Abstract
Context:
Disruption of the Gsα maternal allele leads to severe obesity and insulin resistance in mice and early-onset obesity in patients with pseudohypoparathyroidism (PHP) type 1a. However, insulin resistance and glucose metabolism have not been systematically characterized in patients with PHP1a.
Objective, Design, and Setting:
In a cross-sectional, case-control study, we examined insulin sensitivity, β-cell function, energy expenditure (EE), and sympathetic nervous system activity in adults with PHP1a.
Study Participants:
PHP1a patients (n = 8) and healthy control subjects (n = 24) matched for age (41 ± 7 vs 41 ± 7 years [mean ± SD]), gender, and percent body fat.
Methods:
Insulin sensitivity (SI), acute insulin response to glucose, and disposition index were assessed during a frequently sampled iv glucose tolerance test. Oral glucose insulin sensitivity (OGIS) was measured during a mixed meal. EE was measured using whole-room indirect calorimetry. Body composition was assessed via dual-energy x-ray absorptiometry and sympathetic nervous system activity by measuring 24-hour urinary catecholamine concentrations.
Results:
PHP1a patients were less insulin-sensitive than their matched controls based upon SI and OGIS. Nondiabetic PHP1a patients tended to have a lower SI (P = .09) and reduced OGIS (P = .03). Disposition index, a composite measure of β-cell function, also tended to be lower in patients (P = .07). Total caloric intake, resting EE, total EE, meal-induced thermogenesis, and 24-hour urinary catecholamine concentrations were not significantly different between the groups.
Conclusions:
Adults with PHP-1a have reduced insulin sensitivity compared with their matched controls that may contribute to the pathogenesis of glucose intolerance and diabetes in these patients.
Albright hereditary osteodystrophy (AHO) is caused by heterozygous inactivating mutations of GNAS, the stimulatory G protein α-subunit (Gsα) that mediates hormone-stimulated, receptor-mediated intracellular cAMP formation (1, 2). Due to tissue-specific imprinting, maternal Gsα mutations result in markedly reduced Gsα expression that clinically manifests, in addition to the AHO phenotype, with early-onset obesity and resistance to multiple hormones, including PTH, TSH, GHRH, and gonadotropins in their respective target tissues (pseudohypoparathyroidism type 1a [PHP1a]) (1–5). Severe obesity develops at a very young age and continues into adulthood in PHP1a patients. In contrast, AHO patients who inherit GNAS mutations on the inactive paternal allele do not develop obesity more than the general population (6). These parent-of-origin effects of tissue Gsα imprinting are also observed in mice (7). Mice with disruption of the germline maternal (but not paternal) Gsα allele (E1m−) or central nervous system-specific Gsα deletion on the maternal allele (mBrGs knockout [KO]) develop obesity and insulin-resistant diabetes associated with normal food intake but reduced sympathetic nervous system activity (SNA) and energy expenditure (EE) (8, 9). Likewise, one study showed children with PHP1a to have low levels of serum norepinephrine, consistent with low SNA, even when compared with similarly obese children without PHP1a (10). There have also been solitary case reports of young PHP1a patients that developed obesity without hyperphagia (11) and concomitant obesity and insulin resistance (12).
Based on findings in mouse models, positive energy balance due primarily to reduced EE rather than increased food intake has been proposed to play a key role in the development of obesity in PHP1a (7). Although obesity typically predisposes to insulin resistance, insulin resistance and glucose intolerance occur before the development of obesity in PHP1a mouse models (E1m−, mBrGsKO) (8, 9). Notwithstanding these intriguing findings from mice, insulin resistance and glucose metabolism have not been systematically examined in patients with PHP1a. To that end, in this case-control study, we examined insulin resistance, pancreatic β-cell function, daily EE, and SNA activity in age-, gender-, and percent body fat-matched adult PHP1a patients and healthy controls.
Subjects and Methods
Study design and study subjects
The study protocol was approved by Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases. Written informed consent was obtained from all subjects. Eight adults (7 females and 1 male) were evaluated and genotyped to confirm the diagnosis of PHP1a (6). Each patient was matched to 3 healthy controls based on age, percent body fat, and gender. Age and percent body fat matching was performed using a range of ±5.0 years and ±6%, respectively Participants were admitted to the Metabolic Clinical Research Unit as inpatients for 1 week for metabolic studies.
Study procedures
A detailed description can be found in Supplemental Materials and Methods (published on The Endocrine Society's Journals Online website at http://jcem.endojournals.org).
