Background
The current WHO recommended directly observed therapy short course (DOTS) strategies are insufficient to contain the tuberculosis (TB) epidemic in countries with high HIV prevalence [1–3]. It is unclear how the HIV-related TB epidemics impact the children in affected communities. The tuberculin skin test (TST) is an epidemiological tool to measure TB infection; as the prevalence of TB among young children may be interpreted as incidence, these data can be used to calculate an annual risk of tuberculosis infection (ARTI). While limitations due to the methodological weaknesses inherent in this technique are recognized [4, 5], TST surveys are valuable in identifying low case detection rates in TB programs in communities and in assessing the impact of an HIV-driven TB epidemic [6]. TST surveys have an important role in understanding the impact of these dual epidemics on children.
In the pre-chemotherapy era Styblo described a fixed relationship between ARTI and smear-positive disease incidence [7]. This theory has been criticized [5], and the advent of TB chemotherapy would alter any relationship between ARTI and incidence [4, 5, 8, 9]. TB treatment increases survival rates and reduces person-time of infectiousness in a community, thereby reducing infection risk [4] and subsequently decreasing TB prevalence and incidence. However these factors are all dependent on the performance of the TB control program [4, 5, 8] which may vary over time and place. The relationship between ARTI and TB incidence may again have been altered by the impact of the HIV epidemic on TB. This change is postulated based on the increase in both smear-positive and smear-negative TB cases in areas with high HIV prevalence, and the changes in the duration of infectiousness of HIV-positive TB cases [4, 10]. However there is conflicting evidence as to whether HIV-positive TB cases are as infectious as HIV-negative TB patients [11–13].
There have been few studies on the impact of the HIV epidemic on TB infection in children, and available findings are mixed. One study from Tanzania reported a significant drop in ARTI in 6 to 14 year olds from 1983 to 2003 [14] in the context of an increasing HIV epidemic (HIV prevalence 7–11% [15]). This trend was reported despite increasing TB notification rates [14]. In 1994 a study of Ugandan children reported a drop in ARTI among school going children [16]. In contrast, research from Kenya reported an increased ARTI from 1986 to 1996, associated with an increasing TB epidemic in high HIV prevalence areas [17]. These surveys have reported ARTIs ranging from 0.68% to 1.2% [14, 16, 17], and the variability in ARTI may be due to differing performance of TB control programs in the study communities. There is little recent published tuberculin skin test data from southern Africa where HIV prevalence has reached unprecedented levels [18].
We have previously reported on the growing TB epidemic in a peri-urban township heavily affected by HIV in Cape Town, South Africa. The HIV prevalence among adults in this community is 23% [19] and among adolescents ages 11 to 19 years, 10% [20]. The National TB Control Program, based on WHO DOTS recommendations, is well run with treatment completion and cure rates of sputum positive cases of approximately 80% [21] and case finding rates of 67% in HIV-negative smear-positive patients but lower for HIV-positive smear-positive patients [19]. However, local tuberculosis (TB) notification data shows a rapid escalation in TB notification rates in adults from 789/100,000 in 1996 to levels of over 1,900/100,000 in 2005, with the incidence rates of smear-positive TB in this age group increasing from 326/100,000 in 1996 to 1,307/100,000 in 2005 [3,19]. A cross-sectional TB prevalence survey in 2005 showed an overall smear-positive pulmonary TB prevalence rate of 1,495/100,000, and 0.8% of these cases were untreated [19]. We believe that, in this community with an adequate TB control program, the HIV epidemic is driving the increase in TB incidence [3]. To better understand patterns in the transmission of TB in this context we assessed the prevalence of TB infection in children ages 5 to 17 years in this setting of increasing TB and HIV prevalence.
Methods
The tuberculin survey was performed in a sample of children attending school in the above-mentioned community, which comprises of approximately 13,000 predominantly Xhosa-speaking individuals living in a very poor, high-density residential area. A cross-sectional survey was performed in two stages in school children attending the local government primary school. Children were eligible if they were resident in the community and registered at the local school. Children from grades 1 to 3 at the school were enrolled in October 2006 and children in grade 5 to 7 were enrolled in October 2007. Parental consent, and assent from participants >6 years of age, were obtained prior to enrolment. The survey was performed on the school premises. Basic demographic information was collected on each participant. All participants were examined for the presence of a BCG scar, and participants received the TST regardless of BCG scar status. The WHO-recommended standard of 2TU of PPD (Purified Protein Derivative) RT23 with Tween 80 (Statens Seruminstitut, Copenhagen) was administered intradermally to the volar surface of the left forearm by a trained nurse.
