Abstract
Context:
Transient and permanent postoperative hypoparathyroidism are recognized complications of neck surgery. Postoperative hypoparathyroidism is usually considered permanent when it persists for 6 months; in rare cases, recovery of hypoparathyroidism through 1 year has been described. Recovery of hypoparathyroidism years after diagnosis has not previously been reported.
Objective:
We report four patients being treated with PTH(1–84) in a research protocol who recovered from postoperative hypoparathyroidism many years after onset.
Methods:
Recovery from hypoparathyroidism was established by: 1) serum calcium and PTH levels within the normal range off PTH(1–84) treatment for at least 1 week; 2) requirement for daily calcium supplementation reduced to ≤1 g; and 3) no supplemental active vitamin D therapy.
Results:
Hypoparathyroidism developed in three subjects after repeated neck surgery for primary hyperparathyroidism and in one subject after total thyroidectomy for Graves' disease. Parathyroid tissue autotransplant was performed in two of the four subjects. Two had undetectable PTH levels at study entry, whereas the other two subjects had detectable, although low, PTH levels. Hypoparathyroidism had been present for at least 8 years, and in one case for 16 years. The recovery of parathyroid function followed treatment with PTH(1–84) for 36 to 63 months.
Conclusions:
Although it remains relatively rare, this report documents recovery of long-term postoperative hypoparathyroidism many years after the initial diagnosis. A potential role for exogenous PTH is intriguing with several plausible mechanisms.
Transient and permanent postoperative hypoparathyroidism are recognized complications of neck surgery, due to removal or devascularization of all parathyroid tissue (1–6). This occurs either inadvertently or unavoidably in the course of extensive neck surgery, typically for primary hyperparathyroidism or thyroid cancer. Postoperative hypoparathyroidism is considered to be permanent when it persists for at least 6 months (7), although eventual recovery of hypoparathyroidism through the first postoperative year has been described (8, 9). To our knowledge, there are no reports of parathyroid function being regained after many years of well-documented hypoparathyroidism. In this report, we describe four patients who recovered parathyroid function 8 to 16 years after the diagnosis of postoperative hypoparathyroidism was established. The recovery occurred in the context of PTH(1–84) administration as part of a research protocol.
Subjects and Methods
Study design
All four patients were enrolled in our investigation of recombinant human PTH(1–84) therapy in hypoparathyroidism (10–12). The diagnosis of hypoparathyroidism was established by the simultaneous presence of serum calcium and PTH concentrations below the lower limits of normal on at least two occasions separated by at least 30 days. All subjects had to have documented hypoparathyroidism for at least 2 years and were on stable regimens of supplemental calcium and vitamin D intake. Subjects were excluded if they had any confounding skeletal disorders, were using bone-active medications, were pregnant, or were within 5 years of menopause. The study was approved by the Institutional Review Board of Columbia University Medical Center. All subjects gave written informed consent. Biochemistries were measured by automated techniques. Unless otherwise specified, the normal ranges for the assays are provided in Table 1.
Table 1.
Baseline Characteristics of 52 Subjects With Postsurgical Hypoparathyroidism
| Mean ± SD (Median) | Normal Range | |
|---|---|---|
| Age, y | 48 ± 13 (48) | |
| Sex, n | ||
| Females | 45 | |
| Premenopausal | 28 | |
| Postmenopausal | 17 | |
| Males | 7 | |
| Caucasians, % | 98 | |
| Reason for neck surgery | ||
| Primary hyperparathyroidism | 7 | |
| Thyroid cancer | 23 | |
| Benign thyroid disease | 22 | |
| Duration of hypoparathyroidism, y | 11 ± 11 (6) | |
| Calcium supplement dose, g/d | 2.95 ± 2.1 (2.0) | |
| 1,25-Dihydroxyvitamin D supplement dose, μg/d | 0.81 ± 1.0 (0.50) | |
| Parent vitamin D dose (n = 32), IU/d | 10 842 ± 22 230 (1350) | |
| Hydrochlorothiazide dose (n = 10), mg/d | 34 ± 27 (25) | |
| Serum calcium, mg/dLa | 8.66 ± 0.9 (8.8) | 8.6–10.2 |
| Serum PTH, pg/mL | 3.0 ± 6 (undetectable) | 10–65 |
| Serum phosphate, mg/dL | 4.3 ± 0.8 (4.3) | 2.5–4.5 |
| Urinary calcium excretion, mg/d | 268 ± 130 (255) | 50–250b |
Serum calcium concentration was typically normal as a result of calcium and vitamin D supplementation.
