Abstract
The prefrontal cortex (PFC) plays a central role in preparatory and anticipatory attentional processes. To investigate whether subregions of the PFC play differential roles in these processes we investigated the effect of focal lesions to either lateral prefrontal (lateral PFC; n=11) or orbitofrontal cortex (OFC; n=13) on the contingent negative variation (CNV), an electrophysiological index of preparatory brain processes. The CNV was studied using a Go/NoGo delayed response task where an auditory S1 signaled whether or not an upcoming visual S2 was a Go or a NoGo stimulus. Neither early (500–1000 ms) nor late (3200–3700 ms) phase Go trial CNV amplitude was reduced for any of the patient groups in comparison to controls. However, the lateral PFC group showed enhanced Go trial early CNV and reduced late CNV Go/NoGo differentiation. These data suggests that normal orienting and evaluation as reflected by the CNV is intact after OFC lesions. The enhanced early CNV after lateral PFC damage may be due to failure in inhibition and the reduced late CNV difference wave confirms a deficit in preparatory attention after damage to this frontal subregion.
Keywords: Event-related potentials, Contingent negative variation (CNV), Lateral prefrontal cortex, Orbitofrontal cortex
1. Introduction
The ability to use past or present information in order to guide anticipation of future events is crucial for adaptive behavior in humans. This capacity enables preparation for upcoming events, aiding the choice of appropriate behavioral responses. Anticipatory attention contributes to efficient cognition and behavior by facilitating activation of the sensory and motor brain areas needed to execute an appropriate response at the correct time (Brunia, 1999; Fassbender et al., 2006; Gomez et al., 2001, 2003). The prefrontal cortex (PFC) plays a central role in anticipatory behavior, by exerting top-down control on motor and sensory areas through activation of cortico-cortical and thalamo-cortical circuits (Brunia, 1999). Few studies have, however, specifically addressed the critical role of subregions of PFC for preparatory processes (Basile et al., 1994; Rosahl and Knight, 1995).
Event-Related Potentials (ERPs) are well suited to study preparatory brain processes as they allow direct assessment of brain electrical activity. Furthermore, the millisecond time resolution allows for temporally precise separation of preand post-stimulus activity (Naatanen and Picton, 1987). The contingent negative variation (CNV) is an extensively studied ERP component associated with neural preparatory processes. This slow surface negative potential occurs between two stimuli when the first stimulus (S1) signals information on how to respond to a second stimulus (S2) (Walter et al., 1964). When the interval between the two stimuli is sufficiently long, an early and a late CNV can be identified (Loveless and Sanford, 1974a; Rohrbaugh et al., 1976; Weerts and Lang, 1973). Whereas the amplitude of the early CNV usually peaks about 400–800 ms after S1-onset the late CNV peaks at the time of S2 presentation. The early CNV has a frontal scalp distribution and is thought to reflect an orienting response to S1 (Loveless and Sanford, 1974b; Rohrbaugh et al., 1976). Two related components are typically recorded during S1–S2 tasks; a centroparietal P300 with a latency typically around 300 ms post-S1 and a posterior positive slow wave with a peak latency between those of the P300 and the early CNV. Together, these three components constitute an orienting response to S1 (Kok, 1978). The early CNV additionally reflects more specific stimulus processing or evaluation of information contained in S1 (Gomez et al., 2001; McCarthy and Donchin, 1978). The late CNV has a frontocentral topography and has been linked to cognitive anticipation and motor preparation (Brunia and van Boxtel, 2001). In line with this, it has been suggested that the late CNV reflects a preactivation of the neural resources needed for sensory analysis and response to S2 (Brunia, 1999; Gomez et al., 2001, 2003). The late CNV is also considered a correlate of controlled attentional effort during the expectancy period (Brunia and van Boxtel, 2001; Gomez et al., 2007).
Some CNV-studies have applied a Go/NoGo paradigm, where S1 usually delivers Go/NoGo information, whereas S2 signals that the Go trial response should be given. Specifically, the Go S1 signals a need for motor preparation while NoGo S1 signals that no preparation for S2 is needed. In healthy subjects, a negative slow wave is elicited in both Go and NoGo trials, but CNV amplitudes are typically smaller in NoGo compared to Go trials (Curry, 1980; Rosahl and Knight, 1995; Rugg et al., 1989).
Current models of attention suggest that attention is supported by neural networks involving the anterior cingulum, lateral prefrontal and parietal cortices (Corbetta and Shulman, 2002; Petersen and Posner, 2012). Several studies have confirmed that networks involving fronto-parietal areas contribute to the generation of the CNV. Gomez et al. (2007) investigated the underlying EEG sources of the CNV and localized them in fronto-parietal network nodes including dorsolateral PFC, premotor cortex, superior parietal cortex and the inferior parietal lobule (Gomez et al., 2007). Functional Magnetic Resonance Imaging (fMRI) studies have shown CNV-related activation of thalamo-cortico-striatal networks including thalamus, caudate and putamen, as well as parietal and several frontal areas such as premotor cortex, anterior cingulum, dorsolateral and orbitofrontal PFC (Fan et al., 2007; Nagai et al., 2004). The involvement of anterior cingulate, supplementary motor (SMA), premotor, sensorimotor and parietal areas in the generation of the CNV has been confirmed by magnetoencephalography studies (Hultin et al., 1996; Ioannides et al., 1994; Liu et al., 1998), and the involvement of orbitofrontal and medial frontoparietal areas by a study using subdural electrodes (Ikeda et al., 1996). Finally, increases in single-unit activity and field potentials have been reported in prefrontal and premotor, but not in post-central areas, of monkeys in the delay period of CNV paradigms (di Pellegrino and Wise, 1991; Gemba et al., 1990). In summary, neuroimaging studies suggest an association between CNV and activation of widespread cortical and subcortical areas, but particularly prefrontal and premotor/motor cortices, supporting the notion that CNV is related to controlled attention and motor preparation (Brunia, 1999).
Lesion studies provide causal information about which areas are not only associated with, but also necessary for, normal CNV generation. The literature on the effects of brain injury on the CNV is, however, confounded by the fact that extent and site of damage typically vary across studies and are often not well defined. For a summary of lesion studies, see Table 1. Most studies report enhancement of Go and/or NoGo trial early CNV in patients compared to healthy subjects (Curry, 1980; Rugg et al., 1989; Segalowitz et al., 1992). However, reduced (Zappoli et al., 2002) or no change (Rosahl and Knight, 1995) in early CNV in patients compared to controls has also been reported. The late CNV has been found to be reduced after brain lesions (Rosahl and Knight, 1995; Zappoli et al., 2002). Moreover, reduced amplitude differences between Go and NoGo conditions have been reported (Curry, 1980; Rugg et al., 1989). In studies where the CNV-interval has not been separated into early and late parts, both reduced CNV (Rizzo et al., 1978; Zappoli et al., 2002) and no change in CNV (Low, 1979) have been reported for patients. In summary, brain injury studies of the CNV provide somewhat mixed results. Of note, only the Rosahl and Knight (1995), and Zappoli et al. (2000, 2002) studies specifically investigated the effect of focal lesions to the frontal lobes. Zappoli et al. (2000, 2002) observed that frontal lesions mainly centered in the dorsolateral PFC resulted in reduced or absent early and late CNV amplitudes. However, also post-S1 auditory components were reduced or disrupted. Furthermore, the lesions were extensive, with some covering non-lateral prefrontal and/or extrafrontal regions, thus preventing conclusions on whether the reduced early and late CNV amplitudes observed in the work of Zappoli et al. were a result of lesions solely to the lateral PFC. Thus, Rosahl and Knight provide the only report on the effect of focal lesions to a subregion of the PFC on CNV amplitude. They showed that lesions to the dorsolateral PFC resulted in reduced late CNV amplitudes, whereas early CNV amplitudes were unchanged (Rosahl and Knight, 1995).
Table 1.