The reaction size to the tuberculin was read by a trained reader at a second visit performed 3 days following the inoculation. The presence or absence of a reaction was noted, and, where present, the induration was measured along perpendicular axes using standard calipers.
This study was approved by the University of Cape Town’s Research Ethics Committee and all procedures were in accordance with the ethical standards of this committee and the Declaration of Helsinki, 1975, as revised in 1983. All children with a TST reaction ≥10mm were recalled for investigation for active tuberculosis and children with signs or symptoms of active disease were referred to the local clinic for further management.
Data were analyzed using STATA 9.0 (StataCorp, College Station, Texas). Results reported here are calculated as the mean of the two diameters of the TST reaction: a positive reaction was defined at 10mm and 17.4mm cut-off points in separate analyses. The 10mm cut-off was based on guidelines for infection in clinical settings [22]; 17.4mm cut-off was determined as the mean reaction excluding all non-reactors [23]. ARTI was calculated as 1- (1-prevalence)1/(mean age+0.5). As the age in full years at participants’ last birthday was used, 0.5 was added to the mean age for the calculation of ARTI [23]. The cohort was divided into age groups of 5–8, 9–11, 12–14 and 15–17 years and prevalence and ARTI were calculated overall and for each age group. Bivariate analyses employed Student’s t- and Fisher’s exact tests, as appropriate; for comparison of TST results between different groups, Wilcoxon sum rank tests were used. Multiple logistic regression models were developed to examine factors associated with positive TST results. A chi-square for trend was used to assess for a trend between age and ARTI, as well as for changes in TB notification rates over the 5 year period. All statistical tests are 2-sided at α=0.05.
The number of notified TB cases in children was obtained from the TB register at the Masiphumelele TB clinic, and tuberculosis prevalence rates were calculated using the 1996 South African National Census, and the 2003, 2004 and 2006 house-to-house census performed by the Desmond Tutu HIV Centre. Ratios of TB prevalence and incidence to new TB infections were calculated from the ARTI and from TB prevalence and incidence data already published for this community [3, 19].
Results
Of the 1060 children enrolled in the target grades at the primary school, 1020 were eligible for study participation (96%). Ineligibility was due to living outside of the community (n=18) and having dropped out of school during the course of the year (n=22). Consent was obtained from the parents of 837 children (82% of those eligible). The parents of 44 children refused consent (4%) and 137 children had incorrect locator information in the school register (13%). We enrolled 832 children (99% of consented children). The outstanding five children were not enrolled due to persistent absenteeism. Tuberculin reaction was assessed in all children who were inoculated, except one who relocated before her reaction was assessed. She has thus been excluded from the analysis (n=831).
Table 1 shows the demographic characteristics of the study cohort. Ages ranged from 5 to 17 years, with a mean age of 10.7 years, and 52% of the participants were male. The majority of the children did not have a BCG scar (74%); one child’s BCG scar status was unobservable due to burn scars on the upper arm, and he was thus excluded from analysis involving BCG scar status. In crude analysis, older children were more likely to have BCG scars (p<0.001 comparing children above and below the mean age).
Table 1.