For men, 50–300 mg/d.
Subjects
The patient described as case 1 has been followed since her original diagnosis of primary hyperparathyroidism, and her age is given at the time of study participation. The patients described as cases 2–4 came to our attention as part of our research protocol.
We defined recovery from hypoparathyroidism by: 1) serum calcium and PTH levels within the normal range off PTH(1–84) treatment for at least 1 week; 2) requirement for daily calcium supplementation reduced to ≤1 g; and 3) no supplemental active vitamin D therapy.
Statistical analysis
Characteristics of the subjects were summarized with descriptive statistics, using frequencies and percentages for categorical variables. For continuous variables, mean, SD, and median were calculated.
Results
Database for the hypoparathyroid population
Of the 87 subjects with hypoparathyroidism who have been enrolled in our research protocol using PTH(1–84) as replacement hormone therapy, 52 carry the diagnosis of postoperative hypoparathyroidism. Table 1 shows the baseline characteristics of the entire postoperative cohort as a reference.
Case reports
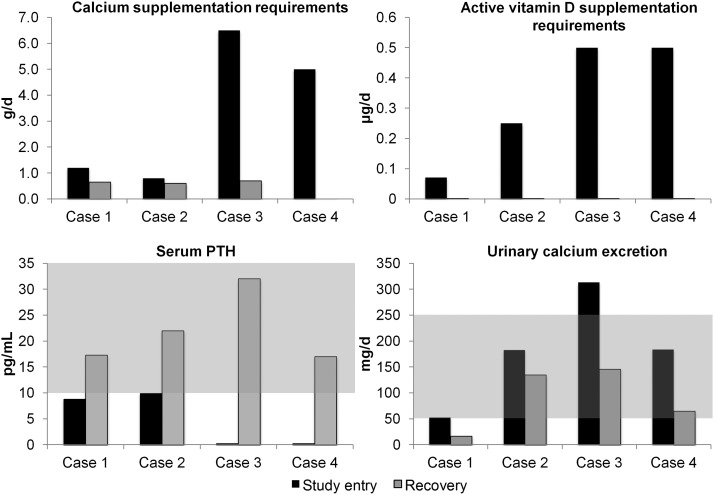
A brief clinical summary of the cases is provided in Table 2. Figure 1 shows calcium and active vitamin D supplement requirements, serum PTH, and urinary calcium excretion for the four patients around the time of study entry and the time of recovery of parathyroid function.
Table 2.
Clinical Summary for the Four Patients With Recovery of Postoperative Hypoparathyroidism
| Case | Age, y | Race | Gender | Original Diagnosis | Autotransplanted Tissue? | Duration of Hypoparathyroidism Before Recovery, y |
|---|---|---|---|---|---|---|
| 1 | 68 | White | Female | Primary hyperparathyroidism | No | 15 |
| 2 | 63 | White | Female | Primary hyperparathyroidism | No | 16 |
| 3 | 39 | White | Female | Graves' disease | Yes, neck | 14 |
| 4 | 47 | White | Female | Primary hyperparathyroidism | Yes, strap muscle | 8 |
Recovery was defined by: 1) serum calcium and PTH levels within the normal range at least 1 week off PTH(1–84) therapy; 2) elemental calcium supplementation <1 g daily; and 3) no supplemental active vitamin D therapy.
Figure 1.
Calcium and active vitamin D supplement requirements, serum PTH, and urinary calcium excretion for the four patients around the time of study entry and the time of recovery of parathyroid function. The normal range for the laboratory values is shaded.
