Abstract
Two-thirds of the world's HIV-infected people live in sub-Saharan Africa and more than 1.5 million of them die annually. As access to antiretroviral treatment (ART) has expanded within the region, early pessimism concerning the delivery of ART using a large-scale public health approach has, at least in the short term, proved to be broadly unfounded. Immunological and virological responses to ART are similar to responses in patients treated in high-income countries. Despite this, however, early mortality rates in sub-Saharan Africa are very high; between 8% and 26% of patients die in the first year of ART, with most deaths occurring in the first few months. Patients typically access ART with advanced symptomatic disease, and mortality is strongly associated with baseline CD4 cell count <50 cells/μL and WHO stage 4 disease (AIDS). Although data are limited, leading causes of death appear to be tuberculosis, acute sepsis, cryptococcal meningitis, malignancy and wasting syndrome. Mortality rates are likely to depend not only on the care delivered by ART programmes, but more fundamentally on how advanced disease is at programme enrolment and the quality of preceding health-care. In addition to improving delivery of ART and providing this free of charge to the patient, strategies to reduce mortality must include earlier diagnosis of HIV infection, strengthening of longitudinal HIV care and timely initiation of ART. Health systems delays in ART initiation must be minimised, especially in patients who present with advanced immunodeficiency.
Keywords: HIV, AIDS, antiretroviral treatment, HAART, ART, mortality, death, Africa
Introduction
Development of highly active antiretroviral treatment (ART) in the mid-1990s revolutionised the care of HIV-infected patients and led to marked reductions in HIV-associated morbidity and mortality in many industrialised countries [1-3]. Over 10 years later, access to ART remains limited in sub-Saharan Africa and other resource-constrained settings where the need is greatest. Although only 10% of the world's population live in sub-Saharan Africa, the region is nevertheless home to around two-thirds of the world's HIV-infected people [4]. In 2007 an estimated 22.5 million adults and children in the region were living with HIV/AIDS and 1.6 million died, representing 76% of global AIDS deaths [4]. Great progress has been made in providing access to ART in sub-Saharan Africa; by April 2007 approximately 1.3 million people were receiving ART - some 28% of the 4.8 million people estimated to be in need [5]. However, in addition to much needed HIV prevention measures, ongoing expansion of capacity to effectively deliver ART long-term to large numbers of people is urgently required.
Early pessimism concerning effective delivery of ART on a large scale using a simplified public health approach has largely proven unfounded, at least in the short term. For example, ART access has been rapidly expanded on a massive scale in Lusaka, Zambia, in Abidjan, Cote D'Ivoire, and on a country-wide scale in Malawi with good early clinical outcomes [6-8]. High levels of treatment compliance and virological suppression have been achieved in both hospital-based and community-based programmes [9-11]. Meta-analyses of data from treatment cohorts have found that the efficacy of ART, as reflected by rates of viral load suppression and CD4 cell count recovery, is similar among patients treated in high-income and resource-limited settings [12, 13]. ART has been demonstrated to be a cost-effective intervention in resource-poor settings [14-16]. Furthermore, ART was estimated to have averted 250,000-350,000 deaths in low and middle income countries in 2005 alone [17].
Despite these positive findings, data from the region suggest that early mortality rates among adults in ART programmes are high [12], contributing to substantial losses in overall patient retention [18]. The ART-LINC collaboration compared outcomes from 18 ART programmes in lower-income settings (predominantly in Africa) with those in 12 HIV cohort studies from Europe and North America [12]. This analysis found that early mortality following initiation of ART was several-fold higher among patients in resource-limited settings compared to that of patients treated in high-income settings, even after adjusting for baseline immunodeficiency [12]. High mortality rates have also been reported by individual cohorts in sub-Saharan Africa. To gain a greater understanding of this problem, we review the mortality rates, temporal distribution, risk factors and causes of death among adult patients accessing ART programmes in sub-Saharan Africa and consider possible strategies to address this.
Search strategy and selection criteria
We searched English-language publications in MEDLINE, using the terms “antiretroviral*”, “HAART”, “ART”, “Africa”, “mortality”, “death”. Searches were completed up to December 2007 to identify published reports from observational ART cohorts in sub-Saharan Africa; those reporting on survival proportions over time and those describing the spectrum of causes of death were selected. Additional articles were identified from references in published papers and abstracts from major international AIDS conferences in the preceding 12 months. For cohorts with multiple presentations of mortality data from different time periods, the most recent report was used. Data from controlled clinical trials were not included in the assessment of mortality rates as these data are unlikely to be generalizable to the scale-up of ART services in the general population. Summary estimates of the hazard ratios for the association of early mortality with CD4 count and with advanced WHO stage was conducted using a random-effects model [19].
Rates and temporal distribution of mortality
Data from 18 published cohort studies containing 39,536 patients treated in countries in west, east and southern African are summarised in Table 1. Patients were mainly receiving public sector treatment. Twelve of the cohorts were based in urban settings and 6 were rural. The median baseline CD4 cell count in these studies ranged between 43 and 147 cells/μL and the median duration of follow-up ranged between 3 and 46 months. The vast majority of these patients were ART-naive and most initiated triple-drug therapy that incorporated two nucleoside analogues and a non-nucleoside reverse transcriptase inhibitor.
Table 1.
Mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa
| Studya | Year | Country | n | Setting | Median(IQR) CD4 count (cells/μL) | Median follow-up (months) | On-treatment survival proportion |
Survival proportion at 12 months of ART |
Temporal distribution of deaths | Losses to follow-up | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12m | 24m | CD4<50a | CD4>50a | |||||||||
| Weidle [25] | 2002 | Uganda | 476 | Urban | 73 (15-187) | 3 | 0.74 | - | 0.67 | 0.82 | 57% deaths in first 3 m | 24% |
| Djomand [30] | 2003 | Cote D'Ivoire | 480 | Urban | [37% <50] | 6 | 0.84 | - | - | - | - | Not reported |
| Seyler [31] | 2003 | Cote D'Ivoire | 101 | Urban | 135 (55-221) | 17 | - | - | 0.80 | 0.96 | 50% deaths in first 3.3 m | Not reported |
| Coetzee[10] | 2004 | S. Africa | 287 | Urban | 43 (13-94) | 13.9 | 0.86 | 0.86 | 0.82 | 0.91 | 71% deaths in first 3 m | 2% |
| Laurent [40] | 2005 | Senegal | 176 | Urban | 144 (58-224) | 30 | - | 0.84 | - | - | Median time to death 9 m | Not reported |
| Wester [11] | 2005 | Botswana | 153 | Urban | 96 (33-165) | 12 | 0.85 | - | 0.76 | 0.90 | Majority of deaths in first 6 m | 8% at 12 mc |
| Lawn[22] | 2006 | S. Africa | 927 | Urban | 100 (47-160) | 7 | 0.91 | 0.90 | 0.85 | 0.94 | 87% between enrolment and first 4 m ART | 2% |
| Ferradini [24] | 2006 | Malawi | 1308 | Rural | 112 (59-176) | 8.3 | 0.81 | 0.72 | - | - | 77% deaths in first 6 m | 5% at 12 mc |
| Etard [23] | 2006 | Senegal | 404 | Urban | 128 (54-127) | 46 | 0.88 | 0.83 | 0.80 | 0.90 | 50% deaths in first 12 m | 2% at 12 m |
| Stringer [6] | 2006 | Zambia | 16,198 | Urban | 147 (69-268) | 7 | 0.82 | - | 0.80 | - | 71% within first 3 m | Not reported |
| Zachariah [35] | 2006 | Malawi | 1507 | Rural | 123 (58-206) | 10b | 0.87 | - | 0.77 | 0.92 | 61% in first 3 m; 79% in first 6m | 3% |
| Makombe [95] | 2007 | Malawi | 4580 | Rural | - | 12 | 0.87 | - | - | - | >90% in first 6 m | 11% at 12 m |
| Bajunirwe [37] | 2007 | Uganda | 398 | Rural | - | - | 0.76 | 0.71 | - | - | - | Not reported |
| De Iaco [32] | 2007 | Burkina Faso | 315 | Urban | 97 | 18 | 0.69 | 0.83 | - | - | Mortality rate 2.3-fold higher in first 6 m ART | Not reported |
| Johannessen [36] | 2007 | Tanzania | 336 | Rural | - | 12 | 0.74 | 0.68 | - | - | 60% deaths in first 3 m | 11% |
| Kambugu [51] | 2007 | Uganda | 559 | Urban | 104 | 12 | 0.86 | - | - | - | 73% in first 6 m | Not reported |
| Moore [39] | 2007 | Uganda | 1120 | Rural | 127 | 24 | 0.92 | 0.91 | - | - | 68% in first 6 m | Not reported |
| Toure [8] | 2008 | Cote D'Ivoire | 10,211 | Urban | 123 (47-207) | 7.7 | - | - | 0.76 | >0.86 | 75% in first 4.6 m | 19% total losses at 12 mc |
CD4 cell counts in cells/μL.
Mean (SD).
Kaplan-Meier estimate.
m = months.
Using product-limit methods, the 12-month survival proportion in patients who were not lost to follow-up ranged between 0.74 and 0.92, with the greatest burden of mortality occurring during the initial months of ART (Table 1). Heterogeneity in outcomes between programmes may reflect differences in both baseline patient characteristics and programme characteristics [20]. Survival proportions were reported at both 12 and 24 months of follow-up in 9 studies and these showed that mortality accruing in the second year of ART was much less than that in the first.
Longitudinal changes in mortality rates were calculated in some studies [21-23]. In a South African cohort, the rate in the first 4 months of ART was 19.1 deaths/100 person-years (100PYs), decreasing to 2.9 deaths/100PYs beyond 4 months and 1.3 deaths/100PYs beyond one year [22]. A similar pattern of mortality was observed in another South African cohort [10] and in both studies rates of viral load suppression <400 copies/ml were high (>93% and >89%) [10, 22]. In other studies, however, substantial mortality accrued between 12 and 24 months of follow-up [23, 24]. Mortality rates in a cohort in Senegal, for example, were 12.5, 6.6 and 4.5 deaths/100PYs in the first, second and third years of treatment respectively [23]. The higher mortality rates beyond the first year of ART in these studies might be explained by lower rates of virological suppression and associated poor immunological recovery, for example [23, 25].
In all 20 cohorts (Table 1), the mortality proportions at 12 months of follow-up (range 8% - 26%) exceeded ART-LINC summary mortality estimate of 6.4% (95% CI 5.1-7.7) [12]. The ART-LINC estimate was derived from 4,810 patients receiving ART between 2001 and 2004 in resource-limited settings and included 3,449 patients in sub-Saharan Africa. Since then, there has been a more than 10-fold increase in the number patients receiving ART in the region whose outcomes have been published. The current literature included in this review suggests that further updated collaborative analyses might show a substantially higher early mortality rate than initially derived from the original ART-LINC cohorts.
Reliability of mortality data
There may be a tendency for mortality rates in the published literature to be lower than those observed in most large-scale ART programmes that haven't published their data because of selective reporting from well-run ART programmes in urban settings with more intensive service delivery and fewer resource constraints. The quality of mortality data is unclear in some reports; mortality is likely to be underestimated to a degree by most cohorts due to misclassification of unascertained patient deaths as ‘losses to follow-up’. The greater the loss to follow-up rate the greater the potential for misclassifications. Rates of loss to follow-up differed greatly between studies, ranging between 2% and 24% (Table 1) and unrecorded mortality among these patients may only be detected by intensive active follow-up [12, 26-28]. The efficacy of active follow-up may vary considerably between cohorts and details concerning the intensity of patient tracing are infrequently reported.
Analysis of the characteristics of losses to follow-up may provide insights into the reliability of programme mortality data. ART cohorts in South African and Cote-D'Ivoire ART with good ascertainment of outcomes found that whereas most deaths occurred in the first months of ART in patients with the lowest baseline CD4 cell counts, losses to follow-up were evenly distributed over time and were not associated with CD4 cell counts [8, 22]. Thus, if a programme reports a high loss to follow-up rate in the first months of ART among patients with the lowest baseline CD4 cell counts, this may be suggestive of high rates of unascertained deaths in this period.
In-programme mortality prior to ART initiation
Very high mortality rates recorded during the initial months of ART may reflect a high mortality rate among individuals who are eligible for ART but have yet to start treatment. This includes individuals enrolled in care who are awaiting treatment as well as those elsewhere in the health care system awaiting referral to HIV treatment services.
