Abstract
Designing nanoscale objects with the potential to perform externally-controlled motion in biological environments is one of the most sought-after objectives in nanotechnology. Different types of chemically and physically-powered motors have been prepared at the macro- and microscale. However, the preparation of nanoscale objects with a complex morphology, and the potential for light-driven motion has remained elusive to date. Here, we go a step forward by designing a nanoscale hybrid with a propeller-resembling shape, which can be controlled by focused light under biological conditions. Our hybrid, hereafter ‘Au@DNA-origami’, consists of a spherical gold nanoparticle with self-assembled, biocompatible, two-dimensional (2D) DNA sheets on its surface. As a first step towards the potential utilization of these nanoscale objects as light-driven assemblies in biological environments, we show that they can be optically trapped, and hence translated and deposited on-demand, and that under realistic trapping conditions the thermally-induced dehybridization of the DNA sheets can be avoided.
Keywords: Gold nanoparticles, DNA origami, nanoscale hybrids, self-assembly, optical tweezers, optical manipulation, optical heating
Externally-powered nanomotors able to perform controlled motion in biological media through the application of an external chemical1 or physical input (e.g. a magnetic field,2 light,3 or ultrasounds4) are envisaged as promising powerful nanomachines.5, 6 Physical triggers are advantageous over chemical ones: (i) the applied input can be turned on and off on-demand, and hence, a higher control over object motion can be achieved;7 and (ii) they overcome the utilization of toxic chemical fuels,8-10 allowing for the realization of controlled motion in a wider range of environments.11 Light is one of the most powerful and versatile physical triggers, and of special interest in the context of bio-related applications, since it can be tuned to operate in the NIR (the so-called biological window). To induce light-controlled rotation of a given object some degree of three-dimensional control is required. The focused light of laser tweezers can provide the trapping force needed to hold the object in a convenient position,12 and also the flux to rotate it when an optical torque is generated on it.13-15 Several examples on light-driven rotation of micron-sized dielectric particles,13, 14, 16, 17 have been reported, while examples at the nanoscale have been mainly restricted to as-synthesized Ag and Au nanorods and nanowires,18 random dimers of spherical Au nanoparticles,18 carbon nanotube bundles,19 or gold nanorods aggregates,19 to name a few. More complex structures, with a propeller-like shape, and the potential for light-driven rotation to the best of our knowledge have only been reported at the microscale, and prepared in a ‘one-by-one’ basis via two-photon polymerization strategies with a spatial resolution in the order of several hundred nanometers.15, 20, 21