Measures of insulin resistance and secretion
Insulin sensitivity indexes were determined by the minimal model using data from the frequently sampled iv glucose tolerance test (FSIVGTT) (13) and a standard mixed meal (14). Data from the mixed meal were available in 7 of 8 PHP1a patients. Insulin sensitivity index from FSIVGTT (SI), oral glucose insulin sensitivity index (OGIS), the acute insulin response to glucose (AIRg), and disposition index (DI) were calculated as previously described (13, 14).
Statistical analyses
A linear mixed-effects model for repeated measures was used to compare outcomes between the 2 groups. Data were logarithm transformed where appropriate before linear regression analyses. Within-paired-groups differences were subsequently evaluated using the post hoc Tukey's honestly significant difference test. Post hoc subgroup differences were evaluated using Student's t or Wilcoxon's signed-rank test, as appropriate. P < .05 was considered to represent statistical significance. The statistical software JMP version 8.1 (SAS Institute Inc) was used for data analysis.
Results
Clinical characteristics and body composition of study subjects
Four of the PHP1a patients were diagnosed with diabetes and treated with metformin (n = 2), sulfonylurea, (n = 1), and pioglitazone (n = 1). These medications were held per protocol for 10 days before the assessment of insulin sensitivity and secretion. PHP1a patients (n = 8) were matched for age, gender, and percent body fat (Table 1). Total lean body mass, body mass index (BMI), and waist circumference were not significantly different (P > .05) between the PHP1a and control groups (Table 1). Although the diabetic and nondiabetic PHP1a (D-PHP1a and ND-PHP1a, respectively) patients were of similar age, the D-PHP1a (and their respective healthy controls) had significantly greater percent fat mass than the ND-PHP1a patients and their respective controls (Supplemental Table 1).
Table 1.
Demographics, Body Composition, Metabolic Parameters, and Incretin Levels in Adult PHP1a Patients and Matched Healthy Control Subjectsa
| Variables | Controls (n = 24) | PHP1a (n = 8) | P value |
|---|---|---|---|
| Age, y | 41 ± 1 | 41 ± 2 | .99 |
| Female/males | 21/3 | 7/1 | |
| Height, cm | 166 ± 2 | 147 ± 2 | <.001 |
| Body weight, kg | 89.9 ± 4.0 | 78.3 ± 5.2 | .34 |
| BMI, kg/m2 | 31.8 ± 1.5 | 35.9 ± 2.2 | .18 |
| Body fat, % | 38.4 ± 1.6 | 39.2 ± 3.1 | .75 |
| Total lean mass, kg | 49.2 ± 2.2 | 43.2 ± 2.5 | .66 |
| Total lean mass, % body weight | 55.4 ± 1.7 | 55.6 ± 1.8 | .94 |
| Waist circumference, cm | 94 ± 3 | 97 ± 7 | .94 |
| Fasting glucose, mmol/L | 5.20 ± 0.09 | 6.56 ± 0.70 | .02 |
| Fasting insulin, pmol/L | 66.5 ± 10.9 | 107.0 ± 25 | .14 |
| Hemoglobin A1c, %) | 5.46 ± 0.11 | 6.20 ± 0.39 | .04 |
| Total cholesterol, mmol/L | 4.88 ± 0.11 | 4.92 ± 0.32 | .74 |
| LDL cholesterol, mmol/L | 3.01 ± 0.13 | 2.93 ± 0.18 | .84 |
| HDL cholesterol, mmol/L | 1.42 ± 0.08 | 1.05 ± 0.04 | .13 |
| Triglycerides, mmol/L | 1.08 ± 0.11 | 2.57 ± 0.69 | .01 |
| Free fatty acids, μEq/L | 626 ± 59 | 693 ± 95 | .84 |
| Fasting GLP-1, pmol/L | 3.03 ± 0.86 | 3.34 ± 0.66 | .82 |
| Fasting GIP, pg/mLb | 25.6 ± 13.7 | 35.5 ± 11.8 | .56 |
| Prandial GLP-1AUC, pmol/min/Lb | 825 ± 163 | 1105 ± 282 | .51 |
| Prandial GIPAUC, ng/min/mLb | 41.4 ± 10.1 | 35.7 ± 10.9 | .66 |
| Fasting glucagon, pg/mLb | 213 ± 18 | 337 ± 60 | .04 |
Values shown are unadjusted means ± SEM. P values indicate significance for comparisons between PHP1a patients and matched control subjects using a linear mixed-effects model.
