Abstract
Context and Objective:
The optimal strategy for inducing fertility in men with congenital hypogonadotropic hypogonadism (CHH) is equivocal. Albeit a biologically plausible approach, pretreatment with recombinant FSH (rFSH) before GnRH/human chorionic gonadotropin administration has not been sufficiently assessed. The objective of the study was to test this method.
Design and Setting:
This was a randomized, open-label treatment protocol at an academic medical center.
Patients and Interventions:
GnRH-deficient men (CHH) with prepubertal testes (<4 mL), no cryptorchidism, and no prior gonadotropin therapy were randomly assigned to either 24 months of pulsatile GnRH therapy alone (inducing endogenous LH and FSH release) or 4 months of rFSH pretreatment followed by 24 months of GnRH therapy. Patients underwent serial testicular biopsies, ultrasound assessments of testicular volume, serum hormone measurements, and seminal fluid analyses.
Results:
rFSH treatment increased inhibin B levels into the normal range (from 29 ± 9 to 107 ± 41 pg/mL, P < .05) and doubled testicular volume (from 1.1 ± 0.2 to 2.2 ± 0.3 mL, P < .005). Histological analysis showed proliferation of both Sertoli cells (SCs) and spermatogonia, a decreased SC to germ cell ratio (from 0.74 to 0.35), and SC cytoskeletal rearrangements. With pulsatile GnRH, the groups had similar hormonal responses and exhibited significant testicular growth. All men receiving rFSH pretreatment developed sperm in their ejaculate (7 of 7 vs 4 of 6 in the GnRH-only group) and showed trends toward higher maximal sperm counts.
Conclusions:
rFSH pretreatment followed by GnRH is successful in inducing testicular growth and fertility in men with CHH with prepubertal testes. rFSH not only appears to maximize the SC population but also induces morphologic changes, suggesting broader developmental roles.
Important testicular development occurs in the first months of life as Sertoli cells (SCs) proliferate in response to the increased FSH levels of a GnRH-induced “minipuberty” (1). The subsequent 3-fold increase in testicular volume (TV) before the pubertal reactivation of the hypothalamic-pituitary-gonadal axis results almost exclusively from SCs and early germ cell proliferation (2). In early puberty, GnRH secretion is reactivated, stimulating both LH and FSH, which induce Leydig cells and SC proliferation and increase testosterone production from the mature Leydig cells. In contrast to the minipuberty, the androgen receptors are now expressed on SCs (3). Thus, intratesticular testosterone can act on SCs in concert with FSH, resulting in the first wave of spermatogenesis, ending SC proliferation (4). Because each SC can only support a finite number of germ cells, achieving a full complement of SCs is critical for spermatogenic capacity and fertility (5). Congenital hypogonadotropic hypogonadism (CHH) (normosmic idiopathic hypogonadotropic hypogonadism or Kallmann syndrome [KS] in the presence of anosmia) is characterized by GnRH deficiency with resulting failure of gonadal maturation and infertility. Men with CHH lack the minipubertal and pubertal periods of SC proliferation and present with prepubertal testes (<4 mL) (6); their lower IB serum levels reflect diminished SC numbers, as is evident histologically (7–9).
Although testosterone therapy induces virilization in men with CHH, it does not stimulate gonadal development. For testicular maturation and fertility, patients are treated with either pulsatile GnRH (stimulating release of endogenous LH and FSH) (10), human chorionic gonadotropin (hCG), or combined FSH and hCG (11). Fertility outcomes with each regimen are variable, with poorer responses in patients with signs of absent minipuberty (prepubertal testes, cryptorchidism, and/or low IB levels) (6, 12). The optimal regiment to maximize the potential for fertility in patients with severe cases, ie, those with testicular volume <4 mL, is not known. Recombinant FSH (rFSH) pretreatment might plausibly maximize the SC population before exposure to hCG or GnRH-induced endogenous LH. Studies in which rFSH was administered to diverse groups of gonadotropin-deficient patients (CHH, congenital hypopituitarism, or after surgery for intracranial tumors) have demonstrated testicular growth and increases in serum IB and anti-Müllerian hormone levels (8, 9, 13, 14), In addition to patient cohort heterogeneity, limitations of these studies include variable degrees of testicular development and cryptorchidism and no assessment of testicular morphology (8, 9, 13, 14). Thus, the aim of the present randomized study was to (1) assess fertility outcomes in men with CHH with or without rFSH pretreatment before GnRH-induced exposure to gonadotropins and (2) isolate the effect of FSH on immature human testes by analyzing both hormonal and histological responses.
