Abstract
Background
Rosiglitazone improves glycemic control for patients with type 2 diabetes, but there remains controversy regarding an observed association with cardiovascular hazard. The cardiovascular effects of rosiglitazone for patients with coronary artery disease (CAD) remain unknown.
Methods and Results
To examine any association between rosiglitazone use and cardiovascular events among patients with diabetes and CAD, we analyzed events among 2368 patients with type 2 diabetes and CAD in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Total mortality, composite death, myocardial infarction (MI), and stroke, and individual incidence of death, MI, stroke, congestive heart failure (CHF) and fractures, were compared during 4.5 yrs of follow-up among patients treated with rosiglitazone vs. patients not receiving a thiazolidinedione using Cox proportional hazards and Kaplan-Meier analyses including propensity matching. After multivariable adjustment, among patients treated with rosiglitazone, mortality was similar (HR 0.83; 95% CI, 0.58 to 1.18) while there was a lower adjusted incidence of composite death, MI, and stroke (hazard ratio (HR) 0.72; 95% confidence interval (CI), 0.55 to 0.93) and stroke (HR 0.36, 95% CI 0.16 to 0.86), and a higher incidence of fractures (HR 1.62, 95% CI 1.05 to 2.51); the incidence of MI (HR 0.77; 95% CI, 0.54 to 1.10) and CHF (HR 1.22, 95%CI, 0.84 to 1.82) were not significantly different. Among propensity matched patients rates of major ischemic cardiovascular events and CHF were not significantly different.
Conclusions
Among patients with type 2 diabetes and CAD in the BARI 2D trial, neither on-treatment nor propensity matched analysis supported an association of rosiglitazone treatment with an increase in major ischemic cardiovascular events.
Keywords: Diabetes mellitus, drugs, coronary disease, myocardial infarction
INTRODUCTION
Rosiglitazone is a member of the thiazolidinedione class of peroxisome-proliferator-activated receptor γ (PPAR-γ) agonists which have favorable effects on glycemic control by reducing insulin resistance,1 a critical factor contributing to hyperglycemia in patients with type 2 diabetes. In early studies thiazolidinediones including rosiglitazone demonstrated effects associated with cardiovascular benefit, including improvement of endothelial dysfunction,2-4 reduction of inflammatory markers,5, 6 and inhibition of atherosclerosis progression.7, 8
More recent meta-analyses of randomized trials and retrospective case-control studies comparing rosiglitazone with placebo or other therapies for type 2 diabetes suggested increased risk of MI or death with rosiglitazone use.9-14 These meta-analyses included predominantly small, short-term, non-adjudicated treatment trials in lower risk populations, each with very few events, prompting controversy regarding their interpretation15. The only completed prospective trial to evaluate safety among patients treated with rosiglitazone, the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) study,16, 17 showed no increase in the rate of cardiovascular or all-cause death due to rosiglitazone. In 2010 the European Medicines Agency (EMA) suspended rosiglitazone from the market,18 the Federal Drug Administration (FDA) issued a safety alert restricting prescription of rosiglitazone,19 and an ongoing major randomized trial to test the cardiovascular safety of rosiglitazone among patients with or at risk for cardiovascular disease was prematurely terminated.20,21
None of the trial data included in the reports suggesting hazard with rosiglitazone focused on patients with diabetes and established CAD, a high risk group for serious complications of both inadequate glycemic control and any potential adverse cardiovascular effects of rosiglitazone or other anti-hyperglycemic therapy. The effect of rosiglitazone on cardiovascular outcomes particularly in patients with established CAD, therefore, remains unknown.
In the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial, 2368 patients with both type 2 diabetes and angiographically documented CAD were randomly assigned to prompt revascularization or initial medical therapy and to insulin-sensitizing drugs or insulin-providing drugs and followed for clinical outcomes for an average of at least 4.5 years. The primary outcomes of BARI 2D have been reported.22 Although thiazolidinedione treatment was not determined by random assignment, during the trial a large number of patients were treated with a thiazolidinedione, the majority of whom received rosiglitazone within the randomly assigned insulin sensitization arm. To examine outcomes associated with rosiglitazone use in this patient population, we present a post hoc comparison of major cardiovascular events, including death, MI, stroke, and CHF, as well as the incidence of fractures, among participants in BARI 2D who did and did not receive rosiglitazone.
METHODS
Study Population and Design
The design of BARI 2D has been previously described.23 Briefly, from January 1, 2001, to March 31, 2005, 2368 participants were enrolled at 49 international clinical sites. Treatment continued until the 6-year visit or until the last visit before December 1, 2008. Eligibility criteria included a diagnosis of both type 2 diabetes and angiographically documented CAD of sufficient severity to warrant consideration of revascularization. All patients had to be candidates for elective percutaneous coronary intervention or coronary-artery bypass graft surgery. Patients were excluded if they required immediate revascularization or had >50% obstruction in the left main artery, a creatinine level >2.0 mg per deciliter (177 μmol/liter), a glycated hemoglobin >13.0%, class III or IV heart failure, hepatic dysfunction, or if they had undergone percutaneous coronary intervention or coronary artery bypass graft surgery within the previous 12 months. The protocol was approved by the institutional review board at the University of Pittsburgh and at each participating site. All patients provided written informed consent. All data were analyzed at the University of Pittsburgh. An independent data and safety monitoring board approved the study protocol and monitored the safety of patients.
Treatments and Rosiglitazone Exposure
Patients were randomly assigned to two treatment strategies in a 2-by-2 factorial design: (1) prompt coronary revascularization and intensive medical therapy or intensive medical therapy and delayed revascularization, if needed, and (2) insulin-sensitizing therapy or insulin-providing therapy. Patients assigned to an insulin-sensitizing strategy were treated with thiazolidinediones or metformin or both, if needed. Patients assigned to an insulin provision strategy were treated with insulin, and/or sulfonylurea or a meglitinide. Patients in the insulin-sensitization group could receive insulin-providing drugs, and patients in the insulin-provision group could receive insulin-sensitizing drugs, if the glycated hemoglobin level could not be maintained below 8.0% with only the protocol assigned drugs.
At entry into BARI 2D, most patients were already receiving anti-diabetic therapy, including 240 patients who were taking rosiglitazone and 204 patients who were taking pioglitazone. Patients entering the trial who were already receiving rosiglitazone but in whom it was discontinued were not considered among the rosiglitazone treated group unless it was reinitiated at a later date. After random assignment to the insulin sensitization vs. insulin provision groups, medical therapy was adjusted by the site diabetologists per study protocol with a goal of achieving a target glycated hemoglobin level of <7.0%. At least one drug from each of the major anti-diabetic drug classes was available during the study at no cost to the patients, including rosiglitazone for patients prescribed a thiazolidinedione. Exposure to rosiglitazone after trial entry or its discontinuation were recorded at each follow up visit for the determination of on-treatment intervals and coincident events. For the propensity matched analysis (see below), patients who were initiated on rosiglitazone within the first 6 months after trial entry were considered exposed (detailed methods in Supplement). Pioglitazone was not available free of charge, but could be and was prescribed, albeit in a small minority of patients. The number of patient-years on pioglitazone was considered insufficient for specific analysis. With a focus on treatment strategy and glycated hemoglobin goals rather than individual agents, many patients had adjustments in medical therapy, including initiation or discontinuation of rosiglitazone, over the course of the trial.
