Abstract
BACKGROUND
Rapid means to identify or rule-out tuberculosis (TB) would permit more efficient management of HIV-infected patients starting antiretroviral treatment (ART).
SETTING
South African township ART clinic.
OBJECTIVE
To assess the diagnostic and prognostic utility of C-reactive protein (CRP) among patients being screened for TB pre-ART.
DESIGN
Patients were enrolled regardless of symptoms and characterized clinically. Serum CRP was measured and sputum and urine samples were subject to multiple assays for TB. Mortality at three months was assessed.
RESULTS
Among 496 patients (median CD4 count, 171 cells/μL), culture-positive TB was diagnosed in 81 (16.3%) patients. CRP concentrations were much higher among TB cases (median, 57.8mg/L; IQR,20.0-202.7) compared to those without TB (6.4mg/L; IQR,2.1-21.8; P<0.001). Very low (<1.5 mg/L) CRP concentrations excluded TB (100% negative predictive value) whereas very high concentrations (>400 mg/L) were strongly predictive of TB (100% positive predictive value). However, these thresholds encompassed only 14.3% and 2.0% of all patients screened, respectively, and only 12.3% of TB cases. TB patients with CRP concentrations ≥50 mg/L were substantially more likely to have poor prognostic characteristics, higher mycobacterial load, disseminated disease and greater mortality risk.
CONCLUSION
CRP concentrations can be used pre-ART to identify groups of patients with very high or very low TB risk, but only in an unacceptably small minority of patients screened. However, CRP concentrations have useful prognostic value in those with HIV-associated TB.
INTRODUCTION
Tuberculosis (TB) is a leading cause of morbidity and mortality in people living with HIV in sub-Saharan Africa, especially among those with advanced immunodeficiency attending antiretroviral treatment (ART) services or admitted to hospital.1 To reduce mortality, intensified case finding is a key intervention.2,3 This aims to either diagnose TB as rapidly as possible (permitting immediate initiation of TB treatment followed by ART) or to rapidly rule-out a diagnosis of TB (permitting more rapid initiation of ART).
Substantial progress has been made in the development of new rapid diagnostic tests for TB with useful accuracy when used for routine screening of patients 3. These include the rapid molecular assay, Xpert MTB/RIF,4,5 and a simple, low-cost, point-of-care lateral flow test for lipoarabinomannan (LAM) in urine called Determine TB-LAM Ag.6,7 However, apart from the national scale-up of Xpert MTB/RIF in South Africa, these tests are at present not widely available in the rest of sub-Saharan Africa and their role remains incompletely defined. Moreover, their predictive value for ruling out TB is limited. 6,7 Screening for HIV-associated TB currently remains a pressing challenge in most settings, with ongoing reliance on sputum smear microscopy and chest radiology which have limited utility in this patient group.3
C-reactive protein (CRP) is an acute phase protein detectable within serum 8 and can now be measured at the point-of-care with results available within a few minutes.9 It has been proposed that this may be useful for TB screening in HIV-infected patients.10,11 Moreover, this marker also has prognostic value in people living with HIV, including those with opportunistic infections such as Pneumocystis jirovecii pneumonia.12-14 We therefore first assessed the predictive value of serum CRP for excluding TB or for identifying TB cases among patients enrolling in an ART service in a township in South Africa. We also assessed the prognostic value among all those testing positive for TB.
METHODS
The ART service in Gugulethu township, Cape Town, and its major burden of TB have previously been reported in detail.15,16 The present study forms part of an ongoing body of work evaluating diagnostic assays for HIV-associated TB for which patient recruitment and laboratory procedures have been described in detail in the parent studies.5,6 Those eligible were new patients referred to the ART clinic aged >18 years, ART-naive and with no current TB diagnosis. All were receiving trimethoprim-sulphmethoxazole prophylaxis.
All participants provided written informed consent and the study was approved by the research ethics committees of the University of Cape Town and the London School of Hygiene & Tropical Medicine, UK. Patients were prospectively recruited between March 2010 and April 2011 and investigated at their first clinic visit. Demographic details were recorded and a standardised World Health Organization (WHO) symptom-screening questionnaire was completed.17 Two sputum samples (one spot specimen and one induced specimen) were obtained where possible as previously reported.18 Urine was also collected and stored at −20 degrees C and blood samples were collected in serum separator tubes and serum stored at −20 degrees C. Chest radiographs were obtained and read using the Chest Radiograph Reading and Recording System (CRRS) 19 for which the lead investigator was accredited.
Laboratory procedures
Laboratory procedures have been described in detail elsewhere.5,6 Briefly, sputum specimens were decontaminated with N-acetyl-L-cysteine and sodium hydroxide and concentrated by centrifugation. Sputum pellets were examined by fluorescence microscopy, liquid culture (Mycobacterial Growth Indicator Tubes, Becton Dickinson, Sparks, Maryland, USA) and the Xpert MTB/RIF assay. Cultures positive for acid-fast bacilli were identified as Mycobacterium tuberculosis complex by line-probe assay.