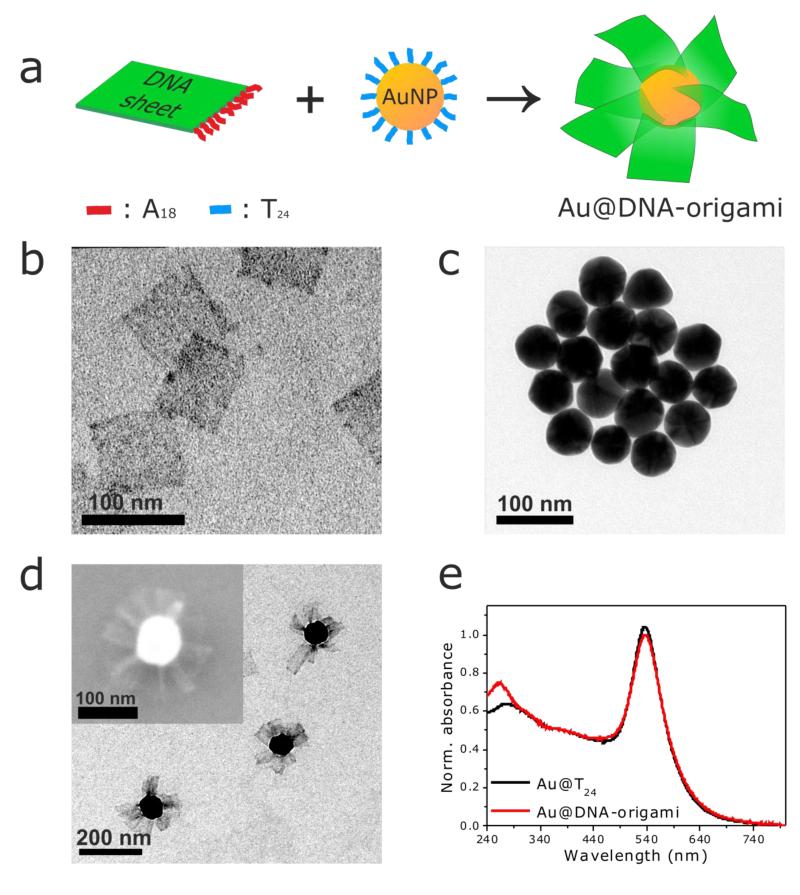
In an effort to fill this gap, here we have designed and prepared a biocompatible propeller-resembling nanoscale hybrid (Au@DNA-origami) through a versatile self-assembly approach (see preparation scheme in Figure 1a) that we introduce in the following. The biocompatible ‘wings’, two-dimensional DNA sheets (80×80 nm, length×width), were prepared by pre-programmed folding of a long single-stranded DNA scaffold via hybridization with rationally designed ‘staple’ oligonucleotides (the so-called DNA origami approach),22 and designed to contain 15 oligonucleotides with an AAAAAAAAAAAAAAAAAA (A18) sequence on one side available for later hybridization (see Figure 1a, Experimental Part, and Figure S1 in the Supporting Information). Excess ‘staple’ strands were separated from the 2D DNA sheets via gel electrophoresis (Figure S2, Supporting Information). Figure 1b contains a representative TEM micrograph of the purified 2D DNA sheets. Spherical Au nanoparticles (60 nm diameter) functionalized with complementary thiolated TTTTTTTTTTTTTTTTTTTTTTTT (T24) oligonucleotides (Figure 1c) were incubated with a 100× excess of 2D DNA sheets in 0.5× TBE buffer ([MgCl2]=11 mM). Self-assembly of the 2D DNA sheets on the Au@T24 nanoparticles gave rise to the formation of an Au@DNA-origami hybrid with a propeller-resembling morphology, consisting of single Au nanoparticles surrounded by multiple 2D DNA sheets on their surface (see TEM and SEM micrographs, Figure 1d). In analogy to other Au/DNA-origami hybrids of varying complexity,23-26 the self-assembly yield is very high: all examined Au@T24 nanoparticles show 2D DNA sheets on their surface (no particle without 2D DNA was found, as determined from TEM imaging). Typically, a hybrid consists of several 2D DNA sheets per Au nanoparticle, though a number estimation cannot be provided given the drying effects associated with electron microscopy imaging, and just a few 2D DNA sheets do not self-assemble on the Au@T24 nanoparticles.
Figure 1.
Preparation and morphological/optical characterization of biocompatible, propeller-resembling, Au@DNA-origami hybrids. (a) Scheme depicting the preparation of Au@DNA-origami hybrids via DNA-directed self-assembly of two-dimensional DNA sheets having A18 ‘handle’ strands (prepared via the DNA origami technique) on DNA-functionalized spherical Au nanoparticles (Au@T24). Representative TEM micrographs of the two-dimensional DNA sheets (b), the Au@T24 nanoparticles (c), and the Au@DNA-origami hybrids (d) (inset: representative SEM image of an Au@DNA-origami hybrid). (e) UV-vis extinction spectra of Au@T24 nanoparticles and Au@DNA-origami hybrids in 0.5× TBE buffer (11 mM MgCl2). The spectra are normalized at 400 nm to facilitate comparison.
