Abstract
Schizotypy is phenotypically associated with neuroticism. To reveal the origin of this association, we assessed 3349 (1449 monozygotic (MZ), 1105 dizygotic (DZ) same-sex and 795 DZ opposite-sex) twins on a 12-item version of Chapman’s Psychosis-Proneness Scales and the short-form of the Eysenck Personality Questionnaire-Revised as measures of schizotypy and neuroticism.
A substantial proportion (.51 with 95% CI from .38 to .64) of the phenotypic correlation of .37 between neuroticism and the perceptual and ideational components of schizotypy was accounted for by shared genetic influences on these two traits. Moreover, a Cholesky decomposition including anhedonia, hypomania and impulsivity fully accounted for the heritable variance in perceptual and ideational components of schizotypy.
These findings suggest a shared genetic etiology between neuroticism and perceptual and ideational components of schizotypy and affect future investigations on the etiology of these phenotypically overlapping traits and affective and psychotic disorders.
Keywords: Perceptual aberration, Magical ideation, Phenotypic correlation, Behavior genetics, Schizotypy, Neuroticism
INTRODUCTION
Schizotypy is defined as schizophrenia-like traits expressed in an attenuated form (Chapman, Chapman, & Raulin, 1978; Venables and Bailes 1994; Vollema and Vandenbosch 1995; Rado 1953; Meehl 1989). Dimensions of schizotypy have been labeled according to their similarity to schizophrenic symptoms (Vollema and Vandenbosch 1995; Kwapil et al. 2008). Cognitive and perceptual anomalies are summarized as positive (Chapman et al., 1978; Eckblad and Chapman 1983) while diminished interest in social interaction and pleasure deficits are regarded as negative schizotypy (Chapman, Chapman, & Raulin, 1976). Other dimensions such as impulsivity and hypomania are also part of the multi-dimensional construct of schizotypy (Vollema and Vandenbosch 1995).
Previous studies indicate heritability estimates (h2) of .33–.53 for positive, .27–.50 for negative features and .28–.58 for hypomania/impulsivity (Linney et al. 2003; Hay et al. 2001; MacDonald et al. 2001; Claridge and Hewitt 1987). Several lines of evidence link schizotypy and the schizophrenia spectrum. Schizotypal personality disorder (SPD) is more common in relatives of schizophrenia spectrum patients than in the general population (Kendler et al. 1993). Moreover, longitudinal studies indicate that schizotypal individuals are at higher risk for developing a psychosis-like pathology (Chapman, Chapman, & Kwapil, 1994), and twin and family studies reveal increased levels of schizotypal traits in first-degree relatives of schizophrenia patients (Kendler and Walsh 1995). Adoption studies similarly report higher rates of schizotypal traits in offspring of schizophrenic patients strengthening evidence for a genetic association between schizophrenia and schizotypal traits (Tienari et al. 2003). In addition, gender differences in schizotypy are similar to those found in schizophrenia, with males presenting more frequently with negative and some evidence for a predominance of positive traits in females (Venables and Bailes 1994).
Neuroticism is a heritable trait (Bouchard 1994) characterized by high tension, irritability, dissatisfaction, shyness, low mood and reduced self-confidence (Eysenck et al. 1985). Females are known to score higher on neuroticism than males (Lynn and Martin 1997). Neuroticism is associated with schizotypy in the general population, especially with the positive features of schizotypy (Barrantes-Vidal et al. 2009; Ettinger et al. 2005). Clinically, neuroticism also acts as both a risk- and as a maintenance-factor for full-blown psychosis (Freeman and Garety 1999). High levels of neuroticism for instance increase odds for psychosis (Van Os and Jones 2001) and are present in patients with schizophrenia (Horan et al. 2008). Additionally, both relatives of patients with schizophrenia and individuals high on schizotypy show enhanced emotional reactivity to daily stressors (Myin-Germeys et al. 2003), score high on neuroticism (Maier et al. 1994), and frequently present with major depressive disorder (Baron and Gruen 1991). Schizotypy increases risk for affective disorders (Verdoux et al. 1999), suggesting the existence of reciprocal associations between schizotypal and affective traits.
Given the oft-replicated association between schizotypy and neuroticism, and the relevance of these traits to the clinical domain, it is important to clarify the nature of this association. Behavioral genetic analyses using monozygotic (MZ) and dizygotic (DZ) twins may be of value. Classical twin designs use correlations between MZ and DZ twins and decompose these into genetic as well as environmental components. The same logic can be applied to the covariation between traits to assess the amount of common genetic etiology between variables. To our knowledge, no study to date has investigated the extent to which the association between schizotypy and neuroticism is of genetic origin. Therefore, based on the strong evidence of a correlation between neuroticism and positive schizotypy we aimed first to replicate the association of these traits. Subsequently, we examined potential gender differences in neuroticism and positive schizotypy. Then, we decomposed this covariance into genetic and environmental components finding significant evidence for shared genes. Driven by the results of the first analysis, a second, more comprehensive analysis was conducted to examine the extent of genetic overlap between positive schizotypy and anhedonic, hypomanic and impulsivity features of schizotypy in addition to neuroticism.