Fasting for GLP-1, GIP, and glucagon and area under the curve values after a mixed meal for GLP-1 and GIP in PHP1a patients and matched controls (n = 6).
Insulin sensitivity, insulin secretion, metabolic parameters, and incretins in study subjects
Fasting plasma glucose and hemoglobin A1c (HbA1c) were significantly higher in PHP1a patients when compared with controls (Table 1). As expected, this is due to a significant increase in HbA1c in the D-PHP1a group as compared with the ND-PHP1a and control groups, which were all similar and in the normal range. Diabetes was reasonably well controlled in the D-PHP1a group (mean HbA1c of 7.1%). Glucose and insulin levels were also significantly increased in the D-PHP1a but not the ND-PHP1a patients (Supplemental Table 1). Fasting levels of triglycerides were higher in PHP1a patients (P = .01), but total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and free fatty acid levels were comparable between the groups (Table 1). The increased triglyceride levels in the PHP1a group primarily reflected an increase in triglyceride levels in the D-PHP1a patients (Supplemental Table 1), whereas there were no differences in total, LDL, or HDL cholesterol levels between the D-PHP1a and ND-PHP1a groups (data not shown).
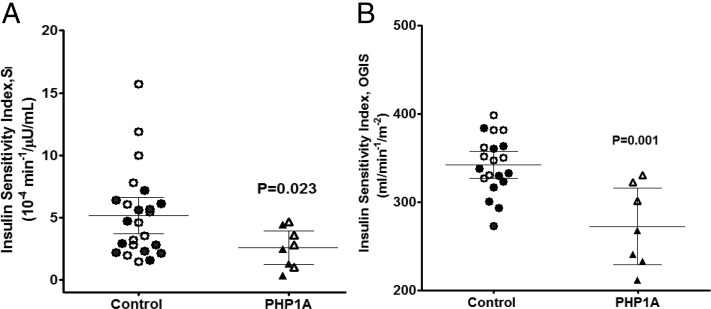
Taken as a whole cohort, PHP1a patients were less insulin-sensitive than their matched controls when measured as SI (Figure 1A) or OGIS (Figure 1B). The differences in SI between the groups remained significant even after adjustment for age and percent body fat (P = .03). When examining only the ND-PHP1a patients, the SI tended to be lower than their matched controls (3.02 ± 0.77 vs 6.22 ± 1.26, P = .09) (Figure 1A), and their mean SI was not significantly different from that of the D-PHP1a patients, which had a mean of 2.14 ± 0.88, despite the fact that D-PHP1a patients had significantly greater percent fat mass than ND-PHP1a patients. In fact, both ND-PHP1a and D-PHP1a patients had a similar reduction in SI (∼50%) compared with their respective controls.
Figure 1.
Insulin sensitivity in adults with PHP1a and matched controls. Insulin sensitivity was measured by FSIVGTT (A) and mixed meal (B). Values shown are unadjusted means ± 95% confidence intervals. P value indicates significance for comparison between PHP1a patients and matched healthy controls using a linear mixed-effects model. Filled symbols represent PHP1a subjects with type 2 diabetes and their respective controls.
ND-PHP1a patients also had a significantly lower OGIS when compared with their respective controls (319 ± 9 vs 359 ± 8 mL/min/m2, P = .03). Although OGIS was significantly higher in ND-PHP1a patients than D-PHP1a patients (319 ± 9 vs 239 ± 12, P = .004), this probably reflects differences in percent fat mass, because the ND-PHP1a controls also had higher OGIS than the D-PHP1a controls (359 ± 8 vs 328 ± 10, P = .03). Although the SI difference in the ND-PHP1a vs controls did not quite reach statistical significance due to small sample size (P = .09), the similarity in SI between ND-PHP1a and D-PHP1a patients and the significantly lower OGIS in ND-PHP1a patients (vs respective controls) provides a strong case that adult PHP1a patients are insulin-resistant independent of obesity.
