Summary
Background
Phosphatidylserine (PS) normally confined to the cytoplasmic leaflet of plasma membrane (PM) is externalized to the exoplasmic leaflet (exPS) during apoptosis where it serves as an “eat-me” signal to phagocytes. In addition, some living cells such as macrophages also express exPS.
Results
A secreted Annexin V (sAnxV::GFP) PS sensor reveals that exPS appears early on apoptotic cells in C. elegans embryos and decreases in older or unengulfed apoptotic cells. This decrease in exPS expression is blocked by loss of CED-7, an ATP binding cassette (ABC) transporter, or TTR-52, a secreted PS binding protein. Phagocytic cells also express exPS, which is dependent on the activity of CED-7, TTR-52, and TTR-52-interacting phagocyte receptor CED-1. Interestingly, a secreted Lactadherin PS sensor (sGFP::LactC1C2) labels apoptotic cells but not phagocytes, prevents sAnxV::GFP from labeling phagocytes, and compromises phagocytosis. Immunoelectron micrographs of embryos expressing sAnxV::GFP or sGFP::LactC1C2 reveal the presence of extracellular PS-containing vesicles between the apoptotic cell and neighboring cells, which are absent or greatly reduced in the ced-7 and ttr-52 mutants, respectively, indicating that CED-7 and TTR-52 promote the generation of extracellular PS vesicles. Loss of the tat-1 gene, which maintains PS asymmetry in the PM, restores phagocyte exPS expression in ced-1, ced-7, and ttr-52 mutants and partially rescues their engulfment defects.
Conclusions
CED-7 and TTR-52 may promote the efflux of PS from apoptotic cells through the generation of extracellular PS vesicles, which lead to exPS expression on phagocytes via TTR-52 and CED-1 to facilitate cell corpse clearance.
Introduction
The major phospholipid constituents of the eukaryotic PM bilayer are asymmetrically distributed between the exo- and cytoplasmic leaflets [1]. The choline containing lipids, phosphatidylcholine (PC) and sphingomyelin (SM), are concentrated in the exoplasmic leaflet, while the aminophospholipids, phosphatidylserine (PS) and phosphatidylethanolamine (PE), are restricted to the cytoplasmic leaflet [1]. During apoptosis, PS is externalized to the exoplasmic leaflet [2, 3], where it promotes interactions between apoptotic and phagocytic cells through bridging molecules or membrane receptors, thereby facilitating phagocytosis [4, 5].
How PS is exposed on the surface of apoptotic cells is not well understood. Activation of the bidirectional phospholipid scramblases or/and activation of some ABC transporters, which promote exoplasmic externalization of phospholipids, have been implicated [3]. In addition, inactivation of aminophospholipid translocases, which flip aminophospholipids from the exoplasmic leaflet to the cytoplasmic leaflet and thus play a role in maintaining asymmetric distribution of aminophospholipids in PM, could contribute to apoptotic exPS expression. Recent studies in C. elegans have provided evidence that two classes of lipid transporters regulate apoptotic exPS expression [6, 7]. For example, one of the eight C. elegans phospholipid scramblases, SCRM-1, is activated by a mitochondrial apoptogenic factor WAH-1 to promote exPS expression in apoptotic germ cells [6], whereas inactivation of one of the six C. elegans aminophospholipid translocases, TAT-1, leads to ectopic PS exposure on the surface of all cells, which triggers stochastic removal of living cells by phagocytosis [7, 8]. CED-7, a C. elegans ABC transporter, was reported to promote PS exposure in C. elegans somatic apoptotic cells, based on a PS sensor derived from the PS-binding protein Lactadherin [9, 10]. However, using a different PS sensor derived from Annexin V, another PS-binding protein [11, 12], CED-7 was shown to have no effect on exPS expression in apoptotic cells. Therefore, examination of these important issues using consistent PS labeling methodologies is necessary to reconcile the differing observations and to advance our understanding of PS externalization during apoptosis.
In some biological events, exPS expression occurs in non-apoptotic cells. For example, PS exposure on the surface of platelets is critical for initiating the blood coagulation cascade [13]. There are reports that PS is also exposed on the surface of some macrophages, which may be important for phagocytosis of PS-expressing target cells [14–16]. However, it is not clear whether this is a general phenomenon for phagocytic cells and whether it occurs in vivo. It is also not understood how PS is exposed on the surface of phagocytes and whether it is important for apoptotic cell clearance.
In this paper, we describe significantly improved techniques for detecting and analyzing exPS expression in C. elegans embryos. We show that exPS not only is detected on the surface of most dying cells, but also consistently appears on the surface of their neighboring cells, one of which acts as a phagocyte. Our genetic analysis identifies a new pathway that not only regulates exPS expression in apoptotic cells but also mediates appearance of PS on the surface of phagocytes. Electron microscopy analysis reveals that extracellular PS vesicles likely mediate the efflux of PS from apoptotic cells and the expression of exPS on phagocytes. Finally, we demonstrate that exPS expression on phagocytes is important for clearance of apoptotic cells.
Results
The Dynamics of exPS Expression During C. elegans Apoptosis
To examine the dynamics of exPS expression during C. elegans apoptosis, we expressed two PS-binding proteins, secreted Annexin V::GFP (sAnxV::GFP; Figure 1A) and secreted GFP::Lactadherin C1 and C2 domains (sGFP::LactC1C2; Figure 1C) under the control of C. elegans heat-shock promoters. Our two PS sensors are expressed at higher levels than the PS sensors described previously [10, 12](Figure S1) and are highly sensitive in labeling apoptotic cells in C. elegans embryos (Table S1). Mutations in Annexin V or Lactadherin that block their ability to bind PS in vitro prevent recognition of apoptotic cells in vivo (Figure 1B and 1D)[17, 18], indicating that they recognize exPS on apoptotic cells.
Figure 1. Detection of exPS in C. elegans Embryos With Two Different PS Sensors and Their Effects on the Removal of Apoptotic Cells.
(A–D) sAnxV::GFP and sGFP::LactC1C2, but not their PS-binding defective mutants, label specifically apoptotic cells. GFP and Nomarski (DIC) images of a wild-type C. elegans bean stage embryo transgenic for PhspsAnxV::GFP (A), PhspsAnxVMut::GFP (B), PhspsGFP::LactC1C2 (C), and PhspsGFP::Lact(mut)C1C2 (D) are shown. Schematic diagrams of the PS sensors are depicted at the bottom, with yellow boxes representing the synthetic signal sequence. Arrows indicate apoptotic cells. Scale bars represent 5 μm.
