Abstract
Objective
Highly sensitive guaiac-based faecal occult blood (Hemoccult SENSA) and Helicobacter pylori stool antigen testing might help detect upper gastrointestinal lesions when appended to a colorectal cancer screening programme with faecal immunochemical testing. We evaluated the diagnostic accuracies of two stool tests in detecting upper gastrointestinal lesions.
Design
Cross-sectional design.
Setting
Hospital-based and community-based screening settings.
Participants
A hospital-based deviation cohort of 3172 participants to evaluate test performance and a community-based validation cohort of 3621 to verify the findings.
Interventions
Three types of stool tests with bidirectional endoscopy as the reference standard.
Outcomes
Sensitivity, specificity and positive and negative likelihood ratios.
Results
For detecting upper gastrointestinal lesions in cases with negative immunochemical tests, the sensitivity, specificity, and positive and negative likelihood ratios of the guaiac-based and H pylori antigen tests were 16.3% (95% CI 13.3% to 19.8%), 90.1% (88.9% to 91.2%), 1.64 (1.31 to 2.07), and 0.93 (0.89 to 0.97), respectively, and 52.5% (48.1% to 56.9%), 80.6% (79.0% to 82.1%), 2.71 (2.41 to 3.04) and 0.59 (0.54 to 0.65), respectively. For detecting upper gastrointestinal lesions in cases with normal colonoscopy, the results of the guaiac-based and H pylori antigen tests were 17.9% (14.8% to 21.5%), 90.1% (88.9% to 91.2%), 1.81 (1.45 to 2.26) and 0.91 (0.87 to 0.95), respectively, and 53.1% (48.6% to 57.4%), 80.7% (79.1% to 82.2%), 2.75 (2.45 to 3.08) and 0.58 (0.53 to 0.64), respectively. Within the community, positive predictive values of the immunochemical and H pylori antigen tests were 36.0% (26.0% to 46.0%) and 31.9% (28.3% to 35.5%), respectively, for detecting lower and upper gastrointestinal lesions, which were similar to expected values.
Conclusions
The H pylori stool antigen test is more accurate than the guaiac-based test in the screening of upper gastrointestinal lesions in a population with high prevalence of H pylori infection and upper gastrointestinal lesions. It is applicable to add the H pylori antigen test to the immunochemical test for pan detection.
Trial registration
NCT01341197 (ClinicalTrial.gov).
Keywords: GASTROENTEROLOGY
Strengths and limitations of this study.
Faecal occult blood tests include guaiac-based tests and immunochemical tests; the former tests for haeme while the latter tests for globin. Because globin can be digested by enzymes in the upper gastrointestinal tract, the immunochemical test is more specific to colorectal diseases. To differentiate lesions in the upper gastrointestinal tract from lesions in the lower gastrointestinal tract, the highly sensitive guaiac-based test (Hemoccult SENSA) combined with the faecal immunochemical test may be helpful. In addition to the highly sensitive guaiac-based test, the Helicobacter pylori stool antigen test may be an alternative choice for mass screening because H pylori is well known as a major cause of peptic ulcers and gastric cancer.
In participants with normal colonoscopies, the highly sensitive guaiac-based test can detect occult blood from the upper gastrointestinal tract in approximately one-fourth of cancerous lesions and the detectability increases with cancer stage. In participants with negative immunochemical tests or normal colonoscopies, the H pylori stool antigen test is more accurate than the highly sensitive guaiac-based test in the detection of upper gastrointestinal lesions.
In community setting, it is applicable to add the H pylori stool antigen test into the colorectal cancer screening with the faecal immunochemical test.
This is the first study to determine the diagnostic accuracy of commercially available stool tests in the detection of upper gastrointestinal lesions and also the first one to evaluate the feasibility of a two-in-one stool test for the simultaneous screening of upper and lower gastrointestinal lesions in the community.
Further randomised controlled trials are needed to confirm the long-term efficacy and cost-effectiveness of using our screening strategy.
Introduction
Upper and lower gastrointestinal diseases have an impact on global health and economics.1 2 Although mass screening can detect early lesions,3 coordination of different methods to simultaneously screen lesions at different depths is required.4 Thus, an efficient alternative screening method is needed.
A campaign against colorectal cancer has suggested that the use of faecal occult blood tests can efficiently reach the population and decrease mortality.5 Faecal occult blood tests include guaiac-based test and faecal immunochemical test (FIT); the former test measures haeme, while the latter test measures globin. Because globin can be digested by enzymes in the upper gastrointestinal tract,6 the guaiac-based test combined with the immunochemical test may help differentiate lesions in the upper gastrointestinal tract from lesions in the lower gastrointestinal tract.7 8
Other than occult blood, stool tests detecting molecular markers are under continuous development; however, most stool tests are not widely available commercially.9 One exception is the Helicobacter pylori stool antigen test (HPSA) because H pylori is well-known as a major cause of peptic ulcers and gastric cancer.10 As the absolute number of patients with gastric cancer is increasing due to advancing age of the global population,11 it makes sense to combine two specific tests (HPSA and FIT) into one panel for the detection of upper as well as lower gastrointestinal lesions.
Beginning in 2004, the Taiwanese Government initiated a nationwide colorectal cancer screening programme using biennial FIT.12 Thus, a unique opportunity exists to evaluate the diagnostic accuracies of the guaiac-based and HPSA tests for detecting upper gastrointestinal lesions, which was the primary aim of our study. Our secondary aim was to evaluate the feasibility of combining two stool tests for pan detection in a real world setting.
Methods
Study design
We recruited consecutive participants from hospital-based and community-based screening sources; the former (deviation cohort) was used to evaluate the test performance and the latter (validation cohort) was to confirm the reproducibility and applicability of the screening strategy in the community. All participants provided signed informed consent before enrolment. Both studies were approved by the Hospital Institutional Review Board (numbers IRB201101016RC and 201205030RIB).
Deviation cohort
Recruitment of study participants
First, beginning on 1 March 2011, we recruited participants at the National Taiwan University Hospital (Health Management Center; Taipei, Northern Taiwan) through advertising messages for cancer screening. Participants >18 years of age who had completed the FIT, guaiac-based test, HPSA and bidirectional endoscopy were included. Cancer detection was the main purpose of the stool test; a small sample of cancers was obtained from a single site. To enrich the deviation cohort with the sample of gastrointestinal tract cancers, we also recruited participants in whom gastrointestinal tract cancers were suspected by the non-invasive screening test, such as radiology and oral inspection, at the Gastroenterologic or Otolaryngologic Clinics at National Taiwan University Hospital. Research staff recruited participants by explaining the purpose and eligibility requirements of the study. The participants were requested to complete three types of stool tests and undergo bidirectional endoscopy; however, those participants who underwent only one endoscopy for detection of a cancerous lesion were still eligible because it was rare (approximately 0.9%) for one subject to have important lesions in the upper as well as lower gastrointestinal tracts in the cancer screening group. All of the stool samples were collected before confirmatory endoscopic diagnoses were available.
In the deviation cohort, we ensured that bleeding was occult by excluding those participants who had overt gastrointestinal bleeding, including haematemesis, tarry stools, melena or hematochezia. We also excluded participants with histories of a gastrectomy and/or colectomy and pregnant or lactating women.