Frozen urine samples were defrosted and retrospectively analysed for the presence of LAM using the commercially available Clearview TB-ELISA (Alere Inc., Waltham, MA, USA). As described previously,20 defrosted urine samples (2.0 mL) were also concentrated by centrifugation, resuspended in 0.75 mL of phosphate buffer and then tested using the Xpert MTB/RIF assay according to the manufacturer’s instructions.
The concentrations of CRP were measured in duplicate serum samples using the Quantikine enzyme-linked immunosorbent assay (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions.
Patient outcomes
Patients were followed up within the routine ART service and patients diagnosed as having TB (by sputum smear, culture or Xpert MTB/RIF) were referred to TB clinics within the township for treatment. ART service patient records were reviewed to determine clinical outcomes.
Definitions and analysis
Analysis was restricted to patients who had a complete set of laboratory data for ≥1 sputum sample, urine diagnostic assays and serum CRP. Patients were defined as having TB if Mycobacterium tuberculosis was cultured from ≥1 sputum sample. The serum CRP concentrations and characteristics of patients with and without TB were compared. Logistic regression was used to identify factors associated with CRP values ≥50 mg/L. The predictive value of CRP to exclude or to identify diagnosis of TB was explored using a series of thresholds. Sensitivity, specificity, predictive values and likelihood ratios associated with these thresholds were calculated and Receiver-Operator Curve (ROC) analysis was done.
To explore the prognostic value of CRP, the characteristics of TB patients stratified by CRP ≥50 mg/L and <50 mg/L (a close approximation to the median value) were defined and the clinical 3-month outcomes of the two groups were compared. Statistical analyses were done using Wilcoxon rank-sum test, t-test, chi-square and Fisher’s exact tests as appropriate. All statistical tests were two-sided at alpha=0.05.
RESULTS
Patients and TB diagnoses
Of all eligible patients enrolled (n=602), 62 could not produce any sputum samples. Complete CRP, sputum and urine results were available for 496 patients. The study participants were predominantly young adults, a majority of whom were female (Table 1). The median CD4 cell count was 171 cells/μL (IQR 98-233), 62.8% of patients had a CD4 cell count of <200 cells/μL and 32.7% of patients had WHO stage 3 or stage 4 (AIDS) disease prior to TB screening.
Table 1.
Baseline patient characteristics
| All patients (n=496) |
Patients with TB (n=81) |
Patients without TB (n=415) |
P-value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, median (IQR) | 33.6 (28.3-40.7) | 32.9 (28.5-39.3) | 33.6 (28.2-40.8) | 0.70 |
| Female | 317 (63.9) | 51 (63.0) | 266 (64.1) | 0.85 |
| BMI, median (IQR) | 23.4 (20.9-27.1) | 21.2 (19.3-26.0) | 23.8 (21.0-27.2) | <0.001 |
|
CD4 counts
(cells per μL)a |
||||
| Median (IQR) | 171 (98-233) | 138 (63-205) | 174 (111-238) | 0.004 |
| CD4 <50 | 62 (12.5) | 18 (22.2) | 44 (10.6) | |
| CD4 50-99 | 63 (12.7) | 11 (13.6) | 52 (12.6) | |
| CD4 100-149 | 90 (18.2) | 16 (19.8) | 74 (17.9) | 0.041 |
| CD4 150-199 | 96 (19.4) | 12 (14.8) | 84 (20.3) | |
| CD4 ≥200 | 184 (37.2) | 24 (29.6) | 160 (38.7) | |
|
Baseline viral load (log
copies per mL), median (IQR)b |
4.6 (4.1-5.0) | 4.8 (4.4-5.3) | 4.5 (4.0-5.0) | <0.001 |
| WHO stage at enrolment | ||||
| 1 or 2 | 334 (67.3) | 45 (55.6) | 289 (69.6) | 0.013 |
| 3 or 4 | 162 (32.7) | 36 (44.4) | 126 (30.4) | |
|
Previous history of
tuberculosis |
131 (26.4) | 16 (19.8) | 115 (27.7) | 0.14 |
|
Positive WHO symptom
screen |
344 (69.4) | 67 (82.7) | 277 (66.8) | 0.004 |
|
Current cough ≥ 2 weeks
Radiological |
102 (20.6) | 21 (25.9) | 81 (19.5) | 0.19 |
|
abnormality consistent
with tuberculosis c |
224 (49.7) | 58 (74.4) | 166 (44.5) | <0.001 |
CD4 cell counts available for 495 patients.
Viral load data available for 494 patients.
