Abstract
Context:
Primary aldosteronism is a heterogeneous group of disorders comprising both sporadic and familial forms. Mutations in the KCNJ5 gene, which encodes the inward rectifier K+ channel 4 (G protein-activated inward rectifier K+ channel 4, Kir3.4), cause familial hyperaldosteronism type III (FH-III) and are involved in the pathogenesis of sporadic aldosterone-producing adenomas.
Objective:
The objective of the study was to characterize the effects of a newly described KCNJ5 mutation in vitro.
Patients and Methods:
The index case is a 62-year-old woman affected by primary aldosteronism, who underwent left adrenalectomy after workup for adrenal adenoma. Exon 1 of KCNJ5 was PCR amplified from adrenal tissue and peripheral blood and sequenced. Electrophysiological and gene expression studies were performed to establish the functional effects of the new mutation on the membrane potential and adrenal cell CYP11B2 expression.
Results:
KCNJ5 sequencing in the index case revealed a new p.Y152C germline mutation; interestingly, the phenotype of the patient was milder than most of the previously described FH-III families. The tyrosine-to-cysteine substitution resulted in pathological Na+ permeability, cell membrane depolarization, and disturbed intracellular Ca2+ homeostasis, effects similar, albeit smaller, to the ones demonstrated for other KCNJ5 mutations. Gene expression studies revealed an increased expression of CYP11B2 and its transcriptional regulator NR4A2 in HAC15 adrenal cells overexpressing KCNJ5Y152C compared to the wild-type channel. The effect was clearly Ca2+-dependent, because it was abolished by the calcium channel blocker nifedipine.
Conclusions:
Herein we describe a new germline mutation in KCNJ5 responsible for FH-III.
Primary aldosteronism (PA) is a heterogeneous group of disorders comprising both sporadic (bilateral adrenal hyperplasia, BAH, and aldosterone-producing adenoma, APA) and familial forms. Three forms of familial hyperaldosteronism (FH) have been reported thus far, named familial hyperaldsteronism types I, II, and III (FH-I to FH-III) (1).
The first family affected by FH-III was described as an early-onset and particularly severe form of PA, with profound hypokalemia, resistant hypertension, and marked BAH (2). Subsequently the molecular basis of FH-III was identified as a germline mutation (p.T158A) in the KCNJ5 gene encoding the G protein-activated inward rectifier K+ channel 4. Adrenal zona glomerulosa cells display high resting K+ conductance, responsible for cell membrane hyperpolarization. Electrophysiological studies demonstrated that the p.T158A KCNJ5 mutation, located near the selectivity filter of the channel, results in loss of ion selectivity and sodium entry, thus leading to membrane depolarization (3). Following these original findings, an additional six families and three germline KCNJ5 mutations (p.G151R, p.G151E, p.I157S) were reported (4–6). Further in vitro studies established that the overexpression of KCNJ5 mutations in HAC15 adrenocortical cells resulted in Na+ and Ca2+ influx, increased transcription of CYP11B2 and of the associated regulatory factors, NR4A2 and NR4A3, thus increasing aldosterone production (7, 8).
Interestingly, mutations in the KCNJ5 gene have also been implicated in the pathogenesis of around 40% of sporadic APA (1).
Herein we describe a patient with a mild form of PA, caused by a newly described germline point mutation (p.Y152C) in the KCNJ5 gene and report the functional characterization of the mutation in vitro.