(E) Summary of sAnxV::GFP cell labeling in cell death deficient mutants. Pre-corpses are cells that were labeled by sAnxV::GFP but did not have the characteristic “cell corpse” morphology. Data are averages ± standard deviation (SD). 20 bean stage embryos were scored.
(F) sGFP::LactC1C2 overexpression causes a defect in apoptotic cell clearance. PhspsAnxV::GFP, PhspsGFP::LactC1C2, or PhspsGFP::Lact(mut)C1C2 transgenic animals were subjected to heat-shock treatment (see Experimental Procedures) and transgenic embryos were then scored for cell corpses (n=15). Error bars represent standard error of mean (SEM). Unpaired two-tailed t test, *P < 0.001 (wild type vs. sGFP::LactC1C2).
sAnxV::GFP labels an average of 12 cells in the bean stage embryos (Figure 1E). The majority of the labeled cells had raised disc-like morphology characteristic of apoptotic cell corpses. But some did not show typical corpse morphology and were sometimes larger than apoptotic cells and more rounded than other living cells (Figure 2A, 4 minutes), suggesting that they could be at an early stage of apoptosis. Indeed, in cell death deficient ced-3(n717), ced-4(n1162), and egl-1(m3082) mutants, we did not observe any cells with exPS (Figure 1E and Figure S1E).
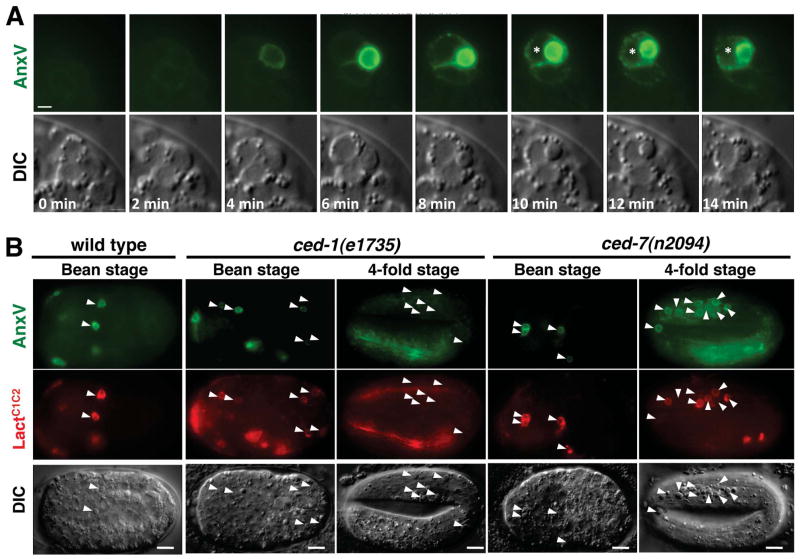
Figure 2. Detection of exPS Expression in Wild-Type and Engulfment-Defective Mutant Embryos.
(A) Time-lapse analysis of exPS expression in C. elegans embryo during apoptosis. A 15-μm Z series (1 μm per section) of smIs76 (PhspsAnxV::GFP) embryo were captured at 2-minute intervals. A single plane from the Z series is shown for DIC and GFP. The apoptotic cell was engulfed at the 10-minute time point by one of the neighboring cells (indicated with *). The scale bar represents 1 μm.
(B) Unengulfed apoptotic cells in ced-7 but not ced-1 mutant embryos retain exPS expression. GFP, Cherry, and DIC images of wild-type, ced-1(e7135), and ced-7(n2094) bean stage or 4-fold stage embryos transgenic for both PhspsAnxV::GFP and PhspsCherry::LactC1C2 are shown. Arrowheads indicate apoptotic cells. Scale bars represent 5 μm.
Using time-lapse microscopy, we analyzed the dynamics of exPS expression during C. elegans apoptosis. sAnxV::GFP was detectable before the dying cell adopted the corpse morphology (Figure 2A, 4 minutes), indicating that PS exposure occurs early during apoptosis. The intensity of sAnxV::GFP on the apoptotic cell reached its maximum at about the same time when the raised disc-like corpse morphology became apparent (Figure 2A, 8 minutes). Shortly after the initial exPS expression by the dying cell, two neighboring cells were also labeled by sAnxV::GFP (Figure 2A, 6 minutes). The intensity of sAnxV::GFP labeling on these cells was approximately two or three fold lower than that on apoptotic cells (Figure S2). The apoptotic cell was engulfed by one of the sAnxV::GFP-positive neighboring cells 6 minutes after its initial exPS expression (Figure 2A, 10 minutes). On average, 1.78 cells adjacent to the apoptotic cells were labeled by sAnxV::GFP (Table S2). In most cases (21 out of 23), one of these sAnxV::GFP-positive neighboring cells engulfed the apoptotic cells (Table S2). These data indicate that the majority of phagocytes are neighboring cells with exPS expression.
CED-7 and TTR-52 Mediate Time-Dependent Loss of exPS From Apoptotic Cells
exPS on apoptotic cells acts as an “eat-me” signal to promote phagocytosis by fostering recognition and interaction between phagocytes and the apoptotic cell [2, 4]. We thus examined whether genes important for removal of apoptotic cells might affect exPS expression. In C. elegans, two parallel engulfment pathways mediate the removal of apoptotic cells, with ced-1, ced-6, ced-7 and ttr-52 acting in one pathway and ced-2, ced-5, ced-10 and ced-12 functioning in the other pathway [8, 19]. During early embryogenesis (bean stage), sAnxV::GFP labeled almost all apoptotic cells in wild-type animals or animals that are defective in one or both of the engulfment pathways (Table 1 and Figure 2B). In later embryonic stages, the percentage of apoptotic cells with exPS decreased (Table 1). At the 4-fold embryonic stage, only 2–10% of unengulfed apoptotic cells had exPS in ced-1/ced-2/ced-5/ced-6/ced-10/ced-12 engulfment-defective mutants (Table 1 and Figure 2B). Because the unengulfed apoptotic cells found in the later embryonic stages of engulfment-deficient mutants were at least several hours old, this observation indicates that exPS generated in the early stage of apoptosis was lost over time. This time-dependent loss of exPS expression in unengulfed apoptotic cells was also observed when sGFP::LactC1C2 or sCherry::LactC1C2 was used as a PS sensor (Table 1 and Figure 2B).