Subjects and Methods
Subjects
Eighteen adult men with CHH were enrolled. All exhibited a complete absence of puberty by age 18, no history of cryptorchidism, prepubertal testes (TV <4 mL), no prior GnRH or gonadotropin therapy, and frankly hypogondal serum testosterone levels (<100 ng/dL, <3 nmol/L) with undetectable serum gonadotropin levels (<1.6 IU/L); levels of other anterior pituitary hormones, results of iron-binding studies, and MRI scans of the hypothalamo-pituitary region were normal. Five subjects were excluded from analysis because of nonadherence to the protocol or nonresponse to GnRH (see Supplemental Materials published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.). The study was approved by the Massachusetts General Hospital Human Research Committee; all subjects provided written informed consent before initiation of study-related procedures.
The baseline evaluation comprised detailed neuroendocrine profiling (6), ultrasound assessment of TV (length × width × height × 0.5236), and testicular biopsy. Subjects were then randomly assigned to either 24 months of pulsatile GnRH therapy (inducing release of endogenous LH and FSH) or 4 months of pretreatment with rFSH (Gonal-F; Merck Serono), 75 to 150 IU SC daily (target serum FSH level, 7–9 IU/L), followed by 24 months of pulsatile GnRH. After rFSH pretreatment, subjects underwent a repeat overnight frequent blood sampling study to assure that they had not undergone spontaneous puberty, repeat testicular ultrasound, and a second testicular biopsy before initiation of pulsatile GnRH as described previously (6). The men randomly assigned to 24 months of GnRH treatment only underwent a repeat biopsy after 4 months of treatment. During the 24 months of GnRH therapy, men had serial clinical assessments and monthly serum hormone measurements (6), including testicular ultrasound examinations at 12, 18, and 24 months. Once subjects were able to produce an ejaculate, monthly seminal fluid analyses were performed according to World Health Organization criteria (15). Until sperm was observed in the ejaculate, all azoospermic samples were centrifuged to ensure that even rare sperm were visualized.
Hormone assays, DNA sequencing, and histology
Measurement of serum hormones (LH, FSH, testosterone, and IB) (6), DNA sequencing (KAL1, GNRH1, GNRHR, KISS1, KISS1R, FGF8, FGFR1, PROK2, PROKR2, TAC3, TACR3, NELF, HS6ST1, and IL17RD) (16) and testicular histology studies (7) were all described previously (Supplemental Materials).
Statistics
Data are presented as means ± SEM, except for tubule diameter (means ± SD). Assay results below the level of detection were assigned the level of detection for analysis. Application of a longitudinal linear mixed-effects model was initially considered for within- and across-group comparisons of the longitudinal mean responses, However, because of the limited sample size, the analysis was performed in a simple manner with careful interpretation to avoid overfitting problems that would have induced bias and imprecision. Thus, we compared mean responses during rFSH pretreatment and final testicular volume and maximal sperm count between groups using a Student t test or a Mann-Whitney rank-sum test when data were not normally distributed.
Results and Discussion
Baseline phenotyping and randomization
Seven men were randomly assigned to rFSH pretreatment followed by pulsatile GnRH (group 1), and 6 received pulsatile GnRH alone (group 2). The groups were similar across all assessed parameters (Supplemental Table 1). Patients had uniformly undetectable LH and FSH levels (<1.6 IU/L), frankly hypogonadal testosterone levels (17 ± 2 ng/dL), and very low serum IB levels (32 ± 8 pg/mL), as well as prepubertal TV (1.0 ± 0.1 mL) and histological hallmarks of immature testes including absent Leydig cells, small seminiferous cords (mean diameter, 110 ± 16 μm; range, 49–184 μm, 360 total cross-section observations), and only immature SCs and spermatogonia (SPGA) (Figure 1, A and D) (7). The lamina propria exhibited thickened basement membrane in 4 of 8 biopsy samples, and increased interstitial fibrosis was evident in 7 of 9 assessed cases (17) (the limited amount of tissue obtainable from prepubertal testes precluded evaluation of all samples for all parameters). Mutations in CHH genes were identified in 5 of 13 subjects (Supplemental Table 1).