Two cohorts in South Africa have reported on deaths occurring in the interval between enrolment of patients into the ART programme and initiation of treatment [21, 22, 29]. In the Cape Town study, this interval of approximately 30 days permitted thorough investigation and treatment of coinfections and preparation of patients for ART, the mortality rate in this interval was very high (approximately 30 deaths/100PYs). Deaths occurring in this short period accounted for 67% of deaths within the first three months from programme enrolment [21]. Similarly, in the Free State cohort, 87% of deaths occurred among patients prior to ART initiation [29]. Thus, it is likely that even short delays in ART initiation are associated with considerable pre-treatment mortality risk. Thus, delays in patient referral, waiting lists for ART initiation and the time taken to prepare patients to start life-long treatment are likely to contribute to overall mortality risk.
Such delays and associated mortality are not typically reported by cohorts and so the optimum period for preparation for ART is therefore unclear. How to balance the need for thorough preparation of patients for life-long therapy with the high risk of death among individuals waiting to start therapy requires urgent research attention. Clearly flexibility in the timing is needed as many patients with advanced immunodeficiency need therapy urgently.
Risk factors for mortality
In the cohorts summarised in Table 1, low baseline CD4 cell count was a strong risk factor for early mortality in all 17 of the 20 cohorts with available data. The summary hazard ratio for the association between CD4 count <50 cells/μL (versus CD4 >50 cells/μL) was 2.5 (95% CI, 1.9-3.2) in studies with appropriate data [10, 11, 21, 24, 25, 30, 31]. A graded association with CD4 cell count was reported in some studies [6, 12, 22] (Figure 2a) but baseline viral load was not found to be an independent risk factor in any of the studies.
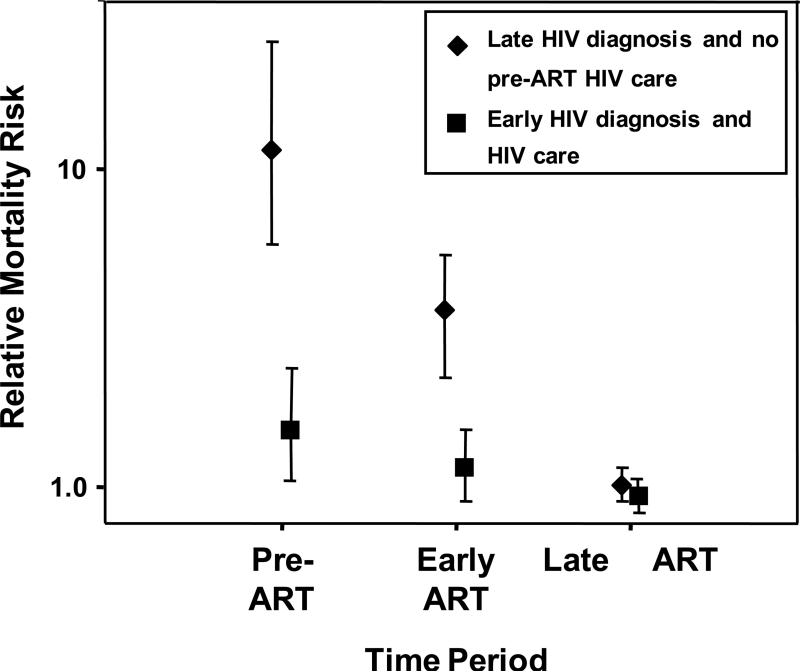
Figure 2.
Hypothetical graph showing relative mortality risk (95% CI) among patients accessing ART with late HIV diagnoses and advanced symptomatic disease (diamonds) versus mortality rates among patients whose HIV was diagnosed early and who received appropriate longitudinal HIV care and timely referral for ART (squares). Mortality risk is shown broken down by period: pre-ART (the interval between enrolment in the ART programme and the time ART is started), early ART (first 4 months of ART) and late ART (beyond 4 months of ART). Data on patients with late diagnoses are based on reference [22] and data from patients with early diagnoses and care are hypothetical, being based approximately on data from references [22, 81, 96].
Symptomatic disease (WHO stages 3 and 4) was associated with mortality in some [6, 8, 12, 21, 32] but not all [11, 23] studies (Figure 2b), possibly reflecting differences in the accuracy of clinical staging or homogeneity of cohorts with respect to this variable. WHO stage 4 disease, however, was found to be a strong predictor of mortality in all studies reporting on this [6, 21, 22, 24, 30, 33-37]. In three studies comparing patients with WHO stage 4 disease at baseline with those with WHO stages 1-3, WHO stage 4 was associated with more than a doubling in the hazard of death (summary hazard ratio, 2.2; 95% CI, 1.5-3.2) [10, 22, 30].
Low body mass index [6, 8, 23, 24, 35, 36, 38, 39] and anaemia [6, 8, 23, 36, 39, 40] were independently associated with mortality in some studies. Anaemia may be associated with a variety of conditions such as extrapulmonary TB, gastrointestinal Kaposi's sarcoma and severe malnutrition or may reflect the direct effects of advanced HIV on haematopoiesis. Male sex was associated with poorer survival in 6 studies [6, 8, 10, 22, 24, 37]; the reasons for this are unknown, but could relate to differences in health seeking behaviour or poorer treatment adherence among men [41].
In contrast to data from high-income settings [2], increasing age was associated with higher mortality risk in only one cohort [8]. The lack of an association may reflect the younger age and narrower age distribution of patients receiving ART in Africa. Alternatively, this may simply reflect the relatively short duration of follow-up in these studies since immunological recovery during the first 4 months of treatment is not age-dependent [42-44].
Patients with TB at enrolment to ART programmes have a high mortality risk [45, 46] and yet paradoxically TB and TB disease activity have not been found to be independent risk factors for mortality during ART [6, 35, 47]. Many deaths, however, may occur before ART is initiated [47, 48] and also much of the true burden of disease may remain unascertained [49]. In contrast, detectable cryptococcal antigen in serum was an independent predictor of early mortality in a rural cohort in Uganda [50].
Although the type of ART regimen and the type of healthcare facility (primary versus secondary) have not been found to be associated with mortality [8, 12, 23-25], programmes in which patients were required to pay for medication were associated with a 4-fold greater mortality risk [12]. This concurs with the findings of a meta-analysis of treatment outcomes in resource-poor settings; due to limitations in finances, patients who had to pay part or all the cost of therapy had an approximately 30% lower probability of having an undetectable viral load at 6 and 12 months compared to patients whose medication was supplied free of charge [13]. In keeping with this, ART adherence has been found to be predictive of mortality [39, 41].