The UV-vis extinction spectrum of Au@DNA-origami (Figure 1e) shows that in the hybrids the absorption peak characteristic of DNA (~260 nm), is more intense than the one of Au@T24 nanoparticles due to the high loading of 2D DNA sheets on the Au surface. No significant change occurs on the Au@T24 plasmon band after self-assembly, confirming the colloidal stability of the Au@DNA-origami hybrids in the TBE buffer. 2D DNA self-assembly on Au@T24 was further confirmed by dynamic light scattering (DLS). The hydrodynamic diameter of Au@DNA-origami is 215 nm (PDI = 0.141, where the PDI is the ‘polydispersity index’) vs. 68 nm (PDI = 0.137) for the Au@T24 nanoparticles. The observed hydrodynamic diameter of Au@DNA-origami of 215 nm agrees very well with the expected size that results from the addition of the TEM dimensions of the individual building-blocks (220 nm). Due to hydrodynamic considerations and thermal fluctuations it is yet likely, that the self-assembled 2D DNA sheets on the Au nanoparticles’ surface are present in solution in a non-fully stretched configuration.
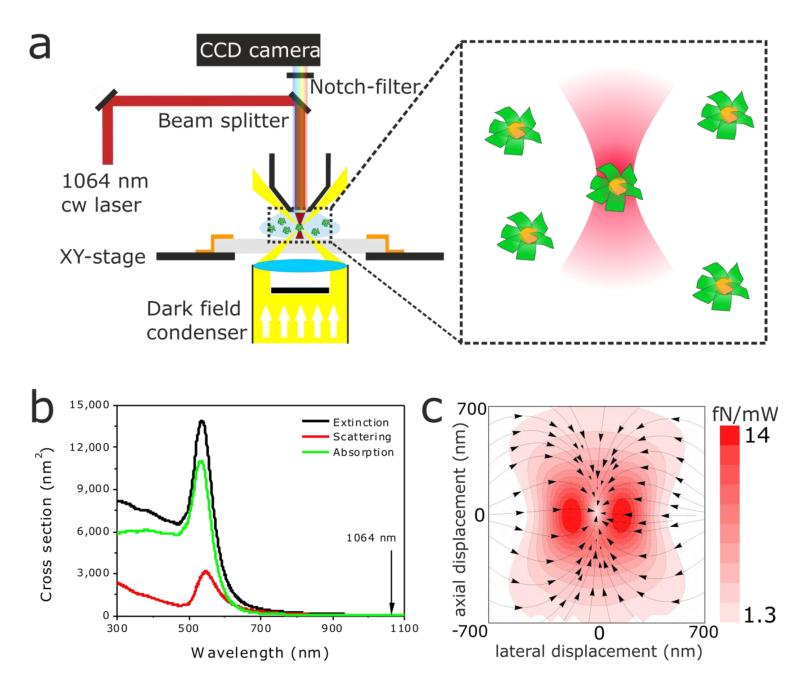
As a necessary first step to evaluate the potential of Au@DNA-origami hybrids for light-controlled rotation, we performed optical trapping experiments in a dark-field microscope (DFM) modified to enable 3D trapping (see Figure 2a, and Experimental Part in the Supporting Information) as described elsewhere.27 Briefly, a diode-pumped solid-state laser operating in continuous wave mode at 1064 nm is coupled to an up-right DFM, and a notch filter is used to filter out light scattered from the trapping laser. For nanoparticles fulfilling the Rayleigh scattering regime (i.e., particle size<< λlight, case of the spherical Au nanoparticles of our Au@DNA-origami hybrids), the forces acting on optically-trapped particles result from a contribution of the absorption and scattering force, and of the gradient force.28, 29 The absorption and scattering forces act in the direction of light propagation, and scale linearly with the particle’s absorption and scattering cross sections, respectively. The gradient force scales linearly with the real part of the particle’s complex polarizability and, with the gradient of ∣E∣2 (E being the electric field), directly related to the beam intensity. The gradient force pushes the particle to the position of highest intensity within the beam. Here we performed off-resonance trapping of Au@DNA-origami hybrids. At 1064 nm the absorption and scattering cross sections of the 60 nm Au nanoparticles forming the Au@DNA-origami hybrids are low (Cabs= 41 nm2, and Csca=42 nm2, see Figure 2b), and with them the absorption and scattering forces. Thus, off-resonance trapping at 1064 nm at the moderate laser powers used in our experiments (see below) the contribution of both forces is low compared to the gradient force, and hence it is possible to induce stable 3D trapping of the Au@DNA-origami hybrids (see force map, Figure 2c).