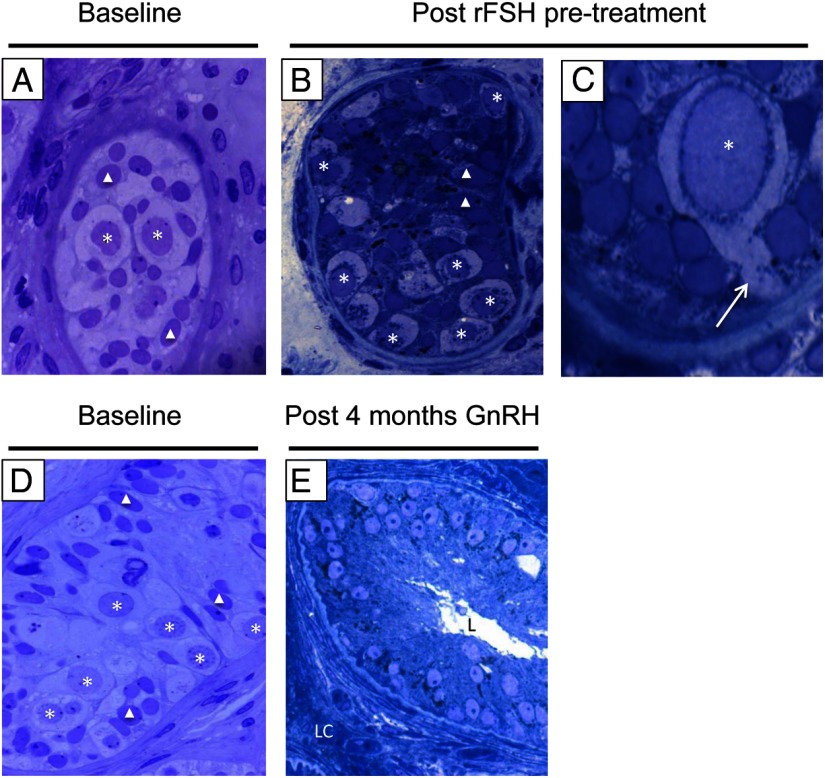
Figure 1.
Testis histology at baseline, after 4 months of rFSH pretreatment, and after 4 months of GnRH. A and D, Light micrographs (×1000) showing immature testes before treatment with few SPGA (asterisks) and SCs (triangles). B, Light micrograph (×1000) after rFSH treatment showing multiple spermatogonia at the basement membrane. C, Light micrograph (×2800) showing a pseudopod (white arrow) extending from a spermatogonium as the cell migrates from the cord center to the basement membrane. Images A, B, and C are from the same subject. E, Light micrograph (×1000) showing Leydig cells (LC) in the interstitium and columnar SCs with nuclei near the base of the tubule and supranuclear cytoplasm extending toward the tubule lumen (L). The SCs are now polarized, and this is a sign of their maturity. Images D and E are from the same subject.
Impact of rFSH pretreatment
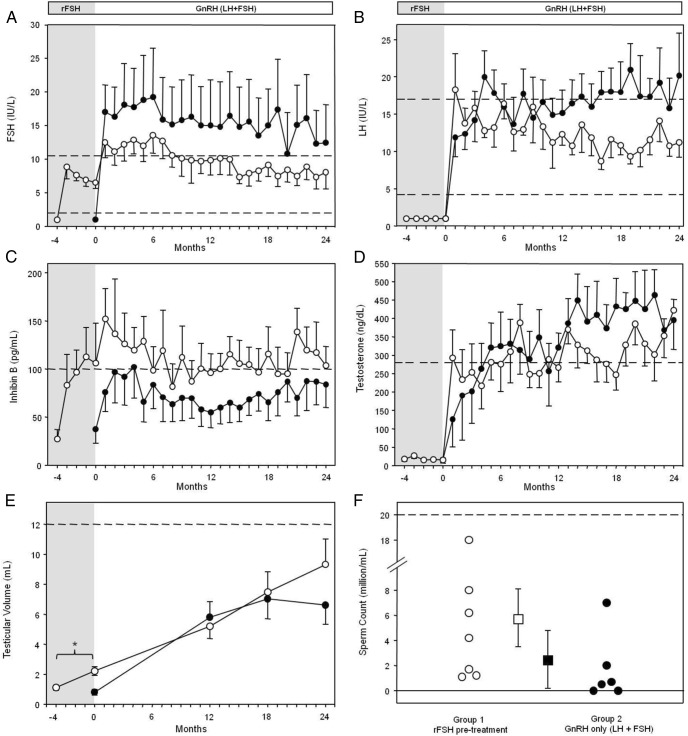
rFSH was well tolerated, and serum FSH levels remained in the target range throughout pretreatment (8.0 ± 0.4 IU/L). Consistent with previous findings (8), IB levels increased significantly (from 29 ± 9 to 107 ± 41 pg/mL within the normal adult range, P < .05). After week 8, IB levels remained elevated and exceeded levels induced by GnRH alone (Figure 2C), suggesting the presence of more IB-secreting SCs in the testes of patients receiving rFSH. TV doubled (from 1.1 ± 0.2 to 2.2 ± 0.3 mL, P < .005), with unchanged LH (<1.6 IU/L) and testosterone (16 ± 3 ng/dL) levels.