Demographic characteristics of sample
| 5 to 8 yrs | 9 to 11 yrs | 12 – 13 yrs | 14 – 17 yrs | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| n [233] | % | n [222] | % | n [237] | % | n [139] | % | p value | n [831] | % | |
| Mean Age | 7.3 years | 9.9 years | 12.5 years | 14.6 years | 10.7 years | ||||||
|
| |||||||||||
| Gender
| |||||||||||
| male | 115 | 49% | 122 | 55% | 110 | 46% | 87 | 63% | 434 | 52% | |
| female | 118 | 51% | 100 | 45% | 127 | 54% | 52 | 37% | 0.014 | 397 | 48% |
|
| |||||||||||
| BCG status
| |||||||||||
| BCG scar | 30 | 13% | 66 | 30% | 87 | 37% | 30 | 22% | 213 | 25% | |
| No BCG scar | 203 | 87% | 155* | 70% | 150 | 63% | 109 | 78% | <0.001 | 617 | 75% |
n = 221: one participant’s BCG scar status was indeterminable due to burn scars
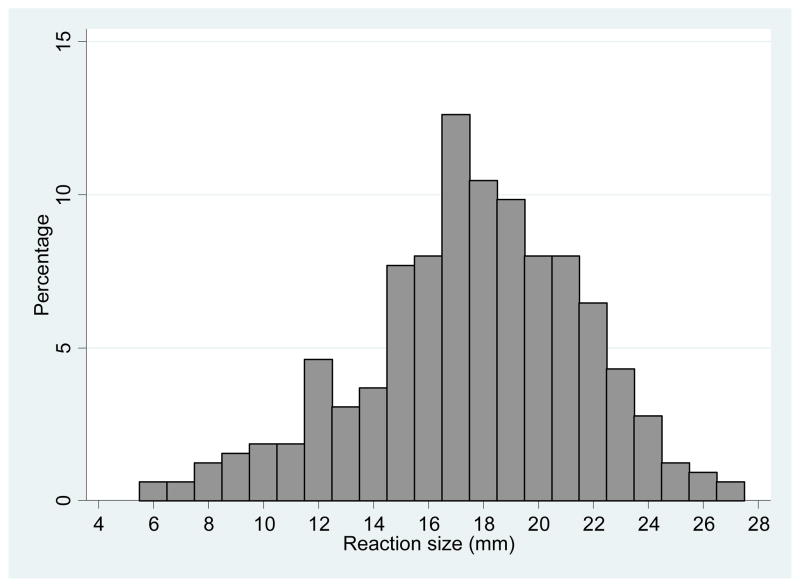
The frequency distribution of reaction sizes for positive TST results is presented in Figure 1. There was no significant difference between the mean reactions of participants with BCG scars compared to those without scars (6.2 vs 7.0mm, respectively, p=0.28).
Figure 1.
Frequency distribution of positive TST reactions
The cut-off point determined by the mean of non-reactors was 17.4mm. At the 10mm cut-off 311 participants (37%) had a positive TST result; at the 17.4mm cut-off 171 participants (21%) had a positive result. At both the 10mm and 17.4 mm cut-off points there was no significant difference in the defined TB positivity by gender (p=0.97 and p=0.69 respectively) or BCG status (p=0.39 and p=0.08 respectively). In a logistic regression model predicting the relative odds of a positive TST result, age was positively associated with a positive TST result at both the 10mm cut-off (adjusted odds ratio (OR) for a 1-year increase in age: 1.19, 95% CI: 1.12 – 1.25; p<0.001), and at the 17.4mm cutoff (adjusted OR 1.19, 95% CI: 1.12 – 1.27).
The total ARTI for this sample was 4.1% at 10mm cut-off, and 2.0% at 17.4mm cut-off. The ARTI did not differ significantly across the age quartiles for the 10mm or the 17.4mm cut-off (p=0.50 and 0.39 respectively). Table 2a and 2b report TB prevalence and ARTI by age quartiles for both cut-off points.
Table 2a.
TB prevalence and ARTI by age quartile for the 10mm cut-off point:
| 10mm cut-off for positivity | |||||
|---|---|---|---|---|---|
| Age Category | Mean Age* | n | TST positive | Prevalence | ARTI% |
| 5–8 yrs | 7.8 | 233 | 61 | 26.2 | 3.8% |
| 9–11 yrs | 10.4 | 222 | 70 | 31.5 | 3.6% |
| 12–13 yrs | 13.0 | 237 | 107 | 45.1 | 4.5% |
| 14–17 yrs | 15.1 | 139 | 73 | 52.5 | 4.8% |
| TOTAL | 11.2 | 831 | 311 | 37.4 | 4.1% |
Table 2b.
TB prevalence and ARTI by age quartile for the 17.4mm cut-off point:
| 17.4mm cut-off for positivity | |||||
|---|---|---|---|---|---|
| Age Category | Mean Age* | n | TST positive | Prevalence | ARTI% |
| 5–8 yrs | 7.8 | 233 | 25 | 10.7 | 1.4% |
| 9–11 yrs | 10.4 | 222 | 39 | 17.6 | 1.8% |
| 12–13 yrs | 13.0 | 237 | 71 | 30.0 | 2.7% |
| 14–17 yrs | 15.1 | 139 | 36 | 25.9 | 2.0% |
| TOTAL | 11.2 | 831 | 171 | 20.6 | 2.0% |
The mean age reported here is the age used for the ARTI calculations, and is based on the mean age for the age category+0.5 years, as described in the methods section.