Case 1
A 68-year-old woman developed hypoparathyroidism after several neck operations for primary hyperparathyroidism. At the time of her first operation in 1985, a left lower parathyroid gland was removed from the thymic region. After persistent postoperative hypercalcemia, she underwent a second procedure in 1992 with removal of a right superior parathyroid gland. The pathology was consistent with hypercellular parathyroid tissue. Hypoparathyroidism ensued after a third operation and removal of a parathyroid adenoma behind the head of the right clavicle. She initially required 4.5 g of calcium and 0.5 μg of 1,25-dihydroxyvitamin D daily for treatment of hypoparathyroidism. She had no history of fractures or nephrolithiasis. At the time of study enrollment in 2004, her regimen included calcium 1.2 g daily, 1,25-dihydroxyvitamin D 0.25 μg twice weekly, and parent vitamin D 50 000 IU every other week. Other medications included losartan and hydrochlorothiazide in combination and quinapril. Family history was significant for an identical twin sister with primary hyperparathyroidism. Laboratory data at study entry demonstrated the following: serum calcium, 9.8 mg/dL; PTH, 8.8 pg/mL; phosphorus, 4.0 mg/dL; 24-hour urine calcium, 53 mg/total volume 750 mL. She was started on PTH(1–84) 100 μg every 3 days. PTH(1–84) was stopped around the time of her month 54 visit in 2009 due to hypercalcemia. At that time, she was taking calcium 1 g daily and vitamin D 50 000 IU every 2 months; active vitamin D had been discontinued. Her serum calcium remained stable off PTH therapy. Five months later, her serum calcium was 10.1 mg/dL with a concurrent PTH of 17.3 pg/mL. Other laboratory tests at that time were noteworthy for serum phosphorus of 3.2 mg/dL and 24-hour urine calcium of 17 mg/total volume 1100 mL. Four years later, she is taking vitamin D 400 IU daily without any calcium or active vitamin D supplementation. Her most recent laboratory tests in January 2013 demonstrated a serum calcium level of 10.1 mg/dL with a concurrent PTH of 30 pg/mL, phosphorus of 3.8 mg/dL (normal, 2.1–4.3), and 24-hour urine calcium of 12 mg/total volume 950 mL.
Case 2
A 63-year-old woman developed hypoparathyroidism after two parathyroid gland resections for primary hyperparathyroidism. In 1984, 3.5 parathyroid glands were removed, and in 1995 a parathyroid adenoma was resected from behind her left ear. At the time of study enrollment in 2005, her therapeutic regimen included calcium 0.8 g daily, 1,25-dihydroxyvitamin D 0.25 μg daily, and vitamin D 50 000 IU twice a week. Other medications included levothyroxine, atorvastatin, and sertraline. She had no history of fractures or nephrolithiasis. Laboratories at study enrollment demonstrated the following: calcium 9.5 mg/dL, PTH 10 pg/mL, 24-hour urine calcium 183 mg/total volume 1450 mL. She started PTH(1–84) at a dose of 100 μg every other day. At month 60, her dose was adjusted to 50 μg daily. She discontinued PTH at month 63 in 2011 due to a diagnosis of breast cancer that was not attributed to study drug. Ten days later, PTH was 22 pg/mL with a concurrent serum calcium of 9.4 mg/dL on calcium 0.3–0.6 g/d and vitamin D 375–750 IU daily; she was not taking any active vitamin D. At the time of the most recent visit in December 2012 almost 2 years off PTH, she was taking calcium 0.6 g/d and required no active or parent vitamin D. Her laboratories from that visit were significant for the following: serum calcium 9.6 mg/dL, PTH 24 pg/mL, phosphorus 3.6 mg/dL, and urine calcium 154 mg/total volume 1310 mL. Her most recent laboratory tests in March 2013 demonstrated a serum calcium level of 9.3 mg/dL.
Case 3
A 39-year-old woman developed hypoparathyroidism after total thyroidectomy for Graves' disease in 1995, despite receiving a parathyroid autotransplant. She had no history of fractures or nephrolithiasis. At the time of study enrollment in 2006, she required calcium 5.2–7.8 g daily and 1,25-dihydroxyvitamin D 0.5 μg daily. Other medications included clonazepam and aspirin. Laboratories at study enrollment were significant for: calcium 9.9 mg/dL, PTH < 3 pg/mL, phosphorus 3.7 mg/dL, 24-hour urine calcium 313 mg/total volume 3100 mL. PTH(1–84) was started at a dose of 100 μg every other day. At month 39, she discontinued PTH because of hypercalcemic symptoms with a regimen consisting of calcium 0.6–0.8 g/d and vitamin D 2000 IU/wk; she had previously stopped all active vitamin D. Laboratories 3 days after discontinuing PTH demonstrated a PTH level of 27 pg/mL with a serum calcium of 9.8 mg/dL. Repeat values 17 days after discontinuing PTH(1–84) were significant for PTH 32 pg/mL with a concurrent serum calcium of 9.2 mg/dL. Laboratories at the time of her last study visit 5 months before discontinuation demonstrated a serum phosphorus of 3.9 mg/dL and 24-hour urine calcium of 146 mg/total volume 2700 mL.