Patients were treated according to current guidelines, with target levels of low-density lipoprotein (LDL) cholesterol of <100 mg per deciliter (2.6 mmol/liter), and blood pressure of 130/80 mmHg or less. Patients were seen monthly for the first 6 months and every 3 months thereafter.
Outcomes
The primary end point of BARI 2D was death from any cause, and the principal secondary end point was a composite of death, MI, or stroke. The definition of MI has been described previously.22, 24 MI was classified by a Core Electrocardiography Laboratory and stroke and cause of death adjudicated by an independent clinical events committee unaware of study-group assignments. Congestive heart failure, defined by the occurrence of one or more of dyspnea on exertion, bilateral pedal edema, fatigue, orthopnea, and paroxysmal nocturnal dyspnea, was ascertained by each site quarterly starting in the second half of 2002. Occurrence of bone fracture was collected retrospectively from patients in June of 2007 and prospectively annually thereafter; both were elicited on the case report forms and site reported.
Statistical Analysis
Analyses included a post hoc comparison of adverse event rates accrued among patients while on treatment with rosiglitazone versus event rates accrued among patients not receiving a thiazolidinedione that included tracking changes in treatment over time. Unadjusted endpoint frequencies were expressed as number of events per 100 patient-years. For outcome comparisons the adjusted and unadjusted hazard ratio (HR) was calculated from Cox proportional hazards regression models analyzing drug use as a time dependent variable. For this analysis, each patient was followed up until six month after his or her last known diabetes prescription. Beyond that point, diabetes therapy was treated as unknown. Only events that occurred while diabetes therapy was known were included. Separate analyses were also performed to examine a potential legacy effect of rosiglitazone treatment by attributing events during exposure and additional events occurring within 3 months or 6 months of discontinuing rosiglitazone to the drug.
An additional analytic method utilized propensity matching to approximate an intention-to-treat approach for the comparison of events among rosiglitazone-treated participants vs. matched participants not treated with a thiazolidinedione. For this analysis, patients assigned to insulin-sensitization therapy who were prescribed rosiglitazone any time in their first 6 monthly visits were matched using a propensity score with similar patients assigned to insulin-provision who were not prescribed thiazolidinedione during the same 6-month period. The propensity score represented a sum of patient characteristics associated with likelihood of being prescribed rosiglitazone during the first 6 months of the trial (see the Supplemental Materials for details). Among the 2207 participants who survived at least 6 months and had a clinic visit documented at 4-6 months after randomization, 748 were categorized as having received rosiglitazone treatment, 96 patients were prescribed pioglitazone during the first 6 months after study entry and were excluded, and 1363 patients comprised the group not prescribed a thiazolidinedione. The propensity for being treated with rosiglitazone was empirically determined by logistic regression models that accounted for use of rosiglitazone prior to study entry and whether the patient had been randomly assigned to the insulin sensitization or insulin provision arms. The full propensity model is shown in Supplemental Tables 1A-D. The number of matched patients in each analysis varied slightly among the outcomes of death, MI, and stroke, due to exclusion of the few patients who had the outcome occur during the first 6 months. For CHF, the number of patients available for analysis was less because the collection of quarterly data on its occurrence was started later, in the second half of 2002.
Outcomes occurring after the 6 month time point were compared between the two propensity-matched groups in time-to-event analyses using Kaplan-Meier and Cox proportional hazards models. Survival analysis consisted of a stratified Cox regression model using a robust sandwich covariance matrix to account for dependence among matched subjects. The adjusted HRs were based on a statistical adjustment for the following baseline variables: diabetes therapy, diabetes duration, glycated hemoglobin level, sex, age, country, obesity, smoking, family history of CAD, previous revascularization, history of transient ischemic attack/non-CAD, atrial fibrillation, abnormal left ventricular ejection fraction, history of MI, history of CHF, either having high low density lipoprotein (LDL) or being on statins, low high density lipoprotein (HDL), either high triglycerides or being on fibrates, prior amputation, albuminuria, and serum creatinine. To estimate the power to detect putative harm or benefit of rosiglitazone in BARI 2D, we considered that the meta-analysis by Nissen and Wolski9 reported rosiglitazone associated with an odds ratio=1.43 for MI and an odds ratio=1.64 for death due to cardiovascular causes. Based on a fixed sample size of 667 per group, the more conservative BARI 2D propensity analysis had 80% power to detect a HR=1.34 (similar to an odds ratio=1.44) and 90% power to detect a HR=1.40 (odds ratio=1.54) of rosiglitazone for death/MI/stroke. Results were considered significant at p < 0.05.
Role of the Funding Sources
The trial was sponsored by the National Institutes of Health, with additional support from multiple industry sponsors, including the manufacturer of rosiglitazone, GlaxoSmithKline, Inc. Industry sponsors had no role in the collection or analysis of the data, had no access to outcome data at any time during the trial, and did not participate in the design or drafting of this manuscript or its content.
RESULTS
Patients Treated With Rosiglitazone
The baseline characteristics of patients treated with rosiglitazone vs. those not treated with a thiazolidinedione during BARI 2D are shown in Table 1. BARI 2D participants showed a high prevalence of risk factors associated with ischemic cardiovascular events, including hypertension in almost 90%, a history of previous MI in nearly one-third, a history of coronary revascularization in one quarter, and recent anginal symptoms in over 60%. There were 992 patients (42%) who were treated with rosiglitazone at some point during the trial, including 885 patients randomly assigned to the insulin sensitization group and 107 patients randomly assigned to the insulin provision group, contributing up to 3025 patient-years of exposure to rosiglitazone for analysis (Table 2). Patients who were treated with rosiglitazone had a higher baseline level of HbA1c, a longer duration of diabetes, more albuminuria, and were marginally younger than patients not treated with a thiazolidinedione.
Table 1.