Table 1.
Mutations in ced-7 and ttr-52 Retain exPS on Unengulfed Apoptotic Cells
| Genotype with smIs76 (PhspAnxV::GFP) | % apoptotic cells with sAnxV::GFP
|
||
|---|---|---|---|
| Bean | 2 fold | 4 fold | |
| wild type | 96±14 | 72±29 | ND |
| ced-1(e1735) | 96±8 | 70±19 | 6±6 |
| ced-2(n1994) | 98±3 | 67±21 | 7±6 |
| ced-5(n1812) | 99±2 | 67±7 | 9±12 |
| ced-6(n2095) | 99±3 | 72±16 | 5±5 |
| ced-7(n2094) | 99±3 | 82±18 | 59±17 |
| ced-7(n1998) | 99±2 | 86±14 | 60±18 |
| ced-7(n2690) | 99±3 | 81±19 | 63±22 |
| ced-10(n3246) | 92±10 | 43±25 | 5±7 |
| ced-12(bz187) | 98±4 | 67±21 | 8±9 |
| ced-12(n3261) | 99±4 | 70±17 | 4±4 |
| ttr-52(tm2087) | 90±4 | 67±4 | 64±3 |
| ced-1(e1735); ced-5(n1812) | 95±20 | 71±12 | 4±7 |
| ced-6(n2095); ced-2(n1994) | 100±0 | 78±19 | 2±3 |
| ced-6(n2095); ced-5(n1812) | 100±0 | 61±18 | 5±8 |
| ced-12(n3261); ced-6(n2095) | 97±7 | 71±20 | 2±2 |
| ced-7(n2094); ced-2(n1994) | 98±6 | 85±15 | 51±20 |
| ced-7(n2094); ced-5(n1812) | 99±1 | 80±15 | 51±22 |
| ced-12(n3261); ced-7(n2094) | 99±2 | 80±14 | 53±21 |
| ced-1(e1735); ttr-52(tm2087) | 97±5 | 79±16 | 61±19 |
| ttr-52(tm2087); ced-5(n1812) | 99±2 | 84±14 | 53±17 |
| ced-7(n2094) ttr-52(tm2087) | 98±4 | 83±13 | 59±11 |
|
| |||
| Genotype with smIs434 [PhspsGFP::LactC1C2] | Bean | 2 fold | 4 fold |
|
| |||
| wild type | 60±18 | 50±18 | ND |
| ced-1(e1735) | 45±19 | 33±7 | 3±5 |
| ced-5(n1812) | 40±17 | 37±13 | 1±1 |
| ced-7(n2094) | 56±12 | 57±13 | 21±11 |
| ttr-52(tm2087) | 63±15 | 61±15 | 33±10 |
The same heat shock treatment and analysis of apoptotic cell labeling were performed as described in Experimental Procedures for all strains. For each strain, the average percentage of cell corpses labeled by sAnxV::GFP or sGFP::LactC1C2 was determined at the indicated embryonic stage (n=15 for each stage). Results are shown as mean ± SD. In wild-type animals, few cell corpses were seen in the 4 fold embryonic stage. ND, not determined.
Interestingly, loss of exPS from unengulfed apoptotic cells is dependent on the activity of ced-7 and ttr-52, as more than 59% of unengulfed apoptotic cells were labeled by sAnxV::GFP in embryos deficient in either ced-7 or ttr-52 (Table 1 and Figure 2B). For example, in the ced-7(n2094) or ttr-52(tm2087) single mutant or in the ced-7(n2094); ced-5(n1812) or ttr-52(tm2087); ced-5(n1812) double mutant, 51–64% of unengulfed apoptotic cells had exPS at the 4-fold embryonic stage, whereas only 2–10% of unengulfed apoptotic cells had exPS in the ced-5(n1812) and other single or double engulfment mutants (Table 1). When we generated new apoptotic cells by activating the expression of cell death initiator EGL-1 in 4-fold egl-1(lf) embryos through heat-shock induction (Phspegl-1), the newly generated apoptotic cells were similarly labeled by sAnxV::GFP (≥ 57%) in egl-1(n3084), ced-1(e1735); egl-1(n3084), ttr-52(tm2087); egl-1(n3084), or ced-7(n2094); egl-1(n3084) embryos (Table S3), providing further evidence that exPS is lost in a time-dependent manner in unengulfed cell corpses through the action of ced-7 and ttr-52. Of note, in all assays using sAnxV::GFP or sGFP::LactC1C2 (Table 1, Table S3, and Figure 2B), we did not observe reduced exPS expression on apoptotic cells in ced-7(lf) mutants. In the case of aged or unengulfed apoptotic cells, we observed a higher percentage of apoptotic cells with exPS in ced-7(lf) and ttr-52(lf) mutants than in other engulfment mutants (Table 1 and Figure 2B). These results indicate that CED-7 does not play a role in externalizing PS in apoptotic cells, but rather, plays a role in reducing exPS expression in aged and unengulfed apoptotic cells
CED-7, TTR-52 and CED-1 Are Required for exPS Expression in Phagocytes
Similarly, we examined whether genes important for removal of apoptotic cells affect exPS expression on phagocytes. 47% of apoptotic cells in wild type embryos were associated with one or more neighboring cells with sAnxV::GFP (Table 2). In contrast, in ced-1(lf), ced-7(lf) or ttr-52(lf) embryos, few apoptotic cells (1–3%) had neighboring cells with sAnxV::GFP. Since ced-1, ced-7 and ttr-52 act in the same engulfment pathway, they may work together to regulate phagocyte exPS expression. The percentage of neighboring cells with exPS was reduced by more than one half (17–21%) in embryos deficient in ced-2, ced-5, ced-10 and ced-12 (Table 2), which act in a different engulfment pathway and encode intracellular signaling proteins that promote phagocyte cytoskeleton rearrangement and membrane extensions that enclose the apoptotic cell [19]. Similar to the ced-2/ced-5/ced-10/ced-12 mutant embryos, exPS expression on neighboring cells was reduced in embryos deficient in ced-6, which encodes an intracellular signaling adaptor for the ced-1/ced-7/ttr-52 engulfment pathway (Table 2)[20]. This indicates that ced-1, ced-7 and ttr-52 are essential for exPS expression on neighboring cells. In comparison, the downstream engulfment-signaling pathway that they activate is not essential but does have a role in promoting exPS expression on neighboring cells, as indicated by the 2-fold reduction in neighboring cells with exPS. In embryos that are deficient in both ced-6 and a component of the ced-2/ced-5/ced-10/ced-12 engulfment pathway, engulfment of apoptotic cells is mostly blocked [19, 21] and virtually no neighboring cell had exPS (Table 2). Therefore, at least one of the two primary, partially redundant engulfment pathways needs to function properly to allow exPS expression on phagocytes.