Chest radiographs were available for 451 patients
Culture-positive TB was diagnosed in 81 patients, giving a TB prevalence of 16.3% (95%CI, 13.2-19.9). The remainder were sputum culture-negative (n=415). Patients with TB had lower CD4 cell counts and were more likely to have advanced WHO stage of disease (Table 1). A positive WHO symptom screen was found in 69.4% of all study participants and 82.7% of TB patients. Any radiological abnormalities consistent with pulmonary TB were observed in just 74.4% of TB patients and were also observed in 44.5% of patients without TB.
CRP concentrations and utility for TB screening
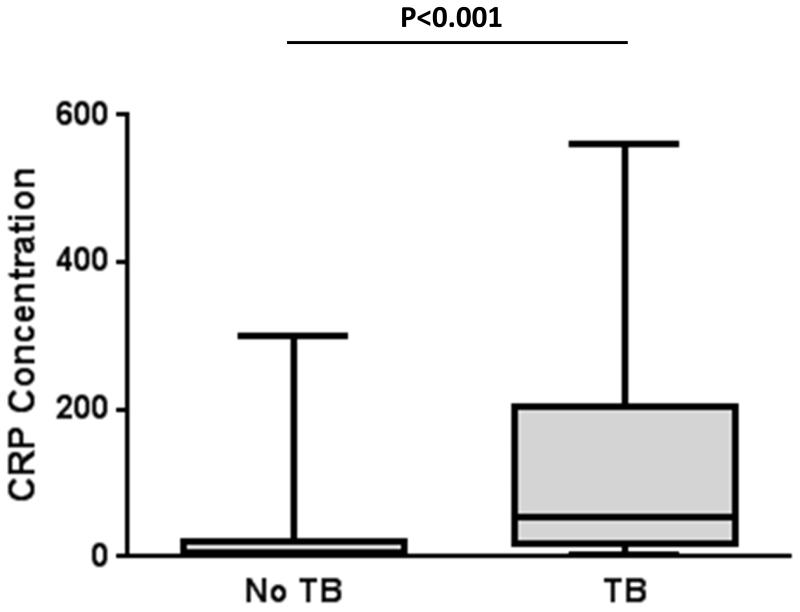
The median serum CRP concentration was much higher in patients with TB (median, 57.8 mg/L; IQR, 20.2-202.7 mg/L) than in patients without TB (median, 6.4 mg/L; IQR 2.1-21.8) as shown in Figure 1 (P<0.001). In multivariate analysis, TB was strongly associated with a serum concentration of CRP ≥50 mg/L and weaker associations were observed with male gender and low CD4 cell counts (Table 2).
Figure 1.
Box and whisker plot showing concentrations of C-reactive protein (CRP) in serum from patient with tuberculosis (TB; n=81) or who were not found to have TB (n=415).The bars, box and whiskers indicate the medians, 25thand 75thcentiles and the ranges, respectively.
Table 2.
Logistic regression analysis showing the relationship between high C reactive protein (CRP) concentrations ≥50 mg/L and a range of patient characteristics.
| Unadjusted OR |
95% CI | p-value | Adjusted OR |
95% CI | p-value | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 1.0 | - | - | 1.0 | - | - |
| Male | 1.76 | 1.11-2.79 | 0.017 | 2.07 | 1.21-3.51 | 0.007 |
| Age (years) | ||||||
| ≥30 | 1.0 | - | - | 1.0 | - | - |
| <30 | 0.99 | 0.61-1.60 | 0.959 | 1.21 | 0.69-2.12 | 0.503 |
| TB status | ||||||
| Negative | 1.0 | - | - | 1.0 | - | - |
| Positive | 9.97 | 5.84-17.03 | <0.001 | 10.00 | 5.75-17.41 | <0.001 |
| CD4 count (cells/μL) | ||||||
| ≥200 | 1.0 | - | - | 1.0 | - | - |
| <200 | 1.95 | 1.17-3.25 | 0.009 | 1.85 | 1.04-3.30 | 0.032 |
| Viral load (log copies/mL) | ||||||
| <4.5 | 1.0 | - | - | 1.0 | - | - |
| ≥4.5 | 1.71 | 1.07-2.74 | 0.023 | 1.17 | 0.69-2.01 | 0.557 |
OR = odds ratio
We explored the utility of a range of CRP threshold concentrations to either rule-out or identify cases of TB (Table 3). At progressively lower CRP concentrations, the negative predictive value for TB increased from 91.1% at a threshold of ≥50 mg/L to 100% at a threshold of ≥1.5 mg/L (Table 3). Thus, a CRP of <1.5 mg/L could be used to reliably exclude a diagnosis of TB but this would only encompass 14.3% of all patients screened. Those with CRP values of <2 mg/L (20.0% of patients screened) had a 4.0% prevalence of TB and those with values of <10 mg/L (50.6% of patients screened) had a prevalence of 4.8%.
Table 3.