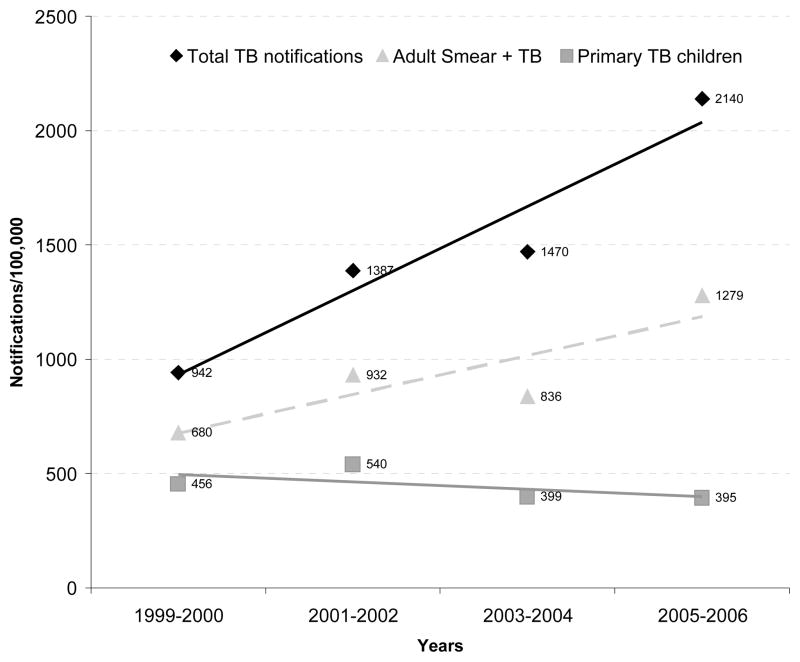
TB notification rates in children ranged from 456/100,000 in 1999 to 395/100,000 in 2005. Figure 2 shows the change in notification rates of total TB cases, smear-positive pulmonary TB in adults and TB in children in this community from 1999 to 2005. Total TB rates in the population and smear-positive TB rates in adults has increased significantly over this period (p<0.001 for both, by test for trend). However, TB notification rates in children have not changed significantly over the same period (p=0.48, by test for trend).
Figure 2.
Changes in notification rates of total TB, smear-positive pulmonary TB in adults and TB in children (under 14 years) in the study community from 1999 to 2005.
The ARTI of 4.1% should result from 4,100/100,000 exposures in a year. Based on the incidence of new smear-positive infections in 2005 (1,459/100,000) [19], we estimated that the ratio of TB incidence to new infections is approximately 1:2.8. Given that 0.8% of smear-positive cases in the community are untreated [19], the ratio of prevalence to new infections is 1: 5.
Discussion
To our knowledge this is the first paper from southern Africa reporting ARTI in the context of a rapidly growing HIV and TB epidemic. ARTI is a measure of tuberculosis infection risk of previously unexposed individuals. A fixed relationship between TB prevalence as a measure of ARTI and incidence of smear-positive disease was proposed in the pre-chemotherapy era [8], although this relationship is increasingly questioned [5]. It is recognized that chemotherapy, TB case-finding and the performance of the TB control program also affect ARTI by modifying the person-time of infectiousness in the community [4, 5, 8, 9]. The HIV epidemic has a profound influence on the epidemiology of tuberculosis. However, TB-HIV co-infected individuals may be less likely to infect their close contacts than HIV-negative TB cases [11, 12]. The relationship between ARTI and HIV-associated TB at a population level is therefore unclear. This study was performed in a well-defined community with detailed information on TB incidence and prevalence, TB control program performance parameters together with longitudinal data on HIV and HIV/TB prevalence.