Case 4
A 47-year-old woman developed hypoparathyroidism after multiple parathyroid gland resections for primary hyperparathyroidism. Her first operation in 2001 was unsuccessful. She underwent a second parathyroid operation in 2002 with a parathyroid implant placed into the strap muscles; she was subsequently diagnosed with postoperative hypoparathyroidism. She had a history of nephrolithiasis while she had hyperparathyroidism, but no kidney stones were apparent after her diagnosis of hypoparathyroidism. At the time of study enrollment in 2007, her supplement requirements included calcium 2–8 g and 1,25-dihydroxyvitamin D 0.5 μg daily. Other medications included valsartan, metoprolol, and albuterol as needed. Laboratories at study enrollment were significant for: calcium 9.3 mg/dL, PTH < 3 pg/mL, phosphorus 4.1 mg/dL, 24-hour urine calcium 184 mg/total volume 2000 mL. She started PTH(1–84) at a dose of 100 μg every other day. At month 18, her dose was adjusted to 100 μg every 4 days. At the time of her month 30 visit, PTH was 20.4 pg/mL with a serum calcium of 8.8 mg/dL 2 days after her last PTH injection off calcium, parent and active vitamin D supplementation. Other laboratories at the time of her month 30 visit demonstrated phosphorus of 4.2 mg/dL and 24-hour urine calcium of 65 mg/total volume 1850 mL. She self-discontinued PTH(1–84) at month 36 of the study due to symptoms of hypercalcemia with normal serum calcium levels. PTH was measured 10 months off PTH(1–84) and was 17 pg/mL with a concurrent serum calcium of 10.0 mg/dL. Her most recent laboratory tests in June 2013 demonstrate a serum calcium of 9.6 mg/dL with a concurrent PTH of 38 pg/mL off calcium and active vitamin D supplementation.
Discussion
In this case series, we report four patients enrolled in our research study of PTH(1–84) therapy who demonstrated recovery from postoperative hypoparathyroidism 8 to 16 years after their initial diagnosis. The plasma half-life of PTH(1–84) is 2.2 hours (13), leading to our definition of recovery in which PTH levels were normal at least 1 week off PTH therapy. At the time of recovery, all subjects had serum calcium and PTH levels within the normal range off PTH therapy.
Cases 1 and 2 had a milder form of the disease at study enrollment as evidenced by their relatively low supplement requirements and detectable, although low, PTH levels. The etiology for the low urine calcium excretion in case 1 is unclear, but use of hydrochlorothiazide may have played a role. Cases 3 and 4 had higher supplement requirements and undetectable PTH levels at the time of study entry. Three of our four subjects (cases 1, 2, and 4) had previously undergone parathyroid surgery for primary hyperparathyroidism, which is a high percentage given the overall number of subjects with postoperative hypoparathyroidism after parathyroid surgery compared to the incidence after thyroid surgery. The reason for this is unclear but may reflect a greater risk for removal or irreversible damage to the parathyroid glands during surgery for large goiters or malignancy. Genetic testing was not performed in the individuals who originally presented with primary hyperparathyroidism. Although it is possible that the recovery of parathyroid function in these patients may represent an early stage of relapse, laboratory results 2 to 4 years after recovery give no indication of relapse, namely hypercalcemia.