Utility of serum C-reactive protein (CRP) threshold concentrations for ruling out or ruling in diagnoses of tuberculosis (TB).
| CRP threshold (mg/L) |
Proportion of total study population (%) |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV1 (95% CI) |
NPV2 (95% CI) |
Positive likelihood ratio |
Negative likelihood ratio |
|---|---|---|---|---|---|---|---|
| ≥1 | 91.1 | 100 (94.4-100) |
10.6 (7.9-14.1) |
17.9 (14.6-21.8) |
100 (90.0-100) |
1.1 (1.1-1.2) |
0 |
| ≥1.5 | 85.7 | 100 (94.4-100) |
17.1 (13.7-21.2) |
19.1 (15.5-23.2) |
100 (93.6-100) |
1.2 (1.2-1.3) |
0 |
| ≥2 | 80.0 | 95.1 (87.2-98.4) |
22.9 (19.0-27.3) |
19.4 (15.7-23.7) |
96.0 (89.4-98.7) |
1.2 (1.1-1.3) |
0.2 (0.1-0.6) |
| ≥5 | 61.7 | 90.1 (81.0-95.3) |
43.9 (39.0-48.8) |
23.9 (19.3-29.1) |
95.8 (91.6-98.0) |
1.6 (1.4-1.8) |
0.2 (0.1-0.4) |
| ≥10 | 49.4 | 85.2 (75.2-91.8) |
57.6 (52.7-62.4) |
28.2 (22.7-34.3) |
95.2 (91.6-97.4) |
2.0 (1.7-2.3) |
0.3 (0.2-0.6) |
| ≥20 | 34.7 | 74.1 (62.9-82.9) |
73.0 (68.4-77.2) |
34.9 (27.9-42.6) |
93.5 (90.1-95.8) |
2.7 (2.2-3.4) |
0.4 (0.2-0.5) |
| ≥50 | 18.5 | 55.6 (44.1-66.5) |
88.7 (85.1-91.5) |
48.9 (38.4-59.5) |
91.1 (87.8-93.6) |
4.9 (3.5-6.8) |
0.5 (0.4-0.6) |
| ≥100 | 9.3 | 34.6 (24.6-46.0) |
95.7 (93.1-97.3) |
60.9 (45.4-74.5) |
88.2 (84.8-91.0) |
8.0 (4.6-13.7) |
0.7 (0.6-0.8) |
| ≥200 | 5.0 | 27.2 (18.1-38.4) |
99.3 (97.7-99.8) |
88.0 (67.7-96.8) |
87.5 (84.1-90.3) |
37.6 (11.5-122.6) |
0.7 (0.6-0.8) |
| ≥300 | 3.2 | 18.5 (11.1-21.0) |
99.8 (98.5-100) |
93.8 (67.7-97.7) |
86.3 (82.7-89.1) |
76.9 (10.3-573.7) |
0.8 (0.7-0.9) |
| ≥400 | 2.0 | 12.3 (6.4-22.0) |
100 (98.9-100) |
100 (65.5-100) |
85.4 (81.9-88.3) |
∞ | 0.9 (0.9-1) |
PPV = positive predictive value.
NPV = negative predictive value
We next assessed the utility of CRP to identify cases of TB. As the CRP threshold was increased from ≥1 mg/L to ≥400 mg/L, the positive predictive value gradually increased from 17.9% to 100%. However, the highest threshold (≥400 mg/L) included only 2.0% of total patients screened and 12.3% of all TB cases (Table 3).
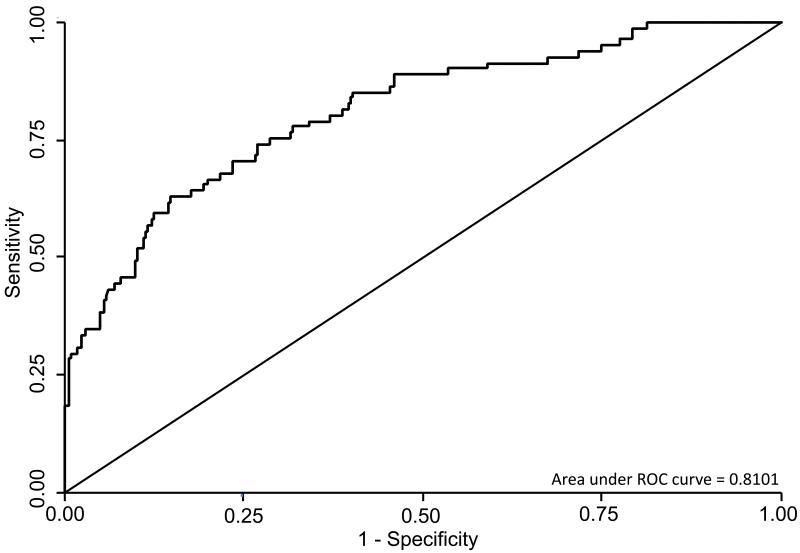
To further explore the diagnostic utility of CRP and the relationship with symptoms, we used receiver operator curve analyses (Figure 2). The area under the curve (AUC) was 0.81 when all patients were included and was similar when the analysis was restricted to patients with a positive WHO symptom screen (n=344; AUC=0.80). A higher AUC was observed when analysis was restricted to patients who reported chronic cough of at least 2 weeks duration (n=102; AUC=0.85).