Studies describing effects of brain injury on the contingent negative variation.
| Main findings | |||||
|---|---|---|---|---|---|
| Study | Lesion etiology (number of patients/controls) |
Design | Early CNV | Late CNV | Not specified/early and late |
| Low (1979) | Tumors (23), cerebral infarctions (8), cerebrovascular insufficiency (4), diffuse atrophy (2), acute subdural hematoma (1) |
Only Go trials |
Not signifycantly reduced over site of lesion |
||
| Controls | ISI: 1.5 s | ||||
| Rizzo et al. (1978) | Traumatic brain injury (TBI) (27) | S2 centered Go/NoGo task |
Overall CNV response attenuated |
||
| Controls (11) | ISI: 1.5 s | ||||
| Curry (1980) | Closed head injury (CHI) (25) Controls (11) |
S1 centered Go/NoGo task |
1/4 of patients: increased in Go and NoGo trials |
1/2: lack of Go/NoGo difference, 1/3: abnormal distribution, 1/12 (auditory task) or 1/5 (visual task): no CNV |
|
| ISI: 1.5 s | |||||
| Rugg et al. (1989) | CHI (20) Controls (20) | S1 centered Go/NoGo task |
Increased in NoGo trials. |
Reduced Go/ NoGo difference |
|
| ISI: 1.5 s | Reduced Go/ NoGo difference |
||||
| Segalowitz et al. (1992) | TBI | Only Go trials |
Enhanced | Reduced | |
| Controls | ISI: 2.3 s | ||||
| Rosahl and Knight (1995) | Etiology: infarction | S1 centered Go/NoGo task |
No change | Reduced over lesioned hemisphere. |
|
| Lesioned area: unilateral dorsolateral PFC (6) |
ISI: 3.0 s | Most prominent effect in Go condition |
|||
| Controls (8) | |||||
| Zappoli et al. (2002) | Etiology: mixed (resection of tumor, lobotomy, resection due to posttraumatic atrophic lesion) |
Only Go trials |
Reduced over ablated area | ||
| Lesioned area: unilateral dorsolateral PFC (9), bilateral dorsomedial (1), unilateral parietotemporal and white matter (1) |
ISI: 2.0 s | ||||
| Controls (10) | |||||
Further clarification of the contribution of distinct brain areas to the generation of the CNV requires lesion studies involving focal, well characterized brain lesions. While the literature points towards lateral PFC as a crucial part of a widespread network contributing to the CNV, the potential differential role of distinct subregions within the PFC has not been described. Critically no studies have addressed the effect of focal orbitofrontal cortex (OFC) lesions.
The main aim of this study was to explore the effects of lesions to the OFC and the lateral PFC on orienting, stimulus evaluation and preparatory attention in a Go/NoGo variant of the classical S1–S2 CNV paradigm. In line with prior reports, we predicted attenuated late CNV amplitude following lateral PFC lesions. Less is known about the role of the OFC in anticipatory attention and response preparation. Although a few studies have reported OFC activity during CNV-generation (Ikeda et al., 1996; Nagai et al., 2004) it is currently not known whether this area is a necessary network node or modulates the CNV. Regarding the early CNV, studies with varied lesion locations due to traumatic head injury suggest injury-related amplitude increases, while the only study including focal lateral PFC lesions found no change in amplitude (Rosahl & Knight, 1995). Taken together, the extant literature did not allow for strong predictions regarding the effects of lateral PFC or OFC lesions on the early CNV. With regard to the late CNV, we hypothesized that lateral PFC lesions would produce attenuated CNV amplitude and reduced differentiation between Go and NoGo trials. Contrarily, we did not expect OFC lesions to influence the late CNV.
2. Results
2.1. Behavioral performance
All three groups had a high hit rate in Go trials (controls: 99.2 (1.0)%, OFC: 98.3 (1.7)%, and lateral PFC: 97.9 (2.0)%) and committed few false alarms in NoGo trials (controls: 5.0 (6.7)%, OFC: 3.1 (4.0)%, and lateral PFC: 4.2 (4.0)%). There were no statistically significant differences between groups on hit rate to S2 in Go trials (F(2,39)=2.47, p<0.098) or on false alarms in NoGo-trials (F(2,39)=0.464, p<0.632). The lateral PFC group had longer reaction times (RT) to S2 in Go-trials compared to the control group (p=0.008). There were no significant differences between the OFC and control group or between the two patient groups in reaction time (controls: 441±68 ms, OFC: 471±73 ms, and lateral PFC: 522±49 ms).
2.2. ERP data
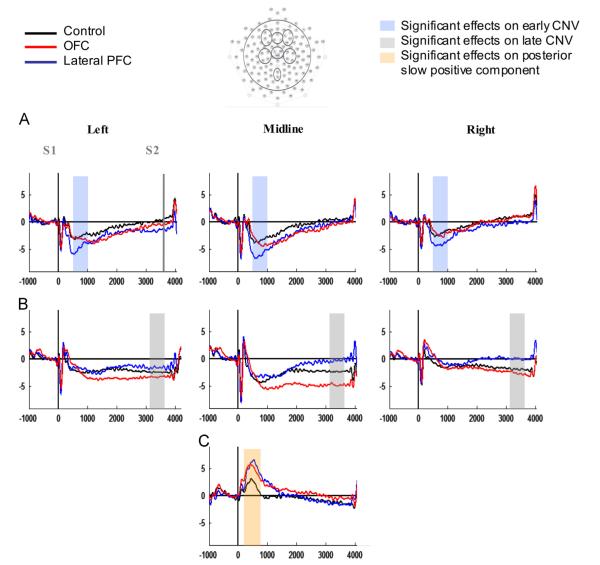
Figs. 4 and 5 show that the overall topographical and temporal characteristics of the CNV were similar across groups. Importantly, however, there were several amplitude differences between the groups at selective regions and time intervals of the S1–S2 interval.
Fig. 4.
Go condition grand average ERPs of the three groups over (A) frontal, (B) central, and (C) parietal electrode groups. The x-axes represent ms in relation to S1-onset and the y-axes represent amplitude in μV. Data were filtered with a 10 Hz lowpass for illustration purposes.
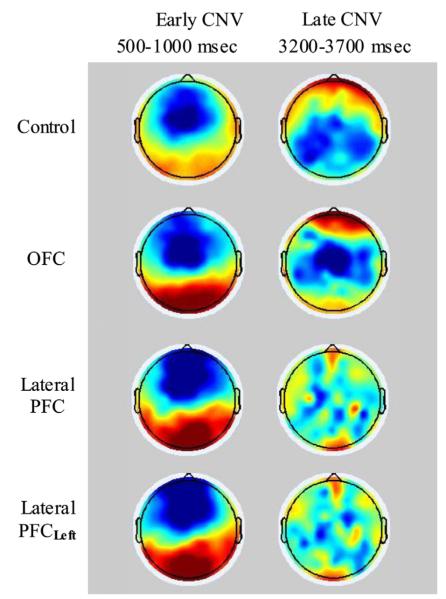
Fig. 5.
Topoplots of the early and late CNV components for the three groups. Data were filtered with a 10 Hz lowpass for illustration purposes. Lateral PFCLeft represent the lateral PFC group when right and left electrodes are exchanged for the patients with right hemisphere lesions so that for the all the lateral PFC patients left hemisphere electrodes are synonymous with lesioned hemisphere.
2.2.1. N1 amplitude
There were no significant differences between the groups in N1 amplitude (F(2, 37)=0.761, p=0.474, η2=0.040), supporting comparable early auditory sensory processing. In all Groups N1 amplitudes were larger over central than frontal regions (Frontal–Central: F(1, 37)=4.746, p=0.036, η2=0.114), and over midline compared to lateral regions (Laterality: F(2, 74)=44.905, p<0.001, η2=0.548). These effects remained significant when the analysis was rerun with left ROIs representing lesioned hemisphere for the lateral PFC group.