METHODS
Sample
An unselected sample of 8,538 twins born 1964–1971 (4,087 males, 4,451 females; 3,378 MZ, 2,896 same-sex DZ (DZss), 2,186 opposite-sex DZ (DZos) and 78 twins of unknown zygosity) drawn from the Australian National Health and Medical Research Council Twin Register (ATR) was targeted in a mailed questionnaire survey conducted in 1989–1991. Despite extensive efforts to re-contact all twins initially targeted, contact details could be generated for 6,122 twins. Those twins contacted were mailed a survey and were followed-up up to 5 times by phone if necessary. A total of 2,294 twin-pairs and 474 individual twins (i.e. 5,062/6,122 contactable twins or 83%) provided any kind of questionnaire data. Of these 5,062 individuals, 3,349 individuals fulfilled our inclusion criteria which are outlined below.
Schizotypy
Schizotypy was assessed with a 12-item version5 of the Chapman Psychosis-Proneness Scales (PPS; Chapman & Chapman, 1985; Eckblad and Chapman 1986). The PPS consists of 4 factors showing good reliability with Cronbach’s alpha around the .80s for all 4 scales (Chapman et al., 1994).
The 12-item version consists of six scales with two items each. Of these, the Magical Ideation (Mag) and Perceptual Aberration (Per) scales assessed positive and the Social and Physical Anhedonia scales (San and Pan, respectively) assessed negative schizotypy. Hypomania (Hyp) and Impulsivity/Non-conformity (Imp) scales targeted elevated mood and impulsive and unconventional behaviors.
The Magical Ideation scale (Eckblad and Chapman 1983) taps beliefs about magical and unrealistic causes and effects. Sample items include items such as “I have wondered whether the spirits of the dead can influence the living”. The Perceptual Aberration scale (Chapman et al., 1978) covers body image and perceptual distortions, an example of an item from this scale is “Sometimes part of my body seems smaller than it really is”. Social Anhedonia (Eckblad et al. 1982) focuses on a lack of interest in experiencing pleasure from interpersonal relationships. Here, a sample item is “I prefer hobbies and leisure activities that do not involve other people”. Physical Anhedonia (Chapman et al., 1976) pertains to a lack of experiencing physical pleasure. Items such as “Trying new foods is something I have always enjoyed” are used here (please note that this item is scored reversed). Hypomania captures features similar to but less severe than those of mania, such as grandiose ideas and elevated mood, latter is captured by items such as “I often have moods where I feel so energetic and optimistic that I feel I could outperform almost anyone or anything”. The Impulsivity/Non-conformity scale (Eckblad and Chapman 1986) captures impulsivity, antisocial behavior and insensitivity to others and is assessed with items such as “I usually find myself doing things ‘on impulse’ ”. All items were answered on a yes/no scale and scored in the direction of higher schizotypy.
Neuroticism
Neuroticism was assessed with the short form of the Eysenck Personality Questionnaire-Revised (Eysenck et al. 1985). All 12 items (e.g. “Does your mood often go up and down?”) were answered on a yes/no scale. Higher scores indicated greater levels of neuroticism. Reliability for the neuroticism scale was good (Cronbach’s α=.81).
Zygosity
Zygosity was determined by response to standard items and checked on a subset through genotyping micro-satellite markers across the genome (Cornes et al. 2005). Errors in zygosity assignment are estimated to be <1%.
Data Preparation
After list-wise deletion of those who did not complete at least 75% of the neuroticism scale (9 or more items) and all of the schizotypy items 3,349 (1,449 MZ, 1,105 DZss and 795 DZos) individual twins remained. The total sample size of 3349 individual twins was composed of complete twin pairs (1,253 complete pairs) as well as of single twins (843 single twins). Excluded (5,062−3,349=1,713) individuals did not differ significantly from included ones in terms of zygosity status, gender distribution or educational level.
Given our exclusion criteria, we allowed for missing items on the neuroticism scale, therefore we used a percentage score. For every twin we calculated a sum score and a count score reflecting the item response and the number of items responded to respectively. Subsequently, we calculated a percentage score (sum score/count score) with higher percentage scores reflecting a higher level of schizotypy or neuroticism.
Item responses were subjected to principal component analysis (PCA) with varimax rotation using PASW Statistics, version 18.0.0. Factors with eigenvalues greater than 1 were retained. Factor scores were extracted, missing factor scores (8%, 5.2%, 2.4% and 1.9% were missing for Perceptual Aberration-Magical Ideation, Hypomania-Impulsivity/Non-conformity, Social Anhedonia and Physical Anhedonia respectively) were replaced by the mean and all corrected for age and gender (MacDonald et al. 2001; McGue and Bouchard 1984). Standardized residuals were used for further analyses.6
Preliminary Analyses
Participation bias was investigated by comparing factor scores of complete pairs with those of individual twins whose co-twin did not participate (3349 twins; 1253 complete pairs, 843 single twins). Effects of zygosity and twin order were examined by comparing MZ and DZ twins with each other and first- and second-born twins with each other, respectively.