Table 2.
Phagocyte exPS Expression Requires ced-1, ced-7, ttr-52, and the Presence of at Least One Phagocytosis Signaling Pathway
| Genotype | % neighboring cells with sAnxV::GFP |
|---|---|
| wild type | 47±7 |
| ced-1(e1735) | 3±5 |
| ced-1(e1735); smIs410 (Pced-1CED-1ΔC) | 19±9 |
| ced-2(n1994) | 21±6 |
| ced-5(n1812) | 19±1 |
| ced-6(n2095) | 20±3 |
| ced-7(n2094) | 2±1 |
| ced-10(n3246) | 17±10 |
| ced-12(n3261) | 20±1 |
| ttr-52(tm2087) | 1±4 |
| ced-5(n1812); ttr-52(tm2087) | 0±0 |
| ced-6(n2095); ced-2(n1994) | 3±5 |
| ced-6(n2095); ced-5(n1812) | 3±6 |
| ced-12(n3261); ced-6(n2095) | 2±4 |
| wild type; smIs437 (PhspsLactC1C2) | 3±5 |
| wild type; smIs441 [PhspsLactC1C2] | 48±14 |
All strains contain the smIs76 (PhspsAnxV::GFP) transgene. For each strain, the average percentage of apoptotic cells that were associated with at least one neighboring cell with sAnxV::GFP was scored visually from at least 15 bean-stage embryos. Results are shown as mean ± SD.
The requirement of ced-1, ced-7, and ttr-52 for neighboring cell exPS expression but not ced-6, which acts downstream in the same pathway, is intriguing. The CED-1 receptor has been shown to recognize apoptotic cells through its extracellular and transmembrane domains and transduce engulfment signals in the phagocyte through its intracellular domain and its cytoplasmic binding partner CED-6 [22, 23]. A truncated CED-1 receptor lacking the intracellular domain, CED-1ΔC, can recognize and bind apoptotic cells but fails to rescue the ced-1(lf) engulfment defect [22]. Interestingly, expression of CED-1ΔC under the control of the endogenous ced-1 promoter (Pced-1 CED-1ΔC) restored exPS expression in neighboring cells in the ced-1(lf) mutant to the level seen in the ced-6(lf) mutant (Table 2), indicating that the CED-1 extracellular and transmembrane domains are sufficient to mediate partial exPS expression on phagocytes independent of its downstream signaling pathway, including CED-6.
exPS on Phagocytes Is Important for Clearance of Apoptotic Cells
Although sGFP::LactC1C2 efficiently recognized apoptotic cells (Figure 1C; Table 1), it did not label neighboring cells -- 0% of neighboring cells were labeled by sGFP::LactC1C2 in wild-type, ced-1(e1735) or ced-2(n1994) embryos (n=15 for each strain; Figure S2B). The inability of sGFP::LactC1C2 to label neighboring cells was not due to its inability to recognize exPS on neighboring cells, as co-expression of sLactC1C2, but not the PS-binding defective sLact(Mut)C1C2 mutant, with sAnxV::GFP also prevented labeling of neighboring cells by sAnxV::GFP (Table 2). These results indicate that sLactC1C2, but not sAnxV::GFP, interferes with the process by which exPS appears on phagocytes.
In mammals, exPS expression on macrophages appears to be important for phagocytosis of apoptotic cells with exPS [14, 15], as pretreatment of macrophages with Annexin V to mask PS blocks apoptotic cell clearance. We found that expression of sGFP::LactC1C2, but not expression of sAnxV::GFP or the PS-binding deficient sGFP::Lact(Mut)C1C2, neither of which blocked exPS expression in neighboring cells, inhibited efficient engulfment of apoptotic cells in C. elegans embryos (Figure 1F), suggesting that exPS expression on phagocytes could be important for cell corpse engulfment.
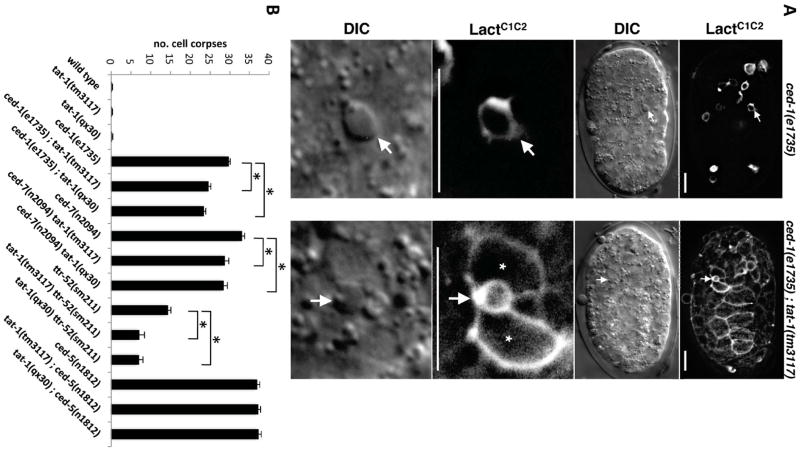
We then examined whether loss of phagocyte exPS expression can be rescued by other means to restore efficient clearance of apoptotic cells. Previously, tat-1 inactivation was shown to result in ectopic exposure of PS on the surface of all living cells in the germline but did not affect PS exposure on the surface of apoptotic cells [7]. Similarly, PS was ectopically exposed on the surface of all somatic cells in ced-1(e1735); tat-1(tm3117) embryos as indicated by sGFP::LactC1C2 labeling (Figure 3A). We thus determined whether PS exposure from the cytoplasmic leaflet of phagocytes was capable of promoting apoptotic cell clearance. We found that the engulfment defects of ced-1(e1735), ttr-52(tm2087), and ced-7(n2094) animals, all of which lacked exPS expression on phagocytes, were partially rescued by two different loss-of-function mutations in tat-1 (qx30 and tm3117)(Figure 3B). In contrast, tat-1 mutations did not reduce the engulfment defect of ced-5(n1812) animals, which still had exPS expression on phagocytes (Table 2). These results provide further supporting evidence that exPS in phagocytes is important for efficient clearance of apoptotic cells.