Risk factors for mortality may alter during the course ART. While mortality during the first 4 months of ART in one cohort was associated with patient characteristics at baseline, mortality beyond this time-point was only associated with the updated absolute CD4 cell count at 4 months and with failure of viral load suppression [22]. These data suggest that the key long-term determinant of mortality is the response to ART as also suggested by more recent findings from Abidjan [38].
Causes of death
Five observational cohort studies from a range of countries in sub-Saharan Africa report on the spectrum of causes of early deaths [21, 23, 35, 39, 51]. The most important causes of death reported include tuberculosis, acute sepsis, cryptococcal meningitis, malignancies (especially Kaposi's sarcoma) and chronic diarrhoea or wasting syndrome.
Diagnostic facilities are limited in many settings, especially for rural cohorts, and no identifiable cause could be identified in a proportion of deaths. No systematic postmortem studies of deaths during ART have been reported and the overall data are therefore limited. Deaths in the first weeks of ART are likely to be caused by conditions that are either pre-existing at programme enrolment or new conditions arising in the context of persisting immunodeficiency. In one study causes of death during the first 4 months of ART were found to reflect those occurring just prior to ART with the addition of deaths due to immune reconstitution disease associated with cryptococcal meningitis and tuberculosis [21].
TB was among the two leading causes of death in 4 of the 5 cohorts, accounting for up to 21% of deaths [21, 23, 39, 51]. However, TB is likely to be an under-reported cause of death. Post-mortem studies have found in the pre-ART era that up to 54% of untreated patients dying from AIDS in Africa have evidence of occult disseminated TB [52, 53] and that this may be a common underlying cause of ‘slim disease’ [54]. Although wasting syndrome (defined as wasting with unexplained fever and / or chronic diarrhoea) was specifically reported as an important cause of death among in only one cohort [21], this may have been more common as ‘chronic diarrhoea’ was also reported as a common cause [35] and wasting was a common risk factor for mortality in many cohorts as described earlier.
The contribution of TB immune reconstitution disease to mortality has yet to fully emerge [55]. While most cases appear to be self-limiting, some are severe and deaths have been reported from cohorts in South Africa and Thailand [56, 57]. However, background mortality rates were also very high among TB patients who did not develop this complication and neither study found immune reconstitution disease to be associated with a significant excess mortality risk. Larger prospective studies are needed to clarify this issue.
Cryptococcal meningitis was among the two leading causes of mortality in 4 of the 5 cohorts [21, 23, 39, 51], accounting for up to 20% of deaths. Many of the deaths from cryptococcal disease in a South African cohort were attributed to immune reconstitution disease [58] and this was a more common cause of death than TB immune reconstitution disease [21]. Many patients developing this complication have previously been treated for cryptococcal meningitis with fluconazole monotherapy [21, 58, 59]. Although this is the standard of care throughout much of Africa, fluconazole is a fungistatic drug with far less efficacy than amphotericin in clearing the organism from cerebrospinal fluid (CSF) and especially so in the context of fluconazole resistance [60]. Persistence of cryptococci or cryptococcal antigen in the CSF is likely to predispose patients to immune reconstitution disease, which has a high mortality risk [58, 59, 61, 62].
Kaposi's sarcoma and other malignancies were among the three leading causes of death in 3 of the 5 cohorts [21, 35, 51], accounting for up to 14% of deaths. Acute sepsis was another important cause of mortality identified in some cohorts despite widespread use of trimethoprim-sulphamethoxazole (co-trimoxazole), [21, 39]. Respiratory disease, acute gastroenteritis or systemic sepsis with no identifiable focus were the most common forms of sepsis and collectively sepsis accounted for up to 19% of deaths. Pneumocystis jiroveci pneumonia was reported in up to 9% of deaths in two Ugandan studies [39, 51] but the basis for these diagnoses is not clear.
Microbiological causes of death from acute bacterial infections have not been reported. In studies of acute sepsis during co-trimoxazole prophylaxis in Abidjan, Cote D'Ivoire [31, 63-65], the predominant pathogens were non-typhoidal salmonella, Escherichia coli, Shigella spp and Streptococcus pneumoniae. Although high rates of co-trimoxazole resistance among non-typhoidal salmonellae and pneumococcal isolates have been reported in Uganda and Malawi [66, 67], prophylaxis with this drug is still effective in reducing mortality in both countries [67, 68].
The most widely used regimens in Africa include stavudine (d4T) and nevirapine, which have the potential for serious toxicity including hepatotoxicity, Stevens-Johnson syndrome and lactic acidosis [69, 70]. However, drug toxicity does not appear to be a major cause of early mortality. Among 226 deaths reported by 4 cohorts, 7 (3.1%) were attributed to ART toxicity [10, 21, 23, 51]. These were comparatively well-resourced programmes with access to biochemical laboratory monitoring; mortality rates may be higher where monitoring is not possible. Analysis of the temporal distribution of drug substitutions due to toxicity shows that nevirapine and zidovudine (AZT) toxicity occur during the first few months of ART whereas stavudine (d4T)-associated toxicity such as lactic acidosis occurs cumulatively from around 6 months onwards [71, 72]. Thus, the contribution of drug toxicity to overall mortality may change over time and may proportionately increase as the risk of opportunistic infections diminishes after the first 6 months of ART.
Deaths associated with nevirapine-induced hepatotoxicity have been reported from South Africa when used concurrently with rifampicin [69] but more data from large cohorts are needed to quantify the risk of concurrent TB treatment. In contrast, efavirenz is very well tolerated [64] and concurrent use of rifampicin and efavirenz in cohorts in South Africa did not result in mortality [47, 73].
Strategies to reduce mortality
Early mortality rates are strongly associated with the degree of immunodeficiency in patients at the time they enrol into ART programmes. Strategies to reduce mortality must therefore focus not only on delivery of care within ART programmes but more fundamentally they must promote early HIV diagnosis and improved pre-ART HIV care (Table 2).
Table 2.