Quantitative Genetic Modeling
The classical twin design decomposes phenotypic correlations between traits into components consistent with additive genetic (A), non-additive genetic (D; e.g. interactions between alleles), common environmental (C; e.g. any environmental factor that creates resemblance between family members) and unique environmental (E; e.g. individual friends but also measurement error) influences. MZ share all while DZ twins share on average half of their genes. MZ twins correlate perfectly for A, D and C, but are uncorrelated for E. DZ twins correlate .5 for A, .25 for D, perfectly for C and are uncorrelated for E. Higher MZ than DZ twin correlations indicate genetic influences. Non-additive genetic and common environmental influences cannot be estimated simultaneously in twins reared together. Therefore, either ADE or ACE models were fitted. Unique environmental influences are reflected in MZ twin correlations as MZ twins correlate perfectly for A and C (the remaining proportion of unexplained variance therefore must be due to unique environment). Model fitting was carried out using the extended structural equation modeling package OpenMx (Boker et al. 2011) and R (R-Development-Core-Team 2010). Nested sub-models were fitted to test the contribution of each variance component (i.e. A, C or E). Comparison of model fit between nested sub-models (e.g. AE) and the full model (ACE) provides information on the importance of the respective component (here: C). Models were fitted using full information maximum likelihood and evaluated by Akaike’s Information Criterion (AIC; Akaike 1987). Thereby, a lower AIC was preferred. The difference in log-likelihood between the full and the nested sub-models is distributed as a χ2-statistic, which together with the difference in degrees of freedom (df) between models is used to test for significance of model fit.
Modeling included three analyses. First, univariate sex limitation modeling was done for neuroticism and Perceptual Aberration-Magical Ideation in data from 3349 twins (1253 complete pairs, 843 single twins). Given frequently reported sex differences between genders, quantitative and qualitative sex differences were examined. Quantitative sex differences are indicated if differences in the amount of A,C or E influences emerge between genders (if e.g. Perceptual Aberration-Magical Ideation is more heritable in males then females), qualitative differences emerge if different as opposed to same A, C or E influences operate between genders (Neale and Maes 2002).
Second, bivariate modeling was performed to estimate the amount of genetic and environmental overlap between positive schizotypy and neuroticism. Finally, multivariate modeling was applied to examine the interrelations between neuroticism and all facets of schizotypy. Both analyses made use of within-trait within-twin, within-trait cross-twin (e.g. neuroticism in twin 1 correlated with neuroticism in twin 2 and vice versa), cross-trait within-twin (e.g. positive schizotypy and neuroticism in twin 1 or 2) and cross-trait cross-twin correlations (e.g. the correlation between positive schizotypy in twin 1 and neuroticism in twin 2 and vice versa). Both bi- and multivariate, analyses used a Cholesky decomposition of the variance-covariance matrices, wherein the number of parameters estimated is equal to the observed parameters.
RESULTS
Principal Component Analysis of Schizotypy
PCA resulted in four factors, the first covering items of Perceptual Aberration and Magical Ideation (Per-Mag). Hypomania and Impulsivity items were combined in a second factor entitled Hypomania-Impulsivity/Non-conformity. Items representing Social Anhedonia and Physical Anhedonia were extracted as third and fourth factors, respectively. Together, these factors explained 45.9% of the variance (15.8% for Perceptual Aberration-Magical Ideation, 11.8 for Hypomania-Impulsivity/Non-conformity, 9.5 for Social Anhedonia, 8.8 for Physical Anhedonia).
Preliminary Analyses
Complete pairs and single twins were compared to test for participation bias. No significant differences on measures of interest emerged (all p>.05) so single twins and complete pairs were merged for further analyses. Effects of twin order (i.e. first born compared to second born) and zygosity (i.e. MZ compared to DZ twins) were also not significant (all p>.05).
Sample Description
The final sample included 3349 twins of Caucasian origin (2506 twins from complete pairs, 843 single twins) consisting of 1449 MZ (923 female (372 complete pairs, 179 single twins), 526 male (209 complete pairs, 108 single twins)), 1105 DZss (684 female (259 complete pairs, 166 single twins), 421 male (140 complete pairs, 141 single twins) and 795 DZos (273 complete pairs, 249 single twins) twins. Mean age of the sample was 23.19 years (SD=2.23; range: 16 to 31 years). Age did not differ significantly between zygosity groups (F[1, 3126]=1.9, p=.17; see Table 1).7
Table 1.
Numbers of twins and sex and age composition.
| MZ | DZss | DZos | ||||
|---|---|---|---|---|---|---|
| Pairs | Single | Pairs | Single | Pairs | Single | |
| Male | 418 | 108 | 280 | 141 | 273 | 92 |
| Age | 23.0 (2.2) | 23.1 (2.0) | 23.0 (2.3) | 23.5 (2.3) | 23.1 (2.1) | 23.5 (2.2) |
| Female | 744 | 179 | 518 | 166 | 273 | 157 |
| Age | 23.3 (2.1) | 23.0 (2.8) | 23.3 (2.2) | 23.2 (2.3) | 23.1 (2.1) | 23.5 (2.2) |
| Total | 1162 | 287 | 798 | 307 | 546 | 249 |
| Age | 23.2 (2.2) | 23.0 (2.6) | 23.2 (2.2) | 23.3 (2.3) | 23.1 (2.1) | 23.5 (2.2) |
Note: MZ=monozygotic, DZss=dizygotic same-sex, DZos=dizygotic opposite-sex. Numbers of twins is shown in bold. Mean age in years (Standard deviations) are reported beneath the number of twins.
Educational level was examined between MZ and DZ twins and between first and second born twins. Results confirmed comparability of both zygosity groups (F[4, 3275]=2.15, p=.07) and of first and second born twins (F[1, 3278]=.02, p=.87). Educational level for the sample was 14.9% high school up to 10 school years, 34.9% high school up to 12 school years, 13.0% achieved a diploma, 13.9% completed a technical college, 19.4% completed a University degree and 1.7% completed postgraduate education (2.1% did not provide data on educational level).