Materials and Methods
Detailed Materials and Methods are provided in the Supplemental Materials and Methods published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Results
Phenotype of the index case
The index case is a 62-year-old African-American woman. She was first diagnosed with hypertension at the age of 48, when she presented for nausea and headache at the Georgia Regents University Emergency Department. She displayed resistant hypertension (180/110 mmHg) on four antihypertensive medications and severe hypokalemia (2.1 mEq/L) despite potassium supplementation. In 2008 she again presented to the Emergency Department for nausea, vomiting, headache, and elevated blood pressure (250/130 mmHg). Abdominal computed tomographic (CT) scanning showed nodularity in the left adrenal gland (several subcentimeter nodules, the largest being 8 mm). Hormonal testing under treatment with angiotensin-converting-enzyme inhibitor revealed plasma aldosterone 24 ng/dL and suppressed plasma renin activity (PRA; <0.6 ng/mL/h). Catecholamine levels and dexamethasone suppression test were normal. The patient underwent adrenal vein sampling, but the right adrenal vein was not cannulated successfully: left adrenalectomy was performed on the basis of CT findings and difficult-to-control blood pressure. Postoperatively, plasma aldosterone levels had decreased, but PRA remained suppressed. Fourteen months after surgery (May 2010), plasma aldosterone increased and PRA remained suppressed. She was not treated with mineralocorticoid receptor antagonists because of reduced renal function that progressed to end-stage renal disease. All 10 siblings and five sons were hypertensive.
Identification of a new KCNJ5 mutation
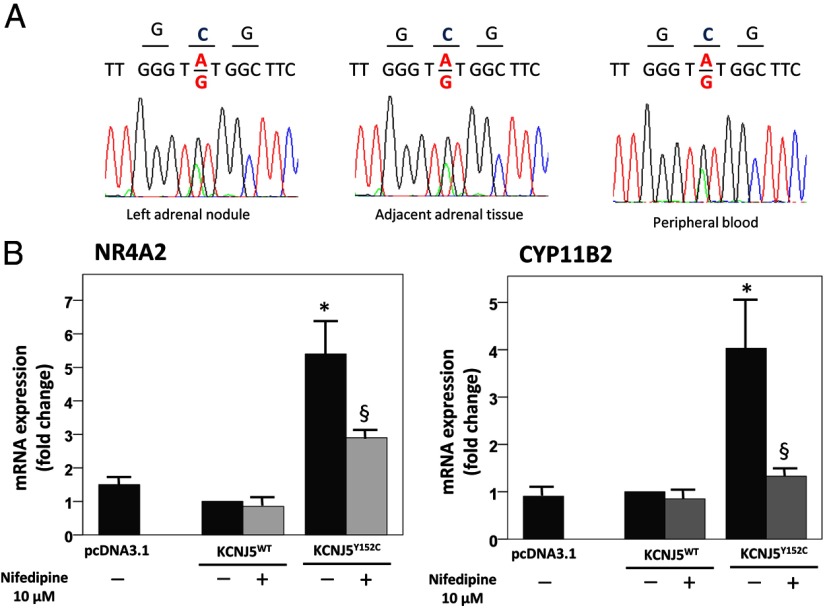
A 610 bp of the exon 1 of the KCNJ5 gene was PCR amplified and sequenced. In both adrenal nodule and adjacent tissue and peripheral blood the heterozygous point mutation c.455A>G was identified, corresponding to a tyrosine (Y) to cysteine (C) substitution at amino acid position 152 (Figure 1A). We also searched for the p.Y152C mutation by single nucleotide polymorphism assays on genomic DNA extracted from patients (n = 100) affected by BAH; we could not identify the 455A>G substitution in any of the analyzed samples.
Figure 1.
(A) Sequences of tumor cDNA, adjacent adrenal tissue, and peripheral blood gDNA of KCNJ5 codons 151–153 showing the c.455A>G substitution resulting in the p.Y152C mutation. (B) Real-time PCR analysis of CYP11B2 and NR4A2 in HAC15 cells overexpressing KCNJ5Y152C compared with cells overexpressing KCNJ5WT and pcDNA3.1 empty vector. Each bar represents the mean ± SE of relative fold change of gene expression in three independent experiments. Each assay was performed in triplicate, and glyceraldehyde-3-phosphate dehydrogenase was used as endogenous control. *, P < .05 compared with WT. §, P < .05 compared with KCNJ5Y152C not treated with nifedipine.