Figure 2.
Hormonal responses of men with CHH to rFSH pretreatment and GnRH treatment. ○, rFSH-pretreated group (group 1, n = 7); ●, GnRH-only group (group 2, n = 6). The shaded region of the graph represents the pretreatment period; limits of normal ranges are shown with dashed horizontal lines. A, Both groups increased serum FSH levels with pulsatile GnRH (releasing endogenous LH + FSH), with no significant differences with long-term treatment. B, Both groups rapidly normalized LH levels with pulsatile GnRH. C, Group 1 serum IB levels increased significantly (*, P < .05) during the rFSH pretreatment (shaded region). D, Pulsatile GnRH was effective in maintaining normal serum T levels with long-term therapy in both groups. E, Group 1 TV doubled after 4 months of pretreatment (*, P < .005); the final TV was not different between groups. F, Maximal sperm counts for study subjects are depicted by circles; 2 subjects in group 2 remained azoospermic. Means ± SEM are shown as squares with error bars.
In animal models, FSH administration induces SC and early germ cell proliferation in immature testes, increasing TV (18). In contrast, hemicastration of adult monkeys elevates endogenous FSH levels without stimulation of SCs or early germ cell replication in the remaining testis (19). Thus, there appears to be a prepubertal window when FSH can induce SC and germ cell proliferation in immature testes, which closes after exposure to androgen production from the Leydig cells; however, these phenomena have not been validated in humans. After 4 months of treatment with rFSH, testes remained devoid of Leydig cells yet displayed changes consistent with a transition toward maturation of the gonads (Figure 1, B and C): (1) increased numbers of SCs and SPGA indicated cell proliferation; (2) similar to prior observations in rats (20), 3 of 7 biopsy samples after rFSH showed rare spermatocytes but no further progression of spermatogenesis; and (3) treatment with rFSH induced morphologic changes such as thinning of the basal membrane in some instances and evidence of cytoskeletal rearrangements including development of tight junctions, SC polarization, and SPGA migration to the basement membrane (Figure. 1C). These findings indicate that FSH plays roles beyond cell proliferation in humans.
Response to pulsatile GnRH
After 4 months of GnRH-stimulated LH + FSH exposure via pulsatile GnRH administration, testicular histological analysis revealed maturational changes with the appearance of Leydig cells and lumen formation, marking the transition from seminiferous cords to tubules (Figure 1E). Tubule diameter (from 128 ± 31 to159 ± 17 μm, not significant (NS)], 400 total cross-section observations) and SC to germ cell ratio (from 0.32 to 0.44, NS, 320 total cross-section observations) were unchanged. As shown in Figure 2, both groups had comparable hormonal responses to GnRH. Further, the GnRH dose needed to stimulate a normal serum testosterone level was similar between groups (370 ± 19 ng/kg [final dose range, 50–600 ng/kg] vs 423 ± 18 ng/kg [final dose range, 50–600 ng/kg], NS). There were no differences in serum LH levels (12.7 ± 0.5 vs 16.5 ± 0.7 IU/L, NS), and both groups maintained normal serum testosterone levels (299 ± 14 vs 330 ± 15 ng/dL, NS). There was a trend for group 1 to have lower serum FSH levels (9.9 ± 0.4 vs 15.6 ± 1.1 IU/L, NS) and higher IB levels (110 ± 5 vs 74 ± 4 pg/mL, NS); however, these differences did not reach statistical significance.