Sex Limitation Twin Modeling
Correlation patterns per gender and zygosity for neuroticism and schizotypy are illustrated in Table 2. As correlations of same and opposite-sex twins differed in magnitude, and to test if these differences were significant, we first tested quantitative and qualitative sex difference models for our two main variables of interest; N and Perceptual Aberration-Magical Ideation. Given the twin correlations in Table 2, we applied models consistent of additive genetic, common and unique environmental influences whenever rDZ>rMZ/2 and models consistent of additive genetic, dominance related and unique environmental influences whenever rDZ<rMZ/2. A test of quantitative differences suggested that models in which in path estimates were constrained to be equal across gender fit to the data well for both neuroticism (Δχ23=.42, p=.94) and for Perceptual Aberration-Magical Ideation (Δχ23=3.15, p=.99), indicating no significant quantitative gender differences. Qualitative sex differences allowing for specific genetic influences on males were examined subsequently. Results again indicated that setting qualitative differences to zero lead to a non-significant drop in fit as compared to an AE homogeneity model for neuroticism (Δχ21=1.69, p=>.99) and for Perceptual Aberration-Magical Ideation (Δχ21=.55, p=>.99). Given the absence of significant gender differences in the current sample, data from DZos and DZss were pooled in subsequent analyses.
Table 2.
Correlations between twins for neuroticism and schizotypy.
| Twin zygosity | N | Neuroticism | Per-Mag | San | Pan | Hyp-Imp |
|---|---|---|---|---|---|---|
| Pairs/ single twins | ||||||
| MZMM | 209/108 | .39** | .25** | .31** | .34** | .38** |
| DZMM | 140/141 | .15 | .19* | .10 | .23** | .08 |
| MZFF | 372/179 | .37** | .27** | .37** | .31** | .21** |
| DZFF | 259/166 | .08 | .15* | .04 | .08 | .03 |
| DZos | 273/249 | .07 | .07 | .04 | .05 | .13* |
| MZ | 1449 | .38** | .26** | .36** | .32** | .27** |
| DZ | 1900 | .09* | .13 | .05 | .10 | .08 |
Note: N=Neuroticism; Per-Mag=Perceptual aberration/Magical ideation, San=Social anhedonia, Pan=Physical anhedonia, Hyp-Imp=Hypomania/Impulsivity; MZMM=male monozygotic, MZFF=female monozygotic, DZMM=male dizygotic, DZFF=female dizygotic, DZos=opposite-sex dizygotic twins; Pearson correlations are reported,
p<.05,
p<.01.
Phenotypic Correlations between Schizotypy factors and Neuroticism
First, correlations between schizotypy and neuroticism were calculated based on the whole sample. Second, correlations between schizotypy factors and neuroticism were calculated separately for each zygosity. At the phenotypic level, neuroticism and Perceptual Aberration-Magical Ideation correlated r=.37 (p<.01). Correlations of neuroticism with Social Anhedonia, Physical Anhedonia and Hypomania-Impulsivity/Non-conformity were low (rSan=.11, p<.01; rPan=.11, p<.01, rHyp-Imp=.05, p<.01).
Table 3 shows the intercorrelations per zygosity group. Cross-twin within-trait correlations in MZ twins range from .26 to .38 (all p<.01). In DZ twins, these correlations were substantially lower suggesting a heritable basis for these traits. Cross-twin cross-trait correlations were significant for MZ twins and were substantially lower in DZ twins, suggesting shared genetic influences among these traits. We next formally modeled these effects.
Table 3.
Correlations between neuroticism and schizotypy factors per zygosity.
| MZ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Twin 1 | Twin 2 | ||||||||||
| Neuroti cism |
Per- Mag |
San | Pan | Hyp- Imp |
N | Per- Mag |