Figure 3. Phagocyte exPS Expression Promotes Apoptotic Cell Clearance.
(A) Inactivation of tat-1 results in PS exposure on the surface of all somatic cells. GFP and DIC images of ced-1(e1735) and ced-1(e1735); tat-1(tm3117) embryos expressing sGFP::LactC1C2 are shown. Bottom panel: Enlarged GFP and DIC images of a single cell corpse from the upper panel (indicated by an arrow) are shown. “*” indicates neighboring cells that surrounded the apoptotic cell and were labeled by sGFP::LactC1C2. Scale bar represents 5 μM.
(B) Loss of tat-1 partially rescues the cell corpse engulfment defects of the ced-1(e1735), ced-7(n2094) and ttr-52(sm211) mutants but not that of the ced-5(n1812) mutant. Cell corpses were scored in 4-fold embryos (n=15). Error bars represent SEM. Unpaired two-tailed t test, compared with respective single mutants, * P < 0.005.
How then does Lactadherin specifically block phagocyte exPS expression and compromise phagocytosis? Interestingly, Lactadherin in vitro can bind to membranes with a PS content below what Annexin V can bind, is a superior competitor to Annexin V for PS binding sites on liposomes, and of particular interest, has a strong preference for highly curved membranes [24, 25]. These distinct PS-binding properties may allow Lactadherin, but not Annexin V, to compete with endogenous PS-binding factors such as TTR-52 for binding to PS-containing particles or vesicles that may mediate PS transfer between the apoptotic cell and the phagocyte (see below)[26].
CED-7 and TTR-52 Promote Generation of Extracellular PS Vesicles
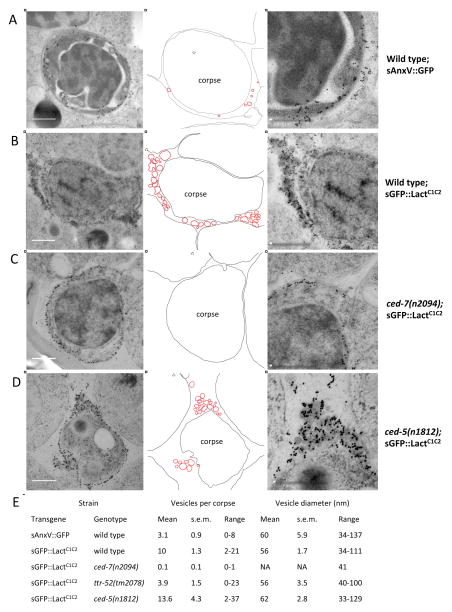
To better understand the mechanisms by which exPS is lost from apoptotic cells and exPS appears on neighboring cells, we performed immuno-electron microscopy (immunoEM) analysis of sAnxV::GFP and sGFP::LactC1C2 embryos, respectively, using an antibody against GFP (see Experimental Procedures). Consistent with the fluorescence microscopy analysis, both sAnxV::GFP- and sGFP::LactC1C2-specific gold particles were found on the surface of apoptotic cells (Figure 4A–D). In addition, only sAnxV::GFP-specific gold particles, but not sGFP::LactC1C2-specific gold particles, were found on the surface of neighboring cells (Figure S3A and S3B), confirming that sGFP::LactC1C2 does not label phagocytes. Importantly, in the extracellular space between the apoptotic cell and neighboring cells, we observed vesicles of 33–137 nm in diameter labeled by sAnxV::GFP- or sGFP::LactC1C2-specific gold particles (Figure 4), indicating that there are extracellular PS-containing vesicles between apoptotic cells and neighboring cells.
Figure 4. Immuno-Electron Microscopy of sAnxV::GFP and sGFP::LactC1C2 Embryos.
(A–D) Representative Immuno-EM micrographs from serial sections of smIs76 [sAnxV::GFP] embryos (A), smIs434 [sGFP::LactC1C2] embryos (B), ced-7(n2094); smIs434 embryos (C), and ced-5(n1812); smIs434 embryos (D). GFP-specific gold particles were observed on membranes of cell corpses and extracellular vesicles. Center column shows traces of the membranes of the cell corpses, extracellular vesicles labeled by gold particles (highlighted in red), and neighboring cells from respective images in the left column. Right column shows higher magnification images of the boxed areas in the center column. Scale bar represents 0.5 μM.
(E) Quantification of the numbers and the diameters of vesicles labeled with gold particles adjacent to a cell corpse. The mean number and diameter of vesicles, the range of the vesicle number and diameter, and SEM are shown. For each genotype, immuno-EM images from at least 10 cell corpses were analyzed. NA: not available.
Although similar in size, significantly more extracellular PS vesicles were observed near apoptotic cells in sGFP::LactC1C2 embryos (10±1.3/per apoptotic cell; Figure 4B and 4E) than in sAnxV::GFP embryos (3±0.9/per apoptotic cell; Figure 4A and 4E). This observation in combination with the findings that sGFP::LactC1C2 did not label the surface of neighboring cells and that co-expression of sLactC1C2 with sAnxV::GFP blocked labeling of neighboring cells by sAnxV::GFP suggest that sLactC1C2 perturbs the interaction of extracellular PS vesicles with neighboring cells, trapping PS vesicles in the extracellular space and thus preventing appearance of exPS on the surface of neighboring cells. These results also indicate that exPS expression in neighboring cells is not due to association with extracellular PS vesicles [27], because sGFP::LactC1C2 embryos had more PS vesicles but no exPS expression in neighboring cells (Figure S3A–S3C).