2.2.1.1. Early CNV amplitude
A main effect of Frontal–Central sites (F(1,37)=12.85, p<0.001, η2=0.258), reflected larger early CNV negativity over frontal compared to central regions, and a main effect of Laterality (F(2,74)=42.45, p<0.001, η2=0.534) indexed largest negativity over the midline across groups (see Table 4 for mean early CNV amplitude per group and ROI). There was no significant main effect of Group in the overall analysis (F(2,37)=0.74, p=0.483, η2=0.039), but a significant interaction between Frontal–Central and Group reflected that the lateral PFC group had enhanced frontal early CNV amplitudes compared to the control group (p=0.016) regardless of Laterality. There was no significant difference between the OFC group and healthy controls, but a trend toward larger early CNV amplitudes for the lateral PFC group compared to the OFC group (p=0.059) over the frontal scalp. The significance/non-significance of the effects remained when the analyses were rerun with left ROIs representing lesioned hemisphere for the lateral PFC group. Entering Total, Verbal and Performance IQ as covariates did not change the effect involving the lateral PFC group.
Table 4.
Early and late CNV amplitudes (SD) over frontal and central regions of interest. Lateral PFCLeft represent the amplitudes of the lateral PFC group when left and right ROIs are exchanged for the patients with right hemisphere lesions, in order for left ROIs to represent lesioned hemisphere for all the lateral PFC patients.
| Control | OFC | Lateral PFC | Lateral PFCLeft | |||
|---|---|---|---|---|---|---|
| Early CNV | Frontal* | Left | −2.4 (2.0) | −2.8 (2.3) | −4.3 (2.3) | −4.4 (2.3) |
| Midline | −3.1 (1.8) | −3.5 (1.9) | −5.5 (2.5) | −5.5 (2.5) | ||
| Right | −1.9 (1.5) | −2.1 (2.0) | −3.8 (2.4) | −3.6 (2.4) | ||
| Central | Left | −2.1 (1.4) | −2.1 (2.1) | −1.5 (2.4) | −1.6 (3.1) | |
| Midline | −3.7 (1.7) | −4.1 (2.2) | −2.9 (2.1) | −2.9 (2.1) | ||
| Right | −1.2 (1.3) | −0.7 (1.4) | −0.3 (2.5) | −0.2 (1.4) | ||
| Late CNV | Frontal | Left | 0.3 (3.0) | −0.4 (3.2) | −1.4 (2.0) | −0.8 (2.2) |
| Midline | 0.4 (3.6) | −0.3 (1.7) | 0.1 (3.4) | 0.1 (3.4) | ||
| Right | 0.9 (2.9) | 1.0 (3.3) | − 0.0 (2.7) | −0.5 (2.7) | ||
| Central* | Left | −2.2 (2.1) | −3.0 (2.4) | −1.5 (3.3) | −1.4 (4.5) | |
| Midline | −2.2 (3.8) | −4.4 (3.7) | −0.3 (2.5) | −0.3 (2.5) | ||
| Right | −1.8 (1.6) | −2.5 (2.4) | −0.0 (4.7) | −0.0 (3.7) |
Plane with significant main effect of Group.
2.2.1.2. Follow-up analysis on ipsi- versus contralesional effects for the lateral PFC group
The intact PFC has been shown to compensate for damage in the lesioned PFC by increases in electrophysiological activity (Voytek et al., 2010a). To investigate the possibility that the increased early CNV amplitudes of the lateral PFC group of the present study could reflect compensatory activity in the intact hemisphere, we checked for ipsi- versus contralesional effects on the difference in early CNV amplitude between the lateral PFC and the control group. A repeated measures ANOVA was run with Hemisphere, with two levels only (left vs. right), as within-subject factor. The analysis was run with left ROIs representing lesioned hemisphere for the lateral PFC group. A lack of significant interaction between Hemisphere and Group (F(1,25)=0.18, p=0.676, η2=0.007) reflected that there was no influence of hemisphere (intact versus lesioned in the lateral PFC group) on the difference in early CNV amplitude between the lateral PFC group and controls. Thus, the results did not provide evidence for compensatory activity in the intact hemisphere and will not be further discussed.
2.2.1.3. Follow-up analyses on lateral frontal subgroups and BA32 lesion
Due to the inconsistency in results regarding early CNV amplitude between the present study and the Rosahl and Knight (1995) study, follow-up analyses were performed on additional lateral PFC lesion variables. The area of largest lateral PFC lesion overlap was slightly more rostral and dorsal in the present study compared to the Rosahl and Knight study. Additionally, the lesions of a large portion (7/11) of the lateral PFC patients in the present study extended into the dorsal anterior cingulum (BA32), an area not affected in the Rosahl and Knight study. This led to the hypothesis that lesions to the most rostral and/or dorsal parts of lateral PFC or to medial frontal PFC could explain the enhanced early CNV of the lateral PFC group. Accordingly, we explored three variables: rostral–caudal lesion, dorsal–ventral lesion, and BA32 lesion volume. However, few lateral PFC lesions could be classified as only dorsal or ventral, preventing analysis. The rostral–caudal variable was investigated using nonparametric independent samples test (comparison of median) with two between subjects levels, and BA32 volume was explored using Pearson product-moment correlation. The two analyses were performed on the frontal and central midline ROIs. One patient could not be grouped as either rostral or caudal and was not included in that analysis. There were no significant differences in early CNV amplitude between the rostral (N=5) and the caudal (N=5) subgroup of the lateral group (frontal: p<0.885; central: p<0.060). The correlation analysis showed a significant positive correlation between early CNV amplitude at the frontal midline ROI and BA32 lesion volume (r=0.630, p=0.038), indicating the smallest early CNV negativity for the patients with largest dorsal anterior cingulum lesion volume.
2.2.2. Late CNV amplitude
There was no significant main effect of Group in the overall analysis (F(2,37)=1.03, p=0.366, η2=0.053) (see Table 4 for mean late CNV amplitude per group and ROI). It appears from Fig. 4 that over the central midline the late CNV of the lateral PFC group is reduced and the late CNV of the OFC group increased compared to that of the control group. However, there were no significant differences between the lateral PFC group and controls, or between the OFC group and controls. Nevertheless, a significant interaction between Frontal–Central and Group (F(2,37)=4.21, p=0.023, η2=0.185) reflected that over central sites the lateral PFC group had reduced late CNV amplitudes compared to the OFC group (p=0.025). The significance/non-significance of the effects were not changed by analyzing the data with left ROIs representing lesioned hemisphere for the lateral PFC group nor by entering Total, Performance or Verbal IQ as covariates in the analyses.
2.2.3. Posterior slow positive component
Fig. 4C indicates enlarged positive slow components over the parietal ROI, peaking around 500 ms post S1 onset, in both patient groups. A main effect of Group (F(2, 37)=4.07, p=0.025) reflected that there was a significant difference between the control group and the OFC group (p=0.040) in posterior slow positive component amplitude, with the OFC group having a larger positivity than the control group (controls: 2.3 μV, SD=1.8; OFC: 4.6 μV, SD=2.2). There was also a trend (p=0.074) towards larger amplitude for the lateral PFC compared to the control group (controls: 2.3 μV, SD=1.8; LFC: 4.4 μV, SD=3.2).
2.2.4. Difference waves
To examine whether the patient groups had altered ability to differentiate preparatory activity in Go versus NoGo trials, early and late CNV difference waves (DW) were investigated over the midline ROI of the plane that showed a significant group difference in the Go-analysis, that is frontal for early CNV and central for late CNV. There were no significant differences between any of the groups in early CNV-DW. Visual inspection (see Fig. 6B) suggested that the lateral PFC group had reduced late DW, reflecting reduced Go–NoGo difference compared to the other two groups. A significant main effect (F(2, 37)=7.71, p=0.002) confirmed that the lateral PFC group had smaller late DW compared to both the OFC group (p<0.001) and controls (p=0.036) (lateral PFC: −0.1 μV, SD=3.7; OFC: −5.5 μV, SD=3.4; controls: −3.5 μV, SD=2.9). The OFC group and controls did not differ significantly.
Fig. 6.
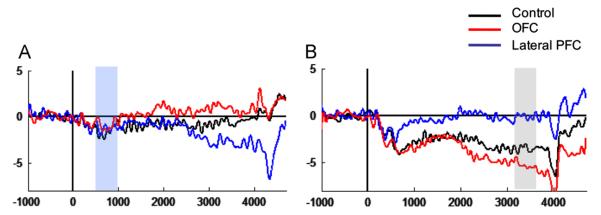
Go minus NoGo difference waves (DW) of the three groups over (A) frontal midline and (B) central midline. The shaded areas illustrate the investigated time interval: The early CNV DW was studied over the frontal midline ROI, the late CNV DW over the central midline ROI. The x-axes represent ms in relation to S1-onset and the y-axes represent amplitude in μV.