San | Pan | Hyp- Imp |
||
| Twin 1 | Neuroticism | .333** | −.142** | −.114** | .019 | .377** | .199** | −.084* | −.077 | .042 | |
| Per-Mag | .397** | −.065 | .022 | −.008 | .184** | .264** | −.014 | .072 | .028 | ||
| San | −.100** | .014 | −.047 | −.049 | −.031 | −.065 | .358* | .038 | −.038 | ||
| Pan | −.096** | .022 | −.035 | .037 | −.016 | .027 | −.030 | .323** | .007 | ||
| Hyp-Imp | .039 | .026 | .046 | −.031 | .064 | .067 | .029 | .057 | .274** | ||
| Twin 2 | N | .094* | .076* | .004 | .028 | .045 | .342** | −.098** | −.125** | .063 | |
| Per-Mag | .040 | .125** | −.012 | −.006 | .058 | .390** | .008 | −.083* | .052 | ||
| San | −.029 | .059 | .050 | .029 | −.008 | −.092** | .025 | −.003 | .060 | ||
| Pan | −.005 | .012 | .019 | .104* | −.005 | −.096** | .004 | .014 | .016 | ||
| Hyp-Imp | .032 | .055 | −.032 | −.014 | .079 | .062 | −.018 | −.038 | .017 | ||
| N | Per-Mag | San | Pan | Hyp-Imp | N | Per-Mag | San | Pan | Hyp-Imp | ||
| Twin 1 | Twin 2 | ||||||||||
| DZ | |||||||||||
Note: Per-Mag=Perceptual aberration/Magical ideation, San=Social anhedonia, Pan=Physical anhedonia, Hyp-Imp=Hypomania/Impulsivity; MZ=monozygotic twins; DZ=dizygotic twins (with grey background); cross-twin within-trait correlations are given in bold, cross-twin cross-trait correlations are underlined
p<.05;
p<.01
Bivariate Twin Modeling on Positive Schizotypy and Neuroticism
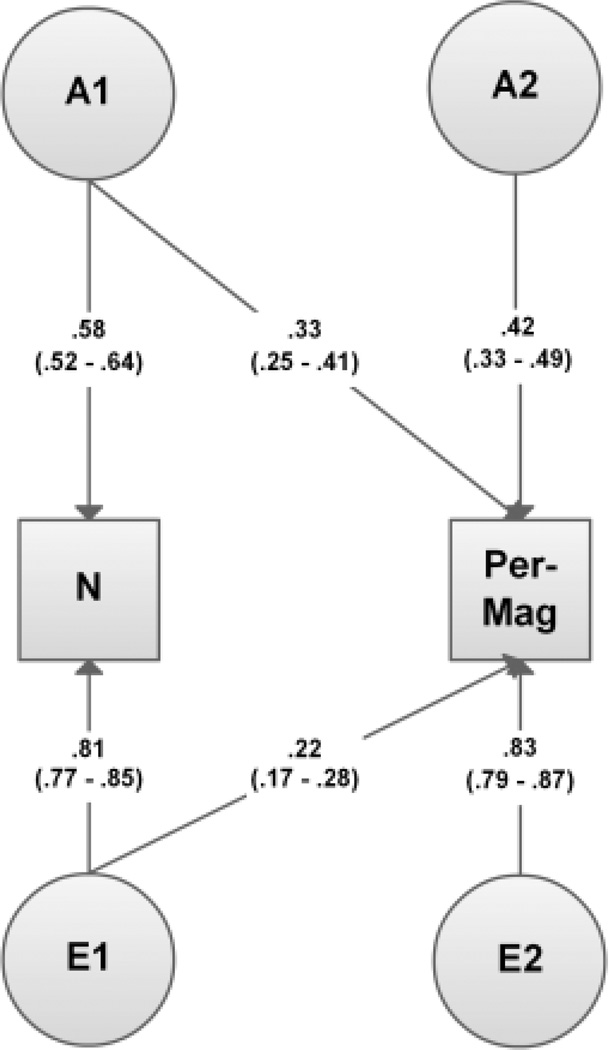
Results of bivariate modeling of positive schizotypy and neuroticism are illustrated in the most parsimonious model depicted in Figure 1. An AE model fit the data with no significant loss of fit as compared to the full ADE model (Δχ23=.55, p>.99). Genetic influences however could not be dropped from the model (see E-Model: Δχ23=129.11, p<.01). Likewise dropping the genetic covariance between neuroticism and Perceptual Aberration-Magical Ideation resulted in a significant loss in model fit (Δχ21=48.55, p<.01), thereby highlighting the importance of a shared additive genetic influence for neuroticism and Perceptual Aberration-Magical Ideation. Unique environmental covariance also could not be dropped without reducing model fitting significantly (Δχ21=58.96, p<.01), indicating that unique environmental influences also act to generate covariance between positive schizotypy and neuroticism. Narrow sense heritability estimates (which can be obtained by squaring the path estimates) are indicated by path loadings on neuroticism (h2=.34; 95% CI .28–.41) and Perceptual Aberration-Magical Ideation (h2=.332 + .422=.29; 95% CI .17–.41).
Figure 1.
Path diagram of most parsimonious model for neuroticism and positive schizotypy.
Note: N=Neuroticism; Per-Mag=Perceptual aberration/Magical ideation, A1/A2=genetic influences, E1/E2=unique environmental influences; bold: standardized path estimates (which need to be squared to obtain variance components), brackets: 95% confidence intervals.
The genetic correlation, an estimate of the correlation between the genetic influences on neuroticism and those on positive schizotypy based on the correlated factor solution, was .62, indicating a large amount of genetic overlap. The proportion of the phenotypic correlation explained by genetic influences was .51 (95% CI .38–.64), indicating that 51% of the phenotypic correlation between neuroticism and Perceptual Aberration-Magical Ideation can be accounted for by shared genetic factors. The unique environmental correlation was estimated at .26. The proportion of phenotypic correlation explained by unique environmental influences was estimated at .49 (95% CI .36–.62).