Figure 2.
Optical trapping setup. (a) Sketch of the experimental setup used for 3D optical trapping of Au@DNA-origami hybrids. (b) Calculated (Mie theory) extinction, scattering, and absorption cross-section of a spherical Au nanoparticle of 60 nm diameter. The vertical line indicates the operating wavelength of the trapping laser (1064 nm). At 1064 nm, Cext= 83 nm2, Csca= 42 nm2, and Cabs= 41 nm2. (c) Force map depicting the total force exerted on a spherical 60 nm Au nanoparticle upon trapping with a focused laser beam at 1064 nm. The color encodes the magnitude of the force, and the arrows, the direction.
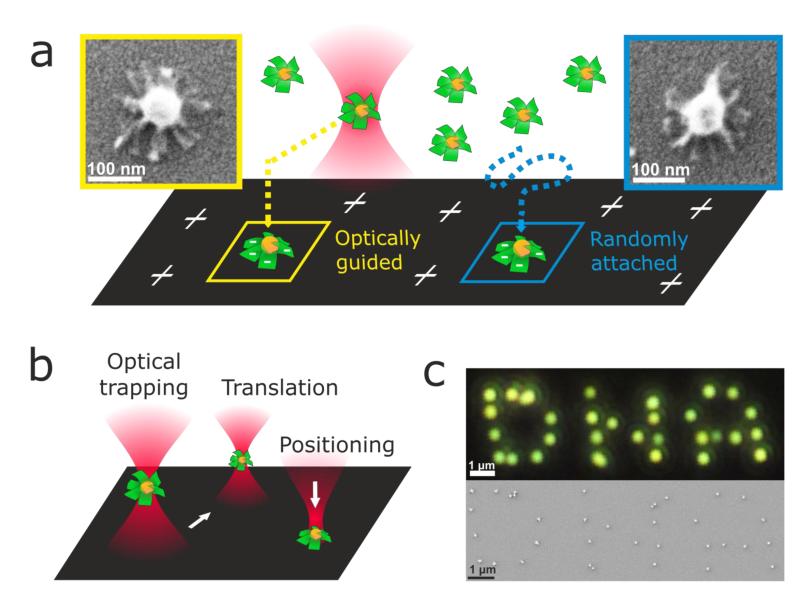
Optothermal effects on optically-trapped metal nanoparticles have been reported,27 and it is also known that double-stranded DNA (like our DNA ‘wings’) can melt if exposed to temperatures higher than its melting temperature,30 and eventually be released from the Au surface.31 To evaluate whether the propeller-resembling morphology of the Au@DNA-origami hybrids is preserved after trapping (i.e., whether the 2D DNA sheets dehybridize or get released from the Au surface), we performed a comparative SEM study on trapped vs. non-trapped Au@DNA-origami hybrids, as sketched in Figure 3a. A plasma-cleaned microscope glass slide was first modified with a cationic polyelectrolyte (polydiallydiaminochloride, PDDA), and used as a substrate on the DFM stage. A highly-diluted Au@DNA-origami dispersion was drop-casted on the substrate, and trapping of individual Au@DNA-origami hybrids was performed as described above at the lowest trapping power possible (68 mW). Trapped hybrids were guided towards the substrate until electrostatic attraction between the negatively-charged Au@DNA-origami (ξ = −14 mV) and the positively-charged substrate overcame the trapping potential (Figure 3a, left). At this point, the trapped Au@DNA-origami hybrids could be selectively immobilized on the substrate at locations that could be easily identified in a scanning electron microscope (SEM), and compared with Au@DNA-origami hybrids undergoing Brownian motion in solution, and spontaneously attaching to the PDDA-modified substrate (Figure 3a, right). As shown in the SEM micrographs (Figure 3a), in both cases the 2D DNA sheets can be clearly identified on the Au nanoparticles, and no significant differences can be observed between trapped vs. non-trapped Au@DNA-origami. These results show that Au@DNA-origami hybrids can be trapped in a 3D optical trap with a non-resonant NIR laser beam, thus allowing, for instance, their precise light-controlled translation and patterning on two-dimensional substrates (see Figures 3b, and 3c).