Figure 2.
Receiver operator curve (ROC) plot showing the relationship between sensitivity and specificity when using C-reactive protein (CRP) as a diagnostic screening test for tuberculosis among patients (n=496) prior to antiretroviral therapy.
Prognostic value of CRP
We next explored the utility of CRP as a prognostic marker in those patients with culture-confirmed TB (n=81). We compared the characteristics of patients with high and low CRP concentrations, using a cut-off of 50 mg/L, which approximated to the median value (Table 4). Patients with high CRP had worse prognostic characteristics, with lower body mass index, lower haemoglobin, lower blood CD4 cell count, higher plasma HIV load and more advanced WHO clinical stage. They were also likely to report of chronic cough.
Table 4.
Characteristics of patients with tuberculosis and either high (≥50 mg/L) or low (<50 mg/L) serum C-reactive protein (CRP) concentration.
| All Patients with tuberculosis (n=81) |
High CRP (≥50 mg/L) (n=45) |
Low CRP (<50 mg/L) (n=36) |
P-value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, median (IQR) | 32.9 (28.5-39.3) | 33.4 (27.1-40.7) | 31.6 (28.9-37.9) | 0.97 |
| Female (%) | 51 (63.0) | 28 (62.2) | 23 (63.9) | 0.88 |
| BMI, median (IQR) | 21.2 (19.3-26.0) | 20.1 (18.0-22.9) | 22.2 (20.9-27.9) | <0.001 |
| History of previous TB | 16 (19.8) | 7 (15.6) | 9 (25.0) | 0.29 |
| Blood Testsa | ||||
| Hemoglobin (g/dL) | 10.8(8.8-12.3) | 9.1 (8-11) | 11.8 (10.9-13.2) | <0.001 |
| White blood cell count (cells/μL) |
5.5 (4.6-7.8) | 5.7 (4.6-9.7) | 5.4 (4.4-6.6) | 0.27 |
| Absolute neutrophil count (×109 cells/L) |
3.3 (2.4-5.0) | 4.1 (2.9-7.0) | 2.9 (2.0-3.5) | 0.002 |
| ALT (iu/L) | 21 (14-32) | 23 (14-43) | 19.5 (13-31) | 0.25 |
| Platelets (×109/L) | 288 (219-382) | 311 (228-441) | 261 (219-359) | 0.14 |
|
CD4 cell countb
(cells/μl) |
||||
| Median (IQR) | 138 (63-205) | 89 (30-146) | 187 (133-229) | <0.001 |
| CD4 <50 | 18 (22.2) | 17 (37.8) | 1 (2.8) | |
| CD4 50-99 | 11 (13.6) | 6 (13.3) | 5 (13.9) | |
| CD4 100-149 | 16 (19.8) | 11 (24.4) | 5 (13.9) | <0.001 |
| CD4 150-199 | 12 (14.8) | 2 (4.4) | 10 (27.8) | |
| CD4 ≥200 | 24 (29.6) | 9 (20.0) | 15 (41.7) | |
| Log viral load (copies/ml), Median (IQR) |
4.8 (4.4-5.3) | 4.9 (4.7-5.5) | 4.7 (4.3-5.0) | 0.011 |
| WHO stage at enrolment | ||||
| 1 or 2 | 45 (55.6) | 17 (37.8) | 28 (77.8) | <0.001 |
| 3 or 4 | 36 (44.4) | 28 (62.2) | 8 (22.2) | |
| WHO symptom screen (any cough, fever, night sweats or weight loss) |
67 (82.7) | 40 (88.9) | 27 (75.0) | 0.100 |
| Current cough ≥ 2 weeks | 21 (25.9) ts. | 16 (35.6) | 5 (13.9) | 0.027 |
| a Blood tests available for 77 patients. |
To explore the relationship between CRP concentration and mycobacterial load, we compared the results of microbiological assays for TB in the high and low CRP patient groups (Table 5). It was striking that sputum samples from the high CRP group were far more likely to test positive by smear microscopy or using Xpert MTB/RIF. The time to culture positivity of sputum was also significantly shorter. In addition, urine samples from patients with high CRP values were also more likely to test positive using the Xpert MTB/RIF assay and the LAM antigen ELISA. Taken together, these data strongly suggest that patients with higher CRP concentrations have higher mycobacterial load and were more likely to have disseminated disease. In marked contrast, there was no association between CRP levels and radiological extent of disease.
Table 5.