The rates of postoperative hypoparathyroidism vary based on surgical expertise and the extent of the surgery. Transient hypoparathyroidism is due to parathyroid “stunning” and usually resolves within a few weeks. Transient hypoparathyroidism is relatively common after thyroid surgery, with rates ranging from 6.9 to 46% (1–3). The reported rates of permanent postoperative hypoparathyroidism are much lower than those for transient hypoparathyroidism, ranging from 0.9 to 1.6% at surgical centers with experienced endocrine surgeons (4–6). Postoperative hypoparathyroidism is usually considered permanent when it persists for at least 6 months, although delayed recovery of hypoparathyroidism through the first postoperative year has been described (8, 9). One surgical cohort documented parathyroid autotransplant function 2.5 and 4 years after reimplantation of cryopreserved parathyroid tissue (14). To our knowledge, there are no case reports describing patients in whom recovery of functional parathyroid tissue occurred more than 4 years after diagnosis.
Halsted (15) first established that parathyroid tissue can survive explant and autotransplantation in dogs in 1907. Published rates of parathyroid autotransplantation survival greater than 80% demonstrate that parathyroid cells are very robust despite relative ischemia (16). Although all our patients were treated with PTH(1–84) for some period of time, we cannot ascertain what role, if any, administration of exogenous PTH played in the recovery of parathyroid function. There are several intriguing mechanisms that could conceivably account for a role of exogenous PTH in the recovery of parathyroid cell function. Calcium and vitamin D are known to negatively regulate parathyroid function (17, 18). Reduced supplement requirements may have decreased these inhibitory actions, resulting in parathyroid recovery. However, this is unlikely because the serum calcium did not fall with a reduction in calcium supplement requirements. Moreover, in the natural history of hypoparathyroidism, periods of hypocalcemia are not infrequent. Their remaining parathyroid tissue would have had ample time to be stimulated by hypocalcemic events that inevitably must have occurred in their long history of frank hypoparathyroidism.
Another potential explanation for the appearance of PTH function after PTH administration might be related to direct trophic effects of PTH on parathyroid tissue, triggering pathways leading to enhanced vascularization. Alternatively, PTH could be influencing parathyroid cell function indirectly by stimulating factors such as vascular endothelial growth factor (VEGF), which has been shown to be necessary for the induction of parathyroid-mediated angiogenesis (19). Administration of PTH(1–34) increases expression of VEGF in human endothelial (20) and osteoprogenitor cells (21). Exogenous PTH(1–84) increases VEGF levels in rat tibial metaphyses, in addition to spatially redistributing small blood vessels (22). Further studies will be necessary to determine whether PTH administration in human subjects results in systemic elevations of VEGF levels and whether this would be sufficient to induce angiogenesis in parathyroid tissue. Finally, it is possible that previously autotransplanted or in situ parathyroid tissue may have gradually become revascularized and functional on its own, in the absence of any possible effect of exogenous PTH administration. If this is the explanation, the time course of recovery is extraordinarily long. Without selective venous sampling, we cannot ascertain without any doubt that parathyroid tissue is indeed the source of circulating PTH. A remote possibility is a nonparathyroid source of the PTH (23).
Whatever mechanism was ultimately operative in these subjects, this case series documents that recovery of postoperative hypoparathyroidism can occur many years after the initial diagnosis. Hypoparathyroid patients who develop hypercalcemia while on therapy should be investigated for recovery of parathyroid function.
Acknowledgments
This work was supported by National Institutes of Health Grant DK069350, Food and Drug Administration Grant 002525, and NPS Pharmaceuticals.
Disclosure Summary: J.P.B. is a consultant for Amgen, Eli Lilly, Radius, NPS Pharmaceuticals, Merck, Warner Chilcott, and GSK and receives research support from NPS Pharmaceuticals and Amgen. M.R.R. receives research support from NPS Pharmaceuticals. No conflicts of interest are reported for the remaining authors.
Footnotes
- VEGF
- vascular endothelial growth factor.