Because CED-7 and TTR-52 mediate both loss of exPS from apoptotic cells and appearance of exPS on neighboring cells and because ABC transporters have been shown to efflux lipids from the exoplasmic leaflet into the extracellular space through lipid vesicles or particles [28, 29], we examined if CED-7 and TTR-52 are involved in the generation of extracellular PS vesicles. In ced-7 mutant embryos expressing sGFP::LactC1C2, no extracellular PS vesicle was found near the apoptotic cells (Figure 4C and 4E and Figures S3D, S3G and S3I), whereas in ced-5 mutant embryos expressing sGFP::LactC1C2, a dozen extracellular PS vesicles (13.6±4.3/per apoptotic cell) were observed (Fig. 4D and 4E and Fig. S3C, S3H and S3I), indicating that loss of extracellular PS vesicles in ced-7(lf) mutants is not due to a general defect in cell corpse engulfment. In ttr-52 mutant embryos expressing sGFP::LactC1C2, significantly fewer extracellular PS vesicles (3.9±1.5/per apoptotic cell) were found near the apoptotic cells and over half of the apoptotic cells (10 out of 17 corpses) had 0–1 PS vesicle (Figure 4E and Figure S3I). In comparison, almost all apoptotic cells (14 out of 15 corpses) in wild type sGFP::LactC1C2 embryos had at least 5 adjacent PS vesicles (Figure S3I). Interestingly, in embryos expressing both sLactC1C2 and TTR-52::mCherry, TTR-52::mCherry-specific gold particles were found on similar extracellular vesicles (Figure S3J), indicating that PS-binding TTR-52 also associates with extracellular PS vesicles and may participate in the generation of these PS vesicles. Altogether, these findings indicate that ced-7 is required and ttr-52 is important for the generation of extracellular PS vesicles, which may mediate the loss of exPS from apoptotic cells and appearance of exPS on neighboring phagocytes.
Discussion
Phagocyte exPS Expression Promotes Apoptotic Cell Clearance
It is well established that exPS expression on apoptotic cells is important for their recognition and clearance by phagocytic cells [2, 4, 5]. However, the generality of phagocyte exPS expression and its potential role in apoptotic cell clearance are controversial. Moreover, the molecular mechanisms controlling exPS expression on apoptotic cells and phagocytes are poorly characterized. Using time-lapse microscopy, we show for the first time that in living C. elegans embryos both apoptotic and neighboring phagocytic cells express exPS during apoptosis, providing strong in vivo evidence for exPS expression by phagocytes. Importantly, sLactC1C2 expression prevents neighboring phagocytes from expressing exPS and compromises cell corpse engulfment. On the other hand, inducing exPS expression on phagocytes by inactivating tat-1 partially rescues the engulfment defects of ced-1, ced-7, and ttr-52 mutants, all of which lack phagocyte exPS expression, but does not rescue the engulfment defect of the ced-5 mutant, which has exPS expression on phagocytes. These results provide strong functional evidence that exPS expression by phagocytes promotes the removal of apoptotic cells.
CED-7 and TTR-52 Promote Loss of exPS from Apoptotic Cells through Generation of Extracellular PS Vesicles
Taking advantage of the well-studied genetic pathways for cell corpse engulfment in C. elegans, we dissect the molecular pathway that regulates exPS expression. Although none of the genes tested in the two primary phagocytosis signaling pathways affects the initial exPS expression on apoptotic cells (Table 1), loss of ced-7 or ttr-52 unexpectedly prevents the time-dependent loss of exPS from apoptotic cells, and in particular, from unengulfed apoptotic cells. This observation reveals for the first time that PS not only is exposed on the surface of dying cells in the early phase of apoptosis but also is actively removed from the surface of apoptotic cells subsequently through a process mediated by the ABC transporter CED-7 and the Transthyretin-like PS-binding protein TTR-52.
How do CED-7 and TTR-52 mediate time-dependent removal of exPS from apoptotic cells? It is well established that ABC transporters can efflux lipids, including cholesterol and phospholipids, from the exoplasmic leaflet into the extracellular space through lipid particles or vesicles [16, 29, 30]. Our observations that PS-containing vesicles are found in the extracellular space between apoptotic cells and phagocytes, but are completely absent in the ced-7 mutant (Figure 4C), are consistent with CED-7 promoting removal of exPS from apoptotic cells through generation of extracellular PS vesicles (Figure 5). However, given that CED-7 is found on the plasma membrane of all cells in C. elegans [31], CED-7 most likely needs to be activated specifically in apoptotic cells to promote this unidirectional PS movement, which likely also requires cooperation of additional extracellular lipid acceptors or carriers similar to apolipoprotein A-I (apoA-I) [30, 32]. Interestingly, mammalian Transthyretin is known to transport hydrophobic ligands in the bloodstream [33] and is found in the high-density lipoprotein (HDL) fraction associating with apoA-I [34]. TTR-52, an extracellular PS-binding protein and a Transthyretin homolog [8], could act as an extracellular PS acceptor or carrier to facilitate the movement of PS and the generation of PS vesicles (Figure 5). In support of this model, TTR-52 is found associating with extracellular PS vesicles near apoptotic cells (Figure S3J), the number of which is reduced significantly in the ttr-52 mutant (Figure 4E).
Figure 5. Models for exPS Expression on Phagocytes in C. elegans.
CED-7, a plasma membrane ABC transporter, acts in the apoptotic cell to promote efflux of PS through the generation of extracellular PS vesicles. TTR-52, an extracellular PS-binding protein, facilitates the generation of PS vesicles and may serve as a PS acceptor or carrier. Two potential mechanisms could result in exPS expression on phagocytes.
(A) TTR-52 also plays a role in mediating the transfer of PS from the PS vesicles to the phagocyte through interacting with the extracellular domain of the CED-1 receptor. sLact interferes with the TTR-52-mediated PS transfer process.
(B) Interaction of TTR-52-containing PS vesicles with CED-1 may result in activation of a PS transporter in the phagocyte, which promotes PS externalization. In this model, sLact may interfere with CED-1/TTR-52 interaction.