Results of investigations in patients with tuberculosis (TB) and either high (≥50 mg/L) or low (<50 mg/L) serum C-reactive protein (CRP) concentration.
| Investigations for TB | All Patients (n=81) |
CRP ≥50 mg/L (n=45) |
CRP <50 mg/L (n=36) |
P-value |
|---|---|---|---|---|
| Microbiological tests | ||||
| Sputum smear positive | 23 (28.4) | 17 (37.8) | 6 (16.7) | 0.036 |
| Xpert sputum positive (2 samples) |
56 (69.1) | 39 (86.7) | 17 (47.2) | <0.001 |
| Xpert sputum positive (1 sample) |
47 (58.0) | 34 (75.6) | 13 (36.1) | <0.001 |
| Determine TB-LAM positive |
24 (29.6) | 22 (48.9) | 2 (5.6) | <0.001 |
| Urine Xpert positive | 15 (18.5) | 12 (26.7) | 3 (8.3) | 0.045 |
| Time to culture positivity median IQR (days) |
16 (11-21) | 14 (10-19) | 17 (14-21.5) | 0.026 |
| Radiological features | ||||
| Any radiological abnormality |
59 (75.6) | 34 (79.1) | 25 (71.4) | 0.43 |
| Central abnormality | 21 (26.9) | 15 (34.9) | 6 (17.1) | 0.079 |
| Pleural abnormality | 16 (20.5) | 12 (27.9) | 4 (11.4) | 0.094 |
| Volume loss | 11 (14.1) | 8 (18.6) | 3 (8.6) | 0.32 |
| Cavitation | 2 (2.6) | 2 (4.7) | 0 | 0.50 |
| Parenchymal abnormality (any zones) |
55 (67.9) | 31 (68.9) | 24 (66.7) | 0.83 |
| Parenchymal abnormality (median number of zones) |
2 (0-4) | 2 (0-4) | 2 (0-4) | 0.96 |
Clinical outcomes
The median time between screening and starting TB treatment in those patients with high CRP (>50 mg/L) was substantially shorter than that of patients with lower CRP values (9.5 days [IQR, 8-18] versus 27 days [9-42]; P=0.026), reflecting the greater likelihood of positive microbiological tests triggering treatment prior to culture confirmation. The time to starting ART, though, was similar (median 28 days versus 35 days, respectively; P=0.13). Despite earlier TB treatment, the high CRP group were more likely to die by 3 months of follow-up (11.1% versus 0%, respectively; P=0.062). The CRP concentrations of the 5 patients who died were 76, 131, 237, 353 and 531 mg/L.
DISCUSSION
In this study, we carefully evaluated the predictive value of CRP during routine screening of patients (regardless of symptoms) for HIV-associated TB prior to starting ART in a South African township clinic. Using a rigorous culture-based gold standard for TB diagnosis, we found that very low CRP thresholds had excellent negative predictive value to effectively rule-out TB but this accounted for only a very small minority of patients screened. Similarly very high CRP thresholds had excellent positive predictive values for TB diagnosis, but only a small minority of TB cases could be identified by these means. ROC analysis did not find any substantial improvement in performance when CRP screening was applied only to patients with a positive WHO symptom screen. Thus, overall CRP lacked diagnostic utility as a screening test. CRP, however, had useful prognostic value. Among confirmed TB cases, high CRP values (≥50 mg/L) were strongly associated with poor prognostic clinical features, higher mycobacterial load, an increased frequency of disseminated TB and higher risk of death.
The prevalence of TB in this and other pre-ART cohorts in southern Africa is so high and the presentation so non-specific that there is a strong argument for investigating all patients for TB regardless of symptoms.16,21 Treatment is needed urgently by those with disease to reduce morbidity, mortality and transmission risk.1,2 Conversely, in those without TB, rapid exclusion of TB is also important so that ART can be started without delay. Failure to achieve this can have adverse consequences for the patient. Inadvertently starting ART in patients with undiagnosed TB can trigger ‘unmasking’ TB immune reconstitution disease 22,23 and even death.24 Conversely, delays in ART initiation while patients are being investigated for possible TB may also result in a high mortality cost.25 Even the new highly promising rapid diagnostics such as Xpert MTB/RIF and Determine TB-LAM have limited sensitivity 6,26 and are therefore an imperfect solution. Any rapid means of ruling in or ruling out TB diagnoses might be very helpful and rapid CRP assessment is now possible at the point-of-care 9.
Serum CRP concentrations are known to correlate strongly with the presence of TB in HIV-infected individuals 27,28 and CRP has been reported as having potential utility for excluding TB in HIV-infected patients with negative sputum smears.10,11 However, in this cohort with a high prevalence of culture-positive TB, CRP could only be used to either rule-in or rule-out diagnoses of TB in a very small proportion of patients screened who had extreme values. Thus, only a small minority of patients would benefit from using this test and cost-benefit analysis is very likely to be unfavourable. A key distinction from previous reports on use of CRP screening in South Africa 10,11 is that in both these reports patients were selected for inclusion on the basis of chronic symptoms (cough >2 weeks was reported by 92% of participants). Our data show that use of a two week cough rule would lead to failure to detect three quarters of cases and that this represents an inappropriate screening tool. We conclude that CRP has very limited diagnostic utility in this clinical setting and that use of rapid and specific microbiological assays should be prioritised.