After 24 months of GnRH, TV had increased significantly (Figure 2E) in both group 1 (from 1.1 ± 0.2 to 9.3 ± 1.7 mL, P < .005) and group 2 (from 0.8 ± 0.2 to 6.6 ± 1.3 mL, P < .005). Neither the final TV nor the increase in TV (8.2 ± 1.6 vs 5.8 ± 1.1 mL) differed significantly between groups. All pretreated subjects (7 of 7) developed sperm in the ejaculate, whereas 2 of 6 men in group 2 remained azoospermic. No significant differences were observed between groups in the maximal sperm count (Figure 2F) (group 1, 5.8 ± 2.3 × 106/mL; range, 1.1–18 × 106/mL; and group 2, 2.6 ± 1.5 × 106/mL; range, 0.5–7.0 × 106/mL) or in time to first appearance of sperm in the ejaculate (16 ± 2.3 vs 11.5 ± 0.6 months, respectively). Neither the baseline IB level nor TV (Supplemental Table 1) was correlated with either the maximal sperm count or the time to development of sperm in the ejaculate. All 4 men in group 1 who were actively seeking fertility were able to conceive (one naturally). Two men in group 2 were actively seeking fertility; one conceived naturally, but in the other several in vitro fertilization attempts failed (Supplemental Table 1).
Despite trends, rFSH was not statistically superior to GnRH-only treatment in either sperm count or fertility, although 2 patients treated with GnRH alone remained azoospermic. A total of 28 patients in each arm were required to identify significant differences between groups enabling detection, with 80% power at a 5% α level 2-sided testing, of (1) a difference of 3.0 mL in final mean TV, (2) an increased fertility rate (sperm in the ejaculate or not) of >98% to rule out a poor rate of <67%, and (3) a difference in mean sperm counts of 4 million/mL. However, enrollment of this magnitude would be a daunting challenge for a single-center study. Previously, among 14 prepubertal men with gonadotropin deficiency (of diverse etiologies) pretreated with variable rFSH regimens before hCG, all 5 men without cryptorchidism who were assessed for fertility developed sperm in the ejaculate; among the 4 GnRH-deficient men (2 with idiopathic hypogonadotropic hypogonadism and 2 with KS and a history of cryptorchidism), all but 1 patient with KS developed sperm (14). Therefore, those men with the most severe forms of GnRH deficiency (ie, cryptorchidism and/or microphallus) may benefit from such rFSH priming. It is notable that the 2 subjects achieving the highest maximal sperm counts were both in the rFSH pretreatment arm and both harbored mutations in PROKR2 (Supplemental Table 1). One might speculate that rFSH could be particularly beneficial for patients with PROKR2 mutations; however, testing of this hypothesis requires further studies.
In conclusion, rFSH pretreatment followed by GnRH is successful in inducing testicular growth, normalizing IB levels, and promoting fertility in men with CHH with prepubertal testes. Because there was no significant change in IB levels during the third and fourth months in patients receiving FSH pretreatment, it seems reasonable to shorten pretreatment to 8 weeks of rFSH to maximize and prime the SC compartment before androgen-induced maturation by GnRH or hCG. However, larger prospective multicenter studies are needed to prove the superiority of pretreatment with FSH in improving fertility outcomes in patients with severe GnRH deficiency with and without cryptorchidism. The power calculations reported herein could be used to guide additional investigations, such as a larger multicenter study, for combined data analysis.
Acknowledgments
We thank Dr Helmut Rennke and his highly skilled team at Brigham and Women's Hospital, the Massachusetts General Hospital (MGH) Clinical Laboratory Research Core and the MGH Clinical Research Center.
This work was supported by Grants R01 HD15788 (to W.F.C.), R01 HD33728 (to M.D.), and M01-RR-01066 from the National Institutes of Health, National Center for Research Resources, General Clinical Research Centers Program.
This study was registered with clinical trial registration number NCT00064987.
Disclosure Summary: Merck Serono Inc. supplied recombinant FSH (Gonal-F). The authors have nothing to disclose.
Footnotes
- CHH
- congenital hypogonadotropic hypogonadism
- hCG
- human chorionic gonadotropin
- KS
- Kallmann syndrome
- NS
- not significant
- rFSH
- recombinant FSH
- SC
- Sertoli cell
- SPGA
- spermatogonia
- TV
- testicular volume.
References
- 1. Cortes D, Müller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int J Androl. 1987;10:589–596 [DOI] [PubMed] [Google Scholar]
- 2. Müller J, Skakkebaek NE. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int J Androl. 1983;6:143–156 [DOI] [PubMed] [Google Scholar]
- 3. Regadera J, Martínez-Garcia F, González-Peramato P, Serrano A, Nistal M, Suárez-Quian C. Androgen receptor expression in Sertoli cells as a function of seminiferous tubule maturation in the human cryptorchid testis. J Clin Endocrinol Metab. 2001;86:413–421 [DOI] [PubMed] [Google Scholar]
- 4. Andersson AM, Müller J, Skakkebaek NE. Different roles of prepubertal and postpubertal germ cells and Sertoli cells in the regulation of serum inhibin B levels. J Clin Endocrinol Metab. 1998;83:4451–4458 [DOI] [PubMed] [Google Scholar]
- 5. Orth JM, Gunsalus GL, Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–794 [DOI] [PubMed] [Google Scholar]
- 6. Pitteloud N, Hayes FJ, Dwyer A, et al. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4128–4136 [DOI] [PubMed] [Google Scholar]
- 7. Kumar PA, Pitteloud N, Andrews PA, et al. Testis morphology in patients with idiopathic hypogonadotropic hypogonadism. Hum Reprod. 2006;21:1033–1040 [DOI] [PubMed] [Google Scholar]
- 8. Young J, Chanson P, Salenave S, et al. Testicular anti-müllerian hormone secretion is stimulated by recombinant human FSH in patients with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:724–728 [DOI] [PubMed] [Google Scholar]
- 9. Bougnères P, François M, Pantalone L, et al. Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. J Clin Endocrinol Metab. 2008;93:2202–2205 [DOI] [PubMed] [Google Scholar]
- 10. Hoffman AR, Crowley WF., Jr Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307:1237–1241 [DOI] [PubMed] [Google Scholar]
- 11. Young J. Approach to the male patient with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2012;97:707–718 [DOI] [PubMed] [Google Scholar]
- 12. Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009;94:801–808 [DOI] [PubMed] [Google Scholar]
- 13. Raivio T, Toppari J, Perheentupa A, McNeilly AS, Dunkel L. Treatment of prepubertal gonadotrophin-deficient boys with recombinant human follicle-stimulating hormone. Lancet. 1997;350:263–264 [DOI] [PubMed] [Google Scholar]
- 14. Raivio T, Wikström AM, Dunkel L. Treatment of gonadotropin-deficient boys with recombinant human FSH: long-term observation and outcome. Eur J Endocrinol. 2007;156:105–111 [DOI] [PubMed] [Google Scholar]
- 15. Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245 [DOI] [PubMed] [Google Scholar]
- 16. Sykiotis GP, Hoang XH, Avbelj M, et al. Congenital idiopathic hypogonadotropic hypogonadism: evidence of defects in the hypothalamus, pituitary, and testes. J Clin Endocrinol Metab. 2010;95:3019–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schell C, Albrecht M, Mayer C, Schwarzer JU, Frungieri MB, Mayerhofer A. Exploring human testicular peritubular cells: identification of secretory products and regulation by tumor necrosis factor-alpha. Endocrinology. 2008;149:1678–1686 [DOI] [PubMed] [Google Scholar]
- 18. Orth JM. The role of follicle-stimulating hormone in controlling Sertoli cell proliferation in testes of fetal rats. Endocrinology. 1984;115:1248–1255 [DOI] [PubMed] [Google Scholar]
- 19. Ramaswamy S, Marshall GR, McNeilly AS, Plant TM. Dynamics of the follicle-stimulating hormone (FSH)-inhibin B feedback loop and its role in regulating spermatogenesis in the adult male rhesus monkey (Macaca mulatta) as revealed by unilateral orchidectomy. Endocrinology. 2000;141:18–27 [DOI] [PubMed] [Google Scholar]
- 20. Vihko KK, LaPolt PS, Nishimori K, Hsueh AJ. Stimulatory effects of recombinant follicle-stimulating hormone on Leydig cell function and spermatogenesis in immature hypophysectomized rats. Endocrinology. 1991;129:1926–1932 [DOI] [PubMed] [Google Scholar]