Abstract
Objectives:
Next-generation sequencing (NGS) allows for high-throughput sequencing analysis of large regions of the human genome. We explored the use of targeted NGS for simultaneous testing for multiple mutations in thyroid cancer.
Design:
A custom panel (ThyroSeq) was designed to target 12 cancer genes with 284 mutational hot spots. Sequencing was performed to analyze DNA from 228 thyroid neoplastic and nonneoplastic samples including 105 frozen, 72 formalin-fixed, and 51 fine-needle aspiration samples representing all major types of thyroid cancer.
Results:
Only 5–10 ng of input DNA was sufficient for successful analysis of 99.6% of samples. The analytical accuracy for mutation detection was 100% with the sensitivity of 3%–5% of mutant allele. ThyroSeq DNA assay identified mutations in 19 of 27 of classic papillary thyroid carcinomas (PTCs) (70%), 25 of 30 follicular variant PTCs (83%), 14 of 18 conventional (78%) and 7 of 18 oncocytic follicular carcinomas (39%), 3 of 10 poorly differentiated carcinomas (30%), 20 of 27 anaplastic (ATCs) (74%), and 11 of 15 medullary thyroid carcinomas (73%). In contrast, 5 of 83 benign nodules (6%) were positive for mutations. Most tumors had a single mutation, whereas several ATCs and PTCs demonstrated two or three mutations. The most common mutations detected were BRAF and RAS followed by PIK3CA, TP53, TSHR, PTEN, GNAS, CTNNB1, and RET. The BRAF mutant allele frequency was 18%–48% in PTCs and was lower in ATCs.
Conclusions:
The ThyroSeq NGS panel allows simultaneous testing for multiple mutations with high accuracy and sensitivity, requires a small amount of DNA and can be performed in a variety of thyroid tissue and fine-needle aspiration samples, and provides quantitative assessment of mutant alleles. Using this approach, the point mutations were detected in 30%–83% of specific types of thyroid cancer and in only 6% of benign thyroid nodules and were shown to be present in the majority of cells within the cancer nodule.
Thyroid cancer is the most common malignancy of endocrine organs, and its incidence is steadily growing in the United States and worldwide (1–4). Thyroid cancer typically occurs in thyroid nodules, which are prevalent in the general population, particularly with increased age. However, most thyroid nodules are benign and the clinical challenge is to accurately identify those nodules that are malignant and need to be surgically removed (5–8). Ultrasound-guided fine-needle aspiration (FNA) of the thyroid nodule followed by cytological examination is a common diagnostic approach that allows detecting cancer or establishing a diagnosis of a benign nodule in most cases. However, in approximately 25% of nodules, the diagnosis can not be reliably established by FNA cytology, hampering clinical management of these patients (6, 9–12). Molecular techniques, ie, a panel of most common mutations in thyroid cancer (BRAF, RAS, RET/PTC, PAX8/PPARγ) (13–16) and Affirma gene expression classifier (17, 18) offer significant diagnostic improvement to FNA cytology, although the accuracy of cancer detection by both approaches needs further enhancement. This may be achieved by testing for a significantly larger group of mutational markers using recently introduced technologies such as next-generation sequencing (NGS).
NGS offers simultaneous sequencing of thousands to millions of short nucleic acid sequences in a massively parallel way and may offer a cost-effective approach for detecting multiple genetic alterations (19, 20). It provides a clear advantage over the conventional sequencing technique, such as Sanger sequencing, by allowing simultaneous analysis of large regions of the genome and offering high sensitivity of mutation detection and quantitative assessment of mutant alleles (21). NGS offers nucleotide sequencing on a different scale including whole-genome sequencing, whole-exome sequencing, and whole-transcriptome sequencing as well as targeted sequencing of multiple specific genomic regions (22–24). Whereas large-scale analyses are essential for discovery projects, targeted panels may offer further advance in routine molecular diagnostics of cancer. In thyroid nodules, such an approach may be helpful to expand the currently existing diagnostic panels of several genes to enable simultaneous testing for multiple mutations.
The most common mutations that occur in papillary thyroid cancer (PTC) are point mutations of the BRAF and RAS genes and RET/PTC and NTRK1 rearrangements, all of which are able to activate the MAPK pathway. These mutually exclusive mutations are found in more than 70% of PTCs (25–28). Follicular thyroid cancer (FTC) harbors either RAS mutations or PAX8/PPARγ rearrangement. These mutations are also mutually exclusive and identified in 70%–75% of follicular carcinomas (29). Genetic alterations involving the phosphatidylinositol-3 kinase/AKT signaling pathway also occur in thyroid tumors, particularly in advanced and dedifferentiating tumors (30–34). Additional mutations known to occur in poorly differentiated and anaplastic carcinomas involve the TP53, AKT1, and CTNNB1 genes (35). Medullary thyroid carcinomas, both familial and sporadic, frequently carry point mutations located in the RET and RAS genes (36, 37). Other somatic mutations, such as those of the TSHR gene, have been reported in some thyroid nodules (38, 39), although their prevalence and diagnostic utility remain unclear.