Visual inspection of the frontal ROI in Fig. 6 suggests a frontal displacement of the DW-effect in the lateral PFC group. A post hoc one-way ANOVA showed a significant difference between the lateral PFC and the OFC group (p=0.026), but no difference between the lateral PFC group and controls (p=0.218) or between the OFC group and controls (p=0.461) (main effect of group: F(2,37)=3.72, p=0.034). A one-way ANOVA on frontal midline NoGo-amplitude also showed a significant difference between the lateral PFC and OFC groups (p=0.014), but no other significant group differences (lateral PFC vs. controls: p=0.334; OFC vs. controls: p=0.196). The lack of significant difference between the lateral PFC group and controls calls for interpretive caution with regard to the frontal negative DW of the lateral group.
2.2.5. Relationship between reaction time and CNV components
There were no significant within-group correlations between RT in successful Go-trials and early or late CNV. However, in the lateral PFC group there was a tendency toward a significant association between RT and late CNV over the right central ROI (r(11)=0.55, p=0.083). Note that the positive correlation indicates increasing RT with decreasing magnitude of the late CNV. There were no significant within-group correlations between early DW and RT, but some significant associations were seen between late DW and RT. In the OFC group smaller Go/NoGo difference was associated with increased RT over the left frontal ROI (r(13)=0.60, p=0.030). A tendency in the same direction was found over the right central ROI (r(13)=0.52, p=0.071).
3. Discussion
Using a combined behavioral, EEG and lesion approach we addressed the role of two major PFC divisions in preparatory attentional and behavioral control. Unilateral lateral PFC lesions resulted in alterations in the early as well as the late CNV component with enhanced early frontal CNV amplitude and decreased late CNV Go/NoGo differentiation. The OFC group did not differ from the healthy controls on any CNV measures, despite predominantly bilateral extensive lesions. A posterior positive slow wave was, however, increased by lesions to the OFC.
3.1. Orienting and evaluation – early CNV and posterior positive slow wave
The present study demonstrates that lateral PFC lesions resulted in enhanced early CNV amplitude, with no effect of OFC lesions on early CNV amplitude. The lateral PFC amplitude increase was not limited to the lesioned hemisphere. The increased early frontal CNV amplitude of patients with lateral PFC lesions contrasts to previous reports on the effect of focal lesions on the CNV (Rosahl and Knight, 1995; Zappoli et al., 2000, 2002). Zappoli et al. reported that patients with dorsolateral PFC lesions had reduced or absent CNV amplitudes over the ablated cortical area. However, the Zappoli cases had extensive lesions often extending to non-lateral frontal and/or extrafrontal areas. The only previous report describing the consequence of focal lesions to a frontal subregion found no effect of lesions to the dorsolateral PFC on early CNV amplitude (Rosahl and Knight, 1995). The area of largest overlap in the lateral PFC lesions of the present study was more rostral and dorsal than in the Rosahl and Knight study. Further, the lesions of a large portion of our patients with lateral PFC lesions extended into the dorsal anterior cingulum (BA32) not involved in the Rosahl and Knight study. This suggests that the increased early CNV amplitude of the lateral PFC group in the present study could be related to lesions to the most rostral and/or dorsal parts of lateral PFC or to medial frontal lesions. Indeed, the anterior cingulum has been implicated in early CNV generation (Gomez et al., 2003; Nagai et al., 2004; Stuss and Alexander, 2007). However, follow-up analyses of lateral PFC subgroups revealed no significant differences between rostral and caudal subgroups on early CNV amplitude. Potential differences between dorsal and ventral subgroups could not be investigated as few lateral PFC lesions could be grouped as either dorsal or ventral. The follow-up analysis on extent of lesion to anterior cingulum showed that larger lesion was associated with smaller, rather than larger early CNV amplitude. Thus, neither the more rostral location, nor the additional lesion in BA32 of the present study compared to the Rosahl and Knight study can explain the differences in effect of lateral PFC lesion on early CNV in the two studies. The smaller early CNV amplitude with larger BA32 lesion within the lateral PFC group is interesting. Stuss et al. showed that superior medial frontal lesions lead to compromised behavior in various tasks probing energizing of attention and further proposed that the CNV could be a neurophysiological correlate of energizing (Stuss and Alexander, 2007; Stuss et al., 1995). The current correlation provides tentative support for this proposition.
Differences in lesion etiology might also contribute to the observed discrepancies regarding early CNV. In the Rosahl and Knight study all lesions were caused by cerebrovascular infarctions. The patients of the present study had lesions due to tumor resections and had burr holes and breaches in the skull where the craniotomies were performed. This could contribute to enhanced ERP amplitudes due to current shunting (Pfurtscheller et al., 1982; Voytek et al., 2010b). However, we do not believe that skull defects can explain the increased early amplitudes of the lateral group. First, if the amplitude enhancements were a result of gaps in the skull, the effects should not be restricted to a single ERP component. The lateral PFC group had increased early CNV amplitude, but not enhanced N1 amplitude. Further, the early CNV amplitude enhancements were not restricted to the lesioned hemisphere. Moreover, the OFC patients in the current study also had burr holes but did not show increased CNV amplitude.
What does this early CNV increase after lateral PFC damage reflect? Curry (1980) interpreted enhanced early CNV amplitudes for head-injured patients in terms of disinhibition. Indeed, the PFC exerts both inhibitory and excitatory control over primary and association cortices (Herrmann and Knight, 2001; Knight et al., 1999). In line with this the enhanced early CNV negativity of the lateral PFC group in the present study might reflect reduced inhibitory control from the lateral PFC, resulting in alterations of electrophysiological correlates of orienting and stimulus evaluation.
Alternatively, Rösler suggested that the amplitude of slow cortical potentials, including the CNV, reflects the amount of resources allocated to a cognitive process (Rosler et al., 1997). The enhanced early CNV amplitude of the lateral PFC group in the present study might reflect an increased allocation of resources to orienting and stimulus evaluation. Although this hypothesis cannot be directly confirmed by the current study, such an early increase of resource allocation might reflect an early compensation for changes in preparatory attention. Increased attention should be an advantage to behavior, thus increased early CNV amplitude should result in faster reaction times. In the present study there were no significant correlation between reaction time and early CNV amplitude. In summary, the early CNV enhancement might reflect an attentional disinhibition or compensatory adaptation to altered processing.
Our finding of comparable early CNV amplitude in the OFC lesioned group and in healthy controls is in line with limited reports of OFC activity during execution of a S1–S2 task (Ikeda et al., 1996; Nagai et al., 2004). Using subdural electrodes placed over the OFC, Ikeda et al. (1996) observed a CNV in the late, but not the early part of the CNV interval. Nagai et al. (2004) on the other hand, reported that the hemodynamic activity observed in brain areas including the OFC mainly reflected the early CNV. However, the OFC activity was not consistently observed at an individual subject level. Thus, there is no consistent evidence pointing to an OFC involvement in early CNV generation. The present study suggests that normal orienting and S1 evaluation as reflected by the early CNV is possible despite extensive OFC lesions.
