Abstract
PLAC1 (placenta-specific 1) is a gene that is placenta specific and transcribed very little, if at all, in any somatic tissue. It is nevertheless expressed in many cancer cell lines. To understand how cancer cells may activate the gene in nonexpressing cells, we found that a model is provided by classical transformation of normal fibroblasts by SV40 T antigen. T antigen derepressed the PLAC1 P1 promoter, with Tp53 and RB exerting critical and opposing actions and nuclear receptors, retinoid X receptor and liver X receptor, sharply increasing the level of expression.
Keywords: large T antigen, transcription, NCOA2, RXRα, LXR, chromatin
Introduction
PLAC1 (placenta-specific 1) gene expression is extraordinarily tissue specific. Among normal tissues, PLAC1 is uniquely expressed in the placenta, at a high level from 6.5 to 13.5 days post coitum in mouse and throughout pregnancy in humans,1, 2 and is thus of interest to reproductive biologists. However, in spite of its selectivity in expression in the normal body, it is also expressed at high levels in a wide variety of cancers3, 4, 5 and, as a result, in a range of commonly used laboratory cell lines. Especially because inhibition of PLAC1 expression in cell lines derived from a breast cancer led to loss of invasiveness and growth,3 how the gene is derepressed in cancer is of particular interest; knowledge of this process might thereby suggest salutary ways to block PLAC1 expression in cancer cells. Consequently, based on its immunogenicity and oncogenic ranking, it is now on the National Cancer Institute list of ‘tumor antigens' that could serve as candidate targets for development of vaccines against breast cancer.6
How can PLAC1 achieve its intriguing selectivity of expression? Tissue-restricted expression is often attributable to the action of tissue-specific transcription factors such as hepatocyte nuclear factor 4 (HNF4) in liver, kidney and pancreas.7 Such factors explain the proximal regulation of the gene, but raise questions of ‘infinite regress': that is, how are the tissue-specific transcription factors themselves regulated? And how are the initiating factors for those transcription factors regulated? Here we have tried to analyze possible activation and derepression pathways for the gene.
The gene structure is conserved between mouse and human, with two promoters, P1 and P2, separated by ∼100 Kb but producing the same protein from a terminal exon.8 Defining the gene structure has facilitated the demonstration that cancer cell lines show a range of preferential usage of P1 and P2, and the activity of both promoters—both endogenously and from transfected reporter constructs—is stimulated by nuclear receptors retinoid X receptor-α (RXRα) and liver X receptor-α (LXRα) or LXRβ. The receptors were shown to bind to cognate promoter binding sites and to lose their activity when the binding sites were mutated.8
On the other hand, the question of how the gene is derepressed in cancer cells (and in placenta) was left open. To find a model to investigate the cancer-based gene activation, we turned to a classical approach, comparing primary cells with the same cells transformed by SV40. The SV40-transformed primary cell lines indeed showed activation of PLAC1, and further transfection studies with SV40 early region or large T (LT) antigen DNA constructs showed that cellular Tp53 and RB have critical—although opposing—effects in determining PLAC1 P1 promoter dynamics and transcription state.
Results
LT activates the P1 promoter in primary cells
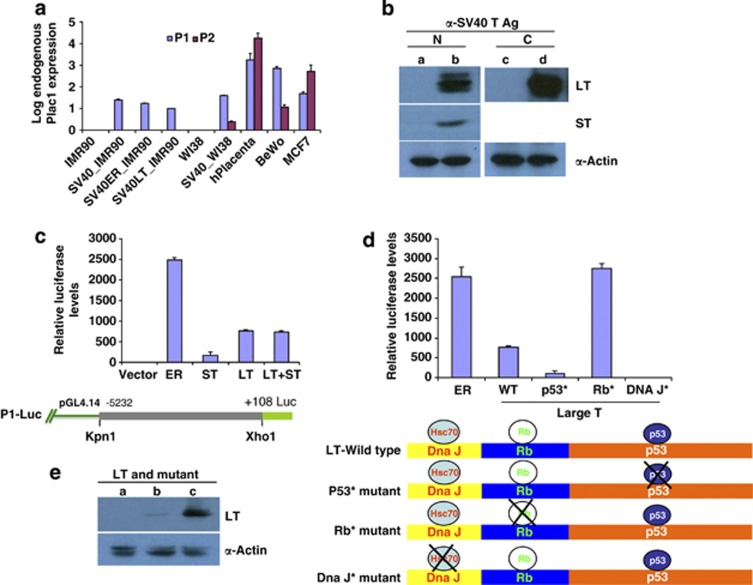
As expected from Northern1 and quantitative reverse transcritption–PCR (qRT–PCR) analyses of normal tissues, WI38 or IMR-90 primary fibroblast cultures do not express PLAC1 (Figure 1a). However, established SV40-transformed cell lines of both cell types express PLAC1 (Figure 1a). The expression in established cell lines was not a secondary effect occurring during outgrowth, because transfection with SV40 early region or just the LT antigen encoded within the early region was sufficient to induce comparable levels of PLAC1 expression (Figure 1a). Figure 1b confirms the expected expression of LT and small T (ST) in IMR-90 cells transfected with the early region (left panel), and the comparable amount of LT expressed in cells transfected with LT.
Figure 1.
Effects of transformation of primary cell lines with SV40, SV40 early region (ER) and LT antigen on endogenous PLAC1 transcription. (a) Endogenous PLAC1 expression was measured by quantitative reverse transcription polymerase chain reaction (qRT-PCR) assays using primers that distinguish the transcript isoforms from P1 and P2 promoters in RNA from placenta, BeWo cells and MCF7 cells.7 PLAC1 transcript levels were below detection in WI38 and IMR-90 cell lines before transformation, but were appreciable following transformation with SV40. Transformation with constructs expressing SV40-ER or SV40-LT induced transcription from the P1 but not the P2 promoter. (b) Western blot analysis of IMR-90 cells transfected with either early region (lane ‘b') or LT expression construct (lane ‘d'). Lanes ‘a' and ‘b' show interaction with N-terminal specific LT antibody (N) that detects both LT and ST expression from the ER. Lanes ‘c' and ‘d' show interaction with a C-terminal-specific (C) LT antibody. Lanes ‘a' and ‘c' are untransfected controls, showing no expression of LT or ST. (c) Co-transfection of P1-Luc construct with ER, ST or LT expressing constructs into BeWo cells. The ER region induced the PLAC1 promoter 2500-fold; LT or LT together with ST induced 600-fold; and ST had only a small effect. P1-Luc construct features are diagrammed below. (d) Effect of mutations in LT on transcription from P1-Luc in BeWo cells. Expression constructs driven by a CAG promoter, carrying mutations in LT at either the Tp53-binding site (p53*), Retinoblastoma protein-binding site (Rb*) or DNA J interaction site (DNAJ*), were co-transfected with P1-Luc construct, and luciferase levels measured thereafter. For comparison, transfections with ER and wild-type LT are shown. Mutation in the Tp53-binding site of LT renders LT unable to induce transcription from P1, whereas mutations in the RB-binding site activate P1 expression to the level seen with ER. (e) Western blots showing comparative expression of wild-type LT (lane ‘b') and LT with mutant p53 binding site (lane ‘c') from BeWo cells transiently transfected with respective constructs. Lane ‘a' contains extract from untransfected cells.