In this study, we evaluate targeted next-generation sequencing as a new approach for testing a broad spectrum of point mutations that occur in thyroid cancer and validate the use of the next-generation sequencing mutational panel (ThyroSeq) in various types of thyroid samples obtained from malignant and benign thyroid nodules.
Materials and Methods
Thyroid samples
Snap-frozen tissue and formalin-fixed, paraffin-embedded (FFPE) tissue from surgically removed thyroid samples and FNA samples were collected at the Department of Pathology, University of Pittsburgh Medical Center, following the University of Pittsburgh Institutional Review Board approval. Fifteen thyroid cancer samples previously positive for BRAF and RAS mutation, three cell lines (HT29, SW620, HT1080) with known mutations in the KRAS, NRAS, BRAF, and TP53 genes, 14 normal thyroid tissue and blood specimens, and a normal HapMap cell line were used for initial validation of the mutational panel. A subsequent analysis was performed on 228 thyroid neoplastic and nonneoplastic specimens including 57 papillary carcinomas (27 classical PTCs and 30 of the follicular variant of papillary carcinoma), 36 follicular carcinomas [18 conventional (cFTC) and 18 oncocytic (oFTC)], 10 poorly differentiated carcinomas, 27 anaplastic carcinomas, 15 medullary carcinomas, and 83 histologically benign hyperplastic nodules. All tumors were classified according to the World Health Organization diagnostic criteria (40). Most of these specimens were either frozen tissues (n = 105) or FFPE tissues (n = 72). In addition, 51 thyroid FNA samples from patients who underwent surgery and yielded surgical diagnosis were included in this study.
DNA isolation
For FFPE tissues, tumor-rich areas (>50% of neoplastic cells) were microdissected using three to four 4-μm unstained histological sections under the stereomicroscopic visualization with an Olympus SZ61 microscope (Olympus) using a hematoxylin and eosin-stained slide for guidance. Genomic DNA was isolated from each target with the DNeasy blood and tissue kit on the automated QIAcube (QIAGEN) instrument according to the manufacturer's instructions. From frozen tissues specimens, DNA was isolated using QIAamp DNA kit (QIAGEN). FNA samples were collected and DNA isolated as previously reported (41).
Next-generation sequencing
For targeted next-generation sequencing analysis, the custom primers were designed using a Life Technologies design tool to generate a pool of 34 primers for amplification of genomic regions of interest. It was used for amplification of isolated DNA in a multiplex PCR. In more details, 10 ng of DNA was amplified by PCR using the premixed primer pool and Ion AmpliSeq HiFi master mix (Ion AmpliSeq Library Kit 2.0). For library construction, the resulting 34 multiplexed amplicons were treated with a FuPa reagent to partially digest primer sequences and phosphorylate the amplicons. The amplicons were then ligated to adapters with the addition of barcodes from the Ion Xpress Barcode Adapters 1–96 kit according to the manufacturer's instructions (Ion AmpliSeq Library Kit 2.0). After ligation, the amplicons underwent nick translation and additional library amplification by PCR to complete the linkage between adapters and amplicons. Library concentration and amplicon size were determined using an Agilent BioAnalyzer high sensitivity DNA kit (Agilent Technologies). Next, multiplexed barcoded libraries were enriched by clonal amplification using emulsion PCR on Ion Sphere particles (Ion PGM Template OT2 200 kit) and loaded on an Ion 318 Chip. Massively parallel sequencing was carried out on a Personal Genome Machine sequencer (Ion Torrent) using the Ion PGM Sequencing 200 Kit version 2 according to the manufacturer's instructions.
Data analysis
After a successful sequencing reaction, the raw signal data were analyzed using Torrent Suite version 3.4.2. The pipeline includes signaling processing, base calling, quality score assignment, adapter trimming, PCR duplicate removal, read alignment to human genome 19 reference, quality control of mapping quality, coverage analysis, and variant calling. After completion of the primary data analysis, a list of detected sequence variants [single nucleotide variants (SNVs) and insertions or deletions (indels)] were compiled in a variant call file format and presented via the web-based user interface. Variant calls were further analyzed using an internally created customized software suite (SeqReporter; University of Pittsburgh Medical Center) that allowed variant filtering and annotation using COSMIC version 64, The Single Nucleotide Polymorphism Database (dbSNP) dbSNP build 137, the Database for Nonsynonymous SNPs' Functional Predictions (dbNSFP) light version 1.3, PolyPhen-2, SIFT, and the University of California, Santa Cruz, genome browser. Amino acid predictions were carried out with in silico prediction algorithms SIFT (42, 43) and PolyPhen-2 (44) to predict potential deleterious effect on protein function. Sequence variant confirmation was performed by conventional techniques including Sanger sequencing, real-time PCR, COLD-PCR, or allele-specific PCR (41, 45, 46). The calculation of analytical accuracy, limits of detection, and assay reproducibility was performed using MedCalc Statistical Software version 9.6.