Immunohistochemistry and microarray analyses of adrenal tissue
Immunohistochemical staining of the adrenal tissue showed nodular structures with diffuse KCNJ5 expression and variable CYP11B2 expression. The adjacent cortex showed a variable degree of glomerulosa hyperplasia (Supplemental Figure 1).
Microarray analysis for steroidogenic enzymes in the adrenal tissue harboring the Y152C mutation showed 1.8-fold higher CYP11B2 levels than normal adrenals. Along with CYP11B2, transcription factors NR4A2 and NR4A3 were also up-regulated (Supplemental Figure 2).
Gene expression studies on HAC15 cells
To investigate the effects of the KCNJ5Y152C mutation in adrenal cell function, HAC15 cells were transfected with pcDNA3.1/KCNJ5Y152C, pcDNA3.1/KCNJ5WT, and pcDNA3.1 empty vector and gene expression was analyzed by real-time PCR.
We observed a significant up-regulation of CYP11B2 (4-fold) and its transcription factor NR4A2 (5.4-fold) in cells overexpressing KCNJ5Y152C compared to KCNJ5WT and cells transfected with empty vector (Figure 1B). No statistically significant difference was observed between HAC15 cells overexpressing KCNJ5WT and pcDNA3.1 mock-transfected cells for either of the two selected genes.
In cells transfected with the mutant KCNJ5, treatment with nifedipine (10 μM) decreased CYP11B2 and NR4A2 overexpression by 90% and 57%, respectively (Figure 1B).
Biophysical properties of KCNJ5Y152C
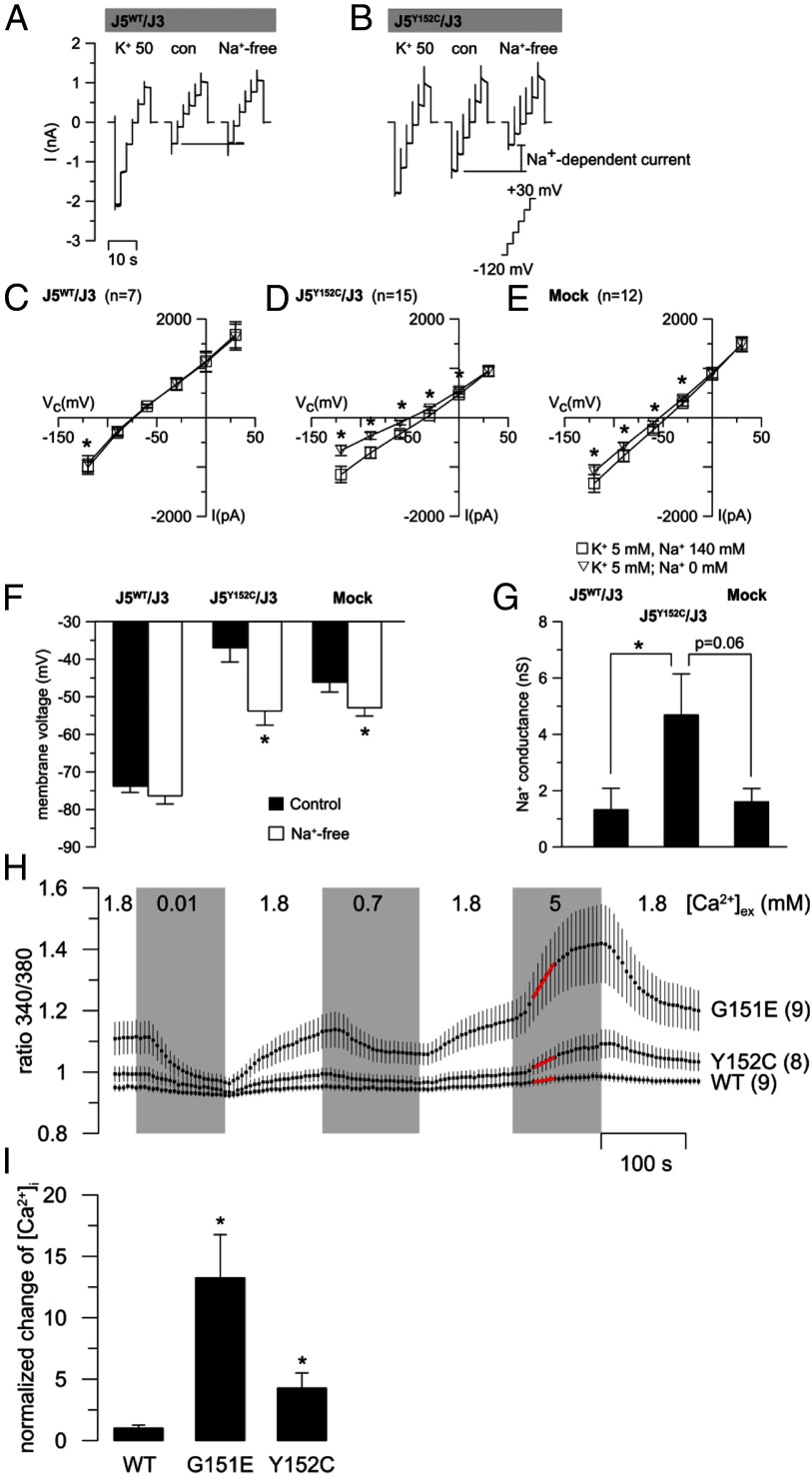
HEK293 cells were transiently cotransfected with mutant or wild-type KCNJ5 and KCNJ3. At high extracellular K+ (50 mM), wild-type transfected cells displayed a large inward (negative) current that was absent after replacement of 45 mM extracellular K+ with Na+ (con; 5 mM K+) (Figure 2A). Replacement of extracellular Na+ by the larger cation N-methyl-d-glucamine (NMDG; Na+ free) had no further effect. Cells expressing KCNJ5Y152C/KCNJ3 also displayed a reduced inward current after replacement of extracellular K+ by Na+ (Figure 