Baseline Characteristics According to Rosiglitazone Use vs. No Thiazolidinedione Treatment During Follow-Up in BARI 2D
| Baseline Characteristic | Total (n=2191) |
No Thiazolidinedione (n=1199) |
Treated With Rosiglitazone (n=992) |
p-value |
|---|---|---|---|---|
|
| ||||
| Female, % | 29 | 28 | 30 | 0.37 |
|
| ||||
| Age, mean±SD | 62.4±8.9 | 62.7±8.8 | 62.0±9.0 | 0.07 |
|
| ||||
| Race, % | 0.46 | |||
| Non-Hispanic, White | 67 | 68 | 65 | |
| Non-Hispanic, Black | 17 | 16 | 18 | |
| Hispanic | 12 | 12 | 12 | |
| Asian + Other | 5 | 4 | 5 | |
|
| ||||
| Education, % | 0.14 | |||
| < High School | 37 | 38 | 35 | |
| High School Graduate | 22 | 22 | 21 | |
| Some Post-High School | 24 | 24 | 25 | |
| Bachelor Degree or Higher | 17 | 15 | 19 | |
|
| ||||
| Geography, % | 0.005 | |||
| USA | 61 | 58 | 65 | |
| Canada | 16 | 17 | 14 | |
| Mexico | 4 | 4 | 4 | |
| Brazil | 16 | 17 | 15 | |
| Czech Republic/Austria | 3 | 4 | 3 | |
|
| ||||
| BMI, % | 0.85 | |||
| Normal (<25) | 9 | 10 | 9 | |
| Overweight (25 to <30) | 35 | 35 | 34 | |
| Class 1 Obesity (30 to <35) |
32 | 31 | 32 | |
| 16 | 15 | 16 | ||
| Class 2 Obesity (35 to <40) |
9 | 9 | 9 | |
|
| ||||
| Cigarette Smoking, % | 0.03 | |||
| Never Smoked | 33 | 31 | 35 | |
| Former Smoker | 55 | 57 | 52 | |
| Current Smoker | 12 | 12 | 13 | |
|
| ||||
| Family History of CAD/sudden cardiac death |
43 | 41 | 45 | 0.12 |
|
| ||||
| Prior PCI, % | 20 | 19 | 20 | 0.40 |
| Prior CABG, % | 6 | 6 | 7 | 0.21 |
| History of Angina within the last 6 weeks, % |
61 | 62 | 60 | 0.40 |
| History of MI, % | 32 | 32 | 32 | 0.85 |
|
| ||||
| History of stroke or TIA, % | 10 | 10 | 10 | 0.95 |
| History of Carotid Artery Disease, % |
8 | 8 | 7 | 0.33 |
| Atrial fibrillation, % | 1 | 1 | 1 | 0.88 |
|
| ||||
| Left ventricular dysfunction, % |
17 | 16 | 18 | 0.31 |
| History of CHF, % | 6 | 7 | 6 | 0.95 |
|
| ||||
| History of Hypertension, % | 88 | 88 | 88 | 0.97 |
|
| ||||
| On statins, % | 75 | 73 | 77 | 0.07 |
| Total cholesterol ≥ 200 mg/dl, % | 19 | 19 | 20 | 0.53 |
| High total cholesterol or on statin, % |
83 | 82 | 85 | 0.05 |
|
| ||||
| LDL-C ≥ 130 mg/dl, % | 14 | 14 | 13 | 0.45 |
| High LDL-C or on statin, % | 82 | 80 | 83 | 0.12 |
|
| ||||
| HDL-C < 40 mg/dl men or < 50 mg/dl women, % |
72 | 72 | 73 | 0.41 |
|
| ||||
| On Fibrate, % | ||||
| Triglycerides ≥ 150 mg/dl, % |
8 | 8 | 9 | 0.18 |
| 50 | 49 | 51 | 0.25 | |
| High triglycerides or on fibrate, % |
52 | 51 | 54 | 0.16 |
| 4 | 3 | 4 | 0.33 | |
| On Gemfibrozil, % | ||||
|
| ||||
| Duration of diabetes, mean±SD |
10.4±8.7 | 10.0±9.1 | 10.8±8.1 | 0.05 |
|
| ||||
| Glycated hemoglobin, mean±SD |
7.65±1.6 | 7.50±1.6 | 7.82±1.6 | <0.0001 |
|
| ||||
| Prior amputation, % | 1 | 1 | 1 | 0.38 |
|
| ||||
| Albuminuria, % | 0.02 | |||
| No albuminuria | 68 | 71 | 65 | |
| Micro albuminuria | 22 | 20 | 25 | |
| Macro albuminuria | 10 | 9 | 10 | |
|
| ||||
| Serum creatinine, mean±SD |
1.04±0.28 | 1.04±0.27 | 1.04±0.28 | 0.54 |
Table 2.
Thiazolidinedione Exposure During Follow-Up in BARI 2D
| Patient-Years of Exposure Among Participants In The Insulin Sensitization Arm |
Patient-Years of Exposure Among Participants In The Insulin Provision Arm |
Total Patient-Years |
|
|---|---|---|---|
| Rosiglitazone | 2895 (54%) | 130 (2%) | 3025 |
| Pioglitazone | 434 (8%) | 48 (1%) | 482 |
| No Thiazolidinedione | 1988 (38%) | 5158 (97%) | 7146 |
In terms of predominant exposure or freedom from exposure to thiazolidinedione drugs during an average of 4.5 years of follow up in BARI 2D, 485 patients were exposed to rosiglitazone during at least 80% of their follow-up, 50 patients were exposed to pioglitazone during at least 80% of their follow-up, and 1404 patients were free of any thiazolidinedione during at least 80% of their follow-up. Exposure to pioglitazone did not reach 500 person-years during follow-up.
Cardiovascular Outcomes Associated With Use of Rosiglitazone
The cardiovascular outcomes associated with rosiglitazone use when comparing events accrued among patients in BARI 2D who were being treated with rosiglitazone with patients who were not receiving a thiazolidinedione are shown in Table 3. During follow-up there were 240 deaths, 57 of which occurred during 3025 patient-years of direct exposure to rosiglitazone compared with 183 deaths during 7146 patient-years free of exposure to a thiazolidinedione. In unadjusted analysis, compared with patients not receiving a thiazolidinedione, patients treated with rosiglitazone experienced a similar rate of all cause death (1.88 vs. 2.56 per 100 patient years, HR 0.77, p=0.08), a significantly lower composite incidence of death, MI and stroke (3.79 vs. 5.81 per 100 patient-years, HR 0.71, p=0.002) and a significantly lower incidence of stroke (0.28 vs. 0.77 per 100 patient-years, HR 0.37, p=0.008); the rate of fatal or non-fatal MI (2.16 vs. 3.16 per 100 patient years, HR 0.76, p=0.06) was not significantly different. Although more frequent among patients taking rosiglitazone, in this analysis the incidence of CHF was not significantly different between patients who were receiving rosiglitazone and those who were not receiving a thiazolidinedione (3.31 vs. 3.07 per 100 patient-years, HR 1.08, p=0.62).
Table 3.