D-PHP1a control subjects were matched for age, percent body fat, and gender but not for the presence of diabetes. Hyperglycemia (diabetic status) may independently affect insulin sensitivity. Thus, in a post hoc analysis, we matched D-PHP1a patients to D-non-PHP1a controls (1:1) for fasting blood glucose levels. Age, percent body fat, and HbA1c levels were not different between the groups (Supplemental Table 2). SI values were comparable between the groups (2.47 ± 0.94 vs 2.14 ± 0.88, P = .82). However, OGIS values were significantly lower in D-PHP1a patients compared with D-non-PHP1a controls (239 ± 12 vs 296 ± 23 mL/min/m2, P = .03, paired t test). These findings together suggest that PHP1a patients have reduced insulin sensitivity independent of their level of obesity or the presence or absence of diabetes.
The fact that SI was similar between D-non-PHP1a and D-PHP1a patients whereas OGIS was higher in ND-PHP1a and D-non-PHP1a than D-PHP1a patients may be due to the fact that factors related to oral administration that do not reflect intrinsic insulin sensitivity, such as gastric emptying, absorption, or incretin responses, might contribute to OGIS and may be specifically altered in patients with diabetes. Alternatively, it is possible that there may be less separation in SI between ND-PHP1a and D-PHP1a patients because the discriminant power of SI in this range may be weak.
Glucose effectiveness, a measure that reflects the combined effect of glucose to enhance glucose uptake and suppress endogenous glucose production at basal insulin levels was similar between the groups (0.013 ± 0.002 minutes−1 for PHP1a vs 0.017 ± 0.002 minutes−1 for controls, P = .35). AIRg, a measure of β-cell function, was also comparable between groups (230 ± 78 μU/mL/min for PHP1a vs 409 ± 94 μU/mL/min for controls, P = .49). However, DI, which takes into account β-cell compensation for the degree of insulin sensitivity (SI), tended to be lower in PHP1a patients (541 ± 202 vs 1782 ± 285 minutes−1 for controls, P = .07). DI tended to be lower in ND-PHP1a patients (P = .11) and was significantly lower in D-PHP1a patients (P = .03), although there were large differences in DI between the 2 respective control groups (Supplemental Table 1). Finally, fasting and postprandial plasma levels of glucagon-like peptide 1 (GLP-1) and gastric inhibitory peptide (GIP) were not different between the groups (Table 1). However, fasting glucagon levels were higher in PHP1a patients compared with control subjects (Table 1). There were no significant differences in GLP-1, GIP, or glucagon levels between the ND-PHP1a and D-PHP1a patients (data not shown).
EE, SNA, and aerobic fitness
Total caloric intake, resting and total EE, and meal-induced thermogenesis were not significantly different between the groups (Supplemental Table 3). The 24-hour urinary catecholamines, a measure of SNA, were not significantly different between PHP1a patients and controls (Supplemental Table 3). Resting heart rate and blood pressure were also not different between the groups. Maximal oxygen consumption was significantly lower in PHP1a patients (Supplemental Table 3). There were no significant differences between ND-PHP1a and D-PHP1a patients in resting EE or urine catecholamine levels (data not shown).
Discussion
In the present study, we show that adults with PHP1a are less insulin-sensitive as compared with healthy controls that were matched to age, sex, and percent body fat. Although it is well recognized that PHP1a patients develop obesity, systematic evaluation of glucose metabolism has not been previously examined. Our cohort of PHP1a patients were less insulin-sensitive and tended to have impaired pancreatic β-cell function that together may have contributed to the high incidence of glucose intolerance and diabetes observed in this group. However, the nondiabetic as well as diabetic PHP1a patients showed a reduction in insulin sensitivity indicating that this finding was not simply a reflection of the higher prevalence of diabetes in our patient cohort. Moreover, it reinforces that insulin resistance precedes and likely contributes to the development of diabetes in these patients.
Age, gender, and adiposity are major determinants of insulin resistance. Therefore, we chose to match our groups for these confounders. BMI is influenced by body proportions, and because short stature is a characteristic feature of AHO, it may not be a reliable measure of adiposity in this group (2, 6). Therefore, we chose to match our cohorts for percent body fat to evaluate obesity-independent metabolic effects of Gsα mutations in humans. The presence of lower insulin sensitivity in PHP1a patients when compared with similarly obese controls indicates that factors beyond obesity contribute to lower insulin sensitivity in these patients and is further supported by the fact that the lower levels of insulin sensitivity in our patients was independent of age and degree of adiposity. The finding that PHP1a patients develop insulin resistance in excess to the extent accounted for by obesity is consistent with observations in a mouse model of PHP1a (mBrGsKO) in which glucose intolerance and insulin resistance develop before the onset of obesity (9). This effect may result from impaired signaling of central melanocortins, which are known to alter peripheral insulin sensitivity and to activate Gsα signaling pathways (7, 15). Low cardiorespiratory fitness (CRF) is associated with insulin resistance, glucose intolerance, and type 2 diabetes (16). PHP1a patients had lower CRF compared with age- and adiposity-matched healthy controls. The role of CRF in lower insulin sensitivity in PHP1a cannot be ascertained in this cross-sectional study. Whether low CRF is observed in younger individuals with PHP1a is also not known. Longitudinal studies that phenotype fatness, fitness, and glucose metabolism may provide additional insights.