Multivariate Twin Modeling of Schizotypy and Neuroticism
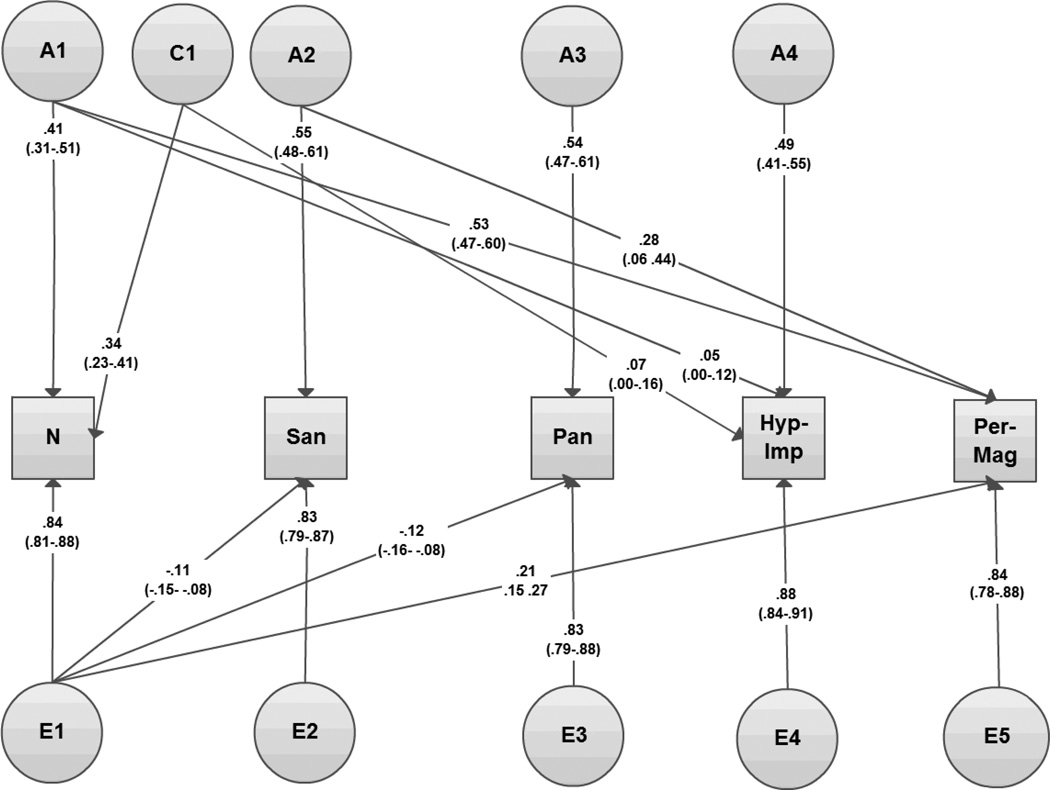
Moving to multivariate modeling, a 5-factor Cholesky decomposition was constructed with neuroticism, Social Anhedonia, Physical Anhedonia, Hypomania-Impulsivity/Non-conformity, and Perceptual Aberration-Magical Ideation entered in that order. To test whether Perceptual Aberration-Magical Ideation had any significant shared genetic or shared environmental effects independent of neuroticism and the other components of schizotypy we attempted to drop the specific genetic and common environmental influences on Perceptual Aberration-Magical Ideation, then both together. In all cases, this could be done with no significant loss of fit (Δχ22=.16, p=.92). Common environmental influences across the traits could be reduced to a single general factor (Δχ211<.16, p>.99) which could not itself be dropped (Δχ25=11.37, p<.05).
The most parsimonious multivariate model is shown in Figure 2 and consisted of specific genetic influences to neuroticism, Social Anhedonia, Physical Anhedonia and Hypomania-Impulsivity/Non-conformity, shared genetic influences from neuroticism to positive schizotypy, a single common environmental component influencing neuroticism and schizotypy, and specific as well as shared unique environmental components. Genetic influences to Perceptual Aberration-Magical Ideation were entirely accounted for by genetic influences shared with neuroticism, Social Anhedonia, Physical Anhedonia and Hypomania-Impulsivity/Non-conformity)
Figure 2.
Path diagram of most parsimonious model for neuroticism and schizotypy.
Note: A1–A5=additive genetic influences, C1=common environmental influences, E1–E5=unique environmental influences; N=Neuroticism, San=Social anhedonia, Pan=Physical anhedonia, Hyp-Imp=Hypomania & Impulsivity/ Non-conformity, Per-Mag=Perceptual aberration & Magical ideation; bold: standardized path estimates, 95% confidence intervals are given below the estimate.
Consistent with the bivariate analysis, the multivariate analysis thus confirmed a strong (.81) genetic correlation between neuroticism and positive schizotypy, indicating large shared genetic influences. The increase in genetic correlation from bivariate (.62) to multivariate (.81) is a reflection of the increase in power (for instance to detect shared environment effects) gained in the multivariate model. These shared genetic influences between neuroticism and positive schizotypy accounted for 46% of the phenotypic association (95% CI 23–67%). The unique environmental correlation between neuroticism and Perceptual Aberration-Magical Ideation was significant, but more moderate at .26, accounting for a further 36% of the shared variance between the two traits. The remaining proportion of phenotypic correlation to be explained (i.e. 18%) was attributable to common environmental influences.
DISCUSSION
The current study examined the nature of the association between neuroticism and schizotypy. First, the well established phenotypic correlation between neuroticism and positive schizotypy was replicated and partitioned into genetic and environmental components. Second, anhedonic, hypomanic and impulsivity components of schizotypy were added to capture the full construct of schizotypy and examine the covariance of these components with neuroticism, and their net effect on the genetic influence to positive schizotypy.