Results
ThyroSeq NGS panel design and validation
The aim of this study was to create a next-generation sequencing approach that would allow the detection of most point mutations and small insertions or deletions (indels) known to occur in thyroid cancer. To achieve this goal, we reviewed the English-language literature (47, 48) and the thyroid-related COSMIC version 63 followed by version 64 records (49), which identified 12 genes with mutations repeatedly found in thyroid cancer (Table 1). A custom library with a pool of 34 primer pairs was designed to sequence 284 hot spot regions accounting for more than 95% of all sequence variants reported at these genes.
Table 1.
Genes and Exons Included in ThyroSeq Panel
| Chromosomes | Genes |
|---|---|
| chr1 | NRAS exons 2, 3 |
| chr3 | CTNNB1 exon 3 |
| chr3 | PIK3CA exons 9, 20 |
| chr7 | BRAF exon 15 |
| chr10 | RET exons 10, 11, 12, 13, 15, 16 |
| chr10 | PTEN exons 5, 6, 7, 8 |
| chr11 | HRAS exons 2, 3 |
| chr12 | KRAS exons 2, 3 |
| chr14 | TSHR exon 10 |
| chr14 | AKT1 exon 3 |
| chr17 | TP53 exons 5, 6, 7, 8, 9 |
| chr20 | GNAS exons 8, 9 |
The performance of this panel was evaluated in 15 thyroid tumors and three cell lines with known genetic alterations and in 15 DNA samples with no mutations. The workflow of the NGS mutation analysis is summarized in Figure 1. ThyroSeq was able to correctly detect all pathogenic mutations in previously positive tumor samples and cell lines, whereas all normal samples and HapMap cell line were negative for mutations, demonstrating a 100% analytical accuracy of mutation detection.
Figure 1.
ThyroSeq work flow. DNA from FFPE tissue, frozen tissue, or FNA samples is amplified for enrichment of 34 target regions in 12 thyroid cancer genes in a multiplex PCR reaction. Then the library is prepared by ligating the PCR amplicons into platform-specific adapters and adding bar codes for specimen multiplexing. Finally, the library is enriched by clonal amplification (emPCR) and sequenced by massively parallel sequencing on the Ion Torrent PGM. The data analysis and variant calling are performed using bioinformatic pipelines followed by a custom SeqReporter algorithm for filtering and annotation of genetic variants.
The analytical sensitivity of the ThyroSeq panel was determined using serial dilutions of thyroid tumor DNA carrying a BRAF, TP53, or NRAS mutation in normal DNA. All three mutations tested were detected down to a 3% allele frequency, which corresponds to 6% of cells with heterozygous mutation.
Analysis of thyroid specimens
Totally, 229 DNA samples from 105 frozen tissues, 72 FFPE tissues, and 52 FNAs were analyzed for mutations using small amount (5–10 ng) of DNA. Only one DNA sample (0.4%) failed library preparation and sequencing analysis, whereas 228 samples were successfully analyzed. Sequence coverage was assessed from the number and distribution of reads across the target DNA regions. The individual samples averaged approximately 180 000 mapped sequence reads with the mean read length of 110 bp, constituting an average of approximately 20 Mb of sequence per sample. The depth of coverage per mutational hot averaged 3753 reads (range 507–6500 reads). No significant difference was observed in the depth of coverage between frozen tissue (average 3546 reads), FFPE (average 3474 reads), and FNA (average 4241 reads) samples.
Overall, 115 mutations were detected in 228 thyroid specimens tested, including 110 mutations in 145 cancer samples (Figure 2) and five mutations in 83 benign nodules. Most mutations (n = 109) were single-nucleotide variants (point mutations), five were small deletions, and one was a complex deletion/mutation. Most tumors were positive for a single mutational event. However, nine tumors demonstrated the simultaneous presence of two to three genetic alterations in the same tumor. All mutations detected by ThyroSeq were confirmed by Sanger sequencing, real-time PCR, or COLD-PCR methods, demonstrating 100% correlations between the techniques (Figure 3).
Figure 2.
Mutational profiles of various types of thyroid cancers identified by ThyroSeq.
Figure 3.