A Genetic Pathway Mediating exPS Expression in Phagocytes
Accompanying the loss of exPS from apoptotic cells is the acquisition of exPS by phagocytes through a mechanism that is also dependent on CED-7 and TTR-52, but in addition, requires the involvement of the CED-1 phagocyte receptor. The observation that TTR-52 interacts directly with the extracellular domain of CED-1 [8] and our surprising finding that the CED-1 extracellular and transmembrane domains are sufficient to mediate exPS appearance on phagocytes suggest that TTR-52 could play a role in mediating the transfer of PS from PS vesicles to CED-1-bearing phagocytes, with CED-1 providing a docking site either to unload PS or to promote fusion of PS vesicles with the phagocyte (Figure 5A)[26]. Of note, transfer of PS from vesicles to the surface of non-apoptotic cells has been documented previously in an in vitro system [35]. In addition, exPS has been shown to mediate membrane fusion in numerous systems, including the fusion of myoblasts into myotubes and HIV infection [36, 37]. Alternatively, interaction of TTR-52-containing PS vesicles with the CED-1 receptor could result in activation of a PS transporter in the phagocyte that promotes PS externalization (Figure 5B) and these two models may not be mutually exclusive. The requirement for at least one functional engulfment pathway to express exPS on phagocytes indicates that the close proximity between the apoptotic cell and the phagocyte initiated and maintained by the two phagocytosis signaling pathways may be a prerequisite for efficient exPS expression on phagocytes mediated through these extracellular PS vesicles.
Finally, exPS expression on phagocytes facilitates apoptotic cell clearance through a yet to be identified mechanism. One possibility is that PS acts as a homotypic ligand to tether the apoptotic cell to the phagocyte through a bipartite PS-binding bridging molecule and thus facilitates the clearance process [4], although RNAi knockdown or deletion of several candidate PS-binding bridging molecules such as homologues of mammalian Annexin I did not yield a detectable engulfment defect (Arur et al., 2003; J.M. and D.X., unpublished results). Alternatively, exPS expression on phagocytes could alter the activity of membrane proteins that are important for cell corpse clearance and thus promote phagocytosis [38]. Altogether, our genetic and cell biological analysis in C. elegans identifies a previously unknown pathway, comprising ced-7, ttr-52, and ced-1, in controlling the expression of exPS on apoptotic cells and phagocytes and a potential phospholipid transfer mechanism that could be important for cell-cell interactions, including phagoctyosis of apoptotic cells.
Experimental Procedures
Strains
All alleles used in this study have been described previously [7, 8, 21], except the ttr-52(tm2087) allele, which contains a 568 base pair deletion that removes most of the coding region except the last exon and is a putative null allele.
Transgenic Animals
Plasmids (at 25 ng/μl) were injected into unc-76(e911) animals [39], using an unc-76 rescuing plasmid (at 50 ng/μl) as a transgenic marker. Integrated transgenes were generated by gamma irradiation [8].
Quantification of Cell Corpses and Corpses Labeled by PS Sensors
The number of cell corpses in the head region of living embryos was scored using Nomarski optics [40]. To score the number of cell corpses labeled by a PS sensor, apoptotic cells were first identified using DIC optics and then examined for the presence of the PS sensor on their surface.
Immuno-Electron Microscopy
Transgenic embryos expressing sGFP::LactC1C2, sAnxV::GFP or TTR-52::mCherry were incubated at 33° C for 30 min to induce expression of the fusion proteins. After kept at 25° C for 2 hours, the embryos were frozen with a HPM 100 high-pressure freezer (Leica Microsystem, Buffalo Grove, IL). Freeze-substitution, low-temperature embedding in HM20 resin, ultramicrotomy, and immunogold labeling were carried out according to Kang (2010)[41]. The anti-GFP antibody was purchased from BioApplications (Pohang, Korea) and the anti-mCherry antibody was provided by Xiaochen Wang. The immunolabeled sections were observed with a Hitachi H-7000 transmission electron microscope (Hitachi America, Inc. Schaumburg, IL).
Supplementary Material
Acknowledgments
We thank Bengt Fadeel and Valerian Kagan for discussions, Roger Tsien, Joel Falke, Zheng Zhou, Michael Hengartner, Jean-Paul Oudinet, Bengt Fadeel, Stan Orrenius, Xiaochen Wang, and Peter Henson for cDNA clones, antibodies, and strains. This work was supported by NIH R01 grants (GM059083 and GM079097), a Burroughs Welcome Fund Award, and a HFSP grant (RGP0016/2005-C) to D.X., and a NSF grant (MCB-0958107) to B.H.K.
Footnotes
Supplemental Information includes three figures, three tables, and additional text and methods.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pomorski T, Hrafnsdottir S, Devaux PF, van Meer G. Lipid distribution and transport across cellular membranes. Semin Cell Dev Biol. 2001;12:139–148. doi: 10.1006/scdb.2000.0231. [DOI] [PubMed] [Google Scholar]
- 2.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 3.Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Henson PM, Bratton DL, Fadok VA. Apoptotic cell removal. Curr Biol. 2001;11:R795–805. doi: 10.1016/s0960-9822(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 5.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Wang J, Gengyo-Ando K, Gu L, Sun CL, Yang C, Shi Y, Kobayashi T, Shi Y, Mitani S, et al. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol. 2007;9:541–549. doi: 10.1038/ncb1574. [DOI] [PubMed] [Google Scholar]
- 7.Darland-Ransom M, Wang X, Sun CL, Mapes J, Gengyo-Ando K, Mitani S, Xue D. Role of C. elegans TAT-1 protein in maintaining plasma membrane phosphatidylserine asymmetry. Science. 2008;320:528–531. doi: 10.1126/science.1155847. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Li W, Zhao D, Liu B, Shi Y, Chen B, Yang H, Guo P, Geng X, Shang Z, et al. Caenorhabditis elegans transthyretin-like protein TTR-52 mediates recognition of apoptotic cells by the CED-1 phagocyte receptor. Nat Cell Biol. 2010 doi: 10.1038/ncb2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen MH, Graversen H, Fedosov SN, Petersen TE, Rasmussen JT. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry. 2000;39:6200–6206. doi: 10.1021/bi992221r. [DOI] [PubMed] [Google Scholar]
- 10.Venegas V, Zhou Z. Two alternative mechanisms that regulate the presentation of apoptotic cell engulfment signal in Caenorhabditis elegans. Mol Biol Cell. 2007;18:3180–3192. doi: 10.1091/mbc.E07-02-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- 12.Zullig S, Neukomm LJ, Jovanovic M, Charette SJ, Lyssenko NN, Halleck MS, Reutelingsperger CP, Schlegel RA, Hengartner MO. Aminophospholipid translocase TAT-1 promotes phosphatidylserine exposure during C. elegans apoptosis. Curr Biol. 2007;17:994–999. doi: 10.1016/j.cub.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 14.Callahan MK, Halleck MS, Krahling S, Henderson AJ, Williamson P, Schlegel RA. Phosphatidylserine expression and phagocytosis of apoptotic thymocytes during differentiation of monocytic cells. J Leukoc Biol. 2003;74:846–856. doi: 10.1189/jlb.0902433. [DOI] [PubMed] [Google Scholar]
- 15.Callahan MK, Williamson P, Schlegel RA. Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ. 2000;7:645–653. doi: 10.1038/sj.cdd.4400690. [DOI] [PubMed] [Google Scholar]
- 16.Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 17.Dubois T, Mira JP, Feliers D, Solito E, Russo-Marie F, Oudinet JP. Annexin V inhibits protein kinase C activity via a mechanism of phospholipid sequestration. Biochem J. 1998;330(Pt 3):1277–1282. doi: 10.1042/bj3301277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao C, Novakovic VA, Head JF, Seaton BA, Gilbert GE. Crystal structure of lactadherin C2 domain at 1.7A resolution with mutational and computational analyses of its membrane-binding motif. J Biol Chem. 2008;283:7230–7241. doi: 10.1074/jbc.M705195200. [DOI] [PubMed] [Google Scholar]
- 19.Reddien PW, Horvitz HR. The engulfment process of programmed cell death in caenorhabditis elegans. Annu Rev Cell Dev Biol. 2004;20:193–221. doi: 10.1146/annurev.cellbio.20.022003.114619. [DOI] [PubMed] [Google Scholar]
- 20.Liu QA, Hengartner MO. Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell. 1998;93:961–972. doi: 10.1016/s0092-8674(00)81202-7. [DOI] [PubMed] [Google Scholar]
- 21.Ellis RE, Jacobson DM, Horvitz HR. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics. 1991;129:79–94. doi: 10.1093/genetics/129.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z, Hartwieg E, Horvitz HR. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 23.Su HP, Nakada-Tsukui K, Tosello-Trampont AC, Li Y, Bu G, Henson PM, Ravichandran KS. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP) J Biol Chem. 2002;277:11772–11779. doi: 10.1074/jbc.M109336200. [DOI] [PubMed] [Google Scholar]
- 24.Shi J, Gilbert GE. Lactadherin inhibits enzyme complexes of blood coagulation by competing for phospholipid-binding sites. Blood. 2003;101:2628–2636. doi: 10.1182/blood-2002-07-1951. [DOI] [PubMed] [Google Scholar]
- 25.Shi J, Heegaard CW, Rasmussen JT, Gilbert GE. Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochim Biophys Acta. 2004;1667:82–90. doi: 10.1016/j.bbamem.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 27.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 28.van Meer G, Halter D, Sprong H, Somerharju P, Egmond MR. ABC lipid transporters: extruders, flippases, or flopless activators? FEBS Lett. 2006;580:1171–1177. doi: 10.1016/j.febslet.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Pohl A, Devaux PF, Herrmann A. Function of prokaryotic and eukaryotic ABC proteins in lipid transport. Biochim Biophys Acta. 2005;1733:29–52. doi: 10.1016/j.bbalip.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu YC, Horvitz HR. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell. 1998;93:951–960. doi: 10.1016/s0092-8674(00)81201-5. [DOI] [PubMed] [Google Scholar]
- 32.Wang N, Lan D, Gerbod-Giannone M, Linsel-Nitschke P, Jehle AW, Chen W, Martinez LO, Tall AR. ATP-binding cassette transporter A7 (ABCA7) binds apolipoprotein A-I and mediates cellular phospholipid but not cholesterol efflux. J Biol Chem. 2003;278:42906–42912. doi: 10.1074/jbc.M307831200. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber G. The evolutionary and integrative roles of transthyretin in thyroid hormone homeostasis. J Endocrinol. 2002;175:61–73. doi: 10.1677/joe.0.1750061. [DOI] [PubMed] [Google Scholar]
- 34.Sousa MM, Berglund L, Saraiva MJ. Transthyretin in high density lipoproteins: association with apolipoprotein A-I. J Lipid Res. 2000;41:58–65. [PubMed] [Google Scholar]
- 35.Liu R, Klich I, Ratajczak J, Ratajczak MZ, Zuba-Surma EK. Erythrocyte-derived microvesicles may transfer phosphatidylserine to the surface of nucleated cells and falsely ‘mark’ them as apoptotic. Eur J Haematol. 2009;83:220–229. doi: 10.1111/j.1600-0609.2009.01271.x. [DOI] [PubMed] [Google Scholar]
- 36.van den Eijnde SM, van den Hoff MJ, Reutelingsperger CP, van Heerde WL, Henfling ME, Vermeij-Keers C, Schutte B, Borgers M, Ramaekers FC. Transient expression of phosphatidylserine at cell-cell contact areas is required for myotube formation. J Cell Sci. 2001;114:3631–3642. doi: 10.1242/jcs.114.20.3631. [DOI] [PubMed] [Google Scholar]
- 37.Callahan MK, Popernack PM, Tsutsui S, Truong L, Schlegel RA, Henderson AJ. Phosphatidylserine on HIV envelope is a cofactor for infection of monocytic cells. J Immunol. 2003;170:4840–4845. doi: 10.4049/jimmunol.170.9.4840. [DOI] [PubMed] [Google Scholar]
- 38.Elliott JI, Surprenant A, Marelli-Berg FM, Cooper JC, Cassady-Cain RL, Wooding C, Linton K, Alexander DR, Higgins CF. Membrane phosphatidylserine distribution as a non-apoptotic signalling mechanism in lymphocytes. Nat Cell Biol. 2005;7:808–816. doi: 10.1038/ncb1279. [DOI] [PubMed] [Google Scholar]
- 39.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Wu YC, Fadok VA, Lee MC, Gengyo-Ando K, Cheng LC, Ledwich D, Hsu PK, Chen JY, Chou BK, et al. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science. 2003;302:1563–1566. doi: 10.1126/science.1087641. [DOI] [PubMed] [Google Scholar]
- 41.Kang BH. Electron microscopy and high-pressure freezing of Arabidopsis. Methods Cell Biol. 2010;96:259–283. doi: 10.1016/S0091-679X(10)96012-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.