Potential strategies to reduce mortality during antiretroviral treatment (ART)
| A. Early HIV diagnosis and longitudinal HIV care pre-ART |
| 1. Promote HIV testing and early diagnosis |
| 2. Strengthen long-term HIV care services prior to ART eligibility |
| 3. Optimise prevention, screening and treatment of opportunistic infections |
| 4. Provide longitudinal clinical and CD4 cell count monitoring to facilitate timely initiation of ART |
| 5. Minimise health system delays in ART initiation |
| B. Delivery of ART |
| 1. Provide ART without charge to the patient |
| 2. Use updated WHO guidelines for ART eligibility |
| 3. Develop locally effective ART adherence strategies |
| 4. Use ART regimens with lower toxicity |
| 5. Opportunistic infections |
| a. Co-trimoxazole prophylaxis during ART |
| b. Optimise screening for TB at ART programme entry |
| c. Optimise diagnosis and management of TB immune reconstitution disease |
| d. Prevent nosocomial transmission of TB |
| e. Consider concurrent isoniazid preventive therapy (IPT) during ART to reduce long term TB incidence (trial data awaited) |
| f. Optimise prevention, diagnosis and treatment of cryptococcal meningitis and immune reconstitution disease |
| 6. Use of laboratory monitoring |
| a. Laboratory monitoring for drug toxicity |
| b. Define appropriate strategies for monitoring response to ART |
Early HIV diagnosis and longitudinal HIV care (Table 2)
Patients entering ART programmes in sub-Saharan Africa have typically had their HIV infection diagnosed following presentation to the health services with advanced symptomatic disease. Such patients have high mortality risk in the period leading up to ART as well as during early ART (Figure 2). Provision of accessible and user-friendly services for serial voluntary counselling and testing (VCT) and CD4 cell count estimation is vital to promote early HIV diagnosis and assessment of ART eligibility. Early diagnosis and initiation of appropriate longitudinal care would probably be associated with much lower mortality risk in the period leading up to ART as well as during the initial months of ART (Figure 2).
Similar to the management of many chronic diseases in Africa, longitudinal care from the time of HIV diagnosis until requirement for ART is often poor. Thus, even those patients who are diagnosed with relatively early HIV infection are often lost to medical care only to later re-enter medical services with advanced disease. Longitudinal care must be strengthened and should include screening for and treatment of opportunistic infections, initiation of co-trimoxazole prophylaxis, isoniazid preventive therapy (IPT), reproductive health care, and serial CD4 cell count assessment until eligibility for ART is reached. This will require strengthening of HIV care services and more widespread availability of CD4 cell count measurement.
Prevention, screening and management of opportunistic infections are needed throughout the HIV care pathway. Co-trimoxazole prophylaxis should be initiated after HIV diagnosis and continued during ART [74] as this is associated with additional gains in life expectancy [16, 75]. Isoniazid preventive therapy (IPT) is an underused intervention in Africa [76]. Key obstacles include the difficulty of excluding active TB in patients with moderate or advanced immunodeficiency. IPT would therefore be more easily used among patients with less advanced disease and yet adherence to treatment among such patients and the infrastructure to efficiently deliver and monitor IPT remain a challenge.
Delivery of ART (Table 2)
National ART programme criteria for ART eligibility require ongoing re-evaluation. Initial WHO guidelines (2002) for resource-poor settings recommended treatment only for those with stage 4 disease or a CD4 cell count <200 cells/μL [77]. These were modified in 2003 [78] and in 2006 [79], bringing them closer to guidelines for high-income settings. However, some national programmes have retained earlier more restrictive eligibility criteria. For example South Africa's national programme has retained the 2002 WHO recommendations despite the fact that under these guidelines much HIV-associated morbidity and mortality occurs prior to eligibility for ART [80, 81]. Studies of when to initiate ART in resource-limited settings are needed. There are currently two trials which aim to address this issue in Cote D'Ivoire and Haiti [82, 83].
As soon as patients are identified as being eligible for ART, prompt referral for ART should be made. Where possible, waiting lists for ART should be minimised and those with highest risk might be prioritised [21, 22]. Supply of medication free of charge to the patient is key [12, 13]. While some international agencies advocate the privatisation of health services and private financing of health services through user fees, this is very unrealistic for most patients living in resource-limited settings who require life-long ART.
The use of regimens in Africa with higher toxicity than those used in the west is due to their low cost and the availability of fixed dose combination formulations. While provision of treatment to the millions of people living with HIV/AIDS who do not yet have treatment access should be the priority, implementation of less toxic regimens is nevertheless desirable.
Although laboratory monitoring and use of point-of-care lactic acid meters may help detect and manage drug adverse effects, no data yet exist to indicate that provision of such monitoring reduces mortality risk. This issue is being studied in the DART (Development of AntiRetroviral Therapy in Africa) trial [84]. Braitstein et al. found no impact of viral load monitoring on mortality in the first year of ART [12]. However, any benefits are only likely to occur during longer term treatment rather than during the first year.
Effective strategies to screen for active TB at entry to ART programmes need to be developed, including detection of the high prevalence of both clinical and sub-clinical disease [45, 46, 85, 86]. Prompt initiation of TB treatment may reduce patient mortality and reduce risks of nosocomial TB transmission. The optimal timing for initiation of ART among TB patients remains unknown and randomised controlled trials of early versus delayed initiation of ART are currently underway [87]. Similar to data from the UK [88, 89] observational data from South Africa strongly suggest that expedited initiation of treatment is needed among those with baseline CD4 cell counts <100 cells/μl in this setting in view of their exceptionally high mortality rate while awaiting ART [90, 91].
Randomised controlled trials are needed to define both the optimal management of moderate and severe TB immune reconstitution disease [55] and to assess the efficacy of concurrent isoniazid prophylaxis in reducing the high rates of TB that persist during ART [45, 92]. In addition, the utility of pre-ART screening for and management of asymptomatic cryptococcal antigenaemia has yet to be defined [50]. Provision of a better standard of care for cryptococcal meningitis using amphotericin might be considered in settings with adequate facilities to administer this drug safely. Guidelines for the prevention, diagnosis and management of cryptococcal immune reconstitution disease are also needed.
Despite early reports that treatment adherence does not pose a major barrier to treatment success in sub-Saharan Africa [93], more recent research suggests that overall adherence rates and retention on ART in sub-Saharan Africa are quite variable and often poor [18, 94]. Adherence is predictive of mortality [41] and development of locally appropriate strategies to promote adherence are central to the success of ART programmes. Research is also needed to determine what cadre of health-care professional is needed to deliver ART effectively with good outcomes.
Conclusions
High early mortality within ART programmes in sub-Saharan Africa has emerged as a key challenge. Between 8% and 26% of patients die in the first year of ART and key issues surrounding this problem are summarised in Table 3. Early death rates threaten the credibility of ART delivery among communities accessing such therapy and among health workers who are responsible for providing this care. While many factors are likely to contribute to this mortality, an over-riding issue is that patients typically present for ART once they have developed advanced symptomatic disease. Much may be done within ART services to potentially reduce this mortality by providing medication free of charge, implementing effective screening, treatment and prevention of opportunistic infections, reinforcing treatment adherence and using regimens with low toxicity. However, despite optimising ART delivery, a proportion of early deaths among patients with very advanced disease is not likely to be preventable. Thus, a more fundamental issue and the greater challenge is the need for early HIV diagnosis and provision of appropriate longitudinal HIV care prior to ART eligibility.