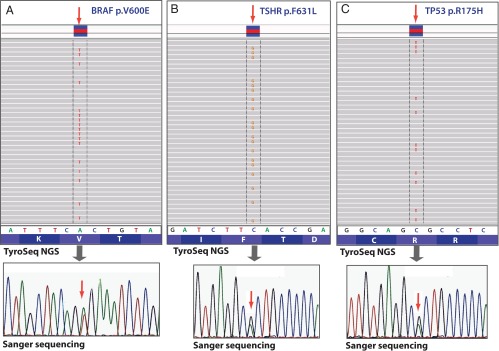
Identification of mutations by ThyroSeq NGS, as visualized in integrative genomic viewer (top panel) and confirmed by Sanger sequencing (bottom panel). A, BRAF V600E mutation in PTC. B, TSHR F631L mutation in FTC. C, TP53 R175H mutation in ATC.
Papillary thyroid carcinomas
A total of 57 PTCs (27 classic PTCs and 30 follicular variant of papillary carcinomas) were analyzed (Figure 2). Mutations were identified in 19 of classic PTCs (70%). The BRAF mutation was the most common type (n = 16), followed by PIK3CA (n = 3), TP53 (n = 2), and NRAS (n = 1). The BRAF mutations included 15 typical p.V600E mutations and one complex mutation BRAF p.V600_R603delVKSR, p.T599I. Seventeen tumors revealed a single mutation, whereas one tumor presented with lung metastases revealed two mutations, BRAF and TP53, and another tumor with known local recurrence was found to be positive for three mutations: BRAF, TP53, and PIK3CA (Figure 2).
Among the follicular variant PTCs, 25 tumors (83%) were positive for mutations. The molecular profile of these tumors was different from classic PTC, with RAS being the most prevalent mutation type (n = 22), followed by BRAF (n = 2) and TSHR (n = 1) mutations. Among the RAS mutations, 16 were in NRAS (14 at codon 61, one at codon 12, and one at codon 13), three in HRAS codon 61, and three in KRAS codon 61. Among the BRAF mutations, one was BRAF p.V600E and one was BRAF p.K601E. Mutations in the TSHR gene were located in exon 10.
Follicular thyroid carcinomas
The analysis was performed on 36 FTCs including 18 conventional and 18 oFTCs. Fourteen cFTCs (78%) were positive mutations including NRAS (n = 9), TSHR (n = 4), and KRAS (n = 1) (Figure 2). All TSHR mutations were located within exon 10, which encodes for the transmembrane domain of the protein. A different genetic profile was observed in oFTCs with only seven tumors (39%) being positive for mutations. Interestingly, the most common genetic alteration detected in our series of oFTCs was the mutation of the TP53 gene, which was found in 22% of these tumors. All TP53 mutations were located in the DNA-binding domain of the gene. In addition, two tumors were positive for RAS mutations and one for an inactivating mutation in the PTEN gene. To determine whether PTEN and TSHR mutations were somatic or germline, normal thyroid tissue from the same cases was also analyzed by conventional Sanger sequencing. PTEN and TSHR mutations were not found in the corresponding normal tissue, indicating their somatic nature.
Poorly differentiated and anaplastic thyroid carcinomas
Ten poorly differentiated thyroid carcinomas (PDCs) and 27 anaplastic thyroid carcinomas (ATCs) were analyzed by the ThyroSeq panel. Only three PDCs (30%) were positive for mutations, with one tumor carrying triple mutation in NRAS, PIK3CA, and GNAS, one tumor positive for BRAF, and one for NRAS mutation (Figure 2). In contrast, 20 ATCs (74%) were found to harbor mutations, including TP53 (n = 8), BRAF (n = 7), RAS (n = 6), PIK3CA (n = 3), PTEN (n = 1), and CTNNB1 (n = 1). Of those, 15 tumors revealed a single mutation and four had two mutations and one tumor had three mutations. The triple-positive case had mutations in the BRAF, TP53, and PIK3CA genes. The BRAF p.V600E mutation was detected in six ATCs, and one ATC was positive for the BRAF p.K601E mutation.
Medullary thyroid carcinomas
Fifteen sporadic medullary carcinomas were analyzed by ThyroSeq, revealing 11 tumors positive for mutations (73%). As expected, the most common mutation was RET p.M918T, followed by RAS mutations (Figure 2). The most common type of RAS mutation was HRAS p.Q61K, followed by KRAS p.G12R and HRAS p.G13R.
Benign hyperplastic nodules
In addition, 83 dominant hyperplastic nodules were studied. Five nodules (6%) were positive for mutations including TSHR (n = 2), KRAS (n = 1), PTEN (n = 1), and GNAS (n = 1) mutations.