Unadjusted Association of Cardiovascular Outcomes of Patients While On Treatment with Rosiglitazone Compared With No Thiazolidinedione During Follow-Up in BARI 2D
| Outcome | Rosiglitazone | No Thiazolidinedione | HR (95% CI) | P Value | ||
|---|---|---|---|---|---|---|
| No. of Events/ Patient-Years of Exposure |
Rate per 100 Patient-Years |
No. of Events/ Patient-Years of Exposure |
Rate per 100 Patient-Years |
|||
| Death | 57/3025 | 1.88 | 183/7146 | 2.56 | 0.77 (0.57, 1.03) | 0.08 |
| Death/MI/Stroke | 105/2769 | 3.79 | 350/6024 | 5.81 | 0.71 (0.57, 0.88) | 0.002 |
| MI | 60/2783 | 2.16 | 193/6102 | 3.16 | 0.76 (0.57, 1.02) | 0.06 |
| Stroke | 8/2883 | 0.28 | 50/6494 | 0.77 | 0.37 (0.17, 0.77) | 0.008 |
| CHF | 64/1936 | 3.31 | 124/4045 | 3.07 | 1.08 (0.80, 1.46) | 0.62 |
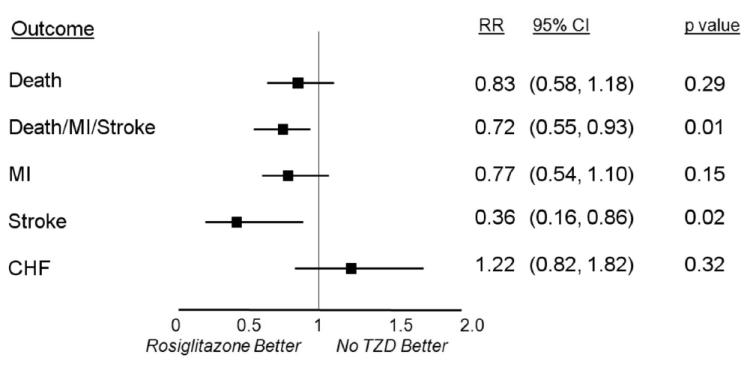
After adjustment for differences in baseline characteristics and use of other anti-diabetes medications (Figure 1), compared with no thiazolidinedione, treatment with rosiglitazone was associated with a similar incidence of all cause death (HR 0.83, 95% CI 0.58 to 1.18, p=0.29), and a significantly lower incidence of the composite of death, MI and stroke (HR 0.72, 95% CI 0.55 to 0.93, p=0.01) and of stroke alone (HR 0.36, 95% CI 0.16 to 0.86, p=0.02). After adjustment, the incidence of MI (HR 0.77, 95% CI 0.54 to 1.10, p=0.15) remained lower and the incidence of CHF higher (HR 1.22, 95% CI 0.82 to 1.82, p=0.32) for patients on treatment with rosiglitazone compared with patients not on a thiazolidinedione, but the differences were not statistically significant. Additional analyses were performed to examine a potential legacy effect of rosiglitazone treatment where events during exposure and additional events occurring within 3 months or 6 months of discontinuing rosiglitazone were included and attributed to the drug. The adjusted HRs of the composite of death, MI and stroke among patients treated with rosiglitazone vs. those not on a thiazolidinedione when including the 3 month and 6 month legacy effects were 0.80 (p=0.08) and 0.85 (p=0.20), respectively.
Figure 1.
Relative risk of adverse cardiovascular outcomes for patients on treatment with rosiglitazone compared with patients not treated with a thiazolidinedione, after adjustment for baseline characteristics and other diabetes-related medications.
Propensity Matched Analysis
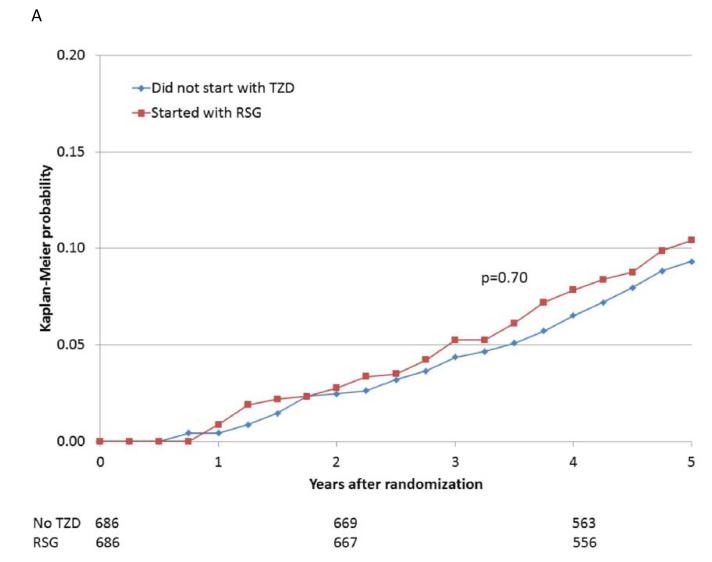
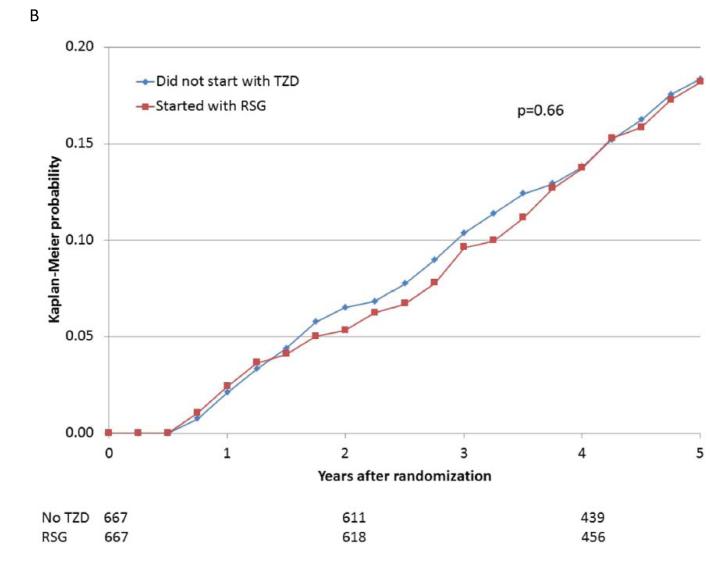
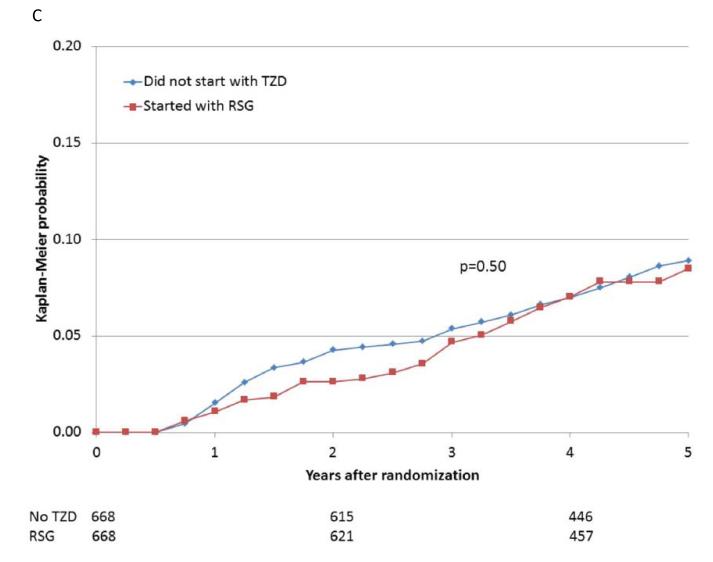
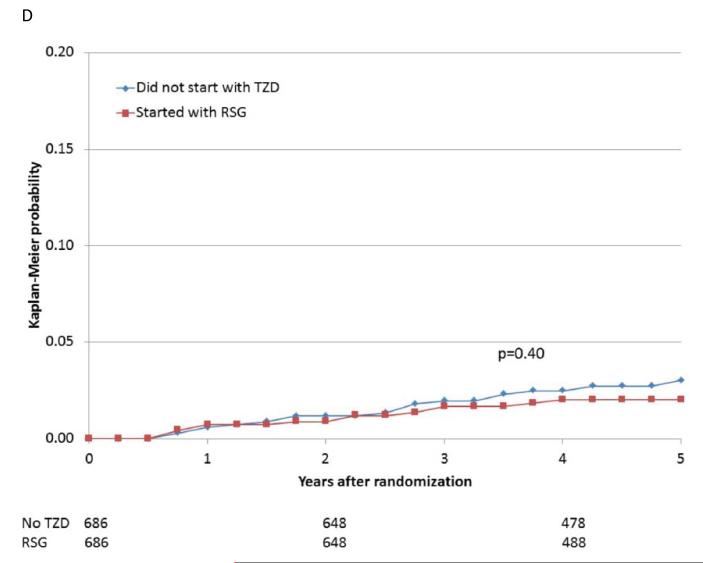
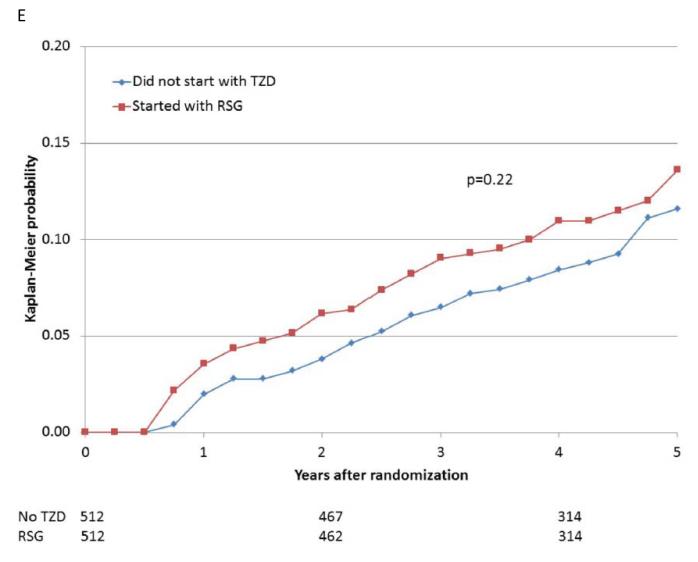
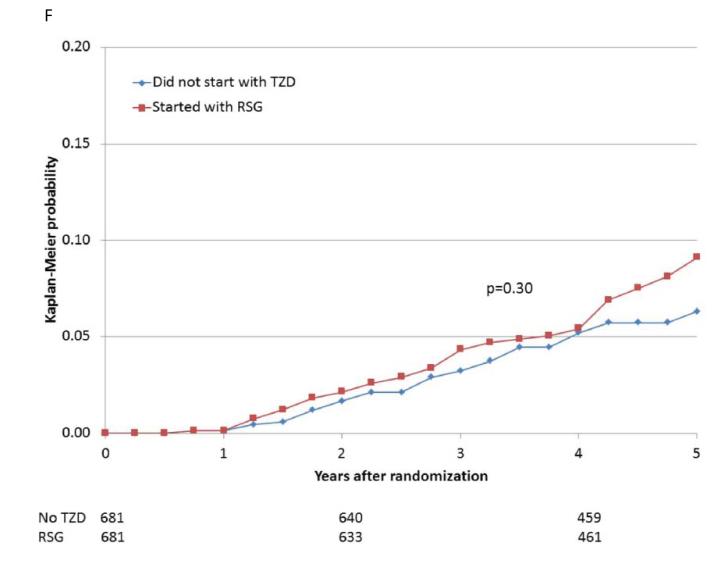
When participants prescribed rosiglitazone during the first 6 months of the trial were propensity-matched with participants who were not prescribed a thiazolidinedione, the baseline characteristics were comparable (Supplemental Table 1E). The 5-year cumulative risks of the specified cardiovascular events for the propensity-matched rosiglitazone and nonthiazolidinedione groups are shown in Table 4 and Figure 2A-E. In these analyses, there were no significant differences observed in the rates of death (HR 1.07), the composite of death, MI, and stroke (HR 0.94), MI (HR 0.88), and stroke (HR 0.75), or in the rate of CHF (HR 1.21), between patients prescribed rosiglitazone vs. those not prescribed a thiazolidinedione.