Figure 3.
Optical trapping, and patterning of Au@DNA-origami hybrids. (a) Sketch depicting an Au@DNA-origami hybrid trapped at 68 mW (lowest power enabling stable trapping) guided to the proximity of a PDDA-modified glass substrate, and immobilized on the surface due to electrostatic attraction (left). Sketch depicting a non-trapped Au@DNA-origami hybrid freely diffusing in solution, and immobilized on the substrate due to electrostatic attraction (right). Inset: color-coded SEM micrographs of the Au@DNA-origami hybrids immobilized on a glass substrate as described. (b) Sketch depicting the manipulation possibilities (translation, two-dimensional positioning) of optically-trapped Au@DNA-origami hybrids. (c) DFM (top), and SEM (bottom) images of a ‘DNA’ pattern consisting of Au@DNA-origami hybrids with a hybrid-to-hybrid distance of ~800 nm. Patterning was performed as sketched in (b).
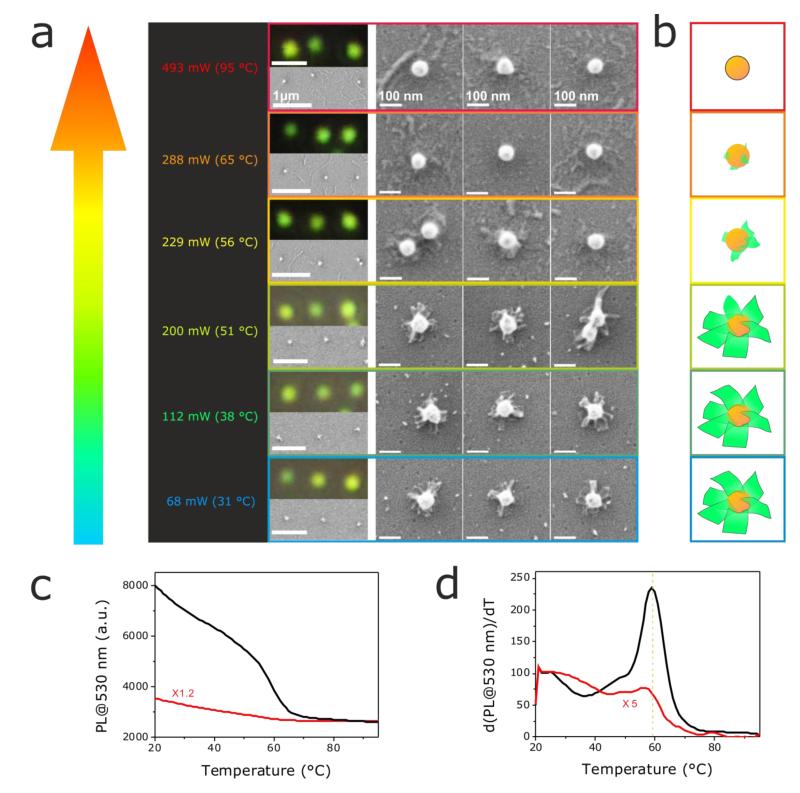
Off-resonant trapping of Au@DNA-origami aims at achieving an efficient 3D trapping, while minimizing optothermal effects in the trap. High laser powers will increase the magnitude of the gradient force, and hence lead to a better trapping. However, this can lead to a non-negligible heating of the 60 nm Au nanoparticles, despite their small absorption cross section at 1064 nm (Cabs= 41 nm2). In order to tackle this issue we performed a systematic evaluation on the impact of the optical trapping conditions on the thermal stability of Au@DNA-origami. We trapped single Au@DNA-origami hybrids with a 1064 nm laser (FWHM = 1.0 μm) operating at various powers (from 68 to 493 mW), and estimated the temperature on the Au surface with a finite element analysis software (see Experimental Part, and Figure S3 in the Supporting Information). Trapped Au@DNA-origami hybrids were immobilized on a PDDA-modified glass substrate as described above, and their morphological assessment was performed via SEM. The results of our investigation are shown in Figure 4a. The trapping power (and with it the temperature on the Au surface) increases upwards from 68 mW (31°C) to 493 mW (95°C), as indicated on the left column. The right images are optical DFM images, lower- and higher-resolution SEM micrographs of series of three Au@DNA-origami hybrids trapped at the corresponding color-coded trapping power. Trapping Au@DNA-origami hybrids at relatively low laser powers, 68 and 112 mW (31°C and 38°C, respectively), does not produce any visible modification or damage of the 2D DNA sheets. At a higher power (200 mW, 51°C on the Au surface) some changes in some of the 2D DNA sheets start to become apparent, becoming very obvious at 229 mW (56°C): the square-like shape of the 2D DNA sheets cannot be distinguished anymore, but a rather clear deformation, and lower electron microscopy contrast (likely related to a partial DNA dehybridization, see discussion below). By increasing the power further to 288 and 493 mW (65°C and 95°C, respectively), no more DNA can be observed at the surface of the Au nanoparticles. Figure 4b contains a schematic depiction of the changes induced on the Au@DNA-origami hybrids at the studied trapping conditions. From our results it seems clear that between 200 and 229 mW (51°C and 56°C, respectively), a drastic morphological change (destruction) of the 2D DNA sheets takes place, suggesting that DNA melting occurs under those trapping conditions.