2B). However, replacement of extracellular Na+ by NMDG (Na+-free) reduced the inward current further, consistent with a pathological Na+ permeability of the mutated channel (Figure 2, B and D). Mock-transfected cells showed a significant albeit smaller Na+ conductance (Figure 2E). The membrane voltage of cells expressing KCNJ5Y152C/KCNJ3 was depolarized due to the pathological Na+ influx through the mutated channel (−37 mV in KCNJ5Y152C/KCNJ3 vs −74 mV in KCNJ5WT/KCNJ3 cells). Accordingly, cells expressing the mutated channel were hyperpolarized when bath Na+ was replaced by NMDG (Figure 2, F and G).
Figure 2.
Basic characteristics of the KCNJ5Y152C mutant channel. (A, B) Representative current traces of a wild-type KCNJ5/KCNJ3 (J5WT/J3; left panel) and of a mutant KCNJ5Y152C/KCNJ3 (J5Y152C/J3; right panel) expressing HEK293 cell are shown. Current traces were recorded at 50 mM extracellular K+ (K+ 50), 5 mM extracellular K+ (con), and after replacement of extracellular Na+ by NMDG (Na+-free). (C–E) I/V curves of similar whole cell experiments as shown in (A) and (B). (F) Effect of Na+ replacement on the membrane voltage. *, Significant differences compared to control conditions. (G) Na+-dependent conductance (calculated from the conductance between −120 and −90 mV from the data shown in [C–E]) was highest in KCNJ5Y152C/KCNJ3 cells. Effects of extracellular Ca2+ concentration on cytosolic Ca2+ concentration. (Fura-2 fluorescence). (H) Mean values of the Fura-2 ratio (340 nm/380 nm) ± SEM in cells expressing KCNJ5WT/KCNJ3 (WT), KCNJ5G151E/KCNJ3 (G151E), and KCNJ5Y152C/KCNJ3 (Y152C) at various extracellular Ca2+ concentrations. Numbers of experiments are shown in parentheses. (I) Initial rate of Fura-2 ratio changes induced by 5 mM extracellular Ca2+. The period labeled with red dots in (H) was used for the linear fitting and values were normalized to that of the wild type. Values are mean values ± SEM. *, Significant differences compared to wild-type channels expressing cells.