Table 4.
Association Of Cardiovascular Outcomes With Rosiglitazone Use In BARI 2D Among Propensity Matched Participants Prescribed vs. Not Prescribed a Thiazolidinedione Within 6 Months of Study Entry.
| Outcome | Rosiglitazone | No Thiazolidinedione | ||||
|---|---|---|---|---|---|---|
| Number of Events/ Patients |
5-Year Cumulative Risk (%)* |
Number of Events/ Patients |
5-Year Cumulative Risk (%)* |
HR (95% CI) | P Value | |
| Death | 74/686 | 10.4 | 72/686 | 9.3 | 1.07 (0.77, 1.48) | 0.70 |
| Death/MI/Stroke | 113/667 | 18.2 | 123/667 | 18.3 | 0.94 (0.73, 1.22) | 0.66 |
| MI | 51/668 | 8.5 | 59/668 | 8.9 | 0.88 (0.61, 1.28) | 0.50 |
| Stroke | 14/686 | 2.0 | 19/686 | 3.0 | 0.75 (0.37, 1.49) | 0.41 |
| CHF | 61/512 | 13.6 | 50/512 | 11.6 | 1·26 (0.87, 1.83) | 0.23 |
estimated from Kaplan-Meier analysis.
Figure 2.
Kaplan-Meier curves during BARI 2D follow-up comparing the incidence of events among propensity-matched patients in the insulin-provision arm who did not start the study with any thiazolidinedione (Did not start with TZD) vs. similar patients in the insulin-sensitization arm who started the study with rosiglitazone (Started with RSG) of (A) all cause death; (B) the composite of death, MI, and stroke; (C) MI (excluding procedure-related MI); (D) stroke; (E) CHF; and (F) fractures. Numbers of patients at risk at baseline, 2 years, and 4 years are shown below each graph.
Fracture Risk Associated With Use of Rosiglitazone
The rate of bone fractures was significantly higher among patients who received rosiglitazone than among patients who were not receiving a thiazolidinedione (adjusted HR 1.62, 95%CI 1.05 to 2.51, p=0.03) (Table 5). When the occurrence of bone fractures was stratified by sex, the magnitude of increase in relative risk appeared greater for women than for men (1.82 vs. 1.47), although the interaction between sex and the increased risk of fracture due to rosiglitazone was not significant when formally tested (p=0.55). When fracture rate was compared using the propensity matched analysis of participants prescribed rosiglitazone compared with participants who were not prescribed a thiazolidinedione within the first 6 months of study entry, there was a trend toward higher adjusted fracture rate observed among women prescribed rosiglitazone (Supplemental Table 2). Kaplan-Meier time-to-event analysis (Figure 2F) suggested that the risk of bone fracture began to increase relatively late after exposure to rosiglitazone.
Table 5.
Association of Fractures Among Patients While On Treatment with Rosiglitazone Compared With No Thiazolidinedione
| Rosiglitazone | No Thiazolidinedione | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of Fractures/ Patient- Years of Exposure |
Rate per 100 Patient- Years |
No. of Fractures/ Patient- Years of Exposure |
Rate per 100 Patient- Years |
Unadjusted RR |
P Value | Adjusted* HR (95% CI) |
P Value |
|
| All Patients | 54/2744 | 1.97 | 86/6309 | 1.36 | 1.45 | 0.03 | 1.62 (1.05, 2.51) | 0.03 |
| Men † | 27/1997 | 1.35 | 45/4472 | 1.01 | 1.32 | 0.26 | 1.47 (0.84, 2.55) | 0.18 |
| Women † | 27/747 | 3.62 | 41/1838 | 2.23 | 1.70 | 0.03 | 1.82 (1.04, 3.19) | 0.04 |
Adjusted for baseline characteristics and other anti-diabetes medications
p interaction of rosiglitazone and gender = 0.55.