The main finding of the study is that regardless of the cut-off used for positivity, the ARTI observed in this study was markedly higher than those documented in other sub-Saharan countries which were heavily affected by HIV (2.0 or 4.1% in our study vs 0.68–1.2% in others). Prevalence of latent TB infection of 70 – 83% among young HIV-uninfected adult controls from similar communities in Cape Town is consistent with this high ARTI rate [24, 25]. With no TST results between 0 and 5mm and little evidence of the cross reaction with environmental mycobacterium that was present in other studies, the high prevalence of TB infection allowed us a unique opportunity to accurately assess ARTI by age strata, providing a measure of prevalence over time. Surprisingly, we found that the ARTI remained constant across the age range of 5–17 years despite children being exposed to a rapidly escalating TB epidemic. This finding is further supported by the TB notification rate in children under 14 years of age remaining unchanged over the past 7 years, although it should be noted that as TB case detection rates are low in this community there may be more TB cases among children than detected by the TB control program.
We have shown previously that HIV is driving the increasing TB epidemic, especially among adults aged 20–40 years in this community, an age group with the highest HIV prevalence [3]. However ARTI has remained stable during a period when adult HIV prevalence has increased from 14% to 23% [3, 19]. In 2005 over 70% of TB patients were co-infected, and TB notification rates in HIV-positive patients were nearly seven fold higher than HIV-negative patients [3]. Therefore it appears that HIV-associated TB can not be the major driver of the ARTI in this community. This would be consistent with the findings in other studies of lower infectivity of HIV-TB co-infected patients [11, 12]. It may also reflect that children are not proportionately affected by the HIV epidemic as their predominant social contacts are peers, parents and teachers.
Despite a significant increase in total PTB and in smear-positive PTB among adults in this community over the past 5 years, the TB incidence among children has remained stable. The ratio of ARTI to new incident smear-positive cases dropped from 1:6 in 1999 to 1:3 in 2006. However the more relevant ratio between ARTI and untreated smear-positive disease was only 1:5 in 2005.
These data are based on a small study sample performed at a single school in one community and therefore they may not be generalisable to other regions.
Since 1960 the South African policy has been to vaccinate all infants with the BCG vaccine [26]. However only 25% of the children in this study had an observable BCG scar, although BCG scarring may be variable [27, 28]. We also found no difference in the distribution of TST results between those children with or without BCG scars, and we therefore included all children in the analysis. This lack of correlation between the presence of a BCG scar and tuberculin reaction has been shown in other studies [28–30].
HIV testing was not performed in this survey as this may have adversely affected parental willingness to consent to their child’s participation. Study participants were born between 1990 to 2001, when the reported HIV prevalence at antenatal clinics ranged from 0.7 to 24% [31]. This was prior to the introduction of the prevention-of-mother-to-child-transmission program. With a transmission rate of approximately 30%, we estimate that 0.2% of the older cohort and up to 7% of the younger children may have been infected with HIV perinatally. However considering the high early mortality rate among infected children [32], it is probable that a maximum of 0.1 to 4% of children in this study sample might be HIV-infected. This is a small percentage and is unlikely to have had a large impact on the TB prevalence. However, any impact of HIV on the prevalence would also be offset by the skin test anergy caused by advanced HIV disease, resulting in false negative TST reactions. Furthermore, it is possible that there is a bias in school attendees, and that non-school attending children may have higher TB infection rates; this may have resulted in an underestimation of the TB prevalence.
An ARTI of 2.0% – 4.1% pa would result in the majority of individuals being latently infected before reaching adulthood, as well as ensuring continued exposure of these individuals to further TB infections. The combination of high latent infection, repeated ongoing exposures and an extremely high lifetime risk of HIV-infection may go a long way to explain the explosive epidemic of HIV/TB [3]. TB control will require measures targeted at decreasing ARTI. The TB control program implements a DOTS policy and reports treatment completion rates >80%. Smear-positive case finding is below WHO targets and could be improved by active case finding among both HIV-negative and HIV-positive individuals. Isoniazid preventative therapy is not routinely used in either TB control or HIV programs and its role needs to be explored. An ART treatment program has been instituted with >30% of HIV-infected individuals on therapy (data not shown). While ART will likely decrease TB rates in the HIV-infected the findings of this study would suggest that this measure is unlikely to positively impact on the ARTI, possibly as this measure may provide limited information about an epidemic in population groups not primarily composed of the participants’ parents and teachers. In this poor community social policies to improve housing and decrease crowding also can not be ignored.