Mutation allele frequency
In addition to the detection of mutations, the NGS analysis provided quantitative assessment of mutation frequency in each sample. Overall, most mutations found in the tumor samples were heterozygous mutations present with high allelic frequency that ranged from 20% to 48% of alleles (which corresponds to 40%–96% of cells with a heterozygous mutation) in tissue samples and from 5% to 46% of the mutant allele (10%–96% of cells with mutation) in FNA samples. Four samples showed low abundance of the mutant allele; they included a hyperplastic nodule that revealed a KRAS mutation found in 5% of alleles (10% of cells with mutation), and three ATCs positive for BRAF V600E, which showed the mutant allele frequency of 5%–8% (10%–16% of cells with mutation). Three TP53 and one PTEN mutations showed allelic frequency of more than 50% (65%–85%), suggesting either a loss of the wild-type allele or amplification of the mutant allele in cancer cells. The allelic frequency of BRAF mutations is summarized in Table 2. In PTCs, the mutant allele frequency was 18%–44% (mean 34%), corresponding to 36%–88% of cells with heterozygous mutation. The frequency of the BRAF mutation was lower in ATCs, with the frequency of mutant allele varying from 5% to 38% (mean 19%). For cancers with concurrent presence of more than one mutation, the allelic frequency of mutant BRAF was similar to other mutations, suggesting that these mutations were present in the same clonal population of cells. In medullary carcinomas, the allelic frequency of RET mutations varied from 39% to 46% of alleles (78%–92% of cells with heterozygous mutation), whereas RAS mutations were seen in 34%–47% of the alleles (68%–94% of cells with heterozygous mutation), indicating that these mutations are present in most tumor cells.
Table 2.
Allelic Frequency of BRAF Mutations in Thyroid Cancers
| Tumor Type | Specimen Type | Mutation Type | BRAF Mutant Allele Frequency | BRAF Mutation Frequencya | Concurrent Mutations (Allele Frequency) |
|---|---|---|---|---|---|
| PTC | FFPE | BRAF V600E | 36% | 72% | |
| PTC | FRZ | BRAF V600E | 44% | 88% | |
| PTC | FRZ | BRAF V600E | 43% | 86% | |
| PTC | FRZ | BRAF V600E | 39% | 78% | |
| PTC | FFPE | BRAF V600E | 37% | 74% | TP53 (40%) |
| PTC | FRZ | BRAF V600E | 36% | 72% | |
| PTC | FRZ | BRAF V600E | 34% | 68% | |
| PTC | FRZ | BRAF V600E | 31% | 62% | |
| PTC | FFPE | BRAF V600E | 29% | 58% | |
| PTC | FFPE | BRAF V600E | 22% | 44% | |
| PTC | FFPE | BRAF V600E | 18% | 36% | |
| PTC | FFPE | BRAF V600E | 35% | 70% | |
| PTC | FRZ | BRAF V600_R603 delVKSR, T599I | 42% | 84% | |
| PTC | FFPE | BRAF V600E | 39% | 78% | PIK3CA (38%), TP53 (31%) |
| PMC (PTC) | FNA | BRAF V600E | 18% | 36% | |
| FLUS (PTC) | FNA | BRAF V600E | 17% | 34% | |
| PTC, FV | FFPE | BRAF K601E | 30% | 60% | |
| PTC, FV | FFPE | BRAF V600E | 29% | 58% | |
| PDC | FRZ | BRAF V600E | 48% | 96% | |
| ATC | FRZ | BRAF V600E | 20% | 40% | |
| ATC | FFPE | BRAF V600E | 23% | 46% | TP53 (60%) |
| ATC | FFPE | BRAF V600E | 34% | 68% | PIK3CA (16%) |
| ATC | FFPE | BRAF K601E | 38% | 76% | |
| ATC | FFPE | BRAF V600E | 5.2% | 10.4% | TP53 (4.9%) |
| ATC | FRZ | BRAF V600E | 5.9% | 11.8% | TP53 (8.8%); PIK3CA (11%) |
| ATC | FRZ | BRAF V600E | 8.0% | 16.0% |
Abbreviations: FLUS, follicular lesion of undetermined significance on FNA; FRZ, frozen tissue; PMC, positive for malignant cells on FNA; FV, follicular variant.
Based on the assumption that the mutation is heterozygous.
Discussion
In this study, we developed a new approach for genotyping thyroid samples using next-generation sequencing. It requires a small amount of DNA and provides simultaneous testing for a broad range of point mutations and indels with high accuracy and sensitivity, offering a valuable tool for a better understanding of thyroid carcinogenesis and clinical genotyping of thyroid nodules.
NGS technology allows sequencing of multiple selected genomic regions and up to the whole genome. Targeted sequencing on Ion Torrent requires a small amount of input DNA because it uses an amplification-based approach for the enrichment of genomic regions. This is of particular importance for FNA samples and small tissue biopsies. In this series, only 1 of 51 routine FNA samples (2%) failed the library preparation and sequencing analysis, suggesting that the vast majority of routine FNA samples should be amendable to such analysis. Moreover, because the amplified sequences are of short size, partially degraded DNA obtained from fixed tissues can be analyzed using this approach. Indeed, the results of this study demonstrate that formalin-fixed and paraffin-embedded samples could be assayed; as little as 5 ng of DNA was sufficient for the analysis. The analysis provided high analytical sensitivity and allowed detecting all mutations previously identified in these samples by traditional Sanger sequencing and other conventional techniques.