Interaction of Rosiglitazone with Other Medications
Co-administration of multiple anti-diabetic and cardiovascular medications was common in BARI 2D. We examined whether there was an interaction between rosiglitazone and other co-administered medications on the long term rates of adverse events associated with rosiglitazone using the on-treatment time-dependent analysis (Supplemental Tables 3 and 4). After adjusting for baseline characteristics and use of other diabetes-related medications, no significant interaction was observed on the composite rate of death, MI, and stroke (Supplemental Table 3), or on individual rates of death, MI, stroke or fractures (data not shown) when rosiglitazone was co-administered with insulin, metformin, gemfibrozil, other fibrates, sulfonylureas, nitrates or angiotensin converting enzyme inhibitors. A significant interaction was observed between rosiglitazone and concurrent metformin on the risk of CHF associated with rosiglitazone (Supplemental Table 4). The relative risk of CHF associated with use of rosiglitazone was significantly greater among patients who were not on metformin (HR 1.89, 95% CI, 1.10 to 3.24, p=0.02) than among patients who were also being treated with metformin (HR 0.85, 95% CI, 0.52 to 1.38, p=0.50; interaction p=0.03).
DISCUSSION
The analyses of long term outcomes from the BARI 2D trial included in this report provide no evidence that use of rosiglitazone is associated with increased rates of major adverse ischemic cardiovascular events among patients with type 2 diabetes and established CAD. In an analysis of events that occurred among all eligible patients during active treatment with rosiglitazone, the incidence of stroke and the composite incidence of death, MI and stroke were significantly lower, and there were nonsignificantly lower rates of non-fatal MI and death, among patients receiving rosiglitazone than patients not treated with a thiazolidinedione. In an analysis of events accrued among propensity matched patients who were prescribed vs. not prescribed rosiglitazone starting during the first 6 months after study entry, the rates of death, MI, and stroke were not significantly different among the two groups of patients.
Because there has been intense focus on the cardiovascular safety of rosiglitazone, we employed two complementary analytic methods of the BARI 2D dataset to rigorously explore possible adverse events attributable to drug exposure. The first utilized Cox regression models to analyze drug use as a time dependent variable, whereby any events occurring among patients while actually receiving rosiglitazone were attributed to the drug. A second analytic method utilized propensity matching, where patients were grouped according to whether they were or were not prescribed rosiglitazone starting within the first 6 months of trial entry after randomization. Patients not prescribed a thiazolidinedione were then propensity matched with patients in the rosiglitazone group, and all events occurring after 6 months until the end of follow-up were then attributed to patients according to their group assignment, whether rosiglitazone had been continued or discontinued later during the trial, to approximate an intention-to-treat approach. From our examination of over 450 adjudicated ischemic cardiovascular events occurring during 4.5 years of follow up in BARI 2D and with at least 2500 patient-years of exposure to rosiglitazone for each endpoint, it is notable that neither analysis suggested that rosiglitazone use was associated with a significant increase in adverse cardiovascular ischemic events, including death or MI.
Each of the two analytic strategies, the on-treatment analysis and the propensity matched analysis, has strengths and limitations. The on-treatment analysis includes cardiovascular outcomes from all of the BARI 2D patients receiving rosiglitazone and those receiving no thiazolidinedione and adjusts for differences in the baseline risk profile among these patients. An on-treatment analysis can produce biased treatment effect estimates when the initiation of drug treatment is influenced by severity of illness or other clinical indications or when the termination of the drug is influenced by adverse events or by markers of poor outcomes. In the context of the BARI 2D trial, half of the patients were assigned to a glycemic strategy of insulin sensitization and half were assigned to a strategy of insulin provision, and initiation of rosiglitazone treatment was generally determined by treatment assignment rather than individual patient indications. In addition, the incorporation of 3-month and 6-month legacy effects attributes subsequent clinical events to rosiglitazone even if the drug were stopped due to complications such as fluid retention. Thus, the biases often seen with on-treatment analyses are minimized by the BARI 2D trial design and the inclusion of the legacy effect. In fact, given the large imbalance of rosiglitazone use concentrated in the insulin sensitizing arm of BARI 2D, a lack of hazard with rosiglitazone is supported by the lack of difference in outcomes observed between the insulin sensitization and insulin provision groups in the overall trial.22 In distinction, the propensity matching approach identifies patient pairs, one insulin sensitization patient who received rosiglitazone in the early phase of BARI 2D and one insulin provision patient who did not receive a thiazolidinedione during the same period, that are similar with respect to the characteristics that are associated with the use of rosiglitazone. The number of patients included in the matched comparison is reduced compared with the full cohort, and as a result, these analyses have less power to detect differences attributable to treatment. However, the key advantage of the propensity-matched analysis is that it mimics a randomization process and an intention-to-treat analysis based on the observed risk factors. Neither the on-treatment nor the propensity analysis methods account for unobserved risk factors that may be related to treatment selection.
Along with cardiovascular ischemic events, additional adverse events previously associated with rosiglitazone use were also examined. Congestive heart failure was observed more frequently among patients receiving rosiglitazone compared with patients not on a thiazolidinedione, consistent with previous reports,25, 26 with a frequency that was significantly greater among patients receiving rosiglitazone who were not also receiving metformin (HR 1.89, p=0.02, Supplemental Table 4). Treatment with rosiglitazone was also associated with a higher incidence of bone fractures, consistent with previous observations reported from the A Diabetes Outcome Progression Trial (ADOPT)27 and RECORD17 trials. Similar to ADOPT and RECORD, while the rate of fracture was higher for both men and women, the relative increase in the risk of fractures associated with rosiglitazone use appeared greater among women than men, although the interaction term was not significant.