References
- 1. Falk SA, Birken EA, Baran DT. Temporary postthyroidectomy hypocalcemia. Arch Otolaryngol Head Neck Surg. 1988;114:168–174 [DOI] [PubMed] [Google Scholar]
- 2. Percival RC, Hargreaves AW, Kanis JA. The mechanism of hypocalcaemia following thyroidectomy. Acta Endocrinol (Copenh). 1985;109:220–226 [DOI] [PubMed] [Google Scholar]
- 3. See AC, Soo KC. Hypocalcaemia following thyroidectomy for thyrotoxicosis. Br J Surg. 1997;84:95–97 [PubMed] [Google Scholar]
- 4. Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery. 2003;133:180–185 [DOI] [PubMed] [Google Scholar]
- 5. Zarnegar R, Brunaud L, Clark OH. Prevention, evaluation, and management of complications following thyroidectomy for thyroid carcinoma. Endocrinol Metab Clin North Am. 2003;32:483–502 [DOI] [PubMed] [Google Scholar]
- 6. Page C, Strunski V. Parathyroid risk in total thyroidectomy for bilateral, benign, multinodular goitre: report of 351 surgical cases. J Laryngol Otol. 2007;121:237–241 [DOI] [PubMed] [Google Scholar]
- 7. Bilezikian JP, Khan A, Potts JT, Jr, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26:2317–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Claussen MS, Pehling GB, Kisken WA. Delayed recovery from post-thyroidectomy hypoparathyroidism: a case report. Wis Med J. 1993;92:331–334 [PubMed] [Google Scholar]
- 9. Sitges-Serra A, Ruiz S, Girvent M, Manjón H, Dueñas JP, Sancho JJ. Outcome of protracted hypoparathyroidism after total thyroidectomy. Br J Surg. 2010;97:1687–1695 [DOI] [PubMed] [Google Scholar]
- 10. Rubin MR, Sliney J, Jr, McMahon DJ, Silverberg SJ, Bilezikian JP. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int. 2010;21:1927–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rubin MR, Dempster DW, Sliney J, Jr, et al. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res. 2011;26:2727–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cusano NE, Rubin MR, McMahon DJ, et al. Therapy of hypoparathyroidism with PTH(1–84): a prospective four-year investigation of efficacy and safety. J Clin Endocrinol Metab. 2013;98:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sikjaer T, Amstrup AK, Rolighed L, Kjaer SG, Mosekilde L, Rejnmark L. PTH (1–84) replacement therapy in hypoparathyroidism: a randomized controlled trial on pharmacokinetic and dynamic effects following 6 months of treatment [published online ahead of print May 3, 2013]. J Bone Miner Res. doi:10.1002/jbmr.1964 [DOI] [PubMed] [Google Scholar]
- 14. Lambert LA, Shapiro SE, Lee JE, et al. Surgical treatment of hyperparathyroidism in patients with multiple endocrine neoplasia type 1. Arch Surg. 2005;140:374–382 [DOI] [PubMed] [Google Scholar]
- 15. Halsted W. Hypoparathyreosis, status parathyreoprivus, and transplantation of the parathyroid glands. Am J Med Sci. 1907;134:1–5 [Google Scholar]
- 16. Moffett JM, Suliburk J. Parathyroid autotransplantation. Endocr Pract. 2011;17(suppl 1):83–89 [DOI] [PubMed] [Google Scholar]
- 17. Habener JF, Rosenblatt M, Potts JT., Jr Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev. 1984;64:985–1053 [DOI] [PubMed] [Google Scholar]
- 18. Cantley LK, Russell J, Lettieri D, Sherwood LM. 1,25-Dihydroxyvitamin D3 suppresses parathyroid hormone secretion from bovine parathyroid cells in tissue culture. Endocrinology. 1985;117:2114–2119 [DOI] [PubMed] [Google Scholar]
- 19. Carter WB, Uy K, Ward MD, Hoying JB. Parathyroid-induced angiogenesis is VEGF-dependent. Surgery. 2000;128:458–464 [DOI] [PubMed] [Google Scholar]
- 20. Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates the endothelial expression of vascular endothelial growth factor. Eur J Clin Invest. 2008;38:798–803 [DOI] [PubMed] [Google Scholar]
- 21. Drake MT, Srinivasan B, Mödder UI, et al. Effects of intermittent parathyroid hormone treatment on osteoprogenitor cells in postmenopausal women. Bone. 2011;49:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prisby R, Guignandon A, Vanden-Bossche A, et al. Intermittent PTH(1–84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J Bone Miner Res. 2011;26:2583–2596 [DOI] [PubMed] [Google Scholar]
- 23. Liu Z, Farley A, Chen L, et al. Thymus-associated parathyroid hormone has two cellular origins with distinct endocrine and immunological functions. PLoS Genet. 2010;6:e1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]