Using this approach, we generated molecular profiles of all main types of thyroid cancer and identified point mutations in 30%–83% of specific cancer types. Among papillary carcinomas, the analysis confirmed contrasting genetic profiles between classic papillary cancer, dominated by BRAF mutations, and the follicular variants of PTCs dominated by RAS mutations. In addition to the most common NRAS codon 61 and HRAS codon 61 mutations previously documented to occur in these tumors (50), we found KRAS codon 61 mutation as the third most common hot spot for RAS mutations in thyroid cancer.
Our analysis of FTCs showed a significant difference between genetic profiles of conventional and oFTCs. Surprisingly, among the cFTCs, somatic TSHR mutations were identified in 22% of cases, making it a common event in this cancer type. One additional TSHR mutation was identified in the follicular variant of PTC, pointing to the association between this gene mutation and follicular-pattern thyroid tumors. Somatic TSHR mutations have been previously reported in rare cases of thyroid carcinoma and in benign hyperfunctioning thyroid nodules (38, 39), although their frequency in a consecutive series of thyroid cancer has not been defined. Of seven TSHR mutations identified in this study, five were found in malignant and two in benign nodules, suggesting that the finding of this mutation in a thyroid nodule may have some association with thyroid cancer, particularly with follicular carcinoma. However, additional studies are required to support this conclusion. Four of seven TSHR mutations found in this study have been reported as gain-of-function mutations (38), although information on the functional status of nodules genotyped in our study was not available.
Another unexpected finding was a significant frequency of TP53 mutations in oncocytic follicular carcinomas. TP53 mutations are known to occur with increasing frequency in dedifferentiating thyroid tumors but not in well-differentiated cancer (51–54). A single case of TP53 mutation in an oncocytic carcinoma has been reported, which was detected by single-strand conformation polymorphism analysis with no further confirmation by sequencing (55). The high frequency of TP53 mutations found in these tumors is not due to a sensitive detection using the NGS approach because these mutations were found at high allelic frequency (>70% of cells with a heterozygous mutation). The presence of the TP53 mutation in approximately 20% of oncocytic follicular cancers raises a possibility that this mutation may occur in a subset of tumors that are prone to dedifferentiation and therefore may be used as a diagnostic and prognostic marker in this cancer type.
The high frequency of mutation detection in thyroid cancers by ThyroSeq is expected to further improve the sensitivity of cancer detection in thyroid nodules with indeterminate FNA cytology that are currently tested using limited (four to seven genes) mutational panels (13, 14). Indeed, due to the inclusion of additional mutations, such as those of TP53 and TSHR, as many as 68% of all tumor types were identified by the ThyroSeq panel to carry at least one point mutation. If combined with the detection of chromosomal rearrangements, such as those of RET/PTC and PAX8/PPARγ, more than 80% of all thyroid cancer would be expected to have at least one mutational event. Importantly, the improved sensitivity is expected to affect both conventional and oncocytic follicular carcinomas, the tumors that can rarely be diagnosed as malignant by FNA cytology.
In addition, the extended mutational profiling using the ThyroSeq panel revealed that 9 of 99 mutation-positive cancers (9%) contained more than one mutation. These tumors included either dedifferentiated tumors (six ATCs and one PDC) or PTCs (n = 2), both with unfavorable prognostic features such as distant metastasis or local tumor recurrence. Most of these tumors had a combination of either BRAF or NRAS mutation, known to be an early driver event in thyroid cancer, and TP53 and/or PIK3CA mutation, which are believed to be late events in tumor clone progression. The occurrence of multiple mutations has been reported before in advanced thyroid cancer (30–34, 56) and was observed in this study. This suggests that in addition to its diagnostic utility, this comprehensive mutational panel may contribute to the prognostication of thyroid cancer, which can be performed preoperatively using FNA samples.
An additional advantage of the NGS approach is in providing quantitative assessment of mutant alleles. Despite strong evidence that BRAF is a driver oncogenic event in thyroid cancer, a recent study reported a low abundance of BRAF mutant allele in most of the PTC samples studied using semiquantitative pyrosequencing, suggesting that BRAF may not be a frequent clonal event in these tumors (57). In fact, most PTCs in that study were found to have 5%–25% of BRAF mutant alleles, which corresponds to less than half of the cells within the tumor-carrying heterozygous mutation of this gene. In the current study, the quantitation of the mutant allele was evaluated using NGS. We observed that in PTCs, the vast majority of tumor samples (80%) had more than 25% of BRAF mutant alleles, corresponding to more than 50% of cells carrying a BRAF V600E heterozygous mutation. Because tumor samples always have some degree of contamination by normal stromal, endothelial, and inflammatory cells, the finding of the BRAF mutation in more than 50% of cells within the target tumor regions provide strong evidence that it is a clonal driver mutation in these tumors. In the remaining 20% of the PTCs, the frequency of mutant alleles was 17%–22%, and none of the 19 mutation-positive tumors contained the amount of mutant allele as low as 5%–10%. In contrast, an unexpected finding in this study was a low abundance of BRAF mutant allele in many ATC samples, most of which revealed less than 25% of mutant BRAF alleles. The reason for this finding is not clear. One of the possibilities is that, as shown by Ryder et al (58), ATCs frequently have dense infiltration by tumor-infiltrating macrophages and other inflammatory cells, which may dilute the level of mutant alleles in these tumor samples. Alternatively, this finding may reflect a complex genetic nature of this undifferentiated and highly aggressive type of thyroid cancer.