Stool tests
Ten days before cancer screening, we mailed one collection card for the highly sensitive guaiac-based test (Hemoccult SENSA Single Slides; Beckman Coulter, Inc, USA) and two sampling tubes for the HPSA (Easy One Step Test; Firstep Bioresearch, Inc, Taiwan) and the FIT (OC-SENSOR; Eiken Chemical Co, Ltd, Tokyo, Japan) to eligible participants. We used the 1-day method for all stool tests and advised participants to start diet and drug restrictions 3 days before they obtained the stool samples and to obtain stool samples within 2 days before starting the bowel preparation. Stool samples were brought to the hospital on the screening day and tested immediately. Two technicians executed the guaiac-based and H pylori stool antigen tests and read the results independently; disagreements were resolved by consensus. For the cancer group, they were also requested to follow the same rule for stool sample collection.
The sensitivity and specificity of the HPSA for the determination of H pylori infection status were 88% and 99%, respectively.13 The FIT was processed at the hospital's central laboratory using an automated reader and the cut-off concentration was set at 100 ng of haemoglobin/mL of buffer (equivalent to 20 μg haemoglobin/g of faeces).14
Bidirectional endoscopy
Endoscopic findings and histological results served as the reference standard. Participants were given polyethylene glycol for bowel preparation, which they drank at least 4 h before endoscopic examinations. This bowel preparation scheme had been shown with less than 5% poor or inadequate preparation in our population.15 The participants were advised to stop anticoagulant or antiplatelet therapy for 7 days. Oesophagogastroduodenoscopy and colonoscopy were performed by nine experienced endoscopists, each with a minimum experience of 5000 colonoscopies. Endoscopic findings were recorded electronically with information on quality of preparation, completeness of colonoscopy, endoscopic findings and whether or not a biopsy was performed. Participants who declined endoscopy or had an incomplete colonoscopy, including failed caecal intubation and poor bowel preparation, were excluded.
Definition of important lesions
Lesions consistent with occult bleeding were defined as important, and were classified into three major categories, as follows in flow diagrams: neoplastic, inflammatory and vascular.7 16 Important upper gastrointestinal lesions included cancer, an ulcer >0.5 cm in diameter, reflux oesophagitis with a severity of Los Angeles grade C or D,17 Barrett's oesophagus, oesophageal varix with a severity of form II or III,18 and gastric antral vascular ectasia. Gastritis and ulcer scars were not included. Important lower gastrointestinal lesions included colorectal cancer, advanced adenomas, ulcers or colitis and angiodysplasia. An advanced adenoma was defined as >10 mm in diameter or having a villous component or high-grade dysplasia.11 Adenomas <10 mm in diameter, hyperplastic polyps and haemorrhoids were not included. All endoscopists were blinded to stool test results before endoscopic examination.
Validation cohort
Recruitment of study subjects
According to the results of the deviation cohort, we found that the H pylori stool antigen test had a better performance in the detection of upper gastrointestinal lesions. We then evaluated the applicability and reproducibility of this strategy in a community population aged 50–69 years (Changhua County, central Taiwan) in the Changhua Community-based Integrated Screening (CHCIS). This integrated screening programme has provided oral inspection for oral/throat cancer, mammography for breast cancer, Pap smear for cervical cancer and FIT for colorectal cancer since 2005.4 In 2012, we added the HPSA into this screening programme and used standard screening indicators, including participation rate, positive rate, referral rate, endoscopic findings, positive predictive value and detection rate, to confirm the applicability of this new strategy in the community and compared the observed and estimated positive predictive values to confirm the reproducibility of the stool test performance.19 The endoscopic diagnoses of gastrointestinal neoplasia were confirmed by histological results under routine medical practice.
Under the auspices of the Changhua County Public Health Bureau and the Health Promotion Administration, Ministry of Health and Welfare, a series of consensus meetings and educational programmes were held for primary care physicians and first-line healthcare workers before implementation. Beginning on 21 April 2012, eligible participants were invited by telephone or postcard from 27 public health units covering a total of 26 townships. Participants with positive results were referred to 15 local gastrointestinal clinics and 9 hospitals in Changhua County for antibiotic treatment and/or endoscopic diagnoses. The sample size was planned to be similar to the hospital-based deviation cohort.
Statistical analysis
Stool test performance and sample size estimation
We began our analyses from evaluations of the guaiac-based and FIT for the screening of lower gastrointestinal lesions. We used test results and colonoscopic findings to construct a 2×2 table and expressed test performance as sensitivity, specificity, positive and negative likelihood ratios and corresponding 95% CIs.
For the detection of upper gastrointestinal lesions, our sample size estimation was based on the fact that the detectability of the guaiac-based test for the upper gastrointestinal lesions should be interpreted together with a negative FIT.7 We anticipated that the prevalence of the upper gastrointestinal lesions would be higher in cases with positive guaiac-based tests, but negative FIT. We were aware that approximately 12% and 4% of a general population would test positive with the guaiac-based and FIT, respectively, so at least 8% of the participants would have positive results for the guaiac-based test, but negative results for the FIT.20 21 We assumed that the prevalence of upper gastrointestinal lesions would be at least 30%, which was 10% higher than that of the general population,6 thus the guaiac-based test would yield a diagnostic OR of at least 1.7. By setting a power of 80% and a 0.05 one-side type 1 error, we determined that an overall sample size of 3138 would be sufficient. To account for 10% participants who might return an inadequate sample, we planned to test 3500 participants.
We also evaluated whether or not the HPSA would be effective under the same testing conditions. We assumed that the prevalence of upper gastrointestinal lesions with occult bleeding would be 10% higher in participants with H pylori infection than the prevalence in the general population. We knew that approximately one-fourth of our population were infected with H pylori and that 88% would test positive.13 Based on the predefined type 1 error and predetermined sample size, the power to reject our null hypothesis was 99%.
In the previous scenario, performance of the guaiac-based test in detecting upper gastrointestinal lesions could be underestimated because some colorectal lesions would test negative by the FIT. Therefore, we also evaluated whether or not we could use the guaiac-based test to guide the use of oesophagogastroduodenoscopy in cases with normal colonoscopy results.16
For different clinical scenarios, we calculated the test performance of the guaiac-based and HPSA in detecting upper gastrointestinal lesions and used the two-sample proportional test to make comparisons between the tests. A two-sided p value <0.05 indicated a significant difference. Statistical analyses were performed using SAS V.9.2. Knowing that the guaiac-based and HPSA tests may detect different lesions of the gastrointestinal tract, we did subgroup analyses according to the anatomic sites and tumour stages22 to determine whether or not test sensitivity would change.
Community validation
Using sensitivity and specificity derived from our hospital-based study and reported prevalence of gastrointestinal lesions in the community, we estimated the positive predictive values of stool tests in the community with the Bayes’ rule23
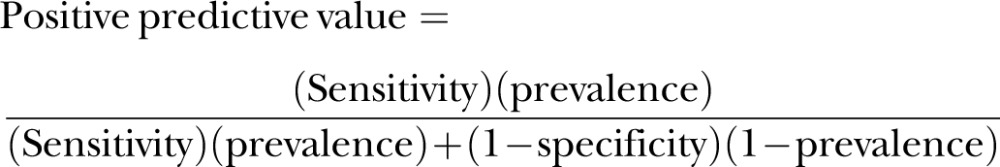 |
We compared the estimated values with observed values using WinBUGS (V.1.4; MRC Biostatistics Unit, Cambridge, UK (see online supplementary appendix figure S1)).