Table 3.
Summary of key issues
| Summary points |
|---|
| • Although virological and immunological responses to ART are similar in patients living in high-income and resource-limited countries, early mortality rates are much higher |
| • 9-26% of patients die during the first year of ART in sub-Saharan Africa. |
| • Most deaths occur in the first few months of ART |
| • A high loss to follow-up rate may conceal true mortality rates |
| • High mortality rates during early ART also reflect high mortality rates in the period preceding ART. |
| • Key risk factors for early mortality include low CD4 cell count, advanced clinical stage of disease, and the need for patients to pay for treatment. |
| • Early deaths largely reflect the spectrum of causes of death prior to ART initiation plus immune reconstitution disease. |
| • Common causes of death are tuberculosis, acute sepsis, cryptococcal meningitis malignancies and wasting syndrome. |
| • Drug adverse effects are a relatively minor cause of early mortality. |
| • Strategies to reduce early mortality include promotion of early HIV diagnosis, strengthening of the patient care pathway pre-ART, timely initiation of ART, provision of ART free of charge to the patient, adherence support, and optimal prevention, screening and management of opportunistic infections. |
Figure 1.
Kaplan Meier plot showing survival proportion over 21 months among patients (n=1235) from the time of entering a community-based antiretroviral treatment (ART) programme in South Africa. The period covered includes the interval between programme enrolment and ART initiation – a median of 33 days. The survival proportions are shown according to (a) baseline CD4 cell count and (b) baseline WHO clinical stage. The graphs show the strong unadjusted association between CD4 cell count, WHO clinical stage and mortality risk in the first year of ART and the low mortality risk in the second year. Data adapted from reference [22].
Acknowledgements
SDL is funded by the Wellcome Trust, London, UK with grant 074641/Z/04/Z. RW is funded in part by the National Institutes of Health, USA, RO1 grant (A1058736-01A1) and a CIPRA grant 1U19AI53217-01. XA is funded in part by the ANRS (Agence nationale de recherches sur le SIDA et les hépatites virales, Paris, France (grant ANRS 1269).
SDL conceptualised and outlined the review with input from RW. SDL did the literature searches and drafted the manuscript. LM calculated the summary hazard estimates. ADH, XA, LM and RW all provided critical input and helped redraft the manuscript into its final form.
References
- 1.Palella FJ, Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d'Arminio MA, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS / WHO AIDS epidemic update 2007. http://data.unaids.org/pub/EPISlides/2007/2007_epiupdate_en.pdf.
- 5.World Health Organization . Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report, April 2007. WHO; Geneva: 2007. [24.04.07]. http://www.who.int/hiv/toronto2006/towardsuniversalaccess.pdf. [Google Scholar]
- 6.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 7.Harries AD, Schouten EJ, Libamba E. Scaling up antiretroviral treatment in resource-poor settings. Lancet. 2006;367:1870–1872. doi: 10.1016/S0140-6736(06)68809-0. [DOI] [PubMed] [Google Scholar]
- 8.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote D'Ivoire: two-year outcomes and determinants. AIDS. 2008 doi: 10.1097/QAD.0b013e3282f768f8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96:315–320. [PubMed] [Google Scholar]
- 10.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 11.Wester CW, Kim S, Bussmann H, Avalos A, Ndwapi N, Peter TF, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40:336–343. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 12.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 13.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–224. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 14.Badri M, Maartens G, Mandalia S, Bekker LG, Penrod JR, Platt RW, et al. Cost-effectiveness of highly active antiretroviral therapy in South Africa. PLoS Med. 2006;3:e4. doi: 10.1371/journal.pmed.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badri M, Cleary S, Maartens G, Pitt J, Bekker LG, Orrell C, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther. 2006;11:63–72. [PubMed] [Google Scholar]
- 16.Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, Walensky RP, et al. Cost-effectiveness of HIV treatment in resource-poor settings--the case of Cote d'Ivoire. N Engl J Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 17.WHO/UNAIDS [March 27, 2006];Press release: HIV infection rates decreasing in several countries but globa; number of people living with HIV continues to rise. 2005 Nov; doi: 10.1111/j.1365-2648.2006.03827.x. http://www.who.int/hiv/epiupdate2005/en/print.html. [DOI] [PubMed]
- 18.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.El-Sadr W, Elul B, Hawken M, Lima J, Macharaia D, Oluyden B, et al. HIV care and treatment in resource-limited settings: challenges and outcomes.. Programme & Abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles. Feb 25-28, 2007; Abstract #534. [Google Scholar]
- 21.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 22.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 23.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 24.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 25.Weidle PJ, Malamba S, Mwebaze R, Sozi C, Rukundo G, Downing R, et al. Assessment of a pilot antiretroviral drug therapy programme in Uganda: patients’ response, survival, and drug resistance. Lancet. 2002;360:34–40. doi: 10.1016/S0140-6736(02)09330-3. [DOI] [PubMed] [Google Scholar]
- 26.Anglaret X, Toure S, Gourvellec G, Tchehy A, Zio L, Zaho M, et al. Impact of vital status investigation procedures on estimates of survival in cohorts of HIV-infected patients from Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2004;35:320–323. doi: 10.1097/00126334-200403010-00015. [DOI] [PubMed] [Google Scholar]
- 27.Yu JK, Chen SC, Wang KY, Chang CS, Makombe SD, Schouten EJ, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bisson G, Ndwapi N, Rollins C, Avalos A, Gross R, Bellamy S, et al. High rates of death among patients lost to follow-up in Botswana's National ART Program; implications for monitoring and evaluation.. Programme & Abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles. Feb 25-28, 2007; Abstract #537. [Google Scholar]