OFC lesions did result in enhanced posterior positive slow wave (see Fig. 4C). The relation between early CNV and a posterior positive slow wave is largely undefined, but Kok (1978) suggested that a posterior slow positivity and an early frontal slow negativity (i.e. early CNV) can be conceived as a biphasic potential fluctuation. He further suggested that together with the P300 potential, these ERP components reflect an orienting response to S1. One possibility is that the increased late positivity reflects deficient inhibitory control from OFC over posterior cortical areas. Indeed, lesions to the OFC have been shown to increase amplitudes over posterior brain regions (Hartikainen et al. 2012; Rule et al., 2002). The present results thus show a differential effect of OFC and lateral PFC lesions on two of the ERP components that according to Kok reflect an orienting response to S1, with lesions to the lateral PFC resulting in enhanced frontal slow negativity, and lesions to the OFC resulting in enhanced posterior slow positivity. This suggests that although the slow positive and negative components might both reflect orienting processes, they have at least partly separate generators and might reflect partly different subprocesses within the broad concept of orienting. Contrary to a hypothesis of partly different sources for the early CNV and posterior positivity, Flores et al. (2009) showed that an early anterior negativity and a posterior positivity observed in the CNV period have one common posterior source in children (Flores et al., 2009). In line with this, van Leeuwen et al. (1998) found posterior sources only for the CNV/P3 complex. This does not necessarily imply one single source also in the adult brain. Due to the late maturation of several brain structures, and the PFC in particular (Casey et al., 2000), the neural substrate of CNV and late positivity waveforms might differ between children and adults. Indeed, in the Flores et al. study, the posterior positivity was not observed in young adults. Alternatively, the single source finding in that study could be attributed to the fact that children have smaller brains and possibly lower skull electrical resistance. Thus, dipoles could possibly expand their influence along the anterior–posterior axis. Also, Gomez et al. (2003) found LORETA-activations in several frontal as well as posterior regions during the early CNV in healthy adults, providing evidence against a single posterior source for the early CNV alone and thus also for the early CNV and posterior positivity together (Gomez et al., 2003).
3.2. Preparation and anticipation – late CNV
The lateral PFC group had no observable late CNV over midline and right hemisphere scalp sites. Altered preparatory processing for the lateral PFC group was supported by the finding of reduced Go/NoGo difference in the late CNV interval compared to both the control group and the OFC group (see Fig. 6B). The control group DW showed negative amplitude, indicating larger amplitude in the Go compared to the NoGo condition, as has previously been reported for this group (Funderud et al., 2012). OFC lesions did not alter DW amplitude. Reduced Go/NoGo differentiation for the lateral PFC group indicates that contrary to healthy controls and OFC patients, this group did not display an enhanced magnitude of preparatory brain processes when response preparation was called for. Reduced CNV amplitude difference between Go and NoGo trials has been observed previously in head-injured patients (Curry, 1980; Rugg et al., 1989) who typically sustain PFC damage. A reduced Go/NoGo DW indicates a deficit in prioritizing attentional resource allocation according to stimulus relevance.
Increased RT in successful Go-trials also characterized the lateral PFC group. Variable or prolonged RTs are commonly observed in patients with lateral PFC lesions (Barcelo and Knight, 2007; Chao and Knight, 1998; Stuss et al., 2003). There was a trend towards a positive correlation between right hemisphere central late CNV amplitude and RT for the lateral PFC patients. Increasing RT with decreasing late CNV amplitude has previously been demonstrated after dorsolateral PFC lesions (Rosahl and Knight, 1995) as well as in healthy subjects (Wascher et al., 1996). We speculate that deficient response preparation, as indexed by the reduced late CNV amplitudes in the Rosahl and Knight study, and reduced late DW in the present study, is expressed behaviorally in slowed RT following lateral frontal injury.
Thus, although lesions to the lateral PFC result in enhanced engagement of neural resources for stimulus orienting and evaluation, the subsequent response preparation does not appear to benefit from this as the resources allocated to preparation are no greater in Go compared to NoGo trials. This could suggest an overall disturbed allocation of attention, consistent with an important role for the lateral PFC in attentional control (Posner and Petersen, 1990).
We are only aware of one neuroimaging study reporting OFC activity associated with the late CNV during performance of a S1–S2 task (Ikeda et al., 1996). Studies of the effect of TBI on CNV amplitude, however, have demonstrated reduced late CNV (Curry, 1980; Rizzo et al., 1978; Segalowitz et al., 1992). Together with the anterior temporal cortex, the OFC is the most prevalent location of lesions after TBI (Mattson and Levin, 1990). The head-injury literature could thus indicate an involvement of OFC in late CNV generation. However, TBI patients also frequently suffer from diffuse axonal injury, and typically present with multifocal frontal as well as extrafrontal lesions (McAllister, 2011). As a result it is difficult to ascribe the late CNV reduction after TBI to OFC damage only. The lack of difference in late CNV amplitude between the OFC group and controls of the present study suggests that intact OFC is not a prerequisite for late CNV generation.
3.3. Conclusion
The findings of this study suggest regional functional specificity within the PFC in preparatory processes. Increased early CNV and a diminished late CNV Go/NoGo differentiation support previous proposals of a critical role for the lateral PFC in CNV generation. The study extends previous knowledge in showing that intact OFC is not a prerequisite for the generation of the CNV complex. However, the OFC group had an increased posterior positive slow wave. The early CNV and positive slow wave findings suggest that lateral PFC and OFC lesions result in disinhibition effects in distinct aspects of attentional orienting. In conclusion, the present data demonstrate a restricted effect of OFC lesions and more general effect of lateral PFC lesions in the neural preparatory processes indexed by the CNV.
4. Experimental procedure
4.1. Participants
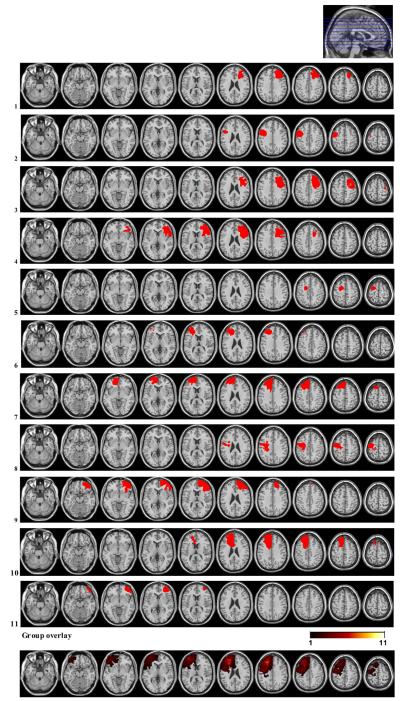
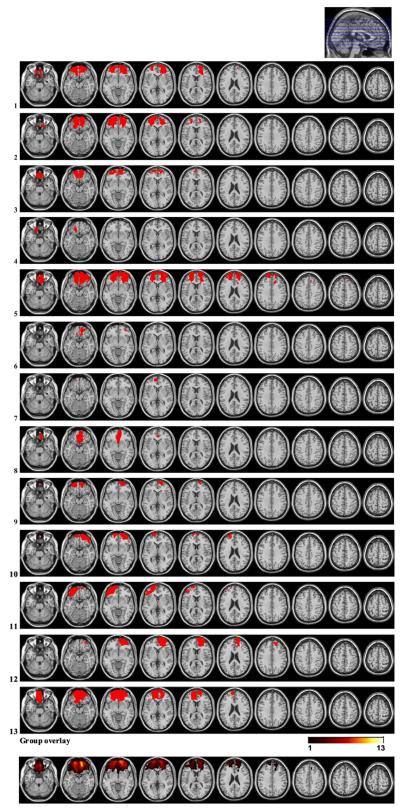
Twenty-four patients with focal frontal lesions and 16 healthy controls were included in the study. The OFC group consisted of 13 patients, 10 with bilateral and 3 with unilateral lesions. In 10 of the OFC patients, lesions were caused by resection of primary cerebral tumor, and in 3 by traumatic brain injury (TBI). The lateral PFC group consisted of 11 patients with unilateral lesions; 5 in the right, and 6 in the left hemisphere, all due to low grade glioma resections. None of the patients had received radiation therapy, whereas one patient in the lateral PFC group had undergone chemotherapy (see Table 2, Figs. 1 and 2 for lesion characteristics). Healthy controls were recruited by advertisement and personal contact. Except for one left-handed subject in each group, all participants were right-handed.
Table 2.