Sensitivity analysis
To solidify the generalisability of our findings, we constructed a decision model using TreeAge Pro 2009 (TreeAge Software, Inc, USA (see online supplementary appendix figure S2)) based on the choice of stool tests, the prevalence of important lesions in the upper gastrointestinal tract, and the prevalence of H pylori infection in participants with or without upper gastrointestinal lesions to determine how changes in population characteristics might affect positive predictive values.23 24 Base-case estimates were derived from our hospital-based study and ranges of sensitivity analyses are shown in online supplementary appendix table S1.13 25 We set a positive predictive value of >10% as a minimal requirement to support screen tests in the community.26
Results
Results of the deviation cohort
Participation rate
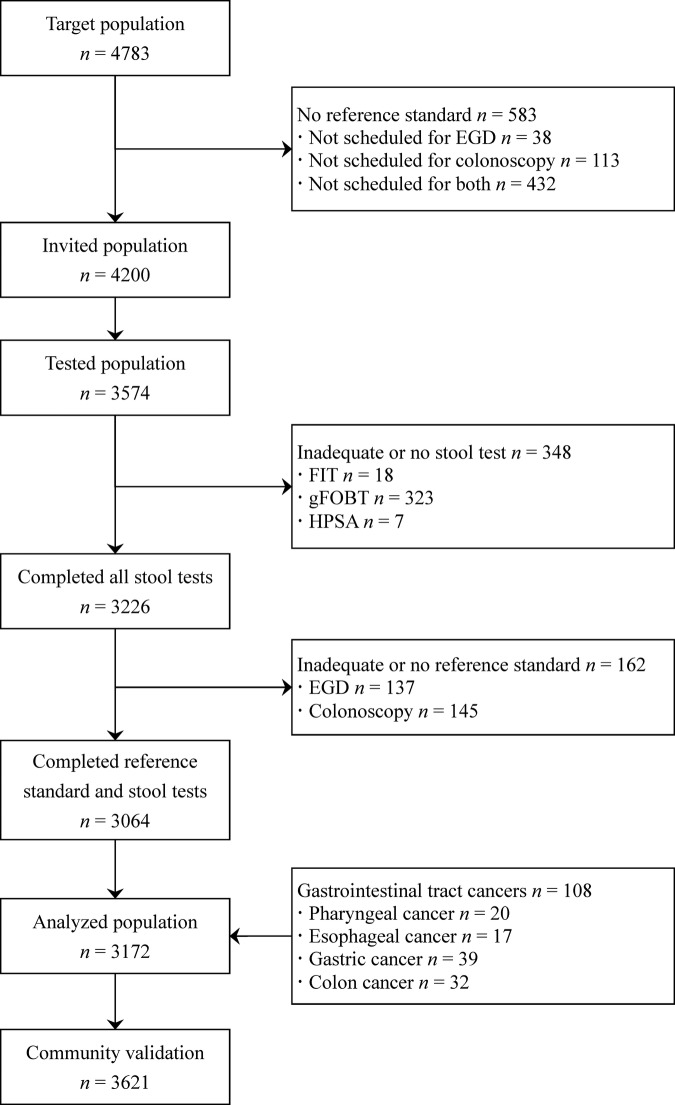
Between 1 March 2011 and 15 February 2012, 4783 participants who underwent cancer screening were evaluated, of which 4200 were invited to participate in the study and 3574 (85.1%) ultimately participated (figure 1). The completion rates for the guaiac-based, HPSA and FIT tests were 91%, 99.8% and 99.5%, respectively. Between 19 July 2011 and 21 November 2012, we also enriched the hospital-based cohort with 108 screen-detected cancers from other sites and all participants completed the stool tests. Our deviation cohort of 3172 participants was comprised of two groups.
Figure 1.
Flow diagram of enrolment. EGD, esophagogastroduodenoscopy; FIT, faecal immunochemical test; gFOBT, guaiac-based faecal occult blood test; HPSA, Helicobacter pylori stool antigen test.;
Results of stool tests and endoscopy
Descriptive results are shown in table 1 and the flow diagrams are shown in figures 2 and 3. Among the cancer screening group (n=3064), the positive rates of the guaiac-based, HPSA and immunochemical tests were 11.3%, 24.2% and 3.1%, respectively, and the prevalence of upper and lower gastrointestinal lesions was 14.5% and 4.6%, respectively. Among the screen-detected cancer group (n=108), 33 participants with upper gastrointestinal cancers did not undergo colonoscopy, while one participant with colon cancer did not undergo oesophagogastroduodenoscopy.
Table 1.
Demographic data, stool test results and endoscopic findings in the hospital-based deviation cohort
| Characteristics | Population, n=3172 |
|---|---|
| Male gender (%) | 1919 (60.5) |
| Age in years (SD; range) | 53.0 (11.7; 19.0–91.8) |
| Positive stool test results (%) | |
| Guaiac-based faecal occult blood test | 397 (12.5) |
| Helicobacter pylori stool antigen test | 789 (24.9) |
| Faecal immunochemical test | 133 (4.2) |
| Important lesions in the upper gastrointestinal tract (%) | 523 (16.5) |
| Categories | |
| Pharyngeal or oesophageal carcinoma | 37 (1.2) |
| Erosive oesophagitis, grade C or D | 46 (1.5) |
| Barrett's oesophagus | 8 (0.3) |
| Oesophageal varices, form II or III | 5 (0.2) |
| Gastric carcinoma | 41 (1.2) |
| Peptic ulcers | 365 (11.5) |
| Gastric antral vascular ectasia | 9 (0.3) |
| Anatomic sites | |
| Pharynx or oesophagus | 96 (3.0) |
| Stomach or duodenum | 439 (13.8) |
| Important lesions in the lower gastrointestinal tract (%) | 174 (5.5) |
| Categories | |
| Colorectal carcinoma | 39 (1.2) |
| Advanced adenoma | 106 (3.3) |
| Colitis or ulcer | 25 (0.8) |
| Angiodysplasia | 4 (0.1) |
| Anatomic sites* | |
| Proximal colon | 98 (3.1) |
| Distal colon | 76 (2.4) |
*Proximal colon was defined as the level above splenic flexure (including splenic flexure). When synchronous colon lesions were found, the anatomic site of the most important one was used to define the location.