- 29.Fairall LR, Bachmann MO, Louwagie GM, van VC, Chikobvu P, Steyn D, et al. Effectiveness of antiretroviral treatment in a South african program: a cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 30.Djomand G, Roels T, Ellerbrock T, Hanson D, Diomande F, Monga B, et al. Virologic and immunologic outcomes and programmatic challenges of an antiretroviral treatment pilot project in Abidjan, Cote d'Ivoire. AIDS. 2003;17(Suppl 3):S5–15. doi: 10.1097/00002030-200317003-00002. S5-15. [DOI] [PubMed] [Google Scholar]
- 31.Seyler C, Anglaret X, koury-Dogbo N, Messou E, Toure S, Danel C, et al. Medium-term survival, morbidity and immunovirological evolution in HIV-infected adults receiving antiretroviral therapy, Abidjan, Cote d'Ivoire. Antivir Ther. 2003;8:385–393. [PubMed] [Google Scholar]
- 32.De Iaco G, Zombre M, Carvalho AC, Capone S, Some E, Pietra V, et al. Factors predicting early death in a cohort of patients with HIV/AIDS in Burkina Faso: the need for earlier diagnosis and antiretroviral treatment (ART).. Abstracts of the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; International AIDS Society. Sydney, Australia. July 2007; Abstract #WEPEB053. [Google Scholar]
- 33.Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21:2483–2491. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 34.Jerene D, Endale A, Hailu Y, Lindtjorn B. Predictors of early death in a cohort of Ethiopian patients treated with HAART. BMC Infect Dis. 2006;6:136. doi: 10.1186/1471-2334-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zachariah R, Fitzgerald M, Massaquoi M, Pasulani O, Arnould L, Makombe S, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 36.Johannessen A, Naman E, Matee M, Gundersen SG, Bruun JN. Risk factors for early mortality on antiretroviral treatment in a rural hospital in Tanzania.. Abstracts of the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; International AIDS Society. Sydney, Australia. July 2007; Abstract #WEPEB054. [Google Scholar]
- 37.Bajunirwe F, Arts EJ, Tisch DJ, Debanne SM, Sethi AK. Survival, adherence to care and antiretroviral treatment (ART) among HIV-infected adults in rural Western Uganda.. Abstracts of the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; International AIDS Society. Sydney, Australia. July 2007; Abstract #WEPEB049. [Google Scholar]
- 38.Moh R, Danel C, Messou E, Ouassa T, Gabillard D, Anzian A, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21:2483–91. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 39.Moore D, Yiannoutos C, Musick B, Downing R, Were W, Degerman R, et al. Determinants of mortality among HIV-infected individuals receiving home-based ART in rural Uganda.. Abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, USA. Feb. 2007; Abstract #34. [Google Scholar]
- 40.Laurent C, Ngom Gueye NF, Ndour CT, Gueye PM, Diouf M, Diakhate N, et al. Long-term benefits of highly active antiretroviral therapy in Senegalese HIV-1-infected adults. J Acquir Immune Defic Syndr. 2005;38:14–17. doi: 10.1097/00126334-200501010-00003. [DOI] [PubMed] [Google Scholar]
- 41.Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 42.Lawn SD, Myer L, Bekker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 44.Bennett KK, DeGruttola VG, Marschner IC, Havlir DV, Richman DD. Baseline predictors of CD4 T-lymphocyte recovery with combination antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:20–26. doi: 10.1097/00126334-200209010-00003. [DOI] [PubMed] [Google Scholar]
- 45.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 46.Moore D, Liechty C, Ekwaru P, Were W, Mwima G, Solberg P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 47.Lawn SD, Myer L, Bekker LG, Wood R. Early mortality in patients with HIV-associated tuberculosis in Africa: implications for time to initiation of treatment.. Programme and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections (CROI); Los Angeles, USA. February 2007; Abstract #0-126. [Google Scholar]
- 48.Zachariah R, Fitzgerald M, Massaquoi M, Acabu A, Chilomo D, Salaniponi FM, et al. Does antiretroviral treatment reduce case fatality among HIV-positive patients with tuberculosis in Malawi? Int J Tuberc Lung Dis. 2007;11:848–853. [PubMed] [Google Scholar]
- 49.Lucas SB, Hounnou A, Koffi K, Beaumel A, Andoh J, De Cock KM. Pathology of paediatric human immunodeficiency virus infections in Cote d'Ivoire. East Afr Med J. 1996;73:S7–S8. [PubMed] [Google Scholar]
- 50.Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, Weidle PJ, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–935. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 51.Kambugu A, Castelnuovo B, Wandera B, Kiragga A, Kamya M. Antiretroviral therapy in an urban African cohort does not prevent significant early mortality.. Abstracts of the 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention; International AIDS Society. Sydney, Australia. July 2007; Abstract #WEPEB055. [Google Scholar]
- 52.Rana F, Hawken MP, Meme HK, Chakaya JM, Githui WA, Odhiambo JA, et al. Autopsy findings in HIV-1-infected adults in Kenya. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:83–85. doi: 10.1097/00042560-199701010-00017. [DOI] [PubMed] [Google Scholar]
- 53.Lucas SB, Hounnou A, Peacock C, Beaumel A, Djomand G, N'Gbichi JM, et al. The mortality and pathology of HIV infection in a west African city. AIDS. 1993;7:1569–1579. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Lucas SB, De Cock KM, Hounnou A, Peacock C, Diomande M, Honde M, et al. Contribution of tuberculosis to slim disease in Africa. BMJ. 1994;308:1531–1533. doi: 10.1136/bmj.308.6943.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–373. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 56.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 57.Manosuthi W, Kiertiburanakul S, Phoorisri T, Sungkanuparph S. Immune reconstitution inflammatory syndrome of tuberculosis among HIV-infected patients receiving antituberculous and antiretroviral therapy. J Infect. 2006;53:357–363. doi: 10.1016/j.jinf.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2052. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 59.Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin Infect Dis. 2006;43:1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- 60.Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 61.Jenny-Avital ER, Abadi M. Immune reconstitution cryptococcosis after initiation of successful highly active antiretroviral therapy. Clin Infect Dis. 2002;35:e128–e133. doi: 10.1086/344467. [DOI] [PubMed] [Google Scholar]