For comparison, Figure 1a also shows the level of endogenous PLAC1 expression from both promoters in the choriocarcinoma-derived BeWo cell line, in MCF-7 cells and in placenta. In placenta, P2 is the preferred promoter, as it is in MCF7 cells; but induction at P1 was preferential in BeWo and highly preferred in transformed WI38 and IMR-90.
Role of LT in derepression of P1 promoter
We used the previously described8 P1 promoter luciferase fusion construct P1-Luc (Figure 1c) for further characterization of LT action in PLAC1 induction. Studies were primarily carried out in BeWo cells, and critical inferences were then confirmed in transfected primary IMR-90 cells.
When the P1-Luciferase plasmid (P1-Luc) was transfected into BeWo cells with and without SV40 constructs, the early region stimulated luciferase reporter activity 2500-fold over control, and LT stimulated the promoter activity up to 600-fold (Figure 1c). ST had very little effect, and co-transfection with both ST and LT together stimulated no more than did LT alone. Therefore, we focused further on the effects of LT.
LT is a multidomain protein that helps to subvert the normal cellular response to viral infection by preventing apoptosis, activating DNA replication and cell division and promoting viral replication.9 It functions via interactions with cellular tumor suppressors Tp53 and RB and a DNA J domain that interacts with HSPA8/HSC70. To further examine its role in stimulating P1 promoter activity, we co-transfected the P1-Luc construct into BeWo cells along with LT that carried mutations in Tp53-binding sites,10 Rb-binding sites11 or DNA J interaction domains.12 We first tested LT mutated for Tp53 binding. Normally, Tp53 is at very low levels in cells and is kept in check by MDM2, an E3 ubiquitin ligase that targets Tp53 for proteasomal degradation.12 In the presence of LT, Tp53 forms a complex that simultaneously stabilizes it and elevates its cellular concentration;13, 14, 15, 16 the complex formation likely helps to deplete it from promoter sites. Mutation in the LT-binding site for TP53 abrogates their interaction, permitting TP53 to bind unimpeded to TP53-responsive promoters and inhibit their transcription. Figure 1d shows that when LT lost its interaction with Tp53 while maintaining intact pRB and DNA J domains, the P1 promoter indeed was no longer stimulated in BeWo cells. We confirmed by western blot analysis that after transient transfection of BeWo cells with plasmids expressing WT LT or LT carrying mutation in Tp53-binding site, the mutant form is expressed (Figure 1e, lane ‘c'), and at a much higher level than wild-type LT.
Next we transfected LT mutated to abolish pRB interaction, with Tp53 and DNA J interacting domains left intact. Under these conditions, RB is expected to carry out its normal cellular functions, including the stimulation of transcription at many sites. P1 promoter activity was in fact activated up to levels seen by transfection with the entire early region (Figure 1c). The more robust upregulation by RB when Tp53 binding to LT is intact may also reflect the absence of additional modulating modifications on pRB, like phosphorylation, that occur when it is bound to LT,17 and this function could be influenced by additional factors such as the 17K protein that is also encoded by the early region.
Finally, mutations in the DNA J9, 18 interaction site completely abolished activation of the PLAC1 promoter (Figure 1d). This is a surprising observation, because the J domain has been found to be dispensable for p53 binding.19 The finding raises the possibility that the J domain has a significant but uncharacterized function independent of RB binding.
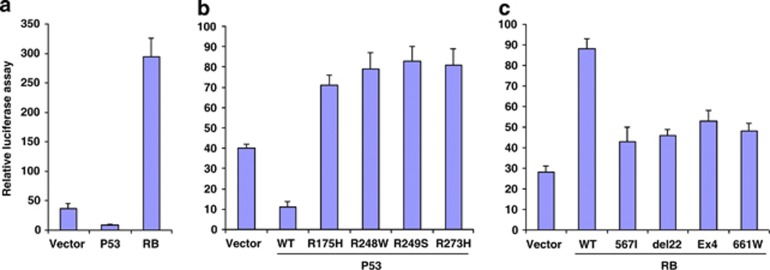
Overall, these experiments indicated that LT exerts a net influence on P1 promoter through interactions with Tp53 and RB, and we thus assessed the direct effects of expression of Tp53 and RB on the promoter in BeWo cells. Figure 2a shows that luciferase levels from the P1-Luc construct transfected into BeWo cells were inhibited when P53 was expressed and were upregulated when RB was transfected. Tp53 and RB are thus confirmed to influence promoter activity independent of the presence of LT.
Figure 2.
Effects of expression of wild-type and mutant forms of Tp53 and RB on P1-Luc activity in BeWo cells. (a) BeWo cells were co-transfected with a P1-Luc construct and a vector carrying no insert or p53 or RB gene. p53 inhibited but RB stimulated the P1 promoter. (b) BeWo cells were co-transfected with P1-Luc and various mutant forms of Tp53 from cancer cell types. Compared with wild-type Tp53, mutated Tp53 failed to repress the promoter. (c) BeWo cells were co-transfected with P1-Luc and mutant forms of RB from cancer cells. Mutated RB failed to activate the promoter as much as wild-type RB.
Notably, several cancer cell lines that express PLAC1 carry mutations in Tp53. When co-transfected with P1-Luc and compared with intact Tp53, Tp53 carrying any of the four frequently found mutations20 was unable to repress promoter expression (Figure 2b). These results imply that the Tp53 mutations most frequently found in cancer are likely sufficient to derepress the promoter (see Discussion).