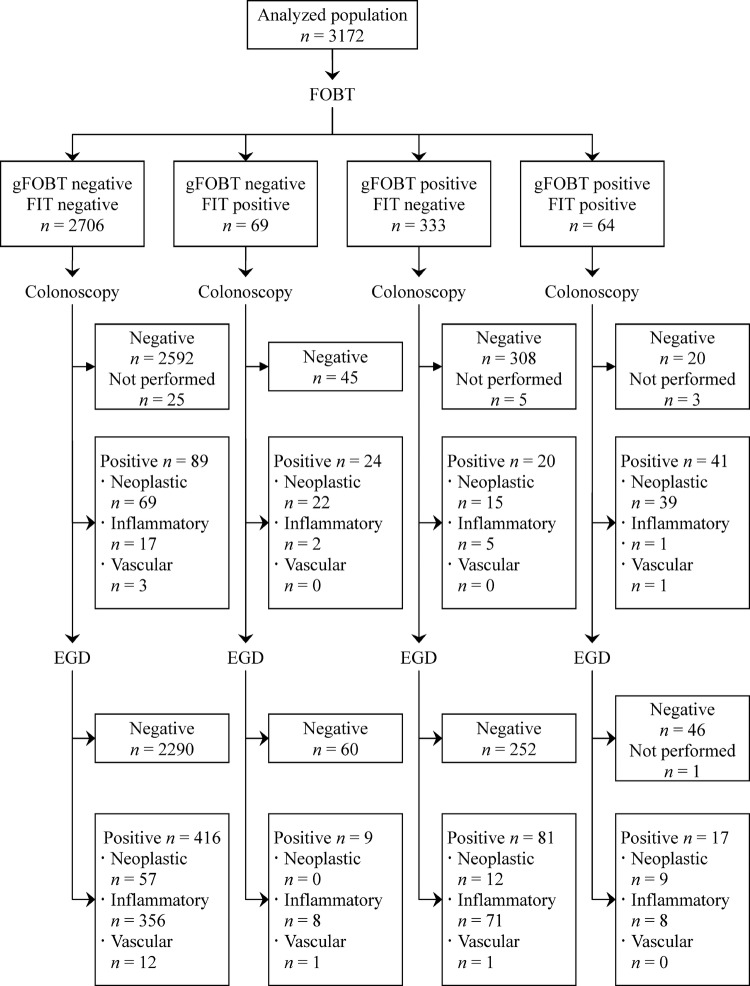
Figure 2.
Flow diagram for the screening using the guaiac-based occult blood test and the faecal immunochemical test according to the Standards for Reporting of Diagnostic Accuracy statement in the hospital-based deviation cohort.
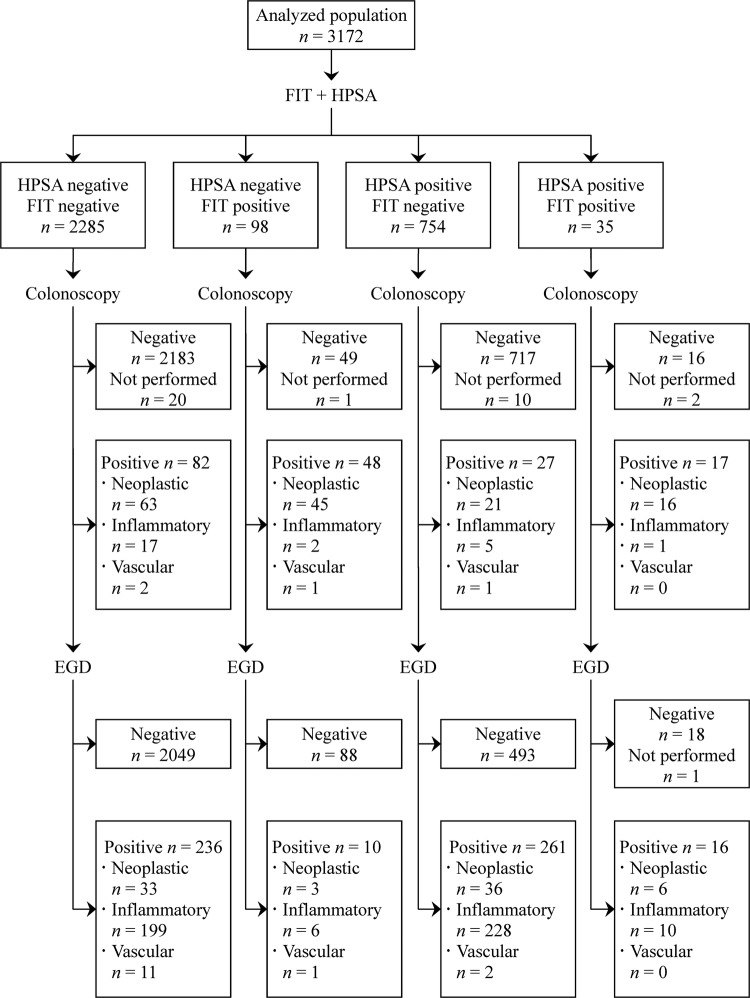
Figure 3.
Flow diagram for the screening using the Helicobacter pylori stool antigen test and the faecal immunochemical test according to the Standards for Reporting of Diagnostic Accuracy statement in the hospital-based deviation cohort.
Overall, the prevalence of upper gastrointestinal lesions was similar between participants with positive and negative results on the FIT (26/133 (19.6%) vs 497/3039 (16.4%), p=0.31). Also, the prevalence of colorectal lesions was similar between participants with positive and negative results on the HPSA (44/789 (5.6%) vs 130/2383 (5.5%), p=0.90). However, the prevalence of upper gastrointestinal lesions was higher in participants with positive results on the guaiac-based test than those with negative results (98/397 (24.7%) vs 425/2775 (15.3%), p<0.001). No serious adverse events occurred related to the endoscopic screening.
In participants with a negative FIT or normal colonoscopy, the prevalence of upper gastrointestinal lesions was higher in participants with a positive guaiac-based test (81/333 (24.3%) and 80/328 (24.4%), respectively) and in participants with a positive HPSA (261/754 (34.6%) and 249/733 (34.0%), respectively) than the overall population (523/3172 (16.5%); all p<0.001).
Stool test performance
To detect colorectal lesions or cancers, the sensitivity and negative likelihood ratio were similar between the guaiac-based and immunochemical tests, while the specificity and positive likelihood ratio of the FIT were significantly higher (table 2, scenario 1, p<0.001).
Table 2.