Previous analyses have suggested an increase in major ischemic cardiovascular events with rosiglitazone therapy. Nissen and Wolski reported a meta-analysis of 42 trials in 20079 and an updated meta-analysis with 14 additional trials in 201010 that included 35,531 subjects randomly assigned to rosiglitazone vs. placebo or an alternative oral anti-diabetes agent. Employing a fixed-effects analytic model, their results suggested that patients treated with rosiglitazone experienced a significantly increased risk of MI. Additional meta-analyses11, 12 and retrospective case-control studies13, 28 also suggested an increased risk of adverse cardiovascular events among patients with type 2 diabetes treated with rosiglitazone. While raising important concerns regarding the cardiovascular safety of rosiglitazone, these reports represent meta-analyses of predominantly short term treatment trials or case-control database analyses in lower risk populations where adverse events were not independently adjudicated, where follow-up may have been imbalanced, where some trials with no events were excluded, and where there was inconsistency across studies in the hazard reported and the estimation of risk.9, 11-13, 28 Moreover, other studies did not suggest increased risk.29, 30 Nevertheless, given these reports, it has been argued that rosiglitazone should be avoided in patients with cardiovascular disease.31 Limited prospective data have been available to address this controversy. The RECORD study16, 17 compared cardiovascular outcomes in patients randomly assigned to receive rosiglitazone combined with metformin or with a sulfonylurea to patients receiving metformin or sulfonylurea alone. Of note, in RECORD only 16.5% of the 4447 participants had a history of ischemic heart disease. At an average of 5.5 years of follow-up, the trial reported no significant difference between the rosiglitazone group and the control group regarding the primary endpoint of cardiovascular death or hospitalization for cardiovascular causes, satisfying the pre-specified criterion for non-inferiority. There were likewise no significant differences in MI (HR 1.14, 95% CI, 0.80 to 1.63, p=0.47) or death from cardiovascular causes (HR 0.84, 95% CI 0.59 to 1.18, p=0.32), but the report concluded that the data were inconclusive for determining whether rosiglitazone was associated with an increase in the risk of MI.17, 32
The current results from BARI 2D contribute prospective longitudinal data regarding outcomes related to treatment with rosiglitazone among high risk patients, all of whom had established CAD documented by coronary angiography. Long term outcomes were prospectively defined and adjudicated by an independent committee blinded to treatment assignment. The current analyses represent the largest experience with rosiglitazone use among patients with type 2 diabetes and CAD in whom long term outcomes are available.
Our analyses did not detect any significant hazard of increased ischemic cardiovascular risk with rosiglitazone treatment despite its use in this particularly vulnerable, higher risk population. Rather, our analysis of adverse events among patients while receiving rosiglitazone showed a lower incidence of the composite of death, MI and stroke and of stroke alone among patients treated with rosiglitazone compared with those not receiving a thiazolidinedione, and lower but not statistically different rates of death or MI. It may be relevant that one potential difference between BARI 2D and other studies reporting cardiovascular effects of rosiglitazone relates to the BARI 2D trial design, where intensive medical therapy was provided for ischemic cardiovascular disease and its risk factors and relief of angina symptoms and lowering of LDL cholesterol and blood pressure was obtained in most patients.22 These results may imply that, in the context of intensive treatment of cardiovascular risk factors, rosiglitazone may provide benefit with retained safety, alone or in combination with other hypoglycemic medications, for high risk patients with type 2 diabetes and established CAD.
Our analyses of drug-drug interactions did not detect any significant interactions of rosiglitazone with nitrates, fibrates, angiotensin converting enzyme inhibitors, metformin, or insulin to suggest these drugs significantly amplify any cardiovascular hazard from rosiglitazone, a concern previously raised by FDA subgroup analyses.11 Within the BARI 2D cohort concurrent treatment with metformin appeared to mitigate the increased risk of CHF observed in metforminnaïve patients receiving rosiglitazone during the trial (RR 0.85, p for interaction=0.03), an interaction that has not been previously reported and that may merit further investigation.
Limitations
There are potentially important limitations to the current analyses and results. As acknowledged above, these were post hoc analyses and the results were not derived from a randomized comparison. Despite careful multivariable adjustment, observed outcomes may be biased by unanticipated and unmeasured confounders, and subject to the potential risks associated with multiple testing. Due to its design employing random assignment to anti-diabetes treatment strategy rather than particular agent(s), antihyperglycemic medical therapy in BARI 2D was complex, with frequent medication additions and adjustments to reach the goal of glycated hemoglobin of < 7%. The majority of patients were receiving more than one anti-diabetes agent and some patients were treated only transiently with rosiglitazone, while a small number were exposed, at different times, to both rosiglitazone and pioglitazone. It is therefore conceivable that the observed lack of ischemic cardiovascular hazards from rosiglitazone could be due to less use of rosiglitazone by investigators in patients they thought might be at increased risk of such events. Nevertheless, the lack of evidence of hazard remained after multivariable adjustment for baseline characteristics and other anti-diabetic medications. In addition, rosiglitazone was provided at no cost by the manufacturer and the effect that free availability of the drug may have had on the prescription and patient adherence to rosiglitazone is unknown.
Conclusions
Among patients with type 2 diabetes and documented CAD in the BARI 2D trial, neither on-treatment nor propensity matched outcome analysis supported an association of rosiglitazone treatment with an increase in major ischemic cardiovascular events.
Supplementary Material
CLINICAL SUMMARY.
Rosiglitazone is a thiazolidinedione that improves glycemic control for patients with type 2 diabetes, but there remains controversy regarding its association with increased cardiovascular hazard, and the cardiovascular effects of rosiglitazone for patients with established coronary artery disease (CAD) remain unknown. The association between rosiglitazone use and cardiovascular events was examined among 2368 patients with type 2 diabetes and CAD in the BARI 2D trial during 4.5 yrs of follow-up using Cox proportional hazards models for on treatment events and using propensity matched analyses. Among patients on treatment with rosiglitazone, there was a significantly lower adjusted incidence of the composite outcome death, myocardial infarction (MI), and stroke, and of stroke, while the incidence of MI, congestive heart failure (CHF), and death were not significantly different. Among propensity matched patients the risk of major ischemic cardiovascular events and CHF were not significantly different. There was a higher incidence of fractures observed among rosiglitazone-treated patients. An interaction was also observed between use of metformin and rosiglitazone that appeared to mitigate an increased risk of CHF with rosiglitazone. The results of these analyses from BARI 2D do not support an association of rosiglitazone treatment with an increase in major ischemic cardiovascular events among patients with type 2 diabetes and established CAD.
Acknowledgments
Funding Sources: This study was funded by the National Heart, Lung and Blood Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, Nos. U01 HL061744, U01 HL061746, U01 HL061748, and U01 HL063804. Significant supplemental funding was provided by GlaxoSmithKline, Collegeville, PA; Bristol-Myers Squibb Medical Imaging, Inc., North Billerica, MA; Astellas Pharma US, Inc., Deerfield, IL; Merck & Co., Inc., Whitehouse Station, NJ, Abbott Laboratories, Inc., Abbott Park, IL; and Pfizer, Inc, New York, NY. Generous support was given by Abbott Laboratories Ltd., Abbott Park, IL; MediSense Products, Mississauga, Canada; Bayer Diagnostics, Tarrytown, NY; Becton, Dickinson and Company, Franklin Lakes, NJ; J. R. Carlson Labs, Arlington Hts., IL; Centocor, Inc., Malvern, PA; Eli Lilly and Company, Indianapolis, IN; LipoScience, Inc., Raleigh, NC; Merck Sante, Lyon, France; Novartis Pharmaceuticals Corporation, East Hanover, NJ; and Novo Nordisk, Inc. Princeton, NJ.
Footnotes
Clinical Trial Registration Information: http://www.clinicaltrials.gov. Identifier: NCT00006305.