In summary, in this study we report the use of the NGS-based ThyroSeq mutational panel for broad, high-throughput genotyping of thyroid samples, which provides new insights into the biology of thyroid cancer and is expected to further improve the accuracy of cancer diagnosis and prognostication in thyroid nodules.
Acknowledgments
This work was supported in part by Grant R01 CA88041 from the National Institutes of Health and by the Richard A. and Leslie A. Snow Fund for Thyroid Cancer Research.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATC
- anaplastic thyroid carcinoma
- cFTC
- conventional FTC
- FFPE
- formalin-fixed, paraffin-embedded
- FNA
- fine-needle aspiration
- FTC
- follicular thyroid cancer
- indels
- insertions or deletions
- NGS
- next-generation sequencing
- oFTC
- oncocytic FTC
- PDC
- poorly differentiated thyroid carcinoma
- PTC
- papillary thyroid cancer.
References
- 1. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 2. Albores-Saavedra J, Henson DE, Glazer E, Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype—papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007;18:1–7 [DOI] [PubMed] [Google Scholar]
- 3. Burgess JR, Tucker P. Incidence trends for papillary thyroid carcinoma and their correlation with thyroid surgery and thyroid fine-needle aspirate cytology. Thyroid. 2006;16:47–53 [DOI] [PubMed] [Google Scholar]
- 4. Colonna M, Guizard AV, Schvartz C, et al. A time trend analysis of papillary and follicular cancers as a function of tumour size: a study of data from six cancer registries in France (1983–2000). Eur J Cancer. 2007;43:891–900 [DOI] [PubMed] [Google Scholar]
- 5. Mazzaferri EL. Thyroid cancer in thyroid nodules: finding a needle in the haystack. Am J Med. 1992;93:359–362 [DOI] [PubMed] [Google Scholar]
- 6. Gharib H. Changing trends in thyroid practice: understanding nodular thyroid disease. Endocr Pract. 2004;10:31–39 [DOI] [PubMed] [Google Scholar]
- 7. Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–3417 [DOI] [PubMed] [Google Scholar]
- 8. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946 [DOI] [PubMed] [Google Scholar]
- 9. Greaves TS, Olvera M, Florentine BD, et al. Follicular lesions of thyroid: a 5-year fine-needle aspiration experience. Cancer. 2000;90:335–341 [PubMed] [Google Scholar]
- 10. Sclabas GM, Staerkel GA, Vassillopoulou-Sellin R, et al. Fine-needle aspiration of the thyroid and correlation with histopathology in a contemporary series of 240 patients. Am J Surg 2003;186:702–709; discussion 709–710 [DOI] [PubMed] [Google Scholar]
- 11. Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:109–142 [DOI] [PubMed] [Google Scholar]
- 12. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111:508–516 [DOI] [PubMed] [Google Scholar]
- 13. Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098 [DOI] [PubMed] [Google Scholar]
- 14. Cantara S, Capezzone M, Marchisotta S, et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95:1365–1369 [DOI] [PubMed] [Google Scholar]
- 15. Ohori NP, Nikiforova MN, Schoedel KE, et al. Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance.” Cancer Cytopathol. 2010;118:17–23 [DOI] [PubMed] [Google Scholar]
- 16. Moses W, Weng J, Sansano I, et al. Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg. 2010;34:2589–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chudova D, Wilde JI, Wang ET, et al. Molecular classification of thyroid nodules using high-dimensionality genomic data. J Clin Endocrinol Metab. 2010;95(12):5296–5304 [DOI] [PubMed] [Google Scholar]
- 18. Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–715 [DOI] [PubMed] [Google Scholar]
- 19. Thomas RK, Nickerson E, Simons JF, et al. Sensitive mutation detection in heterogeneous cancer specimens by massively parallel picoliter reactor sequencing. Nat Med. 2006;12:852–855 [DOI] [PubMed] [Google Scholar]
- 20. Metzker ML. Sequencing technologies—the next generation. Nat Rev Genet. 2010;11:31–46 [DOI] [PubMed] [Google Scholar]
- 21. Meldrum C, Doyle MA, Tothill RW. Next-generation sequencing for cancer diagnostics: a practical perspective. Clin Biochem Rev. 2011;32:177–195 [PMC free article] [PubMed] [Google Scholar]
- 22. Ross JS, Cronin M. Whole cancer genome sequencing by next-generation methods. Am J Clin Pathol. 2011;136:527–539 [DOI] [PubMed] [Google Scholar]
- 23. Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11:685–696 [DOI] [PubMed] [Google Scholar]