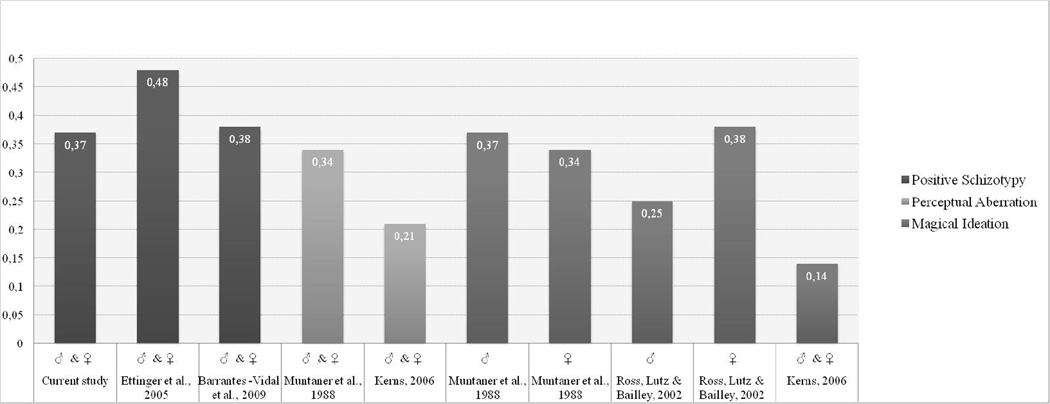
The phenotypic correlation we have obtained (.37) fits nicely with previous findings. Figure 3 illustrates the association between neuroticism and positive schizotypy from various studies. Correlations of moderate size (.38 and .48) were found by Barrantes-Vidal and colleagues (2009) and Ettinger and colleagues (2005), both of these studies reported phenotypic correlation for both gender types pooled. The studies by Muntaner and colleagues (1988), Kerns (2006) and Ross and colleagues (2002) focused on Perceptual Aberration and Magical Ideation separately. Aside from Kerns (2006), who used a different measure to assess neuroticism (that is items from the International Personality Item Pool), all found correlations of moderate size between positive schizotypy and neuroticism similar to our finding.
Figure 3.
Phenotypic correlations between neuroticism and positive schizotypy.
Note: The y-axis depicts Pearson’s r correlations. Previous studies are listed along the x-axis. The exact correlation coefficients are given in white in each column. Please note: The correlations obtained by Muntaner et al. (1998) for Perceptual Aberration was .37 in males and females. ♂ = males, ♀ = females, ♂ & ♀ = males and females pooled.
Bivariate model fitting indicated that the association between positive schizotypy and neuroticism was best described by additive genetic and unique environmental influences. A substantial proportion (51%) of the association between positive schizotypy and neuroticism was accounted for by shared genetic influences, signifying a large genetic basis shared between neuroticism and positive schizotypy. Aside from the proportion of phenotypic association, that was explained by shared genetic factors, findings from the bivariate analysis also point to genetic influences on positive schizotypy which act independently from those on neuroticism. Previous studies examined the nature of individual differences in positive schizotypy and found evidence for significant genetic influences common with other schizotypy factors as well as independent of these (Linney et al. 2003; Hay et al. 2001; MacDonald et al. 2001; Kendler and Hewitt 1992). Despite that half of the genetic influences on positive schizotypy are independent of neuroticism, our study extends previous work by showing that a large proportion of genetic influences on positive schizotypy are shared with neuroticism. This finding from the bivariate analysis combined with previous work on the nature of individual differences in positive schizotypy motivated us to extend our work by a multivariate analysis.
Multivariate analyses included anhedonic, hypomanic and impulsivity components of schizotypy and focused on the genetic and environmental influences to positive schizotypy that could be explained by other schizotypy factors in addition to those captured by neuroticism. Common environmental influences were restricted to a single common factor exerting a modest but significant influence on all traits, including a link between neuroticism and positive schizotypy.
No significant support was found for genetic influences specific to positive schizotypy. Instead, genetic influences on positive schizotypy were shared with neuroticism, and with the anhedonic, hypomanic and impulsivity facets of schizotypy, indicating a complex genetic etiology of positive schizotypy involving neuroticism and the other schizotypy dimensions. As has been mentioned by Loehlin (1996) the use of the Cholesky decomposition requires caution in the interpretation of the results as the order of variables entered into the Cholesky impacts on the outcome. Given the overall aim of decomposing the nature of the overlap between neuroticism and positive schizotypy as well as taking into account previous findings on the nature of positive schizotypy, we entered neuroticism first into the multivariate Cholesky decomposition, followed by the other schizotypy components in order to ensure that all the genetic and environmental influences that can be attributed to these manifest variables are covered first. This allowed us to examine the genetic and environmental influences on positive schizotypy over and above those that are shared with neuroticism, anhedonic, hypomanic and impulsivity components of schizotypy.
In contrast with positive schizotypy, the anhedonic, hypomanic and impulsivity features of schizotypy showed evidence for significant additional specific genetic influences not shared with neuroticism, thereby also providing evidence for a specific genetic etiology of each of these dimensions. The multi-factorial nature of schizotypy at the genetic level has been suggested before (Linney et al. 2003), and evidence at the psychometric level has long established a multi-factorial nature with up to 5 schizotypy factors (Vollema and Vandenbosch 1995). Together with the present data, this suggests that positive schizotypy, while it forms a reliably identifiable part of the schizotypy construct at a psychometric level, may at a genetic level be accounted for by anhedonic, hypomanic and impulsivity components of schizotypy and neuroticism. Supporting this view, studies of the healthy siblings of schizophrenia patients indicate that they have higher levels of negative schizotypy but do not differ on positive schizotypy signs (Clementz et al. 1991; Franke et al. 1993). This might be explained methodologically by different patterns of responding to negative or positive features of schizotypy. Yet it could be that positive schizotypy emerges over time as a result of environmental triggers, building on a biological substrate involving neuroticism, and negative schizotypy.