β-Cell function tended to be reduced in PHP1a patients. Gsα is known to mediate the effects of incretins such as GLP-1 and GIP in β-cells, and total Gsα deficiency in β-cells in mice leads to a severe loss of β-cell mass and insulin-deficient diabetes (17, 18). There were no significant differences in fasting or postprandial levels of circulating GLP-1 or GIP in our study. In contrast, fasting glucagon levels were elevated in PHP1a patients. This is consistent with the finding of normal fasting levels of GLP-1 but glucagon levels that were not inappropriately high for the prevailing level of glycemia in mice with β-cell-specific (βGsKO) or whole pancreas (PGsKO) Gsα deficiency (17, 18). In light of these findings, evaluating incretin effect and/or direct effects of GLP-1 administration on insulin secretion in PHP1a subjects would be intriguing in future studies. Similarly, elevated glucagon levels may contribute to fasting hyperglycemia. Although there is no evidence for Gsα imprinting in β- or α-cells, Gsα haploinsufficiency in these cells of PHP1a patients may contribute to a partial impairment of β-cell function and/or elevated glucagon levels, a finding that needs further confirmation in larger studies.
Studies in mice show that obesity associated with maternal Gsα mutations results from Gsα deficiency in one or more regions of the central nervous system, leading to an impairment of central melanocortins to stimulate SNA and EE (7–9). However, in this study, we were unable to find significant differences in either resting or total EE or global SNA as determined by urinary catecholamine excretion. Differences in SNA to specific tissues that might affect EE such as adipose tissue or muscle may not be discernable by urine catecholamines, which is a measure of global SNA. We were also unable to find differences in heart rate or blood pressure that would reflect SNA to the cardiovascular system. However, meal-induced thermogenesis, another response believed to require central melanocortin activation, tended to be lower in PHP1a patients, similar to what has been observed in children with PHP1a (19). Our findings differ from 2 studies performed in children with PHP1a. In one study, children with PHP1a had a profound reduction in serum norepinephrine compared with similarly obese controls, consistent with lower SNA (10). The other more recent study demonstrated reduced resting EE in children with PHP1a as compared with obese controls (19). It should be noted that in the latter study, the diagnosis of PHP1a was genetically confirmed in only 3 of 6 cases in the study cohort and that 3 of the 6 in the cohort had elevated TSH levels, suggesting that hypothyroidism may have contributed to the observed reduction in resting EE. Nevertheless, differences observed in our study vs the studies in children may reflect the fact that the metabolic differences are more significant in children than in adults, consistent with the fact that the obesity in PHP1a is very early-onset and is more severe in the pediatric than in the adult population (6).
In conclusion, in this study, we systematically examined insulin action and secretion and demonstrate that adults with PHP1a are insulin-resistant. Our data suggest that patients with PHP1a are at higher risk for diabetes than individuals of the same age and degree of adiposity. PHP1a patients may benefit from routine monitoring for glucose intolerance and interventions that improve insulin sensitivity.
Acknowledgments
This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, and U.S. Food and Drug Administration Orphan Products Development Grant R01 FD-R-002568 (to E.L.G.-L.).
Disclosure Summary: There are no potential conflicts of interest relevant to this article.
Footnotes
- AHO
- Albright hereditary osteodystrophy
- AIRg
- acute insulin response to glucose
- BMI
- body mass index
- CRF
- cardiorespiratory fitness
- DI
- disposition index
- D-PHP1a
- diabetic PHP1a
- EE
- energy expenditure
- FSIVGTT
- frequently sampled iv glucose tolerance test
- GIP
- gastric inhibitory peptide
- GLP-1
- glucagon-like peptide 1
- Gsα
- G protein α-subunit
- HbA1c
- hemoglobin A1c
- HDL
- high-density lipoprotein
- KO
- knockout
- LDL
- low-density lipoprotein
- ND-PHP1a
- nondiabetic PHP1a
- OGIS
- oral glucose insulin sensitivity index
- PHP1a
- pseudohypoparathyroidism type 1a
- SI
- insulin sensitivity index from FSIVGTT
- SNA
- sympathetic nervous system activity.