- 24. Beadling C, Neff TL, Heinrich MC, et al. Combining highly multiplexed PCR with semiconductor-based sequencing for rapid cancer genotyping. J Mol Diagn. 2013;15:171–176 [DOI] [PubMed] [Google Scholar]
- 25. Adeniran AJ, Zhu Z, Gandhi M, et al. Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol. 2006;30:216–222 [DOI] [PubMed] [Google Scholar]
- 26. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457 [PubMed] [Google Scholar]
- 27. Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580 [DOI] [PubMed] [Google Scholar]
- 28. Frattini M, Ferrario C, Bressan P, et al. Alternative mutations of BRAF, RET and NTRK1 are associated with similar but distinct gene expression patterns in papillary thyroid cancer. Oncogene. 2004;23:7436–7440 [DOI] [PubMed] [Google Scholar]
- 29. Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPARγ rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88:2318–2326 [DOI] [PubMed] [Google Scholar]
- 30. Garcia-Rostan G, Costa AM, Pereira-Castro I, et al. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. 2005;65:10199–10207 [DOI] [PubMed] [Google Scholar]
- 31. Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2008;93:278–284 [DOI] [PubMed] [Google Scholar]
- 32. Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–1170 [DOI] [PubMed] [Google Scholar]
- 33. Dahia PL, Marsh DJ, Zheng Z, et al. Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res. 1997;57:4710–4713 [PubMed] [Google Scholar]
- 34. Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306 [DOI] [PubMed] [Google Scholar]
- 36. de Groot JW, Links TP, Plukker JT, Lips CJ, Hofstra RM. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev. 2006;27:535–560 [DOI] [PubMed] [Google Scholar]
- 37. Moura MM, Cavaco BM, Pinto AE, Leite V. High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab. 2011;96:E863–E868 [DOI] [PubMed] [Google Scholar]
- 38. Garcia-Jimenez C, Santisteban P. TSH signalling and cancer. Arq Bras Endocrinol Metab. 2007;51:654–671 [DOI] [PubMed] [Google Scholar]
- 39. Nishihara E, Amino N, Maekawa K, et al. Prevalence of TSH receptor and Gsα mutations in 45 autonomously functioning thyroid nodules in Japan. Endocr J. 2009;56:791–798 [DOI] [PubMed] [Google Scholar]
- 40. DeLellis RA, Lloyd RV, Heitz PU, Eng C, eds. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France: IARC Press; 2004 [Google Scholar]
- 41. Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–W457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081 [DOI] [PubMed] [Google Scholar]
- 44. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pang B, Durso MB, Hamilton RL, Nikiforova MN. A novel COLD-PCR/FMCA assay enhances the detection of low-abundance IDH1 mutations in gliomas. Diagn Mol Pathol. 2013;22:28–34 [DOI] [PubMed] [Google Scholar]
- 46. Whitehall V, Tran K, Umapathy A, et al. A multicenter blinded study to evaluate KRAS mutation testing methodologies in the clinical setting. J Mol Diagn. 2009;11:543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580 [DOI] [PubMed] [Google Scholar]
- 48. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Forbes SA, Bindal N, Bamford S, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:D945–D950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE. Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol. 2003;120:71–77 [DOI] [PubMed] [Google Scholar]
- 51. Fagin JA, Matsuo K, Karmakar A, Chen DL, Tang SH, Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Donghi R, Longoni A, Pilotti S, Michieli P, Della Porta G, Pierotti MA. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J Clin Invest. 1993;91:1753–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dobashi Y, Sugimura H, Sakamoto A, et al. Stepwise participation of p53 gene mutation during dedifferentiation of human thyroid carcinomas. Diagn Mol Pathol. 1994;3:9–14 [DOI] [PubMed] [Google Scholar]
- 54. Ito T, Seyama T, Mizuno T, et al. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res. 1992;52:1369–1371 [PubMed] [Google Scholar]
- 55. Ho YS, Tseng SC, Chin TY, Hsieh LL, Lin JD. p53 gene mutation in thyroid carcinoma. Cancer Lett. 1996;103:57–63 [DOI] [PubMed] [Google Scholar]
- 56. Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116 [DOI] [PubMed] [Google Scholar]
- 57. Guerra A, Sapio MR, Marotta V, et al. The primary occurrence of BRAF(V600E) is a rare clonal event in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97:517–524 [DOI] [PubMed] [Google Scholar]
- 58. Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]