Lesion characteristics. Etiology, months post-injury, lesion size, and affected Brodmann Areas (BA).
| Subject | Etiology | Months post-injury | Lesion size (ccm) | BA left hemisphere | BA right hemisphere |
|---|---|---|---|---|---|
| OFC group mean | Total: 47.0 | ||||
| RH: 25.3 | |||||
| LH: 21.7 | |||||
| 1 | Meningioma | 13 | 69.1 | 10, 11, 47 | 10, 11, 32, 46, 47 |
| 2 | Meningioma | 49 | 79.8 | 10, 11, 46, 47 | 10, 11, 47 |
| 3 | Meningioma | 13 | 39.7 | 10, 11, 47 | 10, 11 |
| 4 | Meningioma | 19 | 5.1 | 11 | |
| 5 | Meningioma | 43 | 134.8 | 9, 10, 11, 32, 46, 47 | 9, 10, 11, 32, 46, 47 |
| 6 | Meningioma | 27 | 7.2 | 11 | 11, 47 |
| 7 | Meningioma | 44 | 2.9 | 10, 11 | |
| 8 | LGG | 7 | 28.6 | 11, 25 | 10, 11, 25 |
| 9 | TBI | 44 | 23.6 | 11 | 10, 11 |
| 10 | TBI | 59 | 33.3 | 10, 11, 46 | 10, 11, 47 |
| 11 | TBI | 15 | 41.1 | 10, 11, 38, 45, 46, 47 | 11 |
| 12 | Meningioma | 52 | 48.7 | 9, 10, 11, 32, 46, 47 | |
| 13 | Meningioma | 20 | 96.8 | 10, 11, 24, 25, 32, 47 | 10, 11, 47 |
| Lateral PFC group mean | Total: 46.5 | ||||
| RH: 52.1 | |||||
| LH: 41.9 | |||||
| 1 | LGG | 30 | 34.4 | 8, 9, 32, 44, 45, 46 | |
| 2 | LGG | 27 | 24.8 | 4, 6, 9, 44 | |
| 3 | LGG | 68 | 60.1 | 4, 6, 8, 9, 32, 44, 45, 46 | |
| 4 | LGG | 112 | 72.8 | 6, 9, 32, 44, 45, 46, 47 | |
| 5 | LGG | 9 | 10.1 | 6 | |
| 6 | LGG | 54 | 25.0 | 9, 10, 32, 45, 46, 47 | |
| 7 | LGG | 6 | 77.5 | 6, 8, 9, 10, 11, 24, 32, 46, 47 | |
| 8 | LGG | 104 | 39.9 | 3, 4, 6 | |
| 9 | LGG | 93 | 74.1 | 9, 10, 11, 32, 45, 46, 47 | |
| 10 | LGG | 18 | 74.0 | 6, 8, 9, 10, 24, 32, 46 | |
| 11 | LGG | 14 | 18.9 | 10, 11, 45, 46, 47 | |
Lesions that comprise <0.5 ccm in any given Brodmann Area are not reported. BA=Brodmann Area, RH=right hemisphere, LH=left hemisphere; TBI=traumatic brain injury; LGG=low-grade glioma.
Fig. 1.
Lesion reconstructions of the lateral PFC group. Individual patients (1-11) and group overlay (bottom row). Note that in the group overlay all lesions are portrayed as left-sided. The color code for the group overlay indicates the number of patients with damaged tissue in that area.
Fig. 2.
Lesion reconstructions for the OFC group. Individual patients (1-13) and group overlay (bottom row). The color code for the group overlay indicates the number of patients with damaged tissue in that area.
Patient inclusion was based on presence of focal frontal lobe lesion as indicated on pre-existing structural computer tomography (CT) and/or MRI scans. Inclusion took place at least 6 months after injury or surgery. Table 3 shows that the patient groups were comparable to the healthy control group with regards to age, sex and years of education. Total, Verbal and Performance IQ was estimated based on all 4 subtests of the Wechsler Abbreviated Scale of Intelligence, WASI (Wechsler, 1999). Although the lateral PFC group had lower Total, Performance and Verbal IQ than the control group, and the OFC group had lower Verbal IQ than the control group, all groups had IQ scores within the normal range (see Table 3). Participants with a history of serious psychiatric disease, drug or alcohol abuse requiring treatment, premorbid head injury, pre-/comorbid neurological disease, IQ below 85, substantial aphasia, visual neglect or marked sensory impairment were excluded from participation. Moreover, acceptable signal-to-noise ratio in the EEG recording was a prerequisite for inclusion.
Table 3.
Subject characteristics.
| Post hoc test p-values |
|||||||
|---|---|---|---|---|---|---|---|
| CTR | OFC | lPFC | ANOVA | CTR/OFC | CTR/ lPFC | OFC/lPFC | |
| N (% female) | 16 (38) | 13 (54) | 11 (45) | ||||
| Age in years | 42.6 (12.2) | 49.3 (7.3) | 47.0 (9.1) | ns | |||
| Education in years | 13.2 (2.5) | 12.9 (2.4) | 12.9 (2.2) | ns | |||
| Total IQ | 114.4 (7.4) | 106.1 (12.0) | 101.1 (11.2) | 0.006 | ns | 0.005 | ns |
| Performance IQ | 114.1 (8.4) | 109.5 (13.3) | 102.1 (9.1) | 0.020 | ns | 0.015 | ns |
| Verbal IQ | 110.6 (8.1) | 101.0 (10.4) | 100.5 (13.8) | 0.026 | 0.054 | 0.054 | ns |
Values given are means (±Standard Deviation). CTR=control, lPFC=lateral PFC.
Patients and controls gave written informed consent to participation. Controls were paid 500 NOK (approximately 80 USD) for participation in the entire research program which included neuropsychological assessment, EEG-recording, as well as structural and functional MRI examination. The study was approved by the Regional Committee for Medical Research Ethics, Region South, and was conducted in agreement with the Declaration of Helsinki.
4.2. Lesion reconstruction
Lesion reconstructions were based on structural MRIs obtained after study inclusion. Lesions were outlined by drawing manually on Fluid Attenuated Inversion Recovery (FLAIR) images of each participant’s brain using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/). High-resolution T2-weighted images were used as aids to determine the borders of the lesions. The resulting lesion masks were transferred to normalized space using the Statis tical Parametric Mapping software (SPM.5: http://www.fil.ion.ucl.ac.uk/spm/). Individual participant lesion mask, T1 and FLAIR images were first coregistered to a template T1 image and the resulting transformation parameters subsequently applied to the lesion mask. Lesions were reconstructed under the supervision of a neuroradiologist (PDT), a neurologist (RTK), and a neurosurgeon (TRM). Involved Brodmann areas and lesion volume were calculated using MRIcron.
4.3. Experimental task
The participants were seated 100 cm in front of a computer screen. In each trial, they were presented with a 250 ms binaurally presented tone (S1). After a delay of 3.5 s a white circle (S2) was centrally presented for 250 ms. See Fig. 3A for a schematic presentation of the paradigm. The participants were instructed to press a response button when they saw the circle if S1 was a tone of high pitch (1500 Hz) only, constituting the Go-condition. They were told not to press if S1 was a tone of lower pitch (1000 Hz); NoGo-condition. A black arrow pointing to the right or to the left was embedded in the S2 circle. The arrows indicated that a button-press with the right thumb on the right button of the response box was required if the arrow pointed to the right, and likewise that a left thumb button press was required on the left button if the arrow pointed to the left. Following S2, the participants were given feedback on the screen about their performance (i.e., whether it was correct to press the button or not, along with reaction times). The stimuli were presented in two blocks; each consisting of 30 randomly distributed Go- and 15 NoGo-trials. A short break was given between the blocks. The whole session lasted approximately 20 min.
Fig. 3.
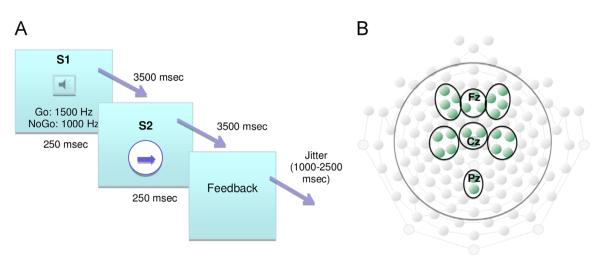
Illustration of experimental task and electrode groups. A. CNV task. B. Region of Interest (ROI) electrode groups. To investigate the CNV, six ROIs were established: right, midline and left frontal, and right, midline and left central. Additionally, one posterior midline ROI was established to investigate the posterior slow positive component.