The current blocked by the K+ channel blocker Ba2+ (Ba2+-sensitive current) displayed the expected rectification of the inward current in wild-type KCNJ5/KCNJ3-expressing cells. In KCNJ5Y152C/KCNJ3 cells, the Ba2+-sensitive current was very small and similar to that of mock-transfected cells (Supplemental Figure 3A). To augment the inwardly rectifying current, the extracellular K+ was increased from 5 to 50 mM. Under these conditions, KCNJ5Y152C/KCNJ3-expressing cells showed a slightly increased Ba2+-sensitive current compared to mock-transfected cells, suggesting that the KCNJ5Y152C mutant is at least partially Ba2+-sensitive (Supplemental Figure 3B) in contrast to KCNJ5G151R and KCNJ5G151E that are Ba2+ insensitive (5).
Disturbed intracellular Ca2+ homeostasis
The effect of the KCNJ5Y152C mutant on the Fura-2 ratio, a measure of intracellular Ca2+ concentration, was studied in transfected HEK293 cells. Under control conditions (extracellular Ca2+ 1.8 mM), the Fura-2 ratio was higher in KCNJ5Y152C/KCNJ3-expressing cells compared with wild-type KCNJ5/KCNJ3 cells but lower than cells expressing the mutant KCNJ5G151E/KCNJ3 (4). The highest increases in cytosolic Ca2+ were observed at 5 mM extracellular Ca2+, where clear differences were evident between cells expressing the mutated KCNJ5 and KCNJ5WT (Figure 2H). This is illustrated by the initial rate of change of the Fura-2 ratio at 5 mM extracellular Ca2+, which shows a strong increase in cytosolic Ca2+ induced by 5 mM extracellular Ca2+ in cells expressing both KCNJ5 mutants compared to the KCNJ5WT (Figure 2I). To further investigate the nature of the increased intracellular Ca2+ levels, Ca2+ was completely removed from the bath solution and replaced by EGTA (Supplemental Figure 4). Under these conditions, intracellular Ca2+ levels of cells expressing KCNJ3/KCNJ5Y152C channels strongly decreased. In cells expressing KCNJ5WT and in nontransfected cells, removal of bath Ca2+ had no such effect. Next, the purinergic agonist ATP (100 μM) was added to the bath under Ca2+-free conditions to stimulate Ca2+ release from inositol trisphosphate sensitive intracellular stores. This store-release of Ca2+ was not modified by the expression of the mutated channels. These data indicate that the increased intracellular Ca2+ of cells expressing KCNJ3/KCNJ5Y152C channels is not caused by increased release of Ca2+ from inositol trisphosphate sensitive stores but reflects pathological transport of Ca2+ across the plasma membrane.
Discussion
Mutations in the KCNJ5 gene have been implicated in the pathogenesis of both sporadic APA and FH-III. Seven FH-III families with four different KCNJ5 mutations have been reported so far (1): with the exception of those carrying the p.G151E mutation, affected members displayed severe hyperaldosteronism and uncontrolled hypertension from childhood, thus requiring bilateral adrenalectomy. By contrast, G151E-affected patients have a milder phenotype, with normal appearing adrenals, treatable with medical therapy (4, 5).
In this study, we describe a patient affected by PA due to a novel germline mutation in KCNJ5. The index case presented with resistant hypertension and hypokalemia. She underwent unilateral adrenalectomy on the basis of adrenal CT findings. KCNJ5 sequencing revealed a point c.455A>G mutation, leading to the Tyr152Cys substitution. Tyrosine 152 belongs to the GlyTyrGly motif of the K+ selectivity filter of the channel and is highly conserved among orthologs and paralogs (3).