CRP is known to have prognostic value among patients living with HIV and in those with HIV-associated opportunistic infections.12-14 We found in those with HIV-associated TB a very strong correlation between high CRP concentrations, poor prognostic features and risk of death. CRP synthesis in the liver is immunologically mediated via interleukin-6 (IL-6) production by macrophages.8 Thus, theoretically, high CRP concentrations could arise from an intense immune response, regardless of pathogen load or alternatively might correlate with high mycobacterial load. This question has not previously been addressed.
By assessing the results of multiple mycobacterial tests done on both sputum and urine samples, it was striking that high CRP correlated with much more frequent and rapid detection of Mycobacterium tuberculosis in clinical samples. These parameters, in turn, reflect mycobacterial load. A total of 15 the patients had direct evidence of disseminated TB, with Mycobacterium tuberculosis bacilli being detected in both sputum and urine samples using culture and/or Xpert MTB/RIF. Of these, 12 (80%) had a CRP concentration ≥50 mg/L. In contrast, CRP was not associated with radiological extent of disease, which poorly reflects mycobacterial load in these patients with advanced immunodeficiency.
Thus, we suspect that the prognostic value of CRP reflects, at least in part, mycobacterial load. It is plausible that higher numbers of bacilli activate greater numbers of macrophages and, in turn, increase secretion of IL-6 thereby upregulating CRP synthesis. A further contributing factor may be the increased risk of sepsis in such patients, evidence of which is common in post-mortem studies of hospitalized patients with HIV-associated TB.29 The slightly higher neutrophil counts of patients with high CRP concentrations may reflect this. Additional interventions might be considered for those with high CRP concentrations, including investigation and/or empiric treatment for sepsis and more intensive clinical follow-up.
Strengths of this study include a well characterized cohort of patients who were investigated regardless of symptoms. A rigorous culture-based gold-standard for diagnosis was used. Multiple assays for TB provided insight into mycobacterial load as well as reducing the likelihood of missing any diagnoses of extrapulmonary TB without pulmonary involvement. Prospective follow-up of patients enabled assessment of the prognostic value of CRP. We only assessed the diagnostic value of CRP at a single time-point and it may have additional diagnostic value if measured serially during empiric TB treatment.30 The negative predictive value of the assay would be higher in cohorts with lower TB prevalence and the positive predictive value of high CRP values may be lower in settings where Pneumocystis jirovecii pneumonia, for example, is more common. Thus, performance may differ in other settings.
In conclusion, we found that CRP had very limited diagnostic utility for either rapidly ruling in or ruling out TB in patients systematically screened pre-ART. However, higher CRP concentrations were found to be associated with poorer prognosis and reflected greater mycobacterial load and higher frequency of disseminated TB.
Acknowledgements
SDL was funded by the Wellcome Trust, London, UK. RW was funded in part by the International Epidemiologic Database to Evaluate Aids with a grant from the National Institute of Allergy and Infectious Diseases (NIAID: 5U01AI069924-02); Cost-Effectiveness of Preventing AIDS Complications (CEPAC) funded by the National Institutes of Health (NIH, 5 R01AI058736-02); USAID Right to Care (CA 674 A 00 08 0000 700) and the South African Centre for Epidemiological Modeling and Analysis (SACEMA).