This study shows high ARTI rates among school-going children in this setting of rapidly growing TB and HIV epidemics. The ARTI observed was markedly higher than those published for other sub-Saharan countries similiarly affected by HIV. The ARTI was constant across the age groups reported, indicating that the HIV-driven TB epidemic has not directly affected children in this community. Novel strategies for reducing the ARTI need to be considered and this highlights the importance of improving understanding of TB transmission in this setting.
Acknowledgments
We gratefully acknowledge the City Bridge and Desmond Tutu HIV Foundations for sponsoring this study, and Prof Bernard Fourie and Dr Karin Weyer of the Medical Research Council for their patient assistance in answering our questions regarding study design. A special thank you to Elaine Sebastian and her clinical team.
Financial Support. National Institutes of Health (Comprehensive Integrated Programme of Research on AIDS) grant 1U19AI053217 to K.M., L.G.B., L.M. and R.W), and NIH CIPRA grant 1U19AI05321 and NIH RO1 grant AI058736-02 to R.W. NIH R01 grant HL090316-01 to R.D.
Footnotes
Potential conflicts of interest. All authors no conflicts
References
- 1.De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis. 1999;3:457–465. [PubMed] [Google Scholar]
- 2.Whalen CC. Failure of directly observed treatment for tuberculosis in Africa: a call for new approaches. Clin Infect Dis. 2006;42:1048–1050. doi: 10.1086/501022. [DOI] [PubMed] [Google Scholar]
- 3.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 4.Rieder HL. Methodological issues in the estimation of the tuberculosis problem from tuberculin surveys. Tuber Lung Dis. 1995;76:114–121. doi: 10.1016/0962-8479(95)90552-9. [DOI] [PubMed] [Google Scholar]
- 5.Rieder H. Annual risk of infection with Mycobacterium tuberculosis. Eur Respir J. 2005;25:181–185. doi: 10.1183/09031936.04.00103804. [DOI] [PubMed] [Google Scholar]
- 6.Menzies D. Tuberculin surveys--why? Int J Tuberc Lung Dis. 1998;2:263–264. [PubMed] [Google Scholar]
- 7.Styblo K. The relationship between the risk of tuberculosis infection and the risk of developing infectious tuberculosis. Bulletin of the International Union Against Tuberculosis. 1985;60:117–119. [Google Scholar]
- 8.Rieder HL. Epidemiologic basis of tuberculosis control. Paris: International Union Against Tuberculosis and Lung Disease; 1999. pp. 1–164. [Google Scholar]
- 9.Cauthen GM, Rieder HL, Geiter LJ. Progress Report 1991. Vol. 1. The Hague: Royal Netherlands Tuberculosis Association; 1991. A model of the relation between age-specific prevalence of tuberculosis infection and incidence of infectious tuberculosis: implications for screening policies. Tuberculosis Surveillance Research Unit of the IUATLD; pp. 1–20. [Google Scholar]
- 10.Borgdorff MW. Annual risk of infection – time for an update? Bulletin of World Health Organisation. 2002;80:501–502. [Google Scholar]
- 11.Espinal MA, Perez EN, Baez J, Henriquez L, Fernandez K, Lopez M, Olivo P, Reingold AL. Infectiousness of Mycobacterium tuberculosis in HIV-1-infected patients with tuberculosis: a prospective study. Lancet. 2000;355:275–280. doi: 10.1016/S0140-6736(99)04402-5. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho AC, DeRiemer K, Nunes ZB, Martins M, Comelli M, Marinoni A, Kritski AL. Transmission of Mycobacterium tuberculosis to contacts of HIV-infected tuberculosis patients. Am J Respir Crit Care Med. 2001;164:2166–2171. doi: 10.1164/ajrccm.164.12.2103078. [DOI] [PubMed] [Google Scholar]
- 13.Cayla JA, Garcia dO, Galdos-Tanguis H, Vidal R, Lopez-Colomes JL, Gatell JM, Jansa JM. The influence of intravenous drug use and HIV infection in the transmission of tuberculosis. AIDS. 1996;10:95–100. doi: 10.1097/00002030-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Egwaga SM, Cobelens FG, Muwinge H, Verhage C, Kalisvaart N, Borgdorff MW. The impact of the HIV epidemic on tuberculosis transmission in Tanzania. AIDS. 2006;20:915–921. doi: 10.1097/01.aids.0000218557.44284.83. [DOI] [PubMed] [Google Scholar]
- 15. [Accessed 10 August 2007];UNAIDS report: AIDS Epidemic Update. 2005 Dec; http://www.unaids.org/epi/2005/doc/report_pdf.asp.