Figure 4.
Influence of laser trapping power on the stability of Au@DNA-origami hybrids. (a) SEM and DFM images of series of three Au@DNA-origami hybrids trapped with a 1064 nm laser (FWHM = 1.0 μm) at varying laser powers (increasing upwards). The calculated temperatures on the Au nanoparticles’ surface are indicated in brackets (left column). SEM and DFM images are color-coded according to the corresponding trapping conditions on the left. (b) Scheme illustrating the morphological evolution of the propeller-resembling Au@DNA-origami hybrids at the trapping powers used in (a). Evolution of the fluorescence (c), and first derivative of the fluorescence (d) of SYBR Green at 530 nm as a function of the solution temperature. The curves correspond to pure 2D DNA sheets (black), and 2D DNA sheets on the spherical Au nanoparticles (Au@DNA origami, red). The scale bars in all DFM images in (a) are 1 μm; and for the low, and high magnification SEM images, as indicated.
To investigate this issue further, we determined the melting temperature of pure 2D DNA sheets, and of 2D DNA sheets self-assembled on the Au nanoparticles. We added a fluorescent dye (SYBR Green, that binds double-stranded DNA) to pure 2D origami sheets, and to Au@DNA-origami hybrids. Following the emission intensity of SYBR Green at 530 nm as a function of the solution temperature, we determined the DNA melting temperature.32 In both cases the fluorescence of SYBR Green gradually decreases as the solution temperature is increased from 20°C to 50°C (Figure 4c). Between 50°C and ~65°C a steep decrease in SYBR Green fluorescence is recorded, suggesting that DNA dehybridization occurs in this temperature range. For temperatures above ~65°C, an additional temperature increase does not produce any additional change in the fluorescence intensity of SYBR Green. The first derivative of the fluorescence intensity of SYBR Green at 530 nm as a function of the solution temperature (Figure 4d), allowed us to determine the exact melting temperature of the pure 2D DNA sheets (~59°C), and of 2D DNA sheets self-assembled on the Au nanoparticles (~56°C). These results are in clear agreement with our results in the optical trap (Figure 4a). When achieving a temperature between 51°C and 56°C in the trap, the 2D DNA sheets get significantly damaged due to a significant melting of the double-stranded DNA forming the sheets. At temperatures higher than 56°C (between 56°C and 65°C), most of the double-stranded DNA dehybridizes, and thus, the 2D DNA sheets on the Au surface can no longer be identified. At this stage, an additional temperature increase in the trap does not produce any noticeable change in the already non-appreciable DNA sheets, in agreement with the results shown in Figures 4c, and 4d.