As with Tp53, various RB mutations are found in cancer. Wild-type RB stimulated the P1 promoter 3.5 fold, whereas the most frequently found RB mutants, pRB;567L, pRB;661W, del22 and pRBΔex4,11 had far less effect, supporting a requirement for wild-type RB for maximum stimulation of the promoter (Figure 2c). Furthermore, because these mutants are specifically unable to bind to E2F,11 it suggests that the PLAC1 promoter is not responsive to E2F1 (see below), and RB would then necessarily affect the promoter through an alternate pathway.
Tp53 occupies a P1 promoter site in IMR-90 cells, whereas Rb shows no direct interaction
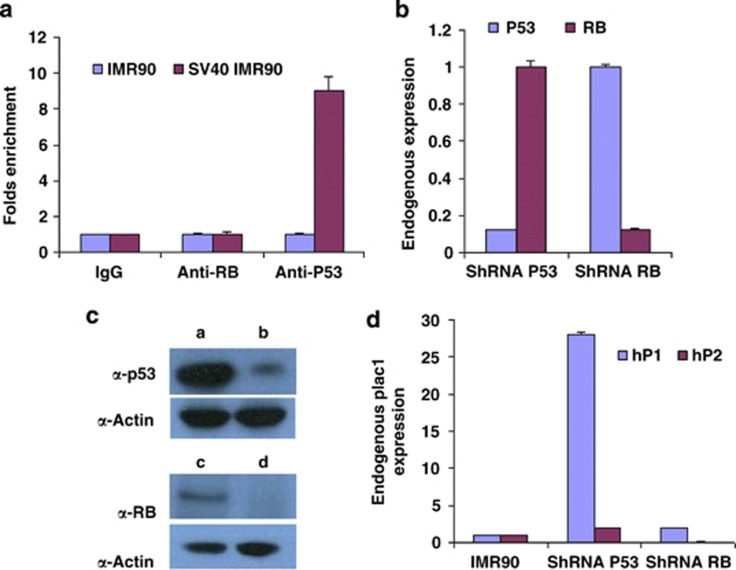
The P1 promoter contains a consensus binding site for Tp53 at -275 base pairs 5′ to the transcription start site, which was thus a candidate site to mediate interaction. To assess whether the site was truly occupied by Tp53, chromatin was immunoprecipitated with anti-Tp53 and anti-RB antibodies from IMR-90 cells and from IMR-90 cells transfected with the SV40 early region. In SV40-transfected cells the promoter region showed enrichment with anti-Tp53 antibody but not anti-RB antibody, consistent with direct association of Tp53 but not RB (Figure 3a).
Figure 3.
ChIP, and effects of shRNA inhibition of Tp53 and RB, on PLAC1 expression in IMR-90 cells. (a) ChIP with antibodies against RB and Tp53. The PLAC1 promoter was enriched for Tp53 but not RB in IMR-90 cells following SV40 transformation, suggesting that only Tp53 interacts directly with the promoter. (b) IMR-90 cells transfected with shRNA against Tp53 or RB. The corresponding targets were reduced, as measured by quantitative polymerase chain reaction (qPCR). (c) Western blot analysis of Tp53 and RB expression following stable shRNA transfections in IMR-90 cells. Lanes ‘a' and ‘c' show Tp53 and RB protein levels, respectively, in untransfected cells. Lanes ‘b' and ‘d' show corresponding protein levels following specific shRNA expression. α-Actin is a loading control. (d) PLAC1 expression measured by qPCR from P1 and P2 promoters in IMR-90 cells following transfection with shRNA against Tp53 or RB. Tp53 inhibition led to derepression of PLAC1 from P1; RB inhibition by shRNA had no effect on PLAC1 transcription.
Because the level of promoter-bound Tp53 in untransfected IMR-90 cells is low (Figure 3a) we tested further whether Tp53 inhibition was nevertheless a major determinant of PLAC1 repression. If so, direct inhibition of Tp53 formation should relieve the repression. Transfection of short hairpin RNA (shRNA) against Tp53 in IMR-90 cells indeed significantly reduced Tp53 transcript and protein levels (Figures 3b and c). In addition, Tp53 inhibition by shRNA did result in derepression of the endogenous PLAC1 gene from the P1 promoter (Figure 3d).
RB acts in concert with nuclear receptor LXR and co-activator NCOA2
Unlike Tp53, inhibition of RB RNA and protein by shRNA (Figures 3b and c) resulted in no change in the levels of P1 PLAC1 activity (Figure 3d) in IMR-90 cells. Instead, RB could act indirectly in any of several established ways.
One route to RB involvement has been established from findings that the pRB family of proteins (pRB, p130, p107), through their shared A and B domains, recruit histone deacetylases and modify chromatin to bring about transcriptional repression.21 However, RB is apparently a positive activator of PLAC1, ruling out such a repressive action.
In an alternative route, RB is bound to E2F, rendering E2F-responsive promoters inactive. When RB dissociates from the RB/E2F (1–4) complex following LT expression, E2F is free to activate E2F-responsive promoters.17, 18 However, the PLAC1 promoter is apparently not E2F responsive (see above), because we see PLAC1 derepression only when LT interaction with RB is disrupted. This would leave a repressive RB/E2F (1–4) interaction intact—the opposite of what is observed.
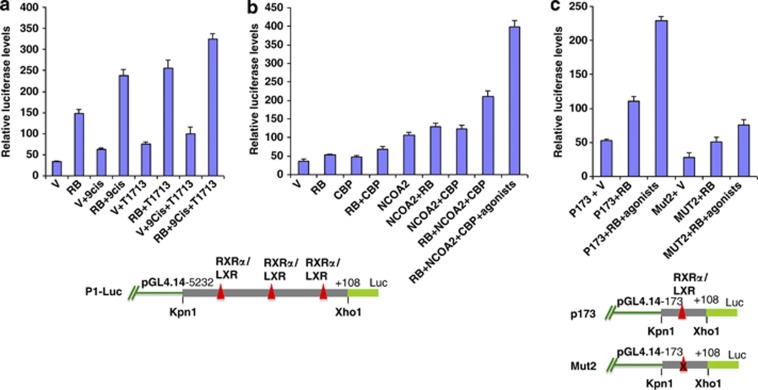
A more likely route to positive action of RB is consistent with results when the effect of RB expression was tested in BeWo cells (Figure 4a). A direct test for transfected RB action in primary cells is not feasible, because expression of RB in primary cells inhibits growth: DNA replication ceases and cells become senescent.22 In BeWo cells, RB transfected by itself gave a fourfold stimulation of luciferase activity. When RXRα and LXRβ agonists were also added, a further 2.5-fold stimulation was seen. Given that either agonist alone gave some additional stimulation (less than twofold) compared with controls in the absence of RB, it remained possible that the nuclear receptor effect was independent of RB action, involving another pathway. However, additional experiments showed that RB and nuclear receptors act jointly, as follows.