- 62.Lortholary O, Fontanet A, Memain N, Martin A, Sitbon K, Dromer F. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19:1043–1049. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 63.Anglaret X, Messou E, Ouassa T, Toure S, koury-Dogbo N, Combe P, et al. Pattern of bacterial diseases in a cohort of HIV-1 infected adults receiving cotrimoxazole prophylaxis in Abidjan, Cote d'Ivoire. AIDS. 2003;17:575–584. doi: 10.1097/00002030-200303070-00013. [DOI] [PubMed] [Google Scholar]
- 64.Danel C, Moh R, Anzian A, Abo Y, Chenal H, Guehi C, et al. Tolerance and acceptability of an efavirenz-based regimen in 740 adults (predominantly women) in West Africa. J Acquir Immune Defic Syndr. 2006;42:29–35. doi: 10.1097/01.qai.0000219777.04927.50. [DOI] [PubMed] [Google Scholar]
- 65.Danel C, Moh R, Minga A, Anzian A, Ba-Gomis O, Kanga C, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–1989. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 66.van Oosterhout JJ, Laufer MK, Graham SM, Thumba F, Perez MA, Chimbiya N, et al. A community-based study of the incidence of trimethoprim-sulfamethoxazole-preventable infections in Malawian adults living with HIV. J Acquir Immune Defic Syndr. 2005;39:626–631. [PubMed] [Google Scholar]
- 67.Mermin J, Ekwaru JP, Liechty CA, Were W, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006;367:1256–1261. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 68.Mwaungulu FB, Floyd S, Crampin AC, Kasimba S, Malema S, Kanyongoloka H, et al. Cotrimoxazole prophylaxis reduces mortality in human immunodeficiency virus-positive tuberculosis patients in Karonga District, Malawi. Bull World Health Organ. 2004;82:354–363. [PMC free article] [PubMed] [Google Scholar]
- 69.Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191:825–829. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 70.Geddes R, Knight S, Moosa MY, Reddi A, Uebel K, Sunpath H. A high incidence of nucleoside reverse transcriptase inhibitor (NRTI)-induced lactic acidosis in HIV-infected patients in a South African context. S Afr Med J. 2006;96:722–724. [PubMed] [Google Scholar]
- 71.Moh R, Danel C, Sorho S, Sauvageot D, Anzian A, Minga A, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Cote d'Ivoire. Antivir Ther. 2005;10:615–624. doi: 10.1177/135965350501000510. [DOI] [PubMed] [Google Scholar]
- 72.Boulle A, Orrell C, Kaplan R, Van Cutsem G, McNally M, Hildebrand K, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antiv Ther. 2007;12:753–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 73.Hoffmann CJ, Charalambous S, Thio CL, Martin DJ, Pemba L, Fielding KL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21:1301–1308. doi: 10.1097/QAD.0b013e32814e6b08. [DOI] [PubMed] [Google Scholar]
- 74.World Health Organisation . Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults. Recommendations for a public health approach. World Health Organisation; Geneva: 2006. [Google Scholar]
- 75.Lowrance D, Makombe S, Clin D, Harries A, Yu J, berle-Grasse J, et al. Lower Early Mortality Rates Among Patients Receiving Antiretroviral Treatment at Clinics Offering Cotrimoxazole Prophylaxis in Malawi. J Acquir Immune Defic Syndr. 2007 Publish Ahead of Print. [PubMed] [Google Scholar]
- 76.Churchyard GJ, Scano F, Grant AD, Chaisson RE. Tuberculosis preventive therapy in the era of HIV infection: overview and research priorities. J Infect Dis. 2007;196(Suppl 1):S52–S62. doi: 10.1086/518662. [DOI] [PubMed] [Google Scholar]
- 77.World Health Organisation . Scaling up Antiretroviral Therapy in Resource-limited Settings: Guidelines for a Public Health Approach; Executive Summary. World Health Organisation; Geneva: 2002. [PubMed] [Google Scholar]
- 78.World Health Organisation . Scaling up Antiretroviral Therapy in Resource-limited Settings: Guidelines for a Public Health Approach; 2003 Revision. World Health Organisation; Geneva: 2002. [Google Scholar]
- 79.World Health Organisation . Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2006 revision. WHO; Geneva: 2006. [PubMed] [Google Scholar]
- 80.Lawn SD, Wood R. National adult antiretroviral therapy guidelines in South Africa: concordance with 2003 WHO guidelines? AIDS. 2007;21:121–122. doi: 10.1097/QAD.0b013e3280117fa5. [DOI] [PubMed] [Google Scholar]
- 81.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–1259. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 82. [Jan 22nd 2008]; http://clinicaltrials.gov/ct2/show/NCT00120510?term=NCT00120510&rank=1.
- 83. [Jan 22nd 2008]; http://clinicaltrials.gov/ct2/show/NCT00495651?term=Temprano&rank=1.
- 84. [Jan 14 2008]; DART trial. http://www.ctu.mrc.ac.uk/dart/summary.asp.
- 85.Wood R, Middelkoop K, Myer L, Grant AD, Whitelaw A, Lawn SD, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mtei L, Matee M, Herfort O, Bakari M, Horsburgh CR, Waddell R, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 87.Blanc FX, Havlir DV, Onyebujoh PC, Thim S, Goldfeld AE, Delfraissy JF. Treatment strategies for HIV-infected patients with tuberculosis: ongoing and planned clinical trials. J Infect Dis. 2007;196(Suppl 1):S46–S51. doi: 10.1086/518658. [DOI] [PubMed] [Google Scholar]
- 88.Dean GL, Edwards SG, Ives NJ, Matthews G, Fox EF, Navaratne L, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS. 2002;16:75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 89.Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–1676. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 90.Lawn SD, Wood R. When should antiretroviral treatment be started in patients with tuberculosis In South Africa? S Afr Med J. 2007;97:412–415. [PubMed] [Google Scholar]
- 91.Lawn SD, Wood R. Optimum time to initiate antiretroviral therapy in patients with HIV-associated tuberculosis: there may be more than one right answer. J Acquir Immune Defic Syndr. 2007;46:121–123. doi: 10.1097/QAI.0b013e3181398d28. [DOI] [PubMed] [Google Scholar]
- 92.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003;17:1369–1375. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 94.Gill CJ, Hamer DH, Simon JL, Thea DM, Sabin LL. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS. 2005;19:1243–1249. doi: 10.1097/01.aids.0000180094.04652.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Makombe SD, Harries AD, Yu JK, Hochgesang M, Mhango E, Weigel R, et al. Outcomes of tuberculosis patients who start antiretroviral therapy under routine programme conditions in Malawi. Int J Tuberc Lung Dis. 2007;11:412–416. [PubMed] [Google Scholar]
- 96.Badri M, Bekker LG, Orrell C, Pitt J, Cilliers F, Wood R. Initiating highly active antiretroviral therapy in sub-Saharan Africa: an assessment of the revised World Health Organization scaling-up guidelines. AIDS. 2004;18:1159–1168. doi: 10.1097/00002030-200405210-00009. [DOI] [PubMed] [Google Scholar]