- 16.Migliori GB, Borghesi A, Spanevello A, Eriki P, Raviglione M, Maciocco G, Morandi A, Ballardini L, Neri M. Risk of infection and estimated incidence of tuberculosis in northern Uganda. Eur Respir J. 1994;7:946–953. [PubMed] [Google Scholar]
- 17.Odhiambo JA, Borgdorff MW, Kiambih FM, Kibuga DK, Kwamanga DO, Ng’ang’a L, Agwanda R, Kalisvaart NA, Misljenovic O, Nagelkerke NJ, Bosman M. Tuberculosis and the HIV epidemic: increasing annual risk of tuberculous infection in Kenya, 1986–1996. Am J Public Health. 1999;89:1078–1082. doi: 10.2105/ajph.89.7.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arabin G, Gartig D, Kleeberg HH. First tuberculosis prevalence survey in KwaZulu. S Afr Med J. 1979;56:434–438. [PubMed] [Google Scholar]
- 19.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, Kaplan G, Huebner R, McIntyre J, Bekker LG. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaspan HB, Berwick JR, Myer L, Mathews C, Flisher AJ, Wood R, Bekker LG. Adolescent HIV prevalence, sexual risk, and willingness to participate in HIV vaccine trials. J Adolesc Health. 2006;39:642–648. doi: 10.1016/j.jadohealth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Cape Town TB Control. Progress report 1997–2003. Health Systems Trust; Cape Town, South Africa: 2004. [Google Scholar]
- 22.The South African Tuberculosis Control Programme. Practical Guidelines. Pretoria, South Africa: South African Department of Health; 2004. [Google Scholar]
- 23.Arnadottir T, Rieder HL, Trebucq A, Waaler HT. Guidelines for conducting tuberculin skin test surveys in high prevalence countries. Tuber Lung Dis. 1996;77 (Suppl 1):1–19. doi: 10.1016/s0962-8479(96)90127-6. [DOI] [PubMed] [Google Scholar]
- 24.Lawn SD, Bangani N, Vogt M, Bekker LG, Badri M, Ntobongwana M, Dockrell HM, Wilkinson RJ, Wood R. Utility of interferon-gamma ELISPOT assay responses in highly tuberculosis-exposed patients with advanced HIV infection in South Africa. BMC Infect Dis. 2007;7:99. doi: 10.1186/1471-2334-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangaka MX, Wilkinson KA, Seldon R, van Cutsem G, Meintjes GA, Morroni C, Mouton P, Diwakar L, Connell TG, Maartens G, Wilkinson RJ. Effect of HIV-1 infection on T-Cell-based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175:514–520. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan M, Fransman D. BCG Vaccination change over in South Africa. Child Health Unit; [Accessed 25 October 2007]. http://web.uct.ac.za/depts/chu/mch16f.rtf. [Google Scholar]
- 27.Friedland IR. The booster effect with repeat tuberculin testing in children and its relationship to BCG vaccination. S Afr Med J. 1990;77:387–389. [PubMed] [Google Scholar]
- 28.Grindulis H, Baynham MI, Scott PH, Thompson RA, Wharton BA. Tuberculin response two years after BCG vaccination at birth. Arch Dis Child. 1984;59:614–619. doi: 10.1136/adc.59.7.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menzies D. What does tuberculin reactivity after bacille Calmette-Guerin vaccination tell us? Clin Infect Dis. 2000;31 (Suppl 3):S71–S74. doi: 10.1086/314075. [DOI] [PubMed] [Google Scholar]
- 30.Great Britain Medical Research Council. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early life. British Medical Journal. 1963;1:973–978. [PMC free article] [PubMed] [Google Scholar]
- 31.National HIV and Syphilis Antenatal Sero-Prevalence Survey in South Africa. Department of Health; Republic of South Africa: 2005. [Accessed 07 September 2007]. http://www.doh.gov.za/docs. [Google Scholar]
- 32.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]