In summary, herein we have demonstrated that biocompatible nanoscale hybrids with a propeller-resembling morphology can be prepared by using the DNA origami technique to produce on-demand ‘wings’ (2D DNA sheets in our case) designed to self-assemble on DNA-modified spherical gold nanoparticles. The Au@DNA-origami hybrids can be trapped with a non-resonant NIR laser beam, and under realistic trapping conditions (< 200 mW, < 51°C) optothermal effects can be minimized, and thus, their propeller-like morphology, preserved. The control over chirality that can be achieved by DNA self-assembly, could enable the construction of objects fulfilling genuine propeller function, and hence our trapping results, together with the biocompatible nature of the DNA ‘wings’, point towards the potential of these Au@DNA-origami hybrids for applications demanding the performance of light-controlled rotation under biological conditions.
Supplementary Material
ACKNOWLEDGMENTS
This work has been financially supported by the DFG through the Nanosystems Initiative Munich (NIM), the ERC through the Advanced Investigator Grant HYMEM, and the EU commission through the Marie Curie Research Training Network ICARUS.
Footnotes
Supporting Information. Experimental part; 2D DNA sheet design (DNA origami); gel electrophoresis purification of the 2D DNA sheets; calculated temperature at the surface of a spherical 60 nm Au nanoparticle. This material is available free of charge via the Internet at http://pubs.acs.org.
Notes The authors declare no competing financial interest.
REFERENCES
- 1.Mirkovic T, Zacharia NS, Scholes GD, Ozin GA. ACS Nano. 2010;4(4):1782–1789. doi: 10.1021/nn100669h. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh A, Fischer P. Nano Lett. 2009;9(6):2243–2245. doi: 10.1021/nl900186w. [DOI] [PubMed] [Google Scholar]
- 3.Padgett M, Bowman R. Nat Photon. 2011;5(6):343–348. [Google Scholar]
- 4.Wang W, Castro LA, Hoyos M, Mallouk TE. ACS Nano. 2012;6(7):6122–6132. doi: 10.1021/nn301312z. [DOI] [PubMed] [Google Scholar]
- 5.Ozin GA, Manners I, Fournier-Bidoz S, Arsenault A. Adv. Mater. 2005;17(24):3011–3018. [Google Scholar]
- 6.Wang J, Manesh KM. Small. 2010;6(3):338–345. doi: 10.1002/smll.200901746. [DOI] [PubMed] [Google Scholar]
- 7.Solovev AA, Smith EJ, Bufon CCB, Sánchez S, Schmidt OG. Angew. Chem.-Int. Edit. 2011;50(46):10875–10878. doi: 10.1002/anie.201102096. [DOI] [PubMed] [Google Scholar]
- 8.Ismagilov RF, Schwartz A, Bowden N, Whitesides GM. Angew. Chem. Int. Ed. 2002;41(4):652–654. [Google Scholar]
- 9.Paxton WF, Kistler KC, Olmeda CC, Sen A, St. Angelo SK, Cao Y, Mallouk TE, Lammert PE, Crespi VH. J. Am. Chem. Soc. 2004;126(41):13424–13431. doi: 10.1021/ja047697z. [DOI] [PubMed] [Google Scholar]
- 10.Fournier-Bidoz S, Arsenault AC, Manners I, Ozin GA. Chem. Commun. 2005;(4):441–443. doi: 10.1039/b414896g. [DOI] [PubMed] [Google Scholar]
- 11.Wang J. ACS Nano. 2009;3(1):4–9. doi: 10.1021/nn800829k. [DOI] [PubMed] [Google Scholar]