Conflict of Interest Disclosures: Richard G. Bach reports research grants from AstraZeneca, Eli Lilly, Bristol-Myers Squibb, Merck/Schering-Plough, and is a consultant (CEC Activity only) for Roche (Significant) and Pfizer (Modest). Alan Garber is a consultant for Halozyme (Modest), Janssen (Modest), Merck, (Significant), Novo Nordisk (Significant), Santarus (Modest), Takeda (Modest), Tethys Bioscience (Modest), is on the Speaker’s Bureau for Janssen (Significant), Merck (Significant), Novo Nordisk (Significant), Santarus (Significant), and has received honoraria from GlaxoSmithKline (Significant). Rodica Pop-Busui reports research grants from Amylin Pharmaceuticals/BMS. The remaining authors have no disclosure to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 2.Kelly AS, Thelen AM, Kaiser DR, Gonzalez-Campoy JM, Bank AJ. Rosiglitazone improves endothelial function and inflammation but not asymmetric dimethylarginine or oxidative stress in patients with type 2 diabetes mellitus. Vasc Med. 2007;12:311–318. doi: 10.1177/1358863X07084200. [DOI] [PubMed] [Google Scholar]
- 3.Esposito K, Ciotola M, Carleo D, Schisano B, Saccomanno F, Sasso FC, Cozzolino D, Assaloni R, Merante D, Ceriello A, Giugliano D. Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes Care. 2006;29:1071–1076. doi: 10.2337/diacare.2951071. [DOI] [PubMed] [Google Scholar]
- 4.Wang TD, Chen WJ, Lin JW, Chen MF, Lee YT. Effects of rosiglitazone on endothelial function, C-reactive protein, and components of the metabolic syndrome in nondiabetic patients with the metabolic syndrome. Am J Cardiol. 2004;93:362–365. doi: 10.1016/j.amjcard.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679–684. doi: 10.1161/01.cir.0000025403.20953.23. [DOI] [PubMed] [Google Scholar]
- 6.Yki-Jarvinen H, Sutinen J, Silveira A, Korsheninnikova E, Fisher RM, Kannisto K, Ehrenborg E, Eriksson P, Hamsten A. Regulation of plasma PAI-1 concentrations in haart-associated lipodystrophy during rosiglitazone therapy. Arterioscler Thromb Vasc Biol. 2003;23:688–694. doi: 10.1161/01.ATV.0000062885.61917.A5. [DOI] [PubMed] [Google Scholar]
- 7.Sidhu JS, Kaposzta Z, Markus HS, Kaski JC. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol. 2004;24:930–934. doi: 10.1161/01.ATV.0000124890.40436.77. [DOI] [PubMed] [Google Scholar]
- 8.Fang CC, Ng Jao YT, Yi C, Yu CL, Chen CL, Wang SP. Angiographic and clinical outcomes of rosiglitazone in patients with type 2 diabetes mellitus after percutaneous coronary interventions: A single center experience. Angiology. 2007;58:523–534. doi: 10.1177/0003319707303587. [DOI] [PubMed] [Google Scholar]
- 9.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 10.Nissen SE, Wolski K. Rosiglitazone revisited an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 11.GlaxoSmithKline [Accessed February 8, 2010];FDA Advisory Committee briefing document: Cardiovascular safety of rosiglitazone. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4308b1-01-sponsor-backgrounder.pdf.
- 12.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: A meta-analysis. JAMA. 2007;298:1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 13.Lipscombe LL, Gomes T, Levesque LE, Hux JE, Juurlink DN, Alter DA. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA. 2007;298:2634–2643. doi: 10.1001/jama.298.22.2634. [DOI] [PubMed] [Google Scholar]
- 14.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, Ali F, Sholley C, Worrall C, Kelman JA. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 15.Diamond GA, Bax L, Kaul S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med. 2007;147:578–581. doi: 10.7326/0003-4819-147-8-200710160-00182. [DOI] [PubMed] [Google Scholar]
- 16.Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV, Grp RS. Rosiglitazone evaluated for cardiovascular outcomes - an interim analysis. New Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 17.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV, Team RS. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): A multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed December 14, 2012];European Medicines Agency recommends suspension of Avandia AaA. 2010 Sep 23; http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/09/WC500096996.pdf.
- 19. [Accessed December 14, 2012];FDA Safety Alert S, 2010: Avandia (rosiglitazone): REMS - Risk of Cardiovascular Events. http://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/uc m226994.htm.
- 20.Punthakee Z, Bosch J, Dagenais G, Diaz R, Holman R, Probstfield J, Ramachandran A, Riddle M, Ryden LE, Zinman B, Afzal R, Yusuf S, Gerstein H. Design, history and results of the thiazolidinedione intervention with vitamin d evaluation (TIDE) randomised controlled trial. Diabetologia. 2012;55:36–45. doi: 10.1007/s00125-011-2357-4. [DOI] [PubMed] [Google Scholar]
- 21. [Accessed December 14, 2012];FDA Statement on Avandia TIDE Trial. 2010 Available at. http://www.fda.gov/Drugs/DrugSafety/ucm219780.htm.
- 22.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks MM, Frye RL, Genuth S, Detre KM, Nesto R, Sobel BE, Kelsey SF, Orchard TJ, Investigators BDT. Hypotheses, design, and methods for the bypass angioplasty revascularization investigation 2 diabetes (BARI 2D) trial. Am J Cardiol. 2006;97:9G–19G. doi: 10.1016/j.amjcard.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Brooks MM, Chaitman BR, Molitch ME, Grp BdS. Therapies for type 2 diabetes and coronary artery disease reply. New Engl J Med. 2009;361:1409–1410. [Google Scholar]
- 25.Delea TE, Edelsberg JS, Hagiwara M, Oster G, Phillips LS. Use of thiazolidinediones and risk of heart failure in people with type 2 diabetes - a retrospective cohort study. Diabetes Care. 2003;26:2983–2989. doi: 10.2337/diacare.26.11.2983. [DOI] [PubMed] [Google Scholar]
- 26.Komajda M, McMurray JJ, Beck-Nielsen H, Gomis R, Hanefeld M, Pocock SJ, Curtis PS, Jones NP, Home PD. Heart failure events with rosiglitazone in type 2 diabetes: Data from the RECORD clinical trial. Eur Heart J. 2010;31:824–831. doi: 10.1093/eurheartj/ehp604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu DH, Heise MA, Aftring RP, Viberti G, Gr PTAS Rosiglitazone-associated fractures in type 2 diabetes - an analysis from a diabetes outcome progression trial (ADOPT) Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- 28.Winkelmayer WC, Setoguchi S, Levin R, Solomon DH. Comparison of cardiovascular outcomes in elderly patients with diabetes who initiated rosiglitazone vs pioglitazone therapy. Arch Intern Med. 2008;168:2368–2375. doi: 10.1001/archinte.168.21.2368. [DOI] [PubMed] [Google Scholar]
- 29.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: A meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 30.Rosen CJ. The rosiglitazone story - lessons from an FDA advisory committee meeting. New Engl J Med. 2007;357:844–846. doi: 10.1056/NEJMp078167. [DOI] [PubMed] [Google Scholar]
- 31.Singh S, Loke YK. The safety of rosiglitazone in the treatment of type 2 diabetes. Expert Opin Drug Saf. 2008;7:579–585. doi: 10.1517/14740338.7.5.579. [DOI] [PubMed] [Google Scholar]
- 32.Retnakaran R, Zinman B. Thiazolidinediones and clinical outcomes in type 2 diabetes. Lancet. 2009;373:2088–2090. doi: 10.1016/S0140-6736(09)61029-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.