Performance and the corresponding 95% CIs of three stool tests in screening important lesions in the lower or upper gastrointestinal tract under three different scenarios in the hospital-based deviation cohort
| Outcome variables | Sensitivity (%) | Specificity (%) | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|
| Scenario 1: screen for lesions in the lower or upper gastrointestinal tract in all participants | ||||
| Guaiac-based faecal occult blood test | ||||
| Important lesions in the lower gastrointestinal tract | 61/174 (35.1; 28.4–42.4) | 2637/2965 (88.9; 87.8–90.0) | 3.17 (2.53–3.98) | 0.73 (0.65–0.82) |
| Colorectal cancers | 34/39 (87.2; 73.3–94.4) | 2770/3133 (88.4; 87.3–89.5) | 7.52 (6.45–8.78) | 0.15 (0.06–0.33) |
| Important lesions in the upper gastrointestinal tract | 98/523 (18.7; 15.6–22.3) | 2350/2648 (88.8; 87.5–89.9) | 1.67 (1.35–2.05) | 0.92 (0.88–0.96) |
| Cancers in the upper gastrointestinal tract | 21/78 (26.9; 18.3–37.7) | 2718/3093 (87.9; 86.7–89.0) | 2.22 (1.52–3.24) | 0.83 (0.73–0.95) |
| Faecal immunochemical test | ||||
| Important lesions in the lower gastrointestinal tract | 65/174 (37.4; 30.5–44.7) | 2900/2965 (97.8; 97.2–98.3) | 17.04 (12.52–23.19) | 0.64 (0.57–0.72) |
| Colorectal cancers | 32/39 (82.1; 67.3–91.0) | 3032/3133 (96.8; 96.1–97.3) | 25.45 (19.99–32.41) | 0.19 (0.10–0.36) |
| Helicobacter pylori stool antigen test | ||||
| Important lesions in the upper gastrointestinal tract | 277/523 (53.0; 48.7–57.2) | 2137/2648 (80.7; 79.2–82.2) | 2.75 (2.45–3.07) | 0.58 (0.53–0.64) |
| Cancers in the upper gastrointestinal tract | 42/78 (53.9; 42.9–64.5) | 2347/3093 (75.9; 74.3–77.4) | 2.23 (1.80–2.77) | 0.61 (0.48–0.77) |
| Scenario 2: screen for lesions in the upper gastrointestinal tract in participants with negative results on the faecal immunochemical test | ||||
| Guaiac-based faecal occult blood test | ||||
| Important lesions in the upper gastrointestinal tract | 81/497 (16.3; 13.3–19.8) | 2290/2542 (90.1; 88.9–91.2) | 1.64 (1.31–2.07) | 0.93 (0.89–0.97) |
| Cancers in the upper gastrointestinal tract | 12/69 (17.4; 10.2–28.0) | 2649/2970 (89.2; 88.1–90.3) | 1.61 (0.96–2.73) | 0.93 (0.83–1.03) |
| H pylori stool antigen test | ||||
| Important lesions in the upper gastrointestinal tract | 261/497 (52.5; 48.1–56.9) | 2049/2542 (80.6; 79.0–82.1) | 2.71 (2.41–3.04) | 0.59 (0.54–0.65) |
| Cancers in the upper gastrointestinal tract | 36/69 (52.2; 40.6–63.5) | 2252/2970 (75.8; 74.2–77.3) | 2.16 (1.71–2.73) | 0.63 (0.49–0.81) |
| Scenario 3: screen for lesions in the upper gastrointestinal tract in participants with normal results on the colonoscopy | ||||
| Guaiac-based faecal occult blood test | ||||
| Important lesions in the upper gastrointestinal tract | 88/492 (17.9; 14.8–21.5) | 2258/2506 (90.1; 88.9–91.2) | 1.81 (1.45–2.26) | 0.91 (0.87–0.95) |
| Cancers in the upper gastrointestinal tract | 21/77 (27.3; 18.6–38.1) | 2606/2921 (89.2; 88.0–90.3) | 2.53 (1.73–3.70) | 0.82 (0.71–0.94) |
| H pylori stool antigen test | ||||
| Important lesions in the upper gastrointestinal tract | 261/492 (53.1; 48.6–57.4) | 2022/2506 (80.7; 79.1–82.2) | 2.75 (2.45–3.08) | 0.58 (0.53–0.64) |
| Cancers in the upper gastrointestinal tract | 41/77 (53.3; 42.2–64.0) | 2217/2921 (75.9; 74.3–77.4) | 2.21 (1.78–2.75) | 0.62 (0.49–0.78) |
In participants with a negative FIT (scenario 2) or normal colonoscopy (scenario 3), the sensitivity and positive and negative likelihood ratios of the HPSA were significantly better than those of the guaiac-based test in detecting upper gastrointestinal lesions (p<0.001), while the specificity was lower. For upper gastrointestinal cancers, the results were similar, except that there was no significant difference between the two tests with respect to the positive and negative likelihood ratios. In participants with a negative FIT, the diagnostic ORs of the guaiac-based and HPSA tests for upper gastrointestinal lesions were 1.77 and 4.60, respectively. The performance of the HPSA was similar between the three scenarios.
Subgroup analysis
The sensitivities of the guaiac-based and FIT tests were similar for different locations and stages of colorectal neoplasms (table 3). The sensitivity of the HPSA was superior to that of the guaiac-based test for stomach/duodenum lesions and stages 1–2 cancers (mainly gastric cancers), while the sensitivity of the guaiac-based test for upper gastrointestinal cancers increased with tumour stage. Note that the sensitivities of the HPSA for gastric lesions and gastric cancer were 60% and 78%, respectively.
Table 3.
The number of positive cases and the corresponding sensitivity of stool tests in the screening of important lesions in the upper or lower gastrointestinal tract, stratified by the anatomic sites and cancer stages in the hospital-based deviation cohort
| Lesions in the lower gastrointestinal tract | Faecal immunochemical test (%) | Guaiac-based fecal occult blood test (%) | All |
|---|---|---|---|
| Location of the lesions | |||
| Overall | 65 (37.4) | 61 (35.1) | 174 |
| Distal colon lesions | 28 (36.8) | 28 (36.8) | 76 |
| Proximal colon lesions | 37 (37.8) | 33 (33.7) | 98 |
| Location of the cancers | |||
| Overall | 32 (82.1) | 34 (87.2) | 39 |
| Distal colon cancers | 17 (94.4) | 17 (94.4) | 18 |
| Proximal colon cancers | 15 (71.4) | 17 (81) | 21 |
| Stage of the cancers | |||
| Stages 1–2 colon cancers | 17 (81) | 17 (81) | 21 |
| Stages 3–4 colon cancers | 15 (83.3) | 17 (94.4) | 18 |
| Lesions in the upper gastrointestinal tract | Helicobacter pylori stool antigen test (%) | Guaiac-based faecal occult blood test (%) | |
| Location of the lesions | |||
| Overall | 261 (53.1) | 88 (17.9) | 492 |
| Stomach and duodenum lesions | 247 (59.4) | 72 (17.3) | 416 |
| Pharynx and oesophagus lesions | 14 (18.4) | 16 (21.1) | 76 |
| Location of the cancers | |||
| Overall | 41 (53.3) | 21 (27.3) | 77 |
| Gastric carcinoma | 31 (77.5) | 10 (25) | 40 |
| Pharynx and oesophagus cancers | 10 (27) | 11 (29.7) | 37 |
| Stage of upper gastrointestinal cancers | |||
| Stages 1–2 upper gastrointestinal cancers | 21 (75) | 5 (17.9) | 28 |
| Stages 3–4 upper gastrointestinal cancers | 20 (40.8) | 16 (32.7) | 49 |
The tumour stage was defined by the American Joint Committee on Cancer seventh edition.22
The colon above the level of the splenic flexure (including splenic flexure) was defined as the proximal colon. Synchronous lesions denoted concurrent proximal and distal lesions of colorectal neoplasms. When multiple lesions were present, participants were categorised according to the highest severity of lesions in the proximal and distal colon.
Note that those who had important lower gastrointestinal lesions have been excluded from the evaluation of test positivity for the upper gastrointestinal lesions. Also note that the majority (25/28, 89.3%) of stages 1–2 cancers were stomach cancers while the majority (34/49, 69.4%) of stages 3–4 cancers were pharynx or oesophagus cancers.
Results of the validation cohort
Applicability and reproducibility
Between 21 April 2012 and 31 December 2012, 6798 participants aged 50–69 years were evaluated, of which 3621 eligible participants were invited to participate. The results are shown in table 4. We estimated that the positive predictive values were 34.5% (95% CI 31.6% to 37.5%) and 35.5% (28.0% to 43.8%), respectively, for the screening of upper and lower gastrointestinal lesions in the community27 28; the results were similar to the observed values of 31.9% (28.3% to 35.5%) and 36.0% (26.0% to 46.0%), respectively. The number of endoscopies needed to find one upper and one lower gastrointestinal lesion was 3.1 (2.8–3.5) and 2.8 (2.1–3.7), respectively.
Table 4.