We are grateful to the Foundation for Innovative New Diagnostics (FIND), Geneva, Switzerland for providing access to the Xpert MTB/RIF assay cartridges with preferential pricing. Alere provided the LAM assays free of charge. None of these sources played any role in the design, conduct, analysis, interpretation or decision to publish these data. We thank sister Pearl Pahlana and the staff of the Hannan Crusaid ART clinic.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare
References
- (1).Lawn SD, Harries AD, Meintjes G, Getahun H, Havlir DV, Wood R. Reducing deaths from tuberculosis in antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2012 doi: 10.1097/QAD.0b013e3283565dd1. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic--when will we act? Lancet. 2010 May 29;375:1906–19. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]
- (3).Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis. 2011;204(Rpl 4):S1159–S1167. doi: 10.1093/infdis/jir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Boehme CC, Nabeta P, Hillemann D, et al. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Lawn SD, Brooks SV, Kranzer K, et al. Screening for HIV-Associated Tuberculosis and Rifampicin Resistance before Antiretroviral Therapy Using the Xpert MTB/RIF Assay: A Prospective Study. PLoS Med. 2011;8:e1001067. doi: 10.1371/journal.pmed.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2012;12:201–9. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Peter JG, Theron G, Smit R, et al. Diagnostic accuracy of a urine LAM strip-test for TB detection in HIV-infected hospitalised patients. Eur Respir J. 2012;7:e39966. doi: 10.1183/09031936.00201711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- (9).Engel MF, Paling FP, Hoepelman AI, van dM V, Oosterheert JJ. Evaluating the evidence for the implementation of C-reactive protein measurement in adult patients with suspected lower respiratory tract infection in primary care: a systematic review. Fam Pract. 2012;29:383–93. doi: 10.1093/fampra/cmr119. [DOI] [PubMed] [Google Scholar]
- (10).Alvarez GG, Sabri E, Ling D, Cameron DW, Maartens G, Wilson D. A model to rule out smear-negative tuberculosis among symptomatic HIV patients using C-reactive protein. Int J Tuberc Lung Dis. 2012;16:1247–51. doi: 10.5588/ijtld.11.0743. [DOI] [PubMed] [Google Scholar]
- (11).Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS ONE. 2011;6:e15248. doi: 10.1371/journal.pone.0015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sage EK, Noursadeghi M, Evans HE, et al. Prognostic value of C-reactive protein in HIV-infected patients with Pneumocystis jirovecii pneumonia. Int J STD AIDS. 2010;21:288–92. doi: 10.1258/ijsa.2010.009551. [DOI] [PubMed] [Google Scholar]
- (13).Drain PK, Kupka R, Msamanga GI, Urassa W, Mugusi F, Fawzi WW. C-reactive protein independently predicts HIV-related outcomes among women and children in a resource-poor setting. AIDS. 2007;21:2067–75. doi: 10.1097/QAD.0b013e32826fb6c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–46. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- (16).Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG, Wood R. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. AIDS. 2010;24:1323–8. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lawn SD, Kerkhoff AD, Pahlana P, Vogt M, Wood R. Diagnostic yield of tuberculosis using sputum induction in HIV-positive patients before antiretroviral therapy. Int J Tuberc Lung Dis. 2012 doi: 10.5588/ijtld.12.0174. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- (19).Dawson R, Masuka P, Edwards DJ, et al. Chest radiograph reading and recording system: evaluation for tuberculosis screening in patients with advanced HIV. Int J Tuberc Lung Dis. 2010;14:52–8. [PMC free article] [PubMed] [Google Scholar]
- (20).Lawn SD, Kerkhoff AD, Vogt M, Wood R. High diagnostic yield of tuberculosis from screening urine samples from HIV-infected patients with advanced immunodeficiency using the Xpert MTB/RIF assay. J Acquir Immune Defic Syndr. 2012;60:289–94. doi: 10.1097/QAI.0b013e318258c6af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bassett IV, Wang B, Chetty S, et al. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 2010;51:823–9. doi: 10.1086/656282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–5. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Breen RA, Smith CJ, Cropley I, Johnson MA, Lipman MC. Does immune reconstitution syndrome promote active tuberculosis in patients receiving highly active antiretroviral therapy? AIDS. 2005;19:1201–6. doi: 10.1097/01.aids.0000176221.33237.67. [DOI] [PubMed] [Google Scholar]
- (24).Lawn SD, Wainwright H, Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophages. AIDS. 2009;23:143–5. doi: 10.1097/QAD.0b013e32831d2a98. [DOI] [PubMed] [Google Scholar]
- (25).Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Lawn SD, Kerkhoff AD, Vogt M, Ghebrekristos Y, Whitelaw A, Wood R. Characteristics and Early Outcomes of Patients With Xpert MTB/RIF-Negative Pulmonary Tuberculosis Diagnosed During Screening Before Antiretroviral Therapy. Clin Infect Dis. 2012;54:1071–9. doi: 10.1093/cid/cir1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lawn SD, Wiktor S, Coulibaly D, Ackah AN, Lal RB. Serum C-reactive protein and detection of tuberculosis in persons co-infected with the human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2001;95:41–2. doi: 10.1016/s0035-9203(01)90328-1. [DOI] [PubMed] [Google Scholar]
- (28).Hosp M, Elliott AM, Raynes JG, et al. Neopterin, beta 2-microglobulin, and acute phase proteins in HIV-1-seropositive and -seronegative Zambian patients with tuberculosis. Lung. 1997;175:265–75. doi: 10.1007/pl00007573. [DOI] [PubMed] [Google Scholar]
- (29).Martinson NA, Karstaedt A, Venter WD, et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS. 2007;21:2043–50. doi: 10.1097/QAD.0b013e3282eea47f. [DOI] [PubMed] [Google Scholar]
- (30).Lawn SD, Obeng J, Acheampong JW, Griffin GE. Resolution of the acute-phase response in West African patients receiving treatment for pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:340–4. [PubMed] [Google Scholar]