As for p.G151E (4), the p.Y152C mutation was associated with a milder phenotype in terms of both clinical and biochemical parameters compared to other FH-III families. In particular, the patient displayed minimal changes at adrenal CT scanning; complete cortisol suppression after dexamethasone administration and aldosterone was produced at a relatively lower rate. However, electrophysiological studies showed that KCNJ5G151E channels were associated with a much larger Na+ conductance compared to other mutated channels and consequent Na+-dependent cell lethality. Therefore, both the G151E mutation with a severe impact on the channel functions and the Y152C mutation with little effect on Na+ conductance are associated with a mild clinical phenotype.
The index case was diagnosed with PA at the age of 48, whereas most FH-III patients were diagnosed before 7 years of age. However, on the basis of the severe target organ damage, with left ventricular hypertrophy and renal function impairment, we can speculate that the patient had been hypertensive for a considerable time before diagnosis.
The electrophysiological consequences of the p.Y152C mutation were similar to those described for other KCNJ5 mutations (3). The overexpression of the mutant channel resulted in cell membrane depolarization, due to a pathological Na+ permeability (albeit smaller than the one observed in other KCNJ5 mutations) (4, 5). Under physiological conditions adrenal zona glomerulosa cell membrane depolarization results in voltage-gated Ca2+ channel opening, which in turn leads to CYP11B2 transcription and aldosterone production.
In agreement with these data, we observed an impairment of cytosolic Ca2+ handling of KCNJ5Y152C-expressing cells (increased intracellular Ca2+ activity and a disturbed capacity to lower intracellular Ca2+) and at least three mechanisms could underlie this phenotype: 1) activation of voltage-gated Ca2+ channels via the depolarization induced by the Na+ influx through KCNJ5Y152C; 2) impairment of Ca2+ extrusion via Na+/Ca2+ exchange mechanisms because intracellular Na+ is increased and the membrane is depolarized; and 3) direct influx of Ca2+ through mutant channels (however this has not yet been shown).
In addition, we showed that overexpressing KCNJ5Y152C in adrenocortical cells resulted in a significant up-regulation of CYP11B2 and its transcription factor NR4A2, thus driving aldosterone production.
In conclusion, we report a new germline KCNJ5 mutation responsible for a milder and late onset form of FH-III. This further broadens the spectrum of the phenotypic presentation of FH-III patients and highlights the importance of considering this disease in apparently late onset familial PA.
Acknowledgments
We are grateful to Professor Celso Gomez-Sanchez (University of Jackson Mississippi) for providing the antibodies for CYP11B2.
This work was supported by the Deutsche Forschungsgemeinschaft (FOR1086 to R.W.) and National Institutes of Health (DK43140 to W.E.R.). S.M. is in receipt of a grant from the Italian Society of Hypertension.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- APA
- aldosterone-producing adenoma
- BAH
- bilateral adrenal hyperplasia
- CT
- computed tomography
- FH
- familial hyperaldosteronism
- NMDG
- N-methyl-d-glucamine
- PA
- primary aldosteronism
- PRA
- plasma renin activity.
References
- 1. Mulatero P, Monticone S, Rainey WE, Veglio F, Williams TA. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol. 2013;9:104–112 [DOI] [PubMed] [Google Scholar]
- 2. Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M, Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93:3117–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mulatero P, Tauber P, Zennaro MC, et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension. 2012;59:235–240 [DOI] [PubMed] [Google Scholar]
- 5. Scholl UI, Nelson-Williams C, Yue P, et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc Natl Acad Sci USA. 2012;109:2533–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charmandari E, Sertedaki A, Kino T, et al. A novel point mutation in the KCNJ5 gene causing primary hyperaldosteronism and early-onset autosomal dominant hypertension. J Clin Endocrinol Metab. 2012;97:E1532–E1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant KCNJ5 T158A expression in HAC-15 cells increases aldosterone synthesis. Endocrinology. 2012;153:1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monticone S, Hattangady NG, Nishimoto K, et al. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab. 2012;97:E1567–E1572 [DOI] [PMC free article] [PubMed] [Google Scholar]