Demographic data, participation rate, positive rate, referral rate, endoscopic findings and stool test performance in the community-based validation cohort
| Characteristics | Invited population, n=3621 |
|---|---|
| Male gender (%) | 941 (26) |
| Age in years (SD; range) | 57.9 (5.3; 50–69) |
| Participation rate (%)* | |
| Helicobacter pylori stool antigen test | 3454/3621 (95.4) |
| Faecal immunochemical test | 3432/3621 (94.8) |
| Positive rate for stool test results (%) | |
| H pylori stool antigen test | 1251/3454 (36.2) |
| Faecal immunochemical test | 152/3432 (4.4) |
| Referral rate (%) | |
| Positive H pylori stool antigen test | 817/1251 (65.3) |
| Antibiotic treatment | 755/817 (92.4) |
| Oesophagogastroduodenoscopy | 643/817 (78.7) |
| Colonoscopy for positive faecal immunochemical test | 89/152 (58.6) |
| Endoscopic findings (%) | |
| Important lesions in the upper gastrointestinal tract | 205/643 (31.9) |
| Categories | |
| Erosive oesophagitis, grade C or D | 7 (1.1) |
| Barrett's oesophagus | 1 (0.2) |
| Oesophageal varices, form II or III | 4 (0.6) |
| Gastric carcinoma | 2 (0.3) |
| Peptic ulcers | 189 (29.4) |
| Gastric antral vascular ectasia | 2 (0.3) |
| Important lesions in the lower gastrointestinal tract (%) | 32/89 (36.0) |
| Categories | |
| Colorectal carcinoma | 2 (2.2) |
| Advanced adenoma | 27 (30.3) |
| Colitis or ulcer | 3 (3.4) |
| Stool test performance (%) | |
| Positive predictive value for gastrointestinal tract lesions | |
| H pylori stool antigen test | 205/643 (31.9) |
| Faecal immunochemical test | 32/89 (36.0) |
| Detection rate for gastrointestinal tract lesions | |
| H pylori stool antigen test | 205/3454 (5.9) |
| Faecal immunochemical test | 32/3432 (0.9) |
*Total number of participants who have returned an adequate stool test sample.
For cancerous lesions, the annual rates were 50.4 and 103.8/100 000 participants aged 50–69 years, respectively, for upper (pharyngeal, oesophageal and gastric cancers) and lower gastrointestinal cancers, which were estimated by searching the databases of the Taiwan Cancer Registry29 and weighting the age and gender distributions of our tested population in the community. The estimated positive predictive values were 0.11% (95% CI 0.08% to 0.12%) and 2.57% (1.99 to 3.20%) for upper and lower gastrointestinal cancers, respectively, and the number of endoscopies needed to find one upper and one lower gastrointestinal cancer was 890 (705–1200) and 39 (31–50), respectively. We expected that approximately 1 of 643 and 2 of 89 participants who underwent endoscopy had upper and lower gastrointestinal cancers, respectively. In the real community-based screening, we detected 2 gastric cancers and 2 colorectal cancers.
Sensitivity analysis
In our study under the scenario of a negative FIT, the positive predictive value of the HPSA was significantly higher than that of the guaiac-based test (see online supplementary appendix figure S3). When the prevalence of upper gastrointestinal lesions was >5%, the positive predictive values of the guaiac-based and HPSA would be >10% (see online supplementary appendix table S2). The positive predictive value of the HPSA would be higher than that of the guaiac-based test when the prevalence of latent H pylori infections in participants with no upper gastrointestinal lesions was <30% (see online supplementary appendix figure S4). Within the reported range of sensitivity and specificity of the HPSA, the above findings were not substantially changed.
Discussion
High cure rates can be achieved for gastrointestinal tract lesions identified in the presymptomatic stage. Colorectal neoplasms are currently screened at the population level. In the present study, we first supported the use of FIT for the screening of colorectal lesions. Second, we evaluated the accuracies of the guaiac-based and HPSA tests for the screening of upper gastrointestinal lesions in cases with negative FIT or normal colonoscopy. Third, we verified our findings by adding the HPSA to the colorectal screening programme in a community population. Taken together, the results of our work contribute new knowledge to the pan detection of gastrointestinal tract diseases.
A recent review suggested that current evidence remained insufficient to recommend for or against routine upper endoscopy for patients with positive stool occult blood tests, but normal colonoscopy, because previous studies have found that upper endoscopic examinations can detect lesions in a wide range 13–45%.30 Such a heterogeneity is related to the fact that the yield rate of oesophagogastroduodenoscopy is subject to the differences in the types of faecal occult blood tests and the population characteristics across different studies. Using the reference standard of bidirectional endoscopies for participants with either positive or negative test results or with guaiac-based or immunochemical tests, our study quantified the accuracy of this finding by showing that in patients with normal colonoscopies, the highly sensitive guaiac-based test can detect occult blood from the upper gastrointestinal tract in approximately one-fourth of cancerous lesions and the detectability increased with cancer stage, thus providing guidance for the use of oesophagogastroduodenoscopy, especially when clinical symptoms/signs, such as iron-deficiency anaemia, are present.31 However, in participants with a negative FIT alone, a non-significant positive likelihood ratio did not support this interpretation. The positivity of FIT was irrelevant to the presence of upper gastrointestinal lesions, similar to the results we previously reported.6
Regarding the use of HPSA, we found that the sensitivities of 60% and 78% for gastric lesions and gastric cancer were non-inferior to those of 35% and 85% for FIT to detect colorectal lesions and colorectal cancers. Our results were consistent with the estimated fraction of 75% for gastric cancers attributable to H pylori infection.32 Screening for H pylori infection is unique because of its once-in-a-lifetime design,33 34 and effective treatments are available.35 36 We invited individuals at average risk for colorectal neoplasms to have the HPSA because in this age range the prevalence of latent H pylori infection is decreased.37 Without previous opportunistic treatment, peptic ulcers and irreversible molecular changes are prevalent after long-term infection.38 Furthermore, the use of non-steroidal anti-inflammatory drugs/aspirin increased (8% in our community population), which would have a synergistic effect with H pylori infection on the risk of peptic ulcers.39 Therefore, it would be appropriate to undergo endoscopy for screening early gastric neoplasms, in addition to the obvious benefit from antibiotic treatment for the peptic ulcer, chronic gastritis and chemoprevention.34
In areas where the prevalence of upper gastrointestinal lesions is high and the prevalence of latent H pylori infection is modest, our sensitivity analyses showed that mass screening with HPSA may be worthwhile. Representative areas may include East Asia, Latin America and East Europe.40–46 The results also may be applied to select populations with high risk, including those with a history of upper gastrointestinal bleeding, those with previous history of peptic ulcer, users of non-steroidal anti-inflammatory drugs, aspirin or clopidogrel, seriously ill patients, elderly participants and immigrants from high-risk areas.10 However, the positive predictive value of the HPSA was lower than that of the guaiac-based test when the prevalence of latent H pylori infection was >30%. Such a circumstance may be observed in areas where the prevalence of upper gastrointestinal lesions was moderate, but the prevalence of latent H pylori infection was high. The representative areas may include India and Africa.47 48
The strengths of this study include large sample size, use of bidirectional endoscopy as the reference standard, evaluation of three tests with high commercial availability, and assessment of applicability and reproducibility in the community. A sensitivity analysis according to the prevalence of upper gastrointestinal lesions and H pylori infection further solidified the generalisability of these findings. Nevertheless, our study had limitations. First, we used the 1-day method for the highly sensitive guaiac-based test. We found that its sensitivity was similar to that of the FIT for detecting colorectal lesions, indicating that such a 1-day test was also an option. However, we might have underestimated the sensitivity of the guaiac-based test for detecting upper gastrointestinal lesions as occult blood was more likely intermittent and unevenly distributed in the stool, requiring multiple samples. However, in our population, this approach must be weighed against the lower completion rate (91%) of using the wooden spatula collection of stool samples for the guaiac-based tests. Second, there is still room for improvement in test performance. Both tests were not able to predict lesions in the upper aerodigestive tract. Distal small bowel lesions might be a possible source for the guaiac or immunochemical positivity.49 Other choices, including the haemeporphyrin test7 50 and the stool-based DNA test,51 52 may be evaluated in future studies. Third, although we have proven the applicability of our two-in-one strategy in a real world setting with a high uptake rate and a fair referral rate, its feasibility would be limited in areas where the resource for endoscopists is constrained or the expense for upper endoscopy is high. Further risk-score based approach with targeted endoscopy may be one potential solution.53 Finally, a low proportion of males was seen in the community-based population because the invitation list was also designed for female cancer screening. Although we have adjusted the age and gender distributions of our community population in the evaluation of test reproducibility, self-selection bias could not be excluded. Also, the present study was not designed in the way to give a definite answer with respect to the mass H pylori screening/eradication strategy. Further randomised trials and cost-effectiveness analyses are warranted to confirm the long-term benefit in adding screening for gastric lesions to the colorectal cancer screening.