References
- 1. Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein α-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705 [DOI] [PubMed] [Google Scholar]
- 2. Mantovani G. Pseudohypoparathyroidism: diagnosis and treatment. J Clin Endocrinol Metab. 2011;96:3020–3030 [DOI] [PubMed] [Google Scholar]
- 3. Weinstein LS, Chen M, Xie T, Liu J. Genetic diseases associated with heterotrimeric G proteins. Trends Pharmacol Sci. 2006;27:260–266 [DOI] [PubMed] [Google Scholar]
- 4. Germain-Lee EL, Groman J, Crane JL, Jan de Beur SM, Levine MA. Growth hormone deficiency in pseudohypoparathyroidism type 1a: another manifestation of multihormone resistance. J Clin Endocrinol Metab. 2003;88:4059–4069 [DOI] [PubMed] [Google Scholar]
- 5. Mantovani G, Maghnie M, Weber G, et al. Growth hormone-releasing hormone resistance in pseudohypoparathyroidism type Ia: new evidence for imprinting of the Gsα gene. J Clin Endocrinol Metab. 2003;88:4070–4074 [DOI] [PubMed] [Google Scholar]
- 6. Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Gsα in the development of human obesity. J Clin Endocrinol Metab. 2007;92:1073–1079 [DOI] [PubMed] [Google Scholar]
- 7. Chen M, Nemechek NM, Mema E, Wang J, Weinstein LS. Effects of deficiency of the G protein Gsα on energy and glucose homeostasis. Eur J Pharmacol. 2011;660:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen M, Wang J, Dickerson KE, et al. Central nervous system imprinting of the G protein Gsα and its role in metabolic regulation. Cell Metab. 2009;9:548–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen M, Gavrilova O, Liu J, et al. Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci U S A. 2005;102:7386–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carel JC, Le Stunff C, Condamine L, et al. Resistance to the lipolytic action of epinephrine: a new feature of protein Gs deficiency. J Clin Endocrinol Metab. 1999;84:4127–4131 [DOI] [PubMed] [Google Scholar]
- 11. Dekelbab BH, Aughton DJ, Levine MA. Pseudohypoparathyroidism type 1A and morbid obesity in infancy. Endocr Pract. 2009;15:249–253 [DOI] [PubMed] [Google Scholar]
- 12. Nwosu BU, Lee MM. Pseudohypoparathyroidism type 1a and insulin resistance in a child. Nat Rev Endocrinol. 2009;5:345–350 [DOI] [PubMed] [Google Scholar]
- 13. Muniyappa R, Sachdev V, Sidenko S, et al. Postprandial endothelial function does not differ in women by race: an insulin resistance paradox? Am J Physiol Endocrinol Metab. 2012;302:E218–E225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rijkelijkhuizen JM, Girman CJ, Mari A, et al. Classical and model-based estimates of β-cell function during a mixed meal vs. an OGTT in a population-based cohort. Diabetes Res Clin Pract. 2009;83:280–288 [DOI] [PubMed] [Google Scholar]
- 15. Obici S, Feng Z, Tan J, Liu L, Karkanias G, Rossetti L. Central melanocortin receptors regulate insulin action. J Clin Invest. 2001;108:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grundy SM, Barlow CE, Farrell SW, Vega GL, Haskell WL. Cardiorespiratory fitness and metabolic risk. Am J Cardiol. 2012;109:988–993 [DOI] [PubMed] [Google Scholar]
- 17. Xie T, Chen M, Weinstein LS. Pancreas-specific Gsα deficiency has divergent effects on pancreatic α- and β-cell proliferation. J Endocrinol. 2010;206:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xie T, Chen M, Zhang QH, Ma Z, Weinstein LS. β-Cell-specific deficiency of the stimulatory G protein α-subunit Gsα leads to reduced β-cell mass and insulin-deficient diabetes. Proc Natl Acad Sci U S A. 2007;104:19601–19606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shoemaker AH, Lomenick JP, Saville BR, Wang W, Buchowski MS, Cone RD. Energy expenditure in obese children with pseudohypoparathyroidism type 1a. Int J Obes (Lond). 2013;37(8):1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]