Figure 4.
Effect of RB on P1-Luc activity in the presence of nuclear receptor RXRα and LXRβ agonists (a) BeWo cells were transfected with P1-Luc construct alone or co-transfected with RB-expressing vector, and incubated with LXR agonist T091713 or RXRα agonist 9-Cis-retinoic acid. Luciferase activity was measured 72 h after transfection. RB itself stimulated fivefold, and in presence of agonists stimulation reached 10-fold over basal levels. The location of nuclear receptor binding sites is indicated below. (b) P1-Luc promoter activity in BeWo cells in the presence of various cofactors. Vectors expressing nuclear receptor co-activators NCOA2 or CBP were co-transfected with or without RB-expressing vector, as indicated, and luciferase levels from the P1 promoter were then measured as in (a). Stimulation by RB was maximal when both CBP and NCOA2 were coexpressed and agonists for RXRα and LXRβ were also added. (c) Activity of wild-type and Mut2 P173-Luc promoter in BeWo cells in the presence of RB alone and RB with agonists. Mut2 construct (below the panel) is a version of P173-Luc with mutations in the RXRα-binding site.7 The Mut2 construct failed to show stimulation by RB with or without agonists.
From literature reports, joint action of RB with nuclear receptors would require co-activators. NCOA2 and other members of its co-activator class have histone acetyltransferase activity and recruit CAAAT binding protein/p300 (CBP/p300).23 We therefore tested the effect of expression of the P1-Luc construct in BeWo cells when nuclear co-receptor NCOA2 and CBP were expressed along with RB. An increase in luciferase activity over RB transfection alone was seen, and increased further to over eightfold when RXRα and LXRβ agonists were also added. These results are consistent with stimulation of P1 promoter activity by RB in conjunction with RXRα and LXRβ and co-activator NCOA2 in the presence of CBP (Figure 4b).
Stimulation by RB is seen even for a shortened version of the minimal promoter, P173-Luc, which contains a single nuclear receptor-binding site (Figure 4c); again, expression was further enhanced twofold in the presence of RXRα and LXR agonists. Decisive linking of RB to nuclear receptors was seen when the promoter was mutated in the RXRα-binding site (Figure 4c, Mut2 construct8), which prevents interaction with the nuclear receptors. The mutation sharply reduced stimulation by both RB and nuclear receptors, supporting the essentiality of the presence of nuclear receptors at the promoter for RB interaction.
Repressive histone methylation patterns are erased following transformation by SV40 early region
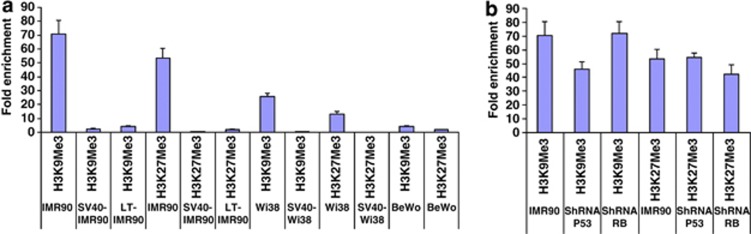
It seemed likely that histone methylation and/or acetylation at specific positions, resulting in heterochromatin formation and repression, could be seen at the endogenous P1 promoter in nonexpressing cells. We checked the chromatin status of the endogenous P1 promoter by immunoprecipitation with antibodies for multiple histone methylation and acetylation modifications in IMR-90, WI38 and BeWo cells. We found that the P1 PLAC1 promoter is enriched for H3K9 and H3K27 trimethyl modifications in IMR-90 and WI38 cells, consistent with repressed promoter status. Upon SV40 early region transfection of IMR-90 and WI38 cells, the promoter was depleted of these modifications (Figure 5a), as expected for transcriptional activation. Basal methylation levels between IMR-90 and WI38 cells differ by approximately threefold, whereas the methylation status in BeWo cells, which already express PLAC1, was similar to the hypomethylated levels in IMR-90 and WI38 cells following SV40 early region transfection; no significant changes in histone acetylation status were observed (results not shown). The changes in repressive methylation status are thus an index of SV40-induced promoter activation, possibly reflecting global LT effects across much of the genome.
Figure 5.
PLAC1 promoter enrichment following chromatin immunoprecipitation with antibodies against histone-modifying enzymes. Antibodies against histone 3 lysine 9 or lysine 27 trimethyl modifications were used to immunoprecipitate chromatin from (a) IMR-90 and WI38 cells with or without SV40 transformation compared with BeWo cells. (b) IMR-90 cells following transfection with shRNAs against Tp53 and RB. The immunoprecipitated chromatin fraction was tested for PLAC1 promoter enrichment by qPCR. Fold enrichment of the promoter was calculated as a ratio compared with control immunoprecipitations with IgG.
To check whether Tp53 and RB had a direct role in bringing about the changes in histone methylation, we checked histone methylation status by inhibiting Tp53 or RB in IMR-90 cells. Results showed some (less than twofold) reduction in H3K9 and H3K27 trimethylation when either Tp53 or RB was inhibited (Figure 5b). However, in contrast, methylation was reduced nearly 70-fold in SV40 or LT transfected primary cells (Figure 5a). This suggests that LT transfection may affect histone demethylation by a process that requires additional proteins or is even independent of Tp53 and RB; alternatively, loss of a small number of critical modifications that are dependent on Tp53 and RB may be sufficient to provide a chromatin conformation adequate for gene expression.
Discussion
Plac1 knockout mice show substantial placental defects, with placental layers showing abnormal differentiation.24 Because the placenta has invasive properties, it has been suggested that cancer cells might hijack placental invasive mechanisms.25 But whatever the possible function of PLAC1 in cancer cells, activation of its promoter is an indispensible first step.
We find that PLAC1 expression follows a paradigm in which highly tissue-restricted transcription involves alleviation of any repression followed by positive amplification of transcription by auxiliary factors. We have shown that SV40 transformation of primary cells WI38 and IMR-90 or transfection with SV40 early region in fact derepresses transcription, and LT is sufficient. A schematic model (Figure 6) outlines a possible mechanism. Consistent with derepression, we see that repressive histone H3K9/K27 trimethylations decline as PLAC1 is activated. A major repressive transcription factor alleviated by SV40 action is Tp53, and we find that Tp53 indeed acts as a repressor of PLAC1 transcription in primary fibroblasts. Tp53 is normally expressed in the cells, and LT expression binds both Tp53 and RB. This could effectively reduce the local concentration at the P1 promoter or render it unable to act effectively as a repressor. The latter option is more likely, because even when transcription was derepressed chromatin immunoprecipitation (ChIP) assays still found Tp53 enriched at the promoter.
Figure 6.
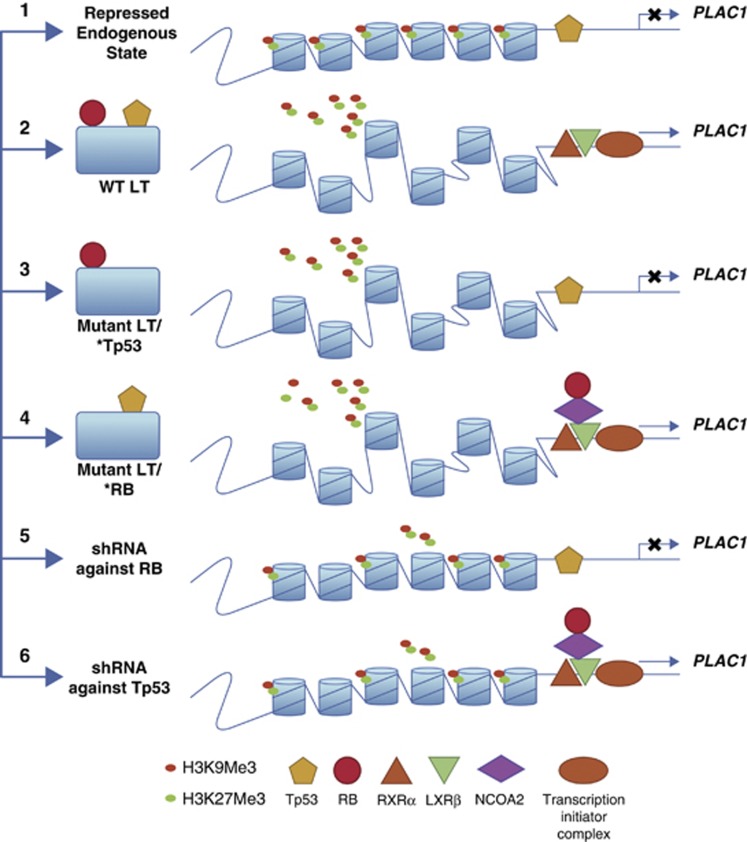
Model for events during PLAC1 derepression by SV40/LT. (1) The repressed status of P1 promoter chromatin in primary cells with histone 3 trimethylated at lysine 3 and lysine 9 position and Tp53 occupying its binding sites. At this stage, PLAC1 is not expressed, with no transcription initiator complex formed. RXRα and LXRβ may or may not occupy their binding sites. (2) Upon direct expression of LT, transfection of SV40-ER, or after SV40 transformation, histone trimethylations are erased, opening the chromatin. RB and Tp53 are bound to LT, resulting in increased stabilization of Tp53 and its cellular sequestration by LT, along with modification(s). This allows transcription machinery to assemble at the promoter; and in cells expressing nuclear receptors RXRα and LXRβ, they would bind to their cognate sites and further boost PLAC1 transcription. (3) When LT with a Tp53-binding site mutation is expressed, it can only interact with RB. Histone methylation patterns are modified, but transcription is still repressed, with Tp53 still bound at the promoter. (4) When LT with an RB-binding domain is expressed, it interacts with Tp53 and removes Tp53 from promoter site. Rb is free to interact with the nuclear receptors in the presence of NCOA2, leading to upregulation of basal transcription levels. (5) When shRNA against RB is expressed, TP53 still occupies the promoter with less than a twofold change in histone trimethylations. PLAC1 remains repressed. (6) When shRNA against Tp53 is expressed, Tp53 occupancy at the promoter is lost, leading to promoter derepression, with assembly of a transcription initiator complex and binding of nuclear receptors. Once again, there is no drastic change in trimethylation status (reduced by less than twofold). These events suggest that complete erasure of trimethylation marks at the promoter is not strictly required, although that might further facilitate the opening of chromatin structure.
WT Tp53 apparently functions in normal fibroblasts independent of SV40 transformation, because PLAC1 expression increased by 25-fold when primary cells were transfected with shRNA against Tp53 (Figure 6). In addition, transfected WT Tp53 inhibited P1-Luc expression, whereas mutant Tp53 variants stimulated transcription (Figures 2a and b). Do all cancer cells with Tp53 mutations derepress PLAC1? In a relevant study, Silva et al.4 surveyed a number of cancer cell lines representing various cancers for expression of PLAC1, and ranked its expression level (see Table 2 in Silva et al.4). We checked these cell lines in the Tp53 mutation database at http://p53.fr/ for the presence of Tp53 mutations, and found that a majority of the cancer cell lines that express high levels of PLAC1 indeed carry mutation in Tp53 (or, as in H929 cells, express high levels of MDM2,26 leading to loss of Tp53 expression). There are, however, also examples of cell lines that carry Tp53 mutation(s) but express very low levels of PLAC1 (for example, MOLT4 and U251).4 Possibly not all mutations in Tp53 lead to derepression of PLAC1 in cancer cells, or additional events are necessary for gene derepression. Nevertheless, activation of PLAC1 when Tp53 is specifically depleted with shRNA argues for direct involvement of Tp53 lesions in PLAC1 derepression.
Inhibition of RB interaction with LT was necessary to bring about its full positive effect on PLAC1 transcription. pRB can act in association with several transcription factors to overcome repression by a negative regulator;27—for example, in the activation of MyoD during myogenesis28 and CBFA1/Runx2 during osteogenesis.29 In some instances, a positive promoter response is brought about indirectly by blocking the action of differentiation inhibitors such as EID-1, ID2 and RBP2.27
In contrast to promoter derepression involving the disarming of Tp53, RB is rather involved in the second, activating phase of expression. Among various possible routes to positive RB action on PLAC1, a likely candidate is based on a co-stimulation model. pRB has been shown to potentiate transcription by interacting with nuclear receptors23 such as HNF4, SF-1 and ERα and ERβ in conjunction with NCOA2 as a cofactor. We previously found that nuclear receptor RXRα and LXRα or LXRβ bind to cognate sites in the promoter to stimulate P1 and P2 promoters in BeWo cells;8 and we find here that RB, analogous to other instances, stimulates PLAC1 P1 promoter activity in the presence of nuclear receptor agonists for RXR and LXR, and is further upregulated when NCOA2 is also expressed (Figure 6b). As expected, transfection of mutant forms of RB normally found in cancer cells stimulated the PLAC1 promoter less.
Because PLAC1 is immunogenic6 and its induction is associated with changes in histone methylation patterns, agents affecting methylation might upregulate its expression in cancers, thereby provoking an augmented immune response with therapeutic potential. However, direct experiments are required to determine whether any increase in PLAC1 levels might also increase tumor aggressiveness or have other negative side effects.
To summarize, a probable scenario for the overall regulation of the gene (Figure 6) now infers that the PLAC1 promoter region in somatic tissues is in a heterochromatic state. In various types of cancer, oncogenic transformation could have an effect like that of LT in fibroblasts, with RB and Tp53 affected in similar ways. For the specific activation of PLAC1 in the placenta, the transcription factor Cdx2, which triggers trophoblast lineage differentiation,30 from which the placenta is derived, opens chromatin access at many loci;31 again having an effect comparable to that of LT (though any involvement of Tp53 and RB has not been investigated in that case). In subsequent transcription initiation, expression would again be augmented in the placenta, as in fibroblasts and BeWo cells, by the positive interactions of co-activators with nuclear receptors, which are localized at the maternal/placental interface.32
Materials and methods
Cell culture
The human placental-derived choriocarcinoma cell line BeWo (CCL-98), human lung epithelial cell line WI38 (CCL-75), SV40 transformed WI38 (VA-13, CCL-75.1) and human breast adenocarcinoma MCF-7 (HTB-22) cells were purchased from ATCC (Manassas, VA, USA). The human lung fibroblast cell line IMR-90 (I90) and SV40 transformed IMR-90 (AG02804) were obtained from Coriell Institute (Camden, NJ, USA). BeWo cells were cultured in F-12k medium with 10% fetal bovine serum, and the other human cell lines in Eagle's minimal essential medium supplemented with 10% fetal bovine serum and 0.01 mg/ml bovine insulin.
Luciferase assay
Luciferase assays were performed as previously described.8 Briefly, BeWo cells in 24-well plastic dishes were transfected with a human PLAC1 P1 promoter construct (P1-Luc) along with indicated cDNA plasmids, and a Renilla Luciferase construct (10 ng/transfection) as an internal control of transfection efficiency. In other controls, vector without insert was co-transfected with the P1 construct. The relative luciferase activity was measured from cells 48 h after transfection, using the Dual-Luciferase reporter assay system (Promega, Madison, WI, USA) on a Victor 1420 multi-label counter (Perkin Elmer, Waltham, MA, USA). To measure the effect of agonists, T091713 (100 nM) and/or 9-Cis-retinoic acid (1 μM) (Sigma, St Louis MO, USA) were added into culture medium 24 h after transfection of plasmids, and luciferase activity measured 24 h thereafter.
ShRNA transfection and western blot analysis
IMR-90 cells (I90-83, Coriell Institute) at passage PDL10 were infected with lentiviral particles from Santa Cruz Laboratories (Santa Cruz, CA, USA) carrying shRNA(h) against Tp53 (Cat. no. sc-29435-V) or Rb (Cat. no. sc-29468-V). Positive cells were selected with Puromycin (2 μg/ml) added 48 h following infection, followed by further growth for 5 days. RNA was isolated from the surviving cells with TRI reagent (Sigma), and real-time PCR assays were used to assess PLAC1 expression. For western blots, cells were solubilized in RIPA buffer with protease inhibitors. After centrifugation at 10 000 × g for 15 min at 4 °C, the supernatant was fractionated on an 8–16% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and proteins were transferred to a nitrocellulose membrane (Thermo Scientific, Lafayette, CO, USA). The membrane was incubated with α-p53 (FL-393, Cat. no. sc-6243) or α-RB (C-15, Cat. no. sc-50; Santa Cruz) antibody at 4 °C overnight. Blots were washed three times with Tris-buffered saline (pH 7.5) containing 1% Triton X-100 before incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies (GE Healthcare, Pittsburgh, PA, USA) for 1 h. Immunoreactive bands were visualized with Enhanced Chemiluminescence Reagent (GE Healthcare).
Chromatin immunoprecipitation (ChIP)
ChIPs were performed as previously described8 with the indicated antibodies, following the protocols provided by the company. Briefly, ∼2 × 107 cells were treated with formaldehyde at a final concentration of 1% for 10 min at room temperature. The crosslinking reaction was terminated by adding glycine to a final concentration of 0.125 M. Treated cells were washed 3 times with phosphate-buffered saline, lysed by adding 1 ml Lysis Buffer (10 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 0.1% Na-Deoxycholate, 0.5% N-lauroylsarcosine and protease inhibitors (Cat. no. 04693132001; Roche Biosciences, Indianapolis, IN, USA) at concentrations recommended by the manufacturer), sonicated 5 times with 15-s pulses at power setting 5, with 2-min intervals of chilling on ice, in a Misonix Sonicator XL2020 (MIsonix Incorporated, Farmingdale, NY, USA) equipped with a microtip. The mixture was then centrifuged at 13 000 r.p.m. in a microfuge for 15 min at 4 °C, and 150 μg of supernatant fraction (chromatin) was incubated with 10 μg of indicated antibodies or with IgG (control) overnight at 4 °C. The antibody–protein–DNA complexes were recovered by adding A/G PLUS-Agarose (Invitrogen, Grand Island, NY, USA). The beads were washed five times with Lysis Buffer. Crosslinking was reversed by incubating the fraction overnight at 67 °C. The beads were removed by centrifugation. The supernatant was extracted with phenol/chloroform/isoamyl alcohol (25:24:1), vortexed thoroughly, and the aqueous phase (DNA) precipitated with ethanol. The resulting DNA was used in Real-time PCR reactions and quantitated using SYBR Green PCR Master Mix (Applied Biosystems, Grand Island, NY, USA). The sequence of the primers used to detect the promoter enrichment was as follows: forward: 5′-CAAGGTAACCCCACGCTCTA-3′, and reverse: 5′-GTTTTCCTCGGGAATGTCTG-3′. The primers amplify a 240-bp fragment between −163 and +76 bases with respect to the transcription start site; this region spans the RXRα-binding sites.8
Quantitation of PLAC1, Tp53 and RB transcripts in human cancer cell lines by one step real-time RT–PCR
RNA was extracted from cells with TRI reagent (Sigma), quantified by measuring A260 absorbance and quality determined by measuring 260/280 absorbance ratios.
Real-time RT–PCR was performed with Taqman one step RT–PCR kits (Life Technologies, Grand Island, NY, USA) on an Applied Biosystems 7900. To distinguish differences in RNA expression levels from P1 and P2 promoters, the following set of primers were used, synthesized by Applied Biosystems: Ex1-realF: 5′-GAGGAGTCTGTCAAGGAGTGC-3′, Ex4-realF: 5′-AAAGGCCACCCCTCTTCAGT-3′, Ex6-realR: 5′-AGCCGTCCAGTGAGGATTTCT-3′, with the reporter oligonucleotide sequence 6Fam-5′-ACTGACAAATTATCCCCAGCTGCCAGAAG-3′-Tamra).
Tp53 and RB transcript levels were determined using Tp53- or RB-specific oligonucleotides (Cat. no. HS.PT.51.22574907G and Cat. no. Rb: HS.PT.51.787090, respectively, from Integrated DNA Technology, Coralville, IA, USA). Standard β2 microglobulin primers were used as an endogenous control (Life Technologies, Cat. no. 4310886E) in all cases. The differences in expression levels were calculated by using the 2ΔΔ CT method and normalized to actin levels.
List of plasmid constructs used in this study
All of the following plasmids were purchased from Addgene (Cambridge, MA, USA), and their descriptions are detailed in the corresponding references (Table 1).
Table 1. List of plasmids used in this study.
| Addgene ID | Plasmids |
|---|---|
| 22883 | pRSVBneoT early region SV409 |
| 9054 | pSG5 Large T delta 434–44412 |
| 9055 | pSG5 Large T K112 |
| 16434 | pCMV-Neo-Bam p53 WT10 |
| 16436 | pCMV-Neo-Bam p53 R175H10 |
| 16437 | pCMV-Neo-Bam p53 R248W10 |
| 16438 | pCMV-Neo-Bam p53 R249S10 |
| 16439 | pCMV-Neo-Bam p53 R273H10 |
| 10720 | pSG5L HA RB11 |
| 10725 | pSG5L HA RB 567L11 |
| 10731 | pSG5L HA RB 661W11 |
| 10730 | pSG5L HA RB del ex411 |
| 10721 | pSG5L HA RB del2211 |
| 13970 | pBABE-puro SV40 LT33 |
| 9048 | pBABE-neo large T H42Q34 |
| 8583 | pBABE-zeo small T34 |
| 16701 | pRC/RSV-m CBP-HA35 |
The left column gives the plasmid ID numbers in Addgene website and the column on the right gives a brief description of the vector and the gene content.
Inserts in pBABE vector containing LT, LTH42Q or ST encoding DNA fragments were PCR amplified using, for LT, forward primer: 5′-atctcgagcaccATGGATAAAGTTTTAAACAGAGAGG-3′ and reverse: R 5′-gcggccgcgcggccgcTTATGTTTCAGGTTCAGGGGGA-3′ and for ST, reverse primer ST_R 5′-gcggccgcgcggccgcTTAGAGCTTTAAATCTCTGTAGGT-3′ in combination with the forward primer for LT. The recovered products were gel-purifed and cloned into the pPyCAG mammalian expression vector (http://www.cdb.riken.jp/pcs/protocol/vector/vector_top.html).
P1-Luc, P173-Luc and Mut2 constructs have been previously described8 and are schematically represented in Figures 1c and 4c. The NCOA2 gene was cloned into the pPyCAG vector at the Spe1/Not1 site by excising the insert with the same enzymes from a PCR Topo Blunt vector carrying NCOA2 (Cat. no. MHS6278-211690481; Thermo Scientific).
The following antibodies were used in western blot (WB) or ChIP assays as indicated (Table 2).
Table 2. List of antibodies used in this study, the type of assay in which they were used and their source.
| Antibodies | Assay | Company |
|---|---|---|
| α-SV40 T Ag N-terminus (Pab 108) | WB | Santa Cruz Laboratories, Santa Cruz, CA, USA |
| α-SV40 T Ag C-terminus (Pab 101) | WB | Santa Cruz Laboratories |
| α– p53 (FL–393) | WB/ChIP | Santa Cruz Laboratories |
| α–RB (C–15) | WB/ChIP | Santa Cruz Laboratories |
| α–H3K9me3 (ab8898) | ChIP | Abcam, Cambridge, MA, USA |
| α–H3K27me3 (07449) | ChIP | Millipore, Billerica, MA, USA |
| α–H3K9ac (ab10812) | ChIP | Abcam |
| α–H3K27ac (ab4729) | ChIP | Abcam |
Acknowledgments
This work was entirely funded by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. We thank Drs Wedong Wang and Paritosh Ghosh for critical reading of the manuscript and suggestions.
The authors declare no conflict of interest.
References
- Cocchia M, Huber R, Pantano S, Chen EY, Ma P, Forabosco A, et al. PLAC1, an Xq26 gene with placenta-specific expression. Genomics. 2000;68:305–312. doi: 10.1006/geno.2000.6302. [DOI] [PubMed] [Google Scholar]
- Fant M, Weisoly DL, Cocchia M, Huber R, Khan S, Lunt T, et al. PLAC1, a trophoblast-specific gene, is expressed throughout pregnancy in the human placenta and modulated by keratinocyte growth factor. Mol Reprod Dev. 2002;63:430–436. doi: 10.1002/mrd.10200. [DOI] [PubMed] [Google Scholar]
- Koslowski M, Sahin U, Mitnacht-Kraus R, Seitz G, Huber C, Türeci O. A placenta-specific gene ectopically activated in many human cancers is essentially involved in malignant cell processes. Cancer Res. 2007;67:9528–9534. doi: 10.1158/0008-5472.CAN-07-1350. [DOI] [PubMed] [Google Scholar]
- Silva WA, Jr, Gnjatic S, Ritter E, Chua R, Cohen T, Hsu M, et al. PLAC1, a trophoblast-specific cell surface protein, is expressed in a range of human tumors and elicits spontaneous antibody responses. Cancer Immun. 2007;7:18–27. [PMC free article] [PubMed] [Google Scholar]
- Dong X-Y, Peng J-R, Ye Y-J, Chen H-S, Zhang L-J, Pang X-W, et al. Plac1 is a tumor-specific antigen capable of eliciting spontaneous antibody responses in human cancer patients. J Int Cancer. 2008;122:2038–2043. doi: 10.1002/ijc.23341. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parviz F, Matullo C, Garrison WD, Savatski L, Adamson JW, Ning G, et al. Hepatocyte nuclear factor 4alpha controls the development of a hepatic epithelium and liver morphogenesis. Nat Genet. 2003;34:292–296. doi: 10.1038/ng1175. [DOI] [PubMed] [Google Scholar]
- Chen Y, Moradin A, Schlessinger D, Nagaraja R. RXRα and LXR activate two promoters in placenta- and tumor-specific expression of PLAC1. Placenta. 2011;32:877–884. doi: 10.1016/j.placenta.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, McClellan AJ, Vartikar J, Marks I, Cantalupo P, Li Y, et al. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SJ, Markowitz S, Fearon ER, Willson JK, Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990;249:912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Sellers WR, Novitch BG, Miyake S, Heith A, Otterson GA, Kaye FJ, et al. Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 1998;12:95–106. doi: 10.1101/gad.12.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalvide J, Stubdal H, DeCaprio JA. The J domain of simian virus 40 large T antigen is required to functionally inactivate RB family proteins. Mol Cell Biol. 1998;18:1408–1415. doi: 10.1128/mcb.18.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M, Maltzman W, Levine AJ. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981;1:101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek TL, Laffin J, Lehman JM, Jacobberger JW. A subset of cells expressing SV40 large T antigen contain elevated p53 levels and have an altered cell cycle phenotype. Cell Prolif. 2000;33:115–125. doi: 10.1046/j.1365-2184.2000.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Deb S, Muñoz RM, Subler MA, Deb SP. The tumor suppressor p53 and the oncoprotein simian virus 40 T antigen bind to overlapping domains on the MDM2 protein. Mol Cell Biol. 1993;13:6849–6857. doi: 10.1128/mcb.13.11.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning W, Rohaly G, Kolzau T, Knippschild U, Maacke H, Deppert W. MDM2 is a target of simian virus 40 in cellular transformation and during lytic infection. J Virol. 1997;71:7609–7618. doi: 10.1128/jvi.71.10.7609-7618.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11:15–23. doi: 10.1006/scbi.2000.0342. [DOI] [PubMed] [Google Scholar]
- Sullivan CS, Tremblay JD, Fewell SW, Lewis JA, Brodsky JL, Pipas JM. Species-specific elements in the large T-antigen J domain are required for cellular transformation and DNA replication by simian virus 40. Mol Cell Biol. 2000;20:5749–5757. doi: 10.1128/mcb.20.15.5749-5757.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjoerup O, Chao H, DeCaprio JA, Roberts TM. pRB-dependent, J domain-independent function of simian virus 40 large T antigen in override of p53 growth suppression. J Virol. 2000;74:864–874. doi: 10.1128/jvi.74.2.864-874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino FP, Marchesi I, Giordano A. On the role of retinoblastoma family proteins in the establishment and maintenance of the epigenetic landscape. J Cell Physiol. 2013;228:276–284. doi: 10.1002/jcp.24141. [DOI] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- Batsché E, Desroches J, Bilodeau S, Gauthier Y, Drouin J. Rb enhances p160/SRC coactivator-dependent activity of nuclear receptors and hormone responsiveness. J Biol Chem. 2005;280:19746–19756. doi: 10.1074/jbc.M413428200. [DOI] [PubMed] [Google Scholar]
- Jackman SM, Kong X, Fant ME. Plac1 (placenta-specific 1) is essential for normal placental and embryonic development. Mol Reprod Dev. 2012;79:564–572. doi: 10.1002/mrd.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old LJ. Cancer is a somatic cell pregnancy. Cancer Immun. 2007;7:19. [PMC free article] [PubMed] [Google Scholar]
- Pichiorri F, Suh S-S, Rocci A, De Luca L, Taccioli C, Santhanam R, et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell. 2010;18:367–381. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Talluri S, Dick FA. Regulation of transcription and chromatin structure by pRB: here, there and everywhere. Cell Cycle. 2012;11:3189–3198. doi: 10.4161/cc.21263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Schneider JW, Condorelli G, Kaushal S, Mahdavi V, Nadal-Ginard B. Interaction of myogenic factors and the retinoblastoma protein mediates muscle cell commitment and differentiation. Cell. 1993;72:309–324. doi: 10.1016/0092-8674(93)90110-c. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Carty SA, Piscopo DM, Lee JS, Wang WF, Forrester WC, et al. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell. 2001;8:303–316. doi: 10.1016/s1097-2765(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–929. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- Verzi MP, Shin H, San Roman AK, Liu XS, Shivdasani RA. Intestinal master transcription factor CDX2 controls chromatin access for partner transcription factor binding. Mol Cell Biol. 2013;33:281–292. doi: 10.1128/MCB.01185-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapin V, Ward SJ, Bronner S, Chambon P, Dollé P. Differential expression of transcripts encoding retinoid binding proteins and retinoic acid receptors during placentation of the mouse. Dev Dyn. 1997;208:199–210. doi: 10.1002/(SICI)1097-0177(199702)208:2<199::AID-AJA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Zhao JJ, Gjoerup OV, Subramanian RR, Cheng Y, Chen W, Roberts TM, et al. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–495. doi: 10.1016/s1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Dessain SK, Brooks MW, King JE, Elenbaas B, Sabatini DM, et al. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22:2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]