In summary, we have demonstrated that, in our population with negative FIT or normal colonoscopy, the HPSA is more accurate than the guaiac-based test in screening for upper gastrointestinal lesions. In a population with prevalent upper and lower gastrointestinal lesions, the HPSA may be appended to the colorectal cancer screening with the FIT.
Supplementary Material
Acknowledgments
The authors would like to thank the nurses at the Health Management Center of National Taiwan University Hospital for their assistance in the hospital-based study and the staff of the Eighth Core Laboratory, Department of Medical Research of National Taiwan University Hospital for technical support for stool tests. The authors also express special thanks to the following clinics and hospitals for their assistance and providing care to the participants of the community-based study in Changhua County: Chang Hong Medicine Clinic, Chang Yu Medicine Clinic, Chen Yen Hsueh Medicine Clinic, Chi Hsu Lien Clinic, Chia Ho Medicine Clinic, Chia Lin Clinic, Ching Hung Clinic, Huang Gung Chin Medicine and Pediatrics Clinic, Feng-An Group Clinic, Lu Tian En Clinic, Shih-Ren Clinic, Wang Chang Hao Medicine Clinic, Wang Jian Lung Medicine Clinic, Wu Shun Yu Medicine Clinic, Ye Teng Hsin Medicine Clinic, Chang-Hua Hospital, Department of Health, Executive Yuan, ROC, Chang Bing Show Chwan Memorial Hospital, Changhua Show Chwan Memorial Hospital, Changhua Christian Hospital, Changhua Christian Hospital-Erlin Branch, Changhua Christian Hospital-Lukang Branch, Han Ming Hospital, Yuan Rung Hospital, and Yuan Sheng Hospital.
Footnotes
Contributors: Y-CL and M-SW have full access to the data in the hospital-based study, take responsibility for the integrity of the data, and the accuracy of the data analysis. Y-CL and H-HC have full access to the community-based study, take responsibility for the integrity of the data and the accuracy of the data analysis. Y-CL, H-MC, Y-PY, C-SL, T-HH, H-HC and M-SW contributed to the study concept and design; Y-CL, H-MC, T-HC, AM-FY, SY-HC, SL-SC, JC-YF, Y-PY, C-SL, T-HH, C-HT, P-HT, C-CC, M-JC, J-ML, W-CL, Y-PL, C-PW, J-YK, H-PW, H-HC and M-SW contributed to acquisition of the data; Y-CL, H-MC, AM-FY, H-HC and M-SW contributed to the drafting of the manuscript; Y-CL, H-MC, HC, J-TL, H-HC and M-SW contributed to the critical revision of the manuscript for important intellectual content; Y-CL, SY-HC, AM-FY and H-HC conducted the statistical analysis; Y-CL, H-MC, AM-FY, SY-HC, SL-SC, JC-YF, Y-PY, H-HC and M-SW were involved in administrative, technical or material support; H-HC and M-SW were involved in study supervision and M-SW had the final responsibility for the decision to submit for publication.
Funding: This study was supported by research grants from the National Center of Excellence for Clinical Trial and Research in the National Taiwan University Hospital, the National Science Council of Taiwan, the Taipei Institute of Pathology, and the Health Promotion Administration, Ministry of Health and Welfare. The funding source had no role in the study design, data collection, analysis, or interpretation, report writing or the decision to submit this paper for publication.
Competing interests: None.
Patient consent: Signed informed consent was obtained from each study participant prior to participation in the study.
Ethics approval: The study protocol was approved by the institutional review board of National Taiwan University Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Globocan 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer, 2010. http://globocan.iarc.fr (accessed 10 Oct 2012) [Google Scholar]
- 2.Bloom DE, Cafiero ET, Jané-Llopis E, et al. The Global Economic Burden of noncommunicable diseases. Geneva: World Economic Forum, 2011 [Google Scholar]
- 3.Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2009;59:27–41 Review. [DOI] [PubMed] [Google Scholar]
- 4.Chen TH, Chiu YH, Luh DL, et al. Taiwan Community-Based Integrated Screening Group Community-based multiple screening model: design, implementation, and analysis of 42,387 participants. Cancer 2004;100:1734–43 [DOI] [PubMed] [Google Scholar]
- 5.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013;369: 1106–14 [DOI] [PubMed] [Google Scholar]
- 6.Chiang TH, Lee YC, Tu CH, et al. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ 2011;183:1474–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rockey DC. Occult gastrointestinal bleeding. N Engl J Med 1999;341:38–46 Review. [DOI] [PubMed] [Google Scholar]
- 8.Rockey DC, Auslander A, Greenberg PD. Detection of upper gastrointestinal blood with fecal occult blood tests. Am J Gastroenterol 1999;94:344–50 [DOI] [PubMed] [Google Scholar]
- 9.Young GP, Bosch LJ. Fecal tests: from blood to molecular markers. Curr Colorectal Cancer Rep 2011;7:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter Study Group. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646–64 [DOI] [PubMed] [Google Scholar]
- 11.Bosman FT, Carneiro F, Hruban RH, et al. eds. WHO classification of tumours of the digestive system. Lyon: IARC, 2010 [Google Scholar]
- 12.Cancer Control and Prevention Health Promotion Administration, Ministry of Health and Welfare. Taiwan, ROC: http://www.hpa.gov.tw/BHPNet/English/Index.aspx (accessed 25 Jul 2013) [Google Scholar]
- 13.Lee YC, Tseng PH, Liou JM, et al. Performance of a one-step fecal sample-based test for diagnosis of Helicobacter pylori infection in primary care and mass screening settings. J Formos Med Assoc 10.1016/j.jfma.2012.05.014 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Chen LS, Liao CS, Chang SH, et al. Cost-effectiveness analysis for determining optimal cut-off of immunochemical faecal occult blood test for population-based colorectal cancer screening (kcis 16). J Med Screen 2007;14:191–9 [DOI] [PubMed] [Google Scholar]
- 15.Chiu HM, Lin JT, Lee YC, et al. Different bowel preparation schedule leads to different diagnostic yield of proximal and nonpolypoid colorectal neoplasm at screening colonoscopy in average-risk population. Dis Colon Rectum 2011;54:1570–7 [DOI] [PubMed] [Google Scholar]
- 16.Rockey DC, Koch J, Cello JP, et al. Relative frequency of upper gastrointestinal and colonic lesions in patients with positive fecal occult-blood tests. N Engl J Med 1998;339:153–9 [DOI] [PubMed] [Google Scholar]