Given that psychometric questionnaire measures of neuroticism and schizotypy are thought to reflect subclinical variation in risk for affective disorder and schizophrenia, respectively, the current findings also imply an overlapping genetic etiology for these two clinical conditions (Fanous and Kendler 2004). Our data thus provide a possible explanation for the frequently observed comorbidity of affective and psychotic disorders. Affective symptoms are seen in up to 75% of schizophrenic patients (Siris 2000). Depressive symptoms are also present prior to the onset of psychosis and have been postulated as an early risk factor of developing schizophrenia (Yung et al. 2004). Our findings suggest that, just as neuroticism and depression largely share a single underlying genetic architecture (Kendler and Myers 2010in press), the clinical comorbidity of affective and schizophrenic disorder may be substantially genetic in origin, a view in line with findings in the largest current GWAS studies (Bergen et al. in press).
Consistent with our speculations on the genetic overlap between schizophrenia and affective disorders, some molecular genetic variants that increase risk for both disorders have already been discovered. Some of the most promising candidates genes for schizophrenia, such as Disrupted-In-Schizophrenia 1, Neuregulin-1and G72 have also been shown to be associated with mood disorder phenotypes (Harrison and Weinberger 2005). These findings provide initial support for the assumed molecular genetic overlap between schizophrenia and affective disorders that may be expected on the basis of our findings. Our findings provide help in the search for susceptibility loci for schizophrenia and affective disorders by referencing to their overlap. Focusing on the overlap might shed light on the molecular genetic basis of either clinical phenotype by making use of the susceptibility loci found for the other phenotype, that is looking at susceptibility loci for schizophrenia in affective disorders and vice versa.
Common environmental influences in our study were weak and accumulated in one component that had to be retained indicating a significant effect of one environmental factor shared between schizotypy and neuroticism. Extending these findings to the clinical domain might similarly indicate common environmental factors underlying schizophrenia and affective disorders. A number of environmental risk factors have been described for both schizophrenia and affective disorders (e.g. early environmental stressors; Agid et al. 1999). These factors might be of relevance in explaining the common environmental component of neuroticism and schizotypy observed in our study.
Limitations
The current findings have to be interpreted in light of their limitations. First, schizotypy was assessed through a small number of items with two to four items per factor. This might have resulted in a higher error of measurement, explaining the rather inflated specific unique environmental estimates and similarly the small overlap in unique environmental components between Perceptual Aberration-Magical Ideation and neuroticism (re=.26) in the bivariate model. However, this short version of the Chapman scales has been validated and its equivalence to the full PPS shown by Hay and colleagues (2001). Also, heritability estimates of positive schizotypy are in line with previous studies (that made use of the full PSS or other schizotypy measures) thereby providing additional support for the construct validity of the short schizotypy scale. Second, the current study used a twin sample and might therefore not generalize to the general population. However, the magnitude of the phenotypic correlation between neuroticism and schizotypy in our study is comparable to findings from previous studies in non-twin populations (Barrantes-Vidal et al. 2009; Ettinger et al. 2005), supporting the generalizability of our findings.
Conclusions
The current study highlights the genetic and environmental structures underlying the observed phenotypic association of schizotypy and neuroticism. Despite specific etiological components to perceptual and ideational features of schizotypy, a substantial shared genetic influence was found between perceptual and ideational features of schizotypy and neuroticism, accounting for over half the phenotypic correlation. Moreover, the genetic components to perceptual aberration and magical ideation could be fully explained by genetic variance in anhedonia, hypomania, impulsivity and neuroticism by means of a Cholesky decomposition. These findings suggest that personality traits resembling the positive schizophrenia spectrum symptoms may be shared to a large amount with genetic factors underlying neuroticism and other schizophrenia spectrum features. The added importance of these findings lies in the possibility that similar genetic overlap could exist between affective disorders and full-blown psychosis.
Acknowledgements
The authors thank David Smyth and Harry Beeby (QIMR) for maintaining data integrity.
Funding
This work was supported by National Health and Medical Research Council (NHMRC) grants to NGM and NIH grants to ACH. Data analysis was supported by the Deutsche Forschungsgemeinschaft (DFG) Emmy Noether program (ET 31/2-1) to UE; a project grant of the Förderprogramm für Forschung und Lehre (FöFoLe) of the Ludwig-Maximilians-University Munich to UE (Reg-Nr. 645); and a travel grant of the Graduate Center at the Ludwig-Maximilians-University Munich to CM.
Footnotes
The 12-item version is based on item reduction of the original PPS. Latter was factor analyzed and the resulting 4 factors were subjected to a reduction procedure based on: high item-total correlations, high frequency of responses in both genders, exclusion of items responded < =10% or >=90%, low loading on factors, limited face validity because of the possibility of ‘faking good’ and a “reasonable level of general acceptance” (see Hay et al. 2001 for details). High correlations between the factors derived from the 12-item version of the PSS and the original PSS scales (.96, .97, .96, .90 for Per-Mag, Hyp-Imp, San and Pan respectively; Hay et al. 2001) were obtained.
The sample analysed here was taken from the same cohort of twins as the sample described by Hay and colleagues. However, due to differences in exclusion criteria, the sample used by Hay and colleagues did not overlap completely with our sample.
Missing data on age was substituted whenever possible (e.g. when the co-twin provided data on zygosity status as well as on age). Two hundred twenty-one twins did not provide data on age. There were no differences in terms of either Perceptual Aberration-Magical Ideation or neