CD25 | Activated T-lymphocytes |
| CD56 | Natural Killer cells |
| Lymphocytes | Total lymphocyte count |
| Monocytes | Total monocyte count |
| WBC | Total white blood cell count |
The meta-analysis procedure also calculates a χ2 value for the heterogeneity in effect size estimates. For any immune cell parameter measured in 3 or more studies with a significant between-study heterogeneity χ2 (p<0.05), we performed a sensitivity analysis. This was done by removing one study at a time and repeating the meta-analysis procedure for that immune cell parameter, to examine its impact on the effect size estimate and heterogeneity χ2.
For descriptive purposes, we also extracted data on correlations between monocytes or lymphocytes and any clinical features in patients, including, age, age of onset of illness, duration of illness, and psychopathology scores.
Results
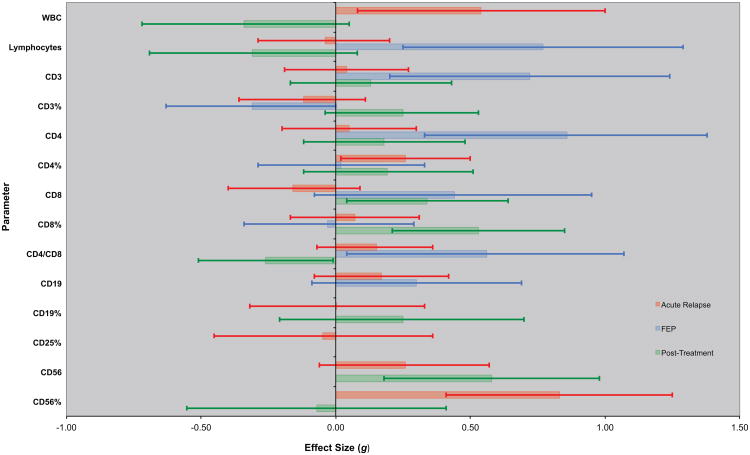
Figure 1 presents effect size estimates with 95% confidence intervals (CIs) by clinical status. These data are also presented in tabular form in the Supplement.
Figure 1. Immune Cell Parameters in Schizophrenia by Clinical Status.
Effect size estimates and 95% CIs for immune cell parameters in cross-sectional studies of acute relapse of psychosis (AR) and drug-naive first-episode psychosis (FEP) versus controls are represented by red and blue bars and error bars, respectively. For AR and FEP, positive effect sizes (bars going to the right) indicate that the parameter was higher in schizophrenia than controls; negative effect sizes (bars going to the left) indicate that levels were higher in controls than in patients with schizophrenia. Similarly, green bars and error bars represent effect size estimates and 95% CIs for the change in levels of immune parameters in longitudinal studies following antipsychotic treatment for an acute exacerbation of psychosis. Positive effect sizes (bars going to the right) indicate that the parameter increased following antipsychotic treatment for acute psychosis; negative effect sizes (bars going to the left) mean that the parameter decreased following antipsychotic treatment. Error bars that exclude 0 are significant at the p<0.05 level.
Acutely Relapsed Inpatients
Blood total white blood cell (WBC) count and the percentage of CD4 and CD56 lymphocytes (CD4% and CD56%) were significantly increased in AR compared to controls (p≤0.04 for each). For a majority of lymphocyte subsets, there was significant heterogeneity in effect size estimates. In sensitivity analyses, the heterogeneity was no longer significant and there was also a significant increase in CD4% (ES=0.35, 95% CI 0.10-0.61, p<0.01), the CD4/CD8 ratio (ES=0.31, 95% CI 0.08-0.54, p<0.01, and absolute CD56 levels (ES=0.63, 95% CI 0.26 - 0.99, p<0.01), a trend for an increase in absolute CD4 levels (ES=0.27, 95% CI −0.01-0.56, p=0.06), and a significant decrease in CD3% (ES=−0.49, 95% CI −0.77 - −0.21, p<0.01) after removing one study ((17) for CD4%, (16) for CD4/CD8, (56) for CD56, (16) for CD4, and (42) for CD3%).
Drug-Naïve First-Episode Psychosis
Effect sizes for FEP were similar in direction and magnitude to those in AR, with a significant increase in absolute CD4 levels and the CD4/CD8 ratio, and a significant decrease in CD3% (p≤0.05 for each). Blood lymphocyte, CD3, and CD4 levels, and the CD4/CD8 ratio were significantly increased (p≤0.03 for each), and there was a decrease in CD3% in FEP versus controls (p=0.05), without significant heterogeneity in these results.
Stable Medicated Outpatients
The CD3% was significantly lower in SO compared to controls (ES=−0.41, 95% CI −0.79 - −0.03, p=0.04), without significant heterogeneity in this results. No other lymphocyte subsets were measured in at least 2 studies of stable medicated outpatients.
Lymphocyte Parameters Following Antipsychotic Treatment for Acute Psychosis
Figure 1 and the Supplement also present effect size estimates with 95% confidence intervals for changes in lymphocyte (or lymphocyte subset) levels following a mean of 9 weeks of antipsychotic treatment for an acute exacerbation of psychosis. Antipsychotic medication was standardized in only one of the seven studies (14%). There was a significant increase in absolute CD8 (p=0.03) and CD56 (p<0.01) levels and the CD8% (p<0.01), and a significant decrease in the CD4/CD8 ratio (p=0.05) following antipsychotic treatment. There was also a trend for a significant decrease in total WBC count following antipsychotic treatment (p=0.09). In a sensitivity analysis, after removing one study (20), the heterogeneity was no longer significant and there was a significant increase in CD3% following antipsychotic treatment (ES=0.46, 95% CI 0.14 - 0.78, p<0.01). In another sensitivity analysis, there was a trend for a significant decrease in absolute CD4 levels following antipsychotic treatment (ES=−0.33, 95% CI −0.67 -0.01, p=0.06) after removing one study (56)
Correlations with Clinical Features
Only 4 of 18 (22%) studies included in the meta-analysis provided data on correlations between immune cell parameters and clinical features, and there were no replicated, significant findings.
Discussion
Taken together, our findings suggest that immune cell parameters in schizophrenia may vary with clinical status. The CD4/CD8 ratio appeared to be a state-related marker, as it was significantly increased in FEP, and significantly decreased following antipsychotic treatment for acute psychosis. In contrast, absolute CD56 levels appeared to be a trait marker, as levels significantly increased following antipsychotic treatment. CD3% may also be a trait marker, as this proportion was significantly decreased in stable medicated outpatients. A paucity of studies investigated correlations between immune cell parameters and clinical features in schizophrenia, which is an important gap in this literature.
We believe ours to be the first systematic, quantitative review of immune cell parameters in schizophrenia. An important strength of our study is that we considered the effects of clinical status and antipsychotic treatment following an acute illness exacerbation. Nonetheless, our results should be interpreted with caution in light of small numbers of studies and subjects, as well as significant heterogeneity across studies (including different assay methodologies and non-standardized antipsychotic treatment). All immune cell parameters were assessed in fewer than 200 patients (and many in fewer than 100) and in 6 or fewer different studies. However, the alterations in drug-naïve first-episode psychosis suggest an association between abnormalities in immune cell parameters and acute exacerbations of schizophrenia that is independent of antipsychotic medications.
There are several other limitations of the present study. Many studies were excluded because either the patient's clinical status or summary data on immune cell parameters were not available. Many of these studies would have otherwise been included in the meta-analysis, and their influence on the results is uncertain. Furthermore, many studies did not control for potential confounding factors that could account for differences in immune cell parameters between different populations at different stages of illness, including age, race or ethnicity, body mass index, smoking, socioeconomic status, and Cortisol (66-69). For example, effects of age and gender were considered in 100% of studies. By contrast, blood was collected at a standardized time of day in only 38% of studies. Even fewer studies considered potential effects of smoking (13%), Cortisol (6%), and body mass index (0%). One study that controlled for many potential confounders reported a significant difference in several immune cell parameters between patients and controls (16). However, this study found that CD19 numbers were positively correlated with the free Cortisol index (as a measure of stress), and CD56 levels were negatively correlated with cotinine (as a measure of smoking). The later result raises the possibility that smoking could confound our findings for CD56.
Another limitation is the potential effect of medication status of the subjects in the AR group. In these studies, blood samples were taken while subjects were still acutely ill, generally on admission or within days of admission. Medication nonadherence would be expected to be common in acutely relapsed inpatients, and indeed many of the studies reported that a large proportion of subjects were drug-free at the time of sampling. However, stratified data based on medication status were generally not available. Two longitudinal studies included in the meta-analysis (20,56) did not find any significant changes in lymphocyte parameters between baseline and day 7 of antipsychotic treatment, suggesting that the effect of medication in the AR group might have been minimal.
We were not able to perform a meta-analysis of the blood mononuclear phagocytic system, however, there is evidence that it may play a role in the pathophysiology of schizophrenia (reviewed in 70). One study (21) also found increased blood levels of IL-1β, IL-6, and TNF-α, and increased levels of S100B, a marker of blood-brain barrier disruption. Another study found a significant decrease (to the same level as in controls) in IL-lβ and TNF-α secretion from isolated blood monocytes following one month of first-generation antipsychotic treatment for an acute relapse of schizophrenia (71). Drexhage et al. (2010) found evidence of a pro-inflammatory gene expression signature in monocytes of patients with recent-onset schizophrenia (72). Furthermore, a retrospective analysis of serial blood samples in patients with schizophrenia found that monocytosis was associated with a worsening of psychotic symptoms, and in some cases the monocytosis resolved with a change in antipsychotic treatment during hospitalization (28). Thus, further studies of the mononuclear phagocyte system, an important source of many pro-inflammatory cytokines that are increased in schizophrenia (14), are warranted.
This study focused only on blood immune cell parameters in schizophrenia, but there is evidence for immune abnormalities in the CSF and brain as well. Nikkila et al. (47) found a significantly increased proportion of CSF macrophages and activated lymphocytes in patients with acute psychosis compared to controls. Another study by the same group also found a significantly increased proportion of CSF macrophages in patients with acute psychosis compared to controls (46). Evidence from PET (73,74) and post-mortem (75,76) studies also support a role for neuroinflammation/microglial activation in schizophrenia
Several other studies support an association between changes in immune cell parameters and acute exacerbations of psychosis. Muller et al. (12) found a significant decrease in CD19 B-lymphocytes in patients treated with risperidone plus placebo and those treated with risperidone plus celecoxib, with a more pronounced decrease in the celecoxib group. Furthermore, the celecoxib group only, the decrease in CD19 cells was significantly positively correlated with the decrease in PANSS negative scale scores. Another study found that at baseline a relative granulocytosis and lymphopenia prospectively predicted poorer recovery in positive, but not negative, symptoms after 6 months of antipsychotic treatment (65). Thus, given the efficacy of adjunctive NSAIDs in improving psychopathology in relapsed patients (7-11), these findings support the plausibility of the hypothesis that immune changes are not a merely artifact of increased stress associated with acute psychosis, but may play a role in the pathophysiology of relapse in schizophrenia. In addition to the association between monocytosis and worsening psychosis mentioned above (28), other studies have found that intra-individual changes in other immune parameters, including CSF IL-2 levels and in vitro IL-2 production may predict relapse in some patients (77,78). Further investigation of potential relapse predictive markers is needed.
Three hypotheses regarding an immune-cytokine basis for schizophrenia have been postulated. The “macrophage-T-lymphocyte theory” proposed that cytokines produced by chronically activated macrophages and T-lymphocytes are the fundamental mediators of schizophrenia (79). Schwarz et al. (80) proposed the “Th2-hypothesis”, which postulates that a shift from Th1-cell (cytotoxic) towards Th2-cell (antibody-dependent) immune responses predominates in schizophrenia. Lastly, the “microglial hypothesis” proposed that activated CNS microglia release pro-inflammatory cytokines and free radicals that cause abnormal neurogenesis, neuronal degradation, and white matter abnormalities contributing to the pathophysiology of schizophrenia (81). Our results inform on these hypotheses. We found abnormal blood lymphocyte parameters, including increased CD4/CD8 ratio in FEP, as well as increased CD56 levels and decreased CD4/CD8 following antipsychotic treatment for relapse. CD4 T-lymphocytes are important sources of IFN-γ and IL-12. IL-12 is also involved in natural killer cell (CD56) activation, and these cells secrete TNF-α and IFN-γ. All of these cytokines were abnormal in our previous metaanalysis (14). Thus, these findings are not inconsistent with the “macrophage-T-lymphocyte theory”. While we found evidence for abnormalities in the CD4/CD8 ratio, studies did not distinguish between T-helper (Th)1 versus Th2 CD4 lymphocytes, limiting our ability to make inferences regarding the “Th2 hypothesis”. We were not able to perform a meta-analysis of the mononuclear phagocyte system, although as noted above, there is evidence of dysfunction broadly consistent with the “microglial hypothesis”
We emphasize that our results should be interpreted with caution in light of a limited number of studies and small sample sizes, between study heterogeneity, and a general lack of consideration of potential confounding factors. However, these findings are of importance as acute relapse of psychosis is common and is associated with adverse outcomes, including increased treatment-resistant symptoms, cognitive decline, and functional disability (82-84). More longitudinal studies of immune cell parameters in schizophrenia are needed evaluate if these abnormalities are specific to illness exacerbations or schizophrenia in general, and whether they are a temporal predictor of relapse, and should control for potential confounding factors. Studies should also simultaneously measure blood cytokines and immune cell subsets, towards better identification of the source (s) of specific cytokines in schizophrenia. For example, one recent study measured intracellular cytokine levels in monocytes of patients with schizophrenia (85). They found significantly lower baseline monocytic IL-6 levels, but significantly increased monocytic intracellular IL-6 production after stimulation with lipopolysaccharide in patients with schizophrenia compared to controls. Correlations between immune cell parameters and clinical features should be routinely assessed in studies, towards better understanding of potential mechanisms between immune dysfunction and psychopathology. Well-replicated findings might suggest novel immunomodulatory treatment strategies. Additionally, stratifying patients based on immune alterations may increase the signal-to-noise ratio of treatment trials of adjunctive anti-inflammatory agents in schizophrenia. Taken together, immune cell parameters may serve as potential biomarkers and therapeutic targets in the etiopathophysiology and clinical course of schizophrenia.
Supplementary Material
Acknowledgments
The authors wish to thank Linda H. Young for assistance.
Footnotes
Disclosures: Dr. Miller, in the past 3 years, Dr. Miller has received grant support from the National Institute of Mental Health (1K23MH098014-01), the GHSU Intramural Scientist Training Program, the GHSU Brain & Behavior and Immunotherapy Discovery Institutes, the University of Oulu (Finland), the Thule Institute of the University of Oulu, and Oy H. Lundbeck Ab; Research support from the National Institutes of Health Clinical Loan Repayment Program; Consultancy fees for surveys from Medefied Europe and Plaza Research, on behalf of Genetech/Roche; Speaker fees for grand rounds lectures from the Maryland Psychiatric Research Center and the Texas A&M University and Scott and White Hospital Department of Psychiatry; Payment for a survey from e-Rewards Medical Market Research.
Dr. Buckley received Grant/Research Support from the National Institute of Mental Health, Janssen Pharmaceutica, Pfizer, and Sunovion, and is a Consultant (Honorarium/Expenses) for the National Institute of Mental Health.
Dr. Mellor received funding support from the NIH (AI083005, AI075165), the Juvenile Diabetes Research Foundation, and the Carlos and Marguerite Mason Trust. Dr. Mellor is a member of the Scientific Advisory Board of NewLink Genetics Inc., and receives compensation for this service.
Ms. Gassama and Dr. Sebastian report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Messias EL, Chen CY, Eaton WW. Epidemiology of schizophrenia: review of findings and myths. Psychiatr Clin North Am. 2007;30:323–38. doi: 10.1016/j.psc.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu PC, Nwulia E, Sawa A. Using bioinformatic tools. Am J Psychiatry. 2009;166:854. doi: 10.1176/appi.ajp.2009.09060908. [DOI] [PubMed] [Google Scholar]
- 3.Jia P, Wang L, Meltzer HY, Zhao Z. Common variants conferring risk of schizophrenia: a pathway analysis of GWAS data. Schizophr Res. 2010;122:38–42. doi: 10.1016/j.schres.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Schizophrenia Consortium. Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefannson H. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller N, Riedel M, Scheppach C, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–34. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 8.Akhondzadeh S, Tabatabaee M, Amini H, et al. Celecoxib as adjunctive therapy in schizophrenia: a doubleblind randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–85. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 9.Laan W, Grobbee DE, Selten JP, et al. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo controlled trial. J Clin Psychiatry. 2010;71:520–7. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- 10.Muller N, Krause D, Dehning S, et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, doubleblind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Chun Chen D, Long Tan Y, et al. A double-blind, placebo-controlled trial of celecoxib added to risperidone in first-episode and drug-naive patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256(Suppl 2):50. [Google Scholar]
- 12.Muller N, Ulmschneider M, Scheppach C, et al. COX-2 inhibition as a treatment approach in schizophrenia: Immunological considerations and clinical effects of celecoxib add-on therapy. Eur Arch Psychiatry Clin Neurosci. 2004;254:14–22. doi: 10.1007/s00406-004-0478-1. [DOI] [PubMed] [Google Scholar]
- 13.Zandi MS, Irani SR, Lang B. Disease-relevant autoantibodies in first episode schizophrenia. J Neurol. 2011;258:686–8. doi: 10.1007/s00415-010-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller B, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fessel WJ, Hirata-Hibi M. Abnormal leukocytes in schizophrenia. Arch Gen Psychiatry. 1963;9:601–13. doi: 10.1001/archpsyc.1963.01720180073010. [DOI] [PubMed] [Google Scholar]
- 16.Steiner J, Jacobs R, Panteli B, et al. Acute schizophrenia is accompanied by reduced T cell and increased B cell immunity. Eur Arch Psychiatry Clin Neurosci. 2010;260:509–18. doi: 10.1007/s00406-010-0098-x. [DOI] [PubMed] [Google Scholar]
- 17.Achiron A, Noy S, Pras E, Lereya J, Hermesh H, Laor N. T-cell subsets in acute psychotic schizophrenic patients. Biol Psychiatry. 1994;35:27–31. doi: 10.1016/0006-3223(94)91163-0. [DOI] [PubMed] [Google Scholar]
- 18.Arolt V, Weitzsch C, Wilke I, Nolte A, Pinnow M, Rothermundt M, Kirchner H. Production of interferon-gamma in families with multiple occurrence of schizophrenia. Psychiatry Res. 1997;66:145–52. doi: 10.1016/s0165-1781(96)03023-5. [DOI] [PubMed] [Google Scholar]
- 19.Baskak SC, Ozsan H, Baskak B, Devrimci Ozgüven H, Kinikli G. Peripheral blood T-lymphocyte and T-lymphocyte subset ratios before and after treatment in schizophrenia patients not taking antipsychotic medication. Turk Psikiyatri Derg. 2008;19:5–12. [PubMed] [Google Scholar]
- 20.Bilici M, Tekelioğlu Y, Efendioğlu S, Ovali E, Ulgen M. The influence of olanzapine on immune cells in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:483–5. doi: 10.1016/S0278-5846(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 21.Carlton E, Falcone T, Batra A, et al. Do systemic inflammation and blood-brain barrier failure play a role in pediatric psychosis? Cleveland Clinic Journal of Medicine. 2009;76:S93a. [Google Scholar]
- 22.Cazzullo CL, Saresella M, Roda K, Calvo MG, Bertrando P, Doria S, Clerici M, Salvaggio A, Ferrante P. Increased levels of CD8+ and CD4+ 45RA+ lymphocytes in schizophrenic patients. Schizophr Res. 1998;31:49–55. doi: 10.1016/s0920-9964(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 23.Chang SH, Chiang SY, Chiu CC, Tsai CC, Tsai HH, Huang CY, Hsu TC, Tzang BS. Expression of anti-cardiolipin antibodies and inflammatory associated factors in patients with schizophrenia. Psychiatry Res. 2011;187:341–6. doi: 10.1016/j.psychres.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 24.Coffey CE, Sullivan JL, Rice JR. T lymphocytes in schizophrenia. Biol Psychiatry. 1983;18:113–9. [PubMed] [Google Scholar]
- 25.Cosentino M, Fietta A, Caldiroli E, Marino F, Rispoli L, Comelli M, Lecchini S, Frigo G. Assessment of lymphocyte subsets and neutrophil leukocyte function in chronic psychiatric patients on long-term drug therapy. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:1117–29. doi: 10.1016/s0278-5846(96)00100-5. [DOI] [PubMed] [Google Scholar]
- 26.Craddock RM, Lockstone HE, Rider DA, Wayland MT, Harris LJ, McKenna PJ, Bahn S. Altered T-cell function in schizophrenia: a cellular model to investigate molecular disease mechanisms. PLoS One. 2007;2:e692. doi: 10.1371/journal.pone.0000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLisi LE, Goodman S, Neckers LM, Wyatt RJ. An analysis of lymphocyte subpopulations in schizophrenic patients. Biol Psychiatry. 1982;17:1003–9. [PubMed] [Google Scholar]
- 28.Dimitrov DH. Correlation or coincidence between monocytosis and worsening of psychosis symptoms in veterans with schizophrenia? Schizophr Res. 2011;126:306–7. doi: 10.1016/j.schres.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Dvoráková M, Zvolský P, Herzog P. Endogenous psychoses and T and B lymphocytes. Folia Haematol Int Mag Klin Morphol Blutforsch. 1980;107:221–8. [PubMed] [Google Scholar]
- 30.Ferguson RM. Effects of psychoactive drugs on in vitro lymphocyte activation. Birth Defects Orig Artic Ser. 1978;14:379–405. [PubMed] [Google Scholar]
- 31.Ganguli R, Rabin BS, Kelly RH, Lyte M, Ragu U. Clinical and laboratory evidence of autoimmunity in acute schizophrenia. Ann N Y Acad Sci. 1987;496:676–85. doi: 10.1111/j.1749-6632.1987.tb35829.x. [DOI] [PubMed] [Google Scholar]
- 32.Ganguli R, Rabin BS. CD5 positive B lymphocytes in schizophrenia: no alteration in numbers or percentage as compared with control subjects. Psychiatry Res. 1993;48:69–78. doi: 10.1016/0165-1781(93)90114-v. [DOI] [PubMed] [Google Scholar]
- 33.Henneberg A, Riedl B, Dumke HO, et al. T-lymphocyte subpopulations in schizophrenic patients. Eur Arch Psychiatry Neurol Sci. 1990;239:283–4. doi: 10.1007/BF01735051. [DOI] [PubMed] [Google Scholar]
- 34.Hornberg M, Arolt V, Wilke I, Kruse A, Kirchner H. Production of interferons and lymphokines in leukocyte cultures of patients with schizophrenia. Schizophr Res. 1995;15:237–42. doi: 10.1016/0920-9964(94)00046-b. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann CA. Lymphocyte subsets and schizophrenia. In: Kurstak E, editor. Virus Immunity and Mental Disorders. Plenum Press; New York: 1987. pp. 307–20. [Google Scholar]
- 36.Kolyaskina GI. Blood lymphocytes in schizophrenia - immunological and virological aspects. Adv Biol Psychiat. 1983;12:142–9. [Google Scholar]
- 37.Kolyaskina GI. Some aspects of immunologic studies in schizophrenia. In: Kurstak E, et al., editors. Virus Immunity and Mental Disorders. Plenum Press; New York: 1987. pp. 285–94. [Google Scholar]
- 38.Maino K, Gruber R, Riedel M, et al. T- and B-lymphocytes in patients with schizophrenia in acute psychotic episode and the course of the treatment. Psychiatry Res. 2007;152:173–80. doi: 10.1016/j.psychres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Masserini C, Vita A, Basile R, et al. Lymphocyte subsets in schizophrenic disorders. Relationship with clinical, neuromorphological and treatment variables. Schizophr Res. 1990;3:269–75. doi: 10.1016/0920-9964(90)90008-u. [DOI] [PubMed] [Google Scholar]
- 40.Mazzarello V, Cecchini A, Fenu G, et al. Lymphocytes in schizophrenic patients under therapy: serological, morphological and cell subset findings. Ital J Anat Embryol. 2004;109:177–188. [PubMed] [Google Scholar]
- 41.McAllister CG, Rapaport MH, Pickar D, Podruchny TA, Christison G, Alphs LD, Paul SM. Increased numbers of CD5+ B lymphocytes in schizophrenic patients. Arch Gen Psychiatry. 1989;46:890–4. doi: 10.1001/archpsyc.1989.01810100032006. [DOI] [PubMed] [Google Scholar]
- 42.Muller N, Ackenheil M, Hofschuster E, et al. Cellular immunity in schizophrenic patients before and during neuroleptic treatment. Psychiatry Res. 1991;37:147–60. doi: 10.1016/0165-1781(91)90072-w. [DOI] [PubMed] [Google Scholar]
- 43.Müller N, Hofschuster E, Ackenheil M, Eckstein R. T-cells and psychopathology in schizophrenia: relationship to the outcome of neuroleptic therapy. Acta Psychiatr Scand. 1993;87:66–71. doi: 10.1111/j.1600-0447.1993.tb03331.x. [DOI] [PubMed] [Google Scholar]
- 44.Müller N, Schlesinger BC, Hadjamu M, Riedel M, Schwarz M, Ackenheil M, Wank R, Gruber R. Increased frequency of CD8 positive gamma/delta T-lymphocytes (CD8+ gamma/delta+) in unmedicated schizophrenic patients: relation to impairment of the blood-brain barrier and HLA-DPA*02011. Schizophr Res. 1998;32:69–71. doi: 10.1016/s0920-9964(98)00036-x. [DOI] [PubMed] [Google Scholar]
- 45.Nikkilä H, Müller K, Ahokas A, et al. Abnormal distributions of T-lymphocyte subsets in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res. 1995;14:215–21. doi: 10.1016/0920-9964(94)00039-b. [DOI] [PubMed] [Google Scholar]
- 46.Nikkilä HV, Müller K, Ahokas A, et al. Accumulation of macrophages in the CSF of schizophrenic patients. Am J Psychiatry. 1999;156:1725–29. doi: 10.1176/ajp.156.11.1725. [DOI] [PubMed] [Google Scholar]
- 47.Nikkilä HV, Müller K, Ahokas A, et al. Increased frequency of activated lymphocytes in the cerebrospinal fluid of patients with acute schizophrenia. Schizophr Res. 2001;49:99–105. doi: 10.1016/s0920-9964(99)00218-2. [DOI] [PubMed] [Google Scholar]
- 48.Nyland H, Naess A, Lunde H. Lymphocyte subpopulations in peripheral blood from schizophrenic patients. Acta Psychiatr Scand. 1980;61:313–8. doi: 10.1111/j.1600-0447.1980.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 49.Printz DJ, Strauss DH, Goetz R, Sadiq S, Malaspina D, Krolewski J, Gorman JM. Elevation of CD5+ B lymphocytes in schizophrenia. Biol Psychiatry. 1999;46:110–8. doi: 10.1016/s0006-3223(98)00307-2. [DOI] [PubMed] [Google Scholar]
- 50.Rabin BS, Ganguli R, Cunnick JE, Lysle DT. The central nervous system--immune system relationship. Clin Lab Med. 1988;8:253–68. [PubMed] [Google Scholar]
- 51.Rothermundt M, Arolt V, Weitzsch C, Eckhoff D, Kirchner H. Immunological dysfunction in schizophrenia: a systematic approach. Neuropsychobiology. 1998;37:186–93. doi: 10.1159/000026501. [DOI] [PubMed] [Google Scholar]
- 52.Rudolf S, Schlenke P, Broocks A, Peters M, Rothermundt M, Arolt V, Kirchner H. Search for atypical lymphocytes in schizophrenia. World J Biol Psychiatry. 2004;5:33–7. doi: 10.1080/15622970410029905. [DOI] [PubMed] [Google Scholar]
- 53.Sasaki T, Nanko S, Fukuda R, et al. Changes of immunological functions after acute exacerbation in schizophrenia. Biol Psychiatry. 35:173–8. doi: 10.1016/0006-3223(94)91149-5. [DOI] [PubMed] [Google Scholar]
- 54.Schattner A, Cori Y, Hahn T, Sirota P. No evidence for autoimmunity in schizophrenia. J Autoimmun. 1996;9:661–6. doi: 10.1006/jaut.1996.0086. [DOI] [PubMed] [Google Scholar]
- 55.Schleifer SJ, Keller SE, Siris SG, Davis KL, Stein M. Depression and immunity. Lymphocyte function in ambulatory depressed patients, hospitalized schizophrenic patients, and patients hospitalized for herniorrhaphy. Arch Gen Psychiatry. 1985;42:129–33. doi: 10.1001/archpsyc.1985.01790250023003. [DOI] [PubMed] [Google Scholar]
- 56.Sperner-Unterweger B, Whitworth A, Kemmler G, et al. T-cell subsets in schizophrenia: a comparison between drug-naïve first episode patients and chronic schizophrenic patients. Schizophr Res. 1999;38:61–70. doi: 10.1016/s0920-9964(98)00175-3. [DOI] [PubMed] [Google Scholar]
- 57.Theodoropoulou S, Spanakos G, Baxevanis CN, Economou M, Gritzapis AD, Papamichail MP, et al. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr Res. 2001;47:13–25. doi: 10.1016/s0920-9964(00)00007-4. [DOI] [PubMed] [Google Scholar]
- 58.Torres KC, Souza BR, Miranda DM, Nicolato R, Neves FS, Barros AG, Dutra WO, Gollob KJ, Correa H, Romano-Silva MA. The leukocytes expressing DARPP-32 are reduced in patients with schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:214–9. doi: 10.1016/j.pnpbp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Vartanian ME, Kolyaskina GI, Lozovsky DV, Burbaeva GS, Ignatov SA. Aspects of humoral and cellular immunity in schizophrenia. Birth Defects Orig Artic Ser. 1978;14:339–64. [PubMed] [Google Scholar]
- 60.Villemain F, Chatenoud L, Galinowski A, Homo-Delarche F, Ginestet D, Loo H, Zarifian E, Bach JF. Aberrant T cell-mediated immunity in untreated schizophrenic patients: deficient interleukin-2 production. Am J Psychiatry. 1989;146:609–16. doi: 10.1176/ajp.146.5.609. [DOI] [PubMed] [Google Scholar]
- 61.Wahlbeck K, Nikkilä H, Rimón R, Ahokas A. Current antipsychotic dose correlates to mononuclear cell counts in the cerebrospinal fluid of psychotic patients. Psychiatry Res. 2000;93:13–9. doi: 10.1016/s0165-1781(99)00125-0. [DOI] [PubMed] [Google Scholar]
- 62.Wilke I, Arolt V, Rothermundt M, et al. Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1996;246:279–284. doi: 10.1007/BF02190280. [DOI] [PubMed] [Google Scholar]
- 63.Zarrabi MH, Zucker S, Miller F, Derman RM, Romano GS, Hartnett JA, Varma AO. Immunologic and coagulation disorders in chlorpromazine-treated patients. Ann Intern Med. 1979;91:194–9. doi: 10.7326/0003-4819-91-2-194. [DOI] [PubMed] [Google Scholar]
- 64.Zorrilla EP, Cannnon TD, Gur RE, et al. Leukocytes and Organ-Nonspecific Autoantibodies in Schizophrenics and Their Siblings: Markers of Vulnerability of Disease? Biol Psychiatry. 1996;40:825–33. doi: 10.1016/0006-3223(95)00598-6. [DOI] [PubMed] [Google Scholar]
- 66.Zorrilla EP, Cannon TD, Kessler J, et al. Leukocyte differentials predict short-term clinical outcome following antipsychotic treatment in schizophrenia. Biol Psychiatry. 1998;43:887–96. doi: 10.1016/s0006-3223(97)00358-2. [DOI] [PubMed] [Google Scholar]
- 65.Hunter J, Schmidt F. Fixed Effects vs. Random Effects Meta-Analysis Models: Implications for Cumulative Research Knowledge. International Journal of Selection and Assessment. 2000;8:275–292. [Google Scholar]
- 66.Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res. 2000;57:497–503. doi: 10.1007/s00011-008-8078-6. [DOI] [PubMed] [Google Scholar]
- 67.Johannsen NM, Priest EL, Dixit VD, Earnest CP, Blair SN, Church TS. Association of white blood cell subfraction concentration with fitness and fatness. Br J Sports Med. 2008;44:588–93. doi: 10.1136/bjsm.2008.050682. [DOI] [PubMed] [Google Scholar]
- 68.Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8:69–83. doi: 10.1080/10253890500100240. [DOI] [PubMed] [Google Scholar]
- 69.Blum KS, Pabst R. Lymphocyte numbers and subsets in the human blood. Do they mirror the situation in all organs? Immunol Lett. 2007;108:45–51. doi: 10.1016/j.imlet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Drexhage RC, Knijff EM, Padmos RC, et al. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10:59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- 71.Kowalski J, Blada P, Kucia K, et al. Neuroleptics normalize increased release of interleukin-1 beta and tumor necrosis factor-alpha from monocytes in schizophrenia. Schizophr Res. 2001;50:169–175. doi: 10.1016/s0920-9964(00)00156-0. [DOI] [PubMed] [Google Scholar]
- 72.Drexhage RC, van der Heul-Nieuwenhuijsen L, Padmos RC, van Beveren N, Cohen D, Versnel MA, Nolen WA, Drexhage HA. Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. IntJ Neuropsychopharmacol. 2010;13:1369–81. doi: 10.1017/S1461145710000799. [DOI] [PubMed] [Google Scholar]
- 73.Doorduin J, de Vries EF, Willemsen AT, et al. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- 74.van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 75.Steiner J, Bielau H, Brisch R, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Steiner J, Mawrin C, Ziegeler A, et al. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112:305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- 77.Ganguli R, Gubbi A. Clinical and immunological characteristics of a subgroup of patients suffering from schizophrenia. In: Henneber AE, Kaschka WP, editors. Immunological alterations in psychiatric diseases Adv Biol Psychiatry. Vol. 18. Basel: Karger; 1997. pp. 35–43. [Google Scholar]
- 78.McAllister CG, van Kammen DP, Rehn TJ, Miller AL, Gurklis J, Kelley ME, Yao J, Peters JL. Increases in CSF levels of interleukin-2 in schizophrenia: effects of recurrence of psychosis and medication status. Am J Psychiatry. 1995;152:1291–1297. doi: 10.1176/ajp.152.9.1291. [DOI] [PubMed] [Google Scholar]
- 79.Smith RS, Maes M. The macrophage-T-lymphocyte theory of schizophrenia: additional evidence. Med Hypotheses. 1995;45:135–141. doi: 10.1016/0306-9877(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 80.Schwarz MJ, Müller N, Riedel M, Ackenheil M. The Th2-hypothesis of schizophrenia: a strategy to identify a subgroup of schizophrenia caused by immune mechanisms. Med Hypotheses. 2001;56:483–486. doi: 10.1054/mehy.2000.1203. [DOI] [PubMed] [Google Scholar]
- 81.Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63:257–265. doi: 10.1111/j.1440-1819.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- 82.Muller N. Mechanisms of Relapse Prevention in Schizophrenia. Pharmacopsychiatry. 2004;37:S141–S147. doi: 10.1055/s-2004-832668. [DOI] [PubMed] [Google Scholar]
- 83.Shepherd M, et al. The natural history of schizophrenia: a five-year follow-up study of outcome and prediction in a representative sample of schizophrenics. Psychol Med Monogr Suppl. 1989;15:1–46. doi: 10.1017/s026418010000059x. [DOI] [PubMed] [Google Scholar]
- 84.Wyatt RJ. Early intervention with neuroleptics may decrease the long-term morbidity of schizophrenia. Schizophr Res. 1991;5:201–202. doi: 10.1016/0920-9964(91)90073-z. [DOI] [PubMed] [Google Scholar]
- 85.Krause DL, Wagner JK, Wildenauer A, Matz J, Weidinger E, Riedel M, Obermeier M, Gruber R, Schwarz M, Muller N. Intracellular monocytic cytokine levels in schizophrenia show an alteration of IL-6. Eur Arch Psychiatry Clin Neurosci. 2012;262:393–401. doi: 10.1007/s00406-012-0290-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.