4.4. EEG recording
EEG-data were acquired using a 128-channel HydroCel Geodesic Sensor Net and Net Amps 300 amplifier (Electrical Geodesics, Eugene, OR). The data were recorded at 250 Hz sampling rate with a 24 bit analog-to-digital converter and a DC to 125 Hz bandpass. Impedance was generally maintained below 50 kΩ, with 100 kΩ as an upper limit (Ferree et al., 2001). All electrodes were referenced to Cz during recording.
4.5. EEG analysis
Continuous EEG data were filtered offline with a 0.01 Hz highpass filter using Net Station, Version 4.3.1 software (Electrical Geodesics, Eugene, OR). Data analysis was carried out using custom-written scripts in MATLAB (Natick, MA) based on EEGLAB (Delorme and Makeig, 2004) functions. A 30 Hz lowpass filter was applied. Bad channels were identified by visual inspection, removed and then interpolated. On average 6 channels per subject were interpolated (controls: 6; lateral PFC: 7; OFC: 7). Eye-movements and blinks were identified and removed using independent component analysis (ICA). The data were rereferenced to average reference (eye channels not included). The re-referenced data were epoched time-locked to S1 onset in segments from −1000 to 4700 ms, with the 500 ms preceding S1 serving as baseline. Trials with incorrect responses and/or amplitude values exceeding 150 μV were rejected.
The CNV is a frontocentral scalp phenomenon, and the following Region of Interest (ROI) electrode groups were established: right, midline and left frontal, and right, midline and left central (see Fig. 3B). Statistical analyses and illustrations were performed on extracted mean values across electrodes in each ROI. Mean amplitudes of the time windows 500–1000 ms and 3200–3700 ms were calculated for each participant to analyze the amplitude of the early and late segments of the CNV. CNVs to Go trials and CNV Go/NoGo difference waves (DWs) were subjected to statistical analysis. The DWs were established by subtracting the NoGo trial waveform from the Go trial waveform. Go condition N1 amplitude was measured at the most negative time point between 60 and 140 ms after S1 onset. In order to measure the posterior positive slow component, peak amplitude (measured as the mean of 100 ms around the peak) of the 200–800 ms post S1 onset interval of Go trials was extracted from a parietal midline ROI (see Figs. 3B and 4C).
4.6. Behavioral data
Investigation of manual reaction time (RT) in successful Go trials was performed by establishing the median of each individual’s RTs to correct Go trials. The mean of the individual median RTs was then computed for each group.
4.7. Statistical analysis
Individual average amplitudes within each ROI and time interval were exported to Statistical Package for the Social Sciences, Version 18.0 (SPSS Inc. Chicago, IL, USA). The Go condition CNV and N1 data were subjected to repeated measures analyses of variance (ANOVAs) with Group (control, OFC vs. lateral PFC) as between-subjects factor and Frontal–Central electrode clusters (frontal vs. central ROI) and Laterality (left, midline vs. right) as within-subject factors. In order to investigate potential effects of lesioned versus non-lesioned hemisphere for the lateral PFC group these analyses were repeated with the same factors and levels, but with right and left electrodes for the 5 patients with right lateral hemisphere lesions exchanged so that for the all the lateral PFC patients left hemisphere ROIs were synonymous with lesioned hemisphere. Analyses that yielded significant interactions between group and plane or laterality resulted in planned contrasts between the levels of the variable. Effects involving differences between patient groups and healthy controls were of primary interest. Due to a difference in Total and Performance IQ between the lateral PFC group and healthy controls, the analyses on early and late CNV were additionally performed with Total- and Performance IQ as covariates. Go/NoGo difference waves over the midline ROI of the plane that showed significant group effects in the Go-condition analysis were subjected to One-Way ANOVAs, with Group as between-subject factor. Likewise, the posterior slow positive component was also examined with a One-Way ANOVA with Group as between-subject factor. Pearson product-moment correlation (two-tailed) analyses were used to investigate the relationship between RT to successful Go-trials and ERP-amplitudes, testing for correlations between RT and early CNV, late CNV and DW at each of the 6 ROIs. Demographic, psychometric, and performance data were analyzed using One-Way ANOVA with Group as between-subject factor. For computations involving more than one degree of freedom, Greenhouse–Geisser epsilon corrected p-values along with uncorrected degrees of freedom are reported. Effect size was computed using eta-squared (η2). Tukey HSD corrected p-values are reported in post hoc analyses. Results are given with a significance level of 0.05.
Acknowledgments
This research is supported by the Southeastern Norway Regional Health Authority (Grants SUN-001-SS and 2008047), the Research Council of Norway (Grant 186504/ V50), and the National Institute of Neurological Disorders and Stroke, USA (NS21135 and PO 40813). The authors would like to thank Bradley Voytek for assistance on ERP analysis and establishment of scripts for analyzing EEG data in Matlab, and Haakon Engen and Clay Campbell Clayworth for support in establishing routines for lesion reconstructions. This work forms part of a doctoral thesis to be submitted to the Department of Psychology at the University of Oslo.
REFERENCES
- Barcelo F, Knight RT. An information-theoretical approach to contextual processing in the human brain: evidence from prefrontal lesions. Cereb. Cortex. 2007;17(Suppl. 1):i51–i60. doi: 10.1093/cercor/bhm111. [DOI] [PubMed] [Google Scholar]
- Basile LF, Rogers RL, Bourbon WT, Papanicolaou AC. Slow magnetic flux from human frontal cortex. Electroencephalogr. Clin. Neurophysiol. 1994;90:157–165. doi: 10.1016/0013-4694(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brunia CH. Neural aspects of anticipatory behavior. Acta Psychol. (Amst.) 1999;101:213–242. doi: 10.1016/s0001-6918(99)00006-2. [DOI] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ. Wait and see. Int. J. Psychophysiol. 2001;43:59–75. doi: 10.1016/s0167-8760(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Contribution of human prefrontal cortex to delay performance. J. Cogn. Neurosci. 1998;10:167–177. doi: 10.1162/089892998562636. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Curry SH. Event-related potentials as indicants of structural and functional damage in closed head injury. Prog. Brain Res. 1980;54:507–515. doi: 10.1016/S0079-6123(08)61668-4. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Wise SP. A neurophysiological comparison of three distinct regions of the primate frontal lobe. Brain. 1991;114:951–978. doi: 10.1093/brain/114.2.951. [DOI] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, McCandliss BD. Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study. J. Neurosci. 2007;27:2272–2282. doi: 10.1523/JNEUROSCI.3470-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Foxe JJ, Garavan H. Mapping the functional anatomy of task preparation: priming task-appropriate brain networks. Hum. Brain Mapp. 2006;27:819–827. doi: 10.1002/hbm.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clin. Neurophysiol. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Flores AB, Digiacomo MR, Meneres S, Trigo E, Gomez CM. Development of preparatory activity indexed by the contingent negative variation in children. Brain Cogn. 2009;71:129–140. doi: 10.1016/j.bandc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Funderud I, Lindgren M, Lovstad M, Endestad T, Voytek B, Knight RT, Solbakk AK. Differential Go/NoGo activity in both contingent negative variation and spectral power. PLoS One. 2012;7:e48504. doi: 10.1371/journal.pone.0048504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemba H, Sasaki K, Tsujimoto T. Cortical field potentials associated with hand movements triggered by warning and imperative stimuli in the monkey. Neurosci. Lett. 1990;113:275–280. doi: 10.1016/0304-3940(90)90597-3. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Delinte A, Vaquero E, Cardoso MJ, Vazquez M, Crommelinck M, Roucoux A. Current source density analysis of CNV during temporal gap paradigm. Brain Topogr. 2001;13:149–159. doi: 10.1023/a:1007816201345. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Flores A, Ledesma A. Fronto-parietal networks activation during the contingent negative variation period. Brain Res. Bull. 2007;73:40–47. doi: 10.1016/j.brainresbull.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Gomez CM, Marco J, Grau C. Preparatory visuo-motor cortical network of the contingent negative variation estimated by current density. Neuroimage. 2003;20:216–224. doi: 10.1016/s1053-8119(03)00295-7. [DOI] [PubMed] [Google Scholar]