- 17.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tajiri T, Yoshida H, Obara K, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc 2010;22:1–9 [DOI] [PubMed] [Google Scholar]
- 19.Benson VS, Atkin WS, Green J, et al. International colorectal cancer screening network. Toward standardizing and reporting colorectal cancer screening indicators on an international level: the international colorectal cancer screening network. Int J Cancer 2012;130:2961–73 [DOI] [PubMed] [Google Scholar]
- 20.Allison JE, Tekawa IS, Ransom LJ, et al. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996;334:155–9 [DOI] [PubMed] [Google Scholar]
- 21.Greenberg PD, Bertario L, Gnauck R, et al. A prospective multicenter evaluation of new fecal occult blood tests in patients undergoing colonoscopy. Am J Gastroenterol 2000;95:1331–8 [DOI] [PubMed] [Google Scholar]
- 22.Edge SB, Byrd DR, Carducci MA, et al. eds. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. 7th edn New York: Springer, 2009 [Google Scholar]
- 23.Altman DG, Bland JM. Diagnostic tests 2: predictive values. BMJ 1994;309:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vecchio TJ. Predictive value of a single diagnostic test in unselected populations. N Engl J Med 1966;274:1171–3 [DOI] [PubMed] [Google Scholar]
- 25.Gisbert JP, de la Morena F, Abraira V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and meta-analysis. Am J Gastroenterol 2006;101:1921–30 [DOI] [PubMed] [Google Scholar]
- 26.Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009;361:170–7 Review. [DOI] [PubMed] [Google Scholar]
- 27.Tseng PH, Lee YC, Chiu HM, et al. Association of diabetes and HbA1c levels with gastrointestinal manifestations. Diabetes Care 2012;35:1053–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu HM, Lee YC, Tu CH, et al. Association between early-stage colon neoplasms and false-negative results from the fecal immunochemical test. Clin Gastroenterol Hepatol 2013;11:832–8 [DOI] [PubMed] [Google Scholar]
- 29.Cancer Statistics. Taiwan Cancer Registry. http://crs.cph.ntu.edu.tw/main.php (accessed 21 Nov 2012)
- 30.Allard J, Cosby R, Del Giudice ME, et al. Gastroscopy following a positive fecal occult blood test and negative colonoscopy: systematic review and guideline. Can J Gastroenterol 2010;24:113–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas WM, Hardcastle JD. Role of upper gastrointestinal investigation in a screening study for colorectal neoplasia. Gut 1990;31:1294–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13:607–15 [DOI] [PubMed] [Google Scholar]
- 33.Lee YC, Lin JT, Wu HM, et al. Cost-effectiveness analysis between primary and secondary preventive strategies for gastric cancer. Cancer Epidemiol Biomarkers Prev 2007;16:875–85 [DOI] [PubMed] [Google Scholar]
- 34.Lee YC, Chen TH, Chiu HM, et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut 2013;62:676–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg ER, Anderson GL, Morgan DR, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet 2011;378:507–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liou JM, Chen CC, Chen MJ, et al. Taiwan Helicobacter Consortium Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2013;381:205–13 [DOI] [PubMed] [Google Scholar]
- 37.Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol 2013. doi:pii: S1044-579X(13)00066-7. 10.1016/j.semcancer.2013.07.004. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Wong BC, Lam SK, Wong WM, et al. ; China Gastric Cancer Study Group Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004;291:187–94 [DOI] [PubMed] [Google Scholar]
- 39.Chan FK, Chung SC, Suen BY, et al. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N Engl J Med 2001;344:967–73 [DOI] [PubMed] [Google Scholar]
- 40.Aro P, Storskrubb T, Ronkainen J, et al. Peptic ulcer disease in a general adult population: the Kalixanda study: a random population-based study. Am J Epidemiol 2006;163:1025–34 [DOI] [PubMed] [Google Scholar]
- 41.Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther 2009;29:938–46 [DOI] [PubMed] [Google Scholar]
- 42.Wang AY, Peura DA. The prevalence and incidence of Helicobacter pylori-associated peptic ulcer disease and upper gastrointestinal bleeding throughout the world. Gastrointest Endosc Clin N Am 2011;21:613–35 [DOI] [PubMed] [Google Scholar]
- 43.World Gastroenterology Organisation World Gastroenterology Organisation Global Guideline: Helicobacter pylori in developing countries. J Clin Gastroenterol 2011;45:383–8 [DOI] [PubMed] [Google Scholar]
- 44.Musumba C, Jorgensen A, Sutton L, et al. The relative contribution of NSAIDs and Helicobacter pylori to the aetiology of endoscopically-diagnosed peptic ulcer disease: observations from a tertiary referral hospital in the UK between 2005 and 2010. Aliment Pharmacol Ther 2012;36:48–56 [DOI] [PubMed] [Google Scholar]
- 45.Sánchez-Delgado J, Gené E, Suárez D, et al. Has H. pylori prevalence in bleeding peptic ulcer been underestimated? A meta-regression. Am J Gastroenterol 2011;106:398–405 [DOI] [PubMed] [Google Scholar]
- 46.Goss PE, Lee BL, Badovinac-Crnjevic T, et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol 2013;14:391–436 [DOI] [PubMed] [Google Scholar]
- 47.Dutta AK, Chacko A, Balekuduru A, et al. Time trends in epidemiology of peptic ulcer disease in India over two decades. Indian J Gastroenterol 2012;31:111–15 [DOI] [PubMed] [Google Scholar]
- 48.Agha A, Graham DY. Evidence-based examination of the African enigma in relation to Helicobacter pylori infection. Scand J Gastroenterol 2005;40:523–9 [DOI] [PubMed] [Google Scholar]
- 49.Annibale B, Capurso G, Baccini F, et al. Role of small bowel investigation in iron deficiency anaemia after negative endoscopic/histologic evaluation of the upper and lower gastrointestinal tract. Dig Liver Dis 2003;35:784–7 [DOI] [PubMed] [Google Scholar]
- 50.Harewood GC, McConnell JP, Harrington JJ, et al. Detection of occult upper gastrointestinal tract bleeding: performance differences in fecal occult blood tests. Mayo Clin Proc 2002;77: 23–8 [DOI] [PubMed] [Google Scholar]
- 51.Nagasaka T, Tanaka N, Cullings HM, et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst 2009;101:1244–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahlquist DA. Next-generation stool DNA testing: expanding the scope. Gastroenterology 2009;136:2068–73 [DOI] [PubMed] [Google Scholar]
- 53.Asaka M, Kato M, Graham DY. Strategy for eliminating gastric cancer in Japan. Helicobacter 2010;15:486–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.