- 12.Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S. Opt. Lett. 1986;11(5):288–290. doi: 10.1364/ol.11.000288. [DOI] [PubMed] [Google Scholar]
- 13.Friese MEJ, Nieminen TA, Heckenberg NR, Rubinsztein-Dunlop H. Nature. 1998;394(6691):348–350. [Google Scholar]
- 14.Paterson L, MacDonald MP, Arlt J, Sibbett W, Bryant PE, Dholakia K. Science. 2001;292(5518):912–914. doi: 10.1126/science.1058591. [DOI] [PubMed] [Google Scholar]
- 15.Galajda P, Ormos P. Appl. Phys. Lett. 2001;78(2):249–251. [Google Scholar]
- 16.Higurashi E, Sawada R, Ito T. Appl. Phys. Lett. 1998;72(23):2951–2953. [Google Scholar]
- 17.Bingelyte V, Leach J, Courtial J, Padgett MJ. Appl. Phys. Lett. 2003;82(5):829–831. [Google Scholar]
- 18.Tong L, Miljković VD, Käll M. Nano Lett. 2009;10(1):268–273. doi: 10.1021/nl9034434. [DOI] [PubMed] [Google Scholar]
- 19.Jones PH, Palmisano F, Bonaccorso F, Gucciardi PG, Calogero G, Ferrari AC, Maragó OM. ACS Nano. 2009;3(10):3077–3084. doi: 10.1021/nn900818n. [DOI] [PubMed] [Google Scholar]
- 20.Galajda P, Ormos P. Appl. Phys. Lett. 2002;80(24):4653–4655. [Google Scholar]
- 21.Péter G, Pál O. Journal of Optics B: Quantum and Semiclassical Optics. 2002;4(2):S78. [Google Scholar]
- 22.Rothemund PWK. Nature. 2006;440(7082):297–302. doi: 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- 23.Gu HZ, Chao J, Xiao SJ, Seeman NC. Nature. 2010;465(7295):202–U86. doi: 10.1038/nature09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding BQ, Deng ZT, Yan H, Cabrini S, Zuckermann RN, Bokor J. J. Am. Chem. Soc. 2010;132(10):3248–3249. doi: 10.1021/ja9101198. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z, Jacovetty EL, Liu Y, Yan H. Angew. Chem.-Int. Edit. 2011;50(9):2041–2044. doi: 10.1002/anie.201006818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuzyk A, Schreiber R, Fan ZY, Pardatscher G, Roller EM, Hogele A, Simmel FC, Govorov AO, Liedl T. Nature. 2012;483(7389):311–314. doi: 10.1038/nature10889. [DOI] [PubMed] [Google Scholar]
- 27.Ohlinger A, Nedev S, Lutich AA, Feldmann J. Nano Lett. 2011;11(4):1770–1774. doi: 10.1021/nl2003544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansen PM, Bhatia VK, Harrit N, Oddershede L. Nano Lett. 2005;5(10):1937–1942. doi: 10.1021/nl051289r. [DOI] [PubMed] [Google Scholar]
- 29.Dienerowitz M, Mazilu M, Reece PJ, Krauss TF, Dholakia K. Opt. Express. 2008;16(7):4991–4999. doi: 10.1364/oe.16.004991. [DOI] [PubMed] [Google Scholar]
- 30.Stehr J, Hrelescu C, Sperling RA, Raschke G, Wunderlich M, Nichtl A, Heindl D, Kurzinger K, Parak WJ, Klar TA, Feldmann J. Nano Lett. 2008;8(2):619–623. doi: 10.1021/nl073028i. [DOI] [PubMed] [Google Scholar]
- 31.Huschka R, Zuloaga J, Knight MW, Brown LV, Nordlander P, Halas NJ. J. Am. Chem. Soc. 2011;133(31):12247–12255. doi: 10.1021/ja204578e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castro CE, Kilchherr F, Kim DN, Shiao EL, Wauer T, Wortmann P, Bathe M, Dietz H. Nat. Methods. 2011;8(3):221–229. doi: 10.1038/nmeth.1570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.