- Hartikainen KM, Ogawa KH, Knight RT. Orbitofrontal cortex biases attention to emotional events. J. Clin. Exp. Neuropsychol. 2012;34:588–97. doi: 10.1080/13803395.2012.666231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Knight RT. Mechanisms of human attention: event-related potentials and oscillations. Neurosci. Biobehav. Rev. 2001;25:465–476. doi: 10.1016/s0149-7634(01)00027-6. [DOI] [PubMed] [Google Scholar]
- Hultin L, Rossini P, Romani GL, Hogstedt P, Tecchio F, Pizzella V. Neuromagnetic localization of the late component of the contingent negative variation. Electroencephalogr. Clin. Neurophysiol. 1996;98:435–448. doi: 10.1016/0013-4694(96)95507-8. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Luders HO, Collura TF, Burgess RC, Morris HH, Hamano T, Shibasaki H. Subdural potentials at orbitofrontal and mesial prefrontal areas accompanying anticipation and decision making in humans: a comparison with Bereitschafts potential. Electroencephalogr. Clin. Neurophysiol. 1996;98:206–212. doi: 10.1016/0013-4694(95)00239-1. [DOI] [PubMed] [Google Scholar]
- Ioannides AA, Fenwick PB, Lumsden J, Liu MJ, Bamidis PD, Squires KC, Lawson D, Fenton GW. Activation sequence of discrete brain areas during cognitive processes: results from magnetic field tomography. Electroencephalogr. Clin. Neurophysiol. 1994;91:399–402. doi: 10.1016/0013-4694(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta. Psychol. (Amst.) 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Kok A. The effect of warning stimulus novelty on the P300 and components of the contingent negative variation. Biol. Psychol. 1978;6:219–233. doi: 10.1016/0301-0511(78)90024-8. [DOI] [PubMed] [Google Scholar]
- Liu AK, Belliveau JW, Dale AM. Spatiotemporal imaging of human brain activity using functional MRI constrained magnetoencephalography data: Monte Carlo simulations. Proc. Natl. Acad. Sci. USA. 1998;95:8945–8950. doi: 10.1073/pnas.95.15.8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless NE, Sanford AJ. Effects of age on the contingent negative variation and preparatory set in a reaction-time task. J. Gerontol. 1974a;29:52–63. doi: 10.1093/geronj/29.1.52. [DOI] [PubMed] [Google Scholar]
- Loveless NE, Sanford AJ. Slow potential correlates of preparatory set. Biol. Psychol. 1974b;1:303–314. doi: 10.1016/0301-0511(74)90005-2. [DOI] [PubMed] [Google Scholar]
- Low M. Event-related potentials and the electroencephalogram in patients with proven brain lesions. Prog. Clin. Neurophysiol. 1979;6:258–264. [Google Scholar]
- Mattson AJ, Levin HS. Frontal lobe dysfunction following closed head injury. A review of the literature. J. Nerv. Ment. Dis. 1990;178:282–291. doi: 10.1097/00005053-199005000-00002. [DOI] [PubMed] [Google Scholar]
- McAllister TW. Neurobiological consequences of traumatic brain injury. Dialogues Clin. Neurosci. 2011;13:287–300. doi: 10.31887/DCNS.2011.13.2/tmcallister. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. Brain potentials associated with structural and functional visual matching. Neuropsychologia. 1978;16:571–585. doi: 10.1016/0028-3932(78)90085-4. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Fenwick PB, Trimble MR, Dolan RJ. Brain activity relating to the contingent negative variation: an fMRI investigation. Neuroimage. 2004;21:1232–1241. doi: 10.1016/j.neuroimage.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Auer L, Oberbauer R. The influence of skull defects and reperfusion after extra-intracranial arterial bypass surgery on the sensorimotor EEG rhythm. J. Neurol. Neurosurg. Psychiatry. 1982;45:1106–1112. doi: 10.1136/jnnp.45.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rizzo PA, Amabile G, Caporali M, Spadaro M, Zanasi M, Morocutti C. A CNV study in a group of patients with traumatic head injuries. Electroencephalogr. Clin. Neurophysiol. 1978;45:281–285. doi: 10.1016/0013-4694(78)90012-3. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh JW, Syndulko K, Lindsley DB. Brain wave components of the contingent negative variation in humans. Science. 1976;191:1055–1057. doi: 10.1126/science.1251217. [DOI] [PubMed] [Google Scholar]
- Rosahl SK, Knight R. Role of prefrontal cortex in generation of the contingent negative variation. Cereb. Cortex. 1995;5:123–134. doi: 10.1093/cercor/5.2.123. [DOI] [PubMed] [Google Scholar]
- Rosler F, Heil M, Roder B. Slow negative brain potentials as reflections of specific modular resources of cognition. Biol. Psychol. 1997;45:109–141. doi: 10.1016/s0301-0511(96)05225-8. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Cowan CP, Nagy ME, Milner AD, Jacobson I, Brooks DN. CNV abnormalities following closed head injury. Brain. 1989;112(Pt. 2):489–506. doi: 10.1093/brain/112.2.489. [DOI] [PubMed] [Google Scholar]
- Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cogn. Affect. Behav. Neurosci. 2002;2:264–270. doi: 10.3758/cabn.2.3.264. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, A U, Dywan J. CNV evidence for the distinctiveness of frontal and posterior neural processes in traumatic brain-injured population. J. Clin. Exp. Neuropsychol. 1992;14:545–565. doi: 10.1080/01688639208402844. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philos. Trans. R. Soc. London B Biol. Sci. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: the frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, Picton TW. A multidisciplinary approach to anterior attentional functions. Ann. N. Y. Acad. Sci. 1995;769:191–211. doi: 10.1111/j.1749-6632.1995.tb38140.x. [DOI] [PubMed] [Google Scholar]
- van Leeuwen TH, Steinhausen HC, Overtoom CC, Pascual-Marqui RD, van’t Klooster B, Rothenberger A, Sergeant JA, Brandeis D. The continuous performance test revisited with neuroelectric mapping: impaired orienting in children with attention deficits. Behav. Brain Res. 1998;94:97–110. doi: 10.1016/s0166-4328(97)00173-3. [DOI] [PubMed] [Google Scholar]
- Voytek B, Davis M, Yago E, Barcelo F, Vogel EK, Knight RT. Dynamic neuroplasticity after human prefrontal cortex damage. Neuron. 2010a;68:401–408. doi: 10.1016/j.neuron.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Secundo L, Bidet-Caulet A, Scabini D, Stiver SI, Gean AD, Manley GT, Knight RT. Hemicraniectomy: a new model for human electrophysiology with high spatio-temporal resolution. J. Cogn. Neurosci. 2010b;22:2491–2502. doi: 10.1162/jocn.2009.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: an electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. http://dx.doi.org/10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Wascher E, Verleger R, Jaskowski P, Wauschkuhn B. Preparation for action: an ERP study about two tasks provoking variability in response speed. Psychophysiology. 1996;33:262–272. doi: 10.1111/j.1469-8986.1996.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence: The Psychological Corporation. San Antonio, TX: 1999. [Google Scholar]
- Weerts TC, Lang PJ. The effects of eye fixation and stimulus and response location on the contingent negative variation (CNV) Biol. Psychol. 1973;1:1–19. doi: 10.1016/0301-0511(73)90010-0. [DOI] [PubMed] [Google Scholar]
- Zappoli R, Versari A, Zappoli F, Chiaramonti R, Zappoli Thyrion GD, Grazia Arneodo M, Zerauschek V. The effects on auditory neurocognitive evoked responses and contingent negative variation activity of frontal cortex lesions or ablations in man: three new case studies. Int. J. Psychophysiol. 2000;38:109–144. doi: 10.1016/s0167-8760(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Zappoli R, Zappoli F, Picchiecchio A, Chiaramonti R, Grazia Arneodo M, Zappoli Thyrion GD, Zerauschek V. Frontal and parieto-temporal cortical ablations and diaschisis-like effects on auditory neurocognitive potentials evocable from apparently intact ipsilateral association areas in humans: five case reports. Int. J. Psychophysiol. 2002;44:117–142. doi: 10.1016/s0167-8760(01)00197-0. [DOI] [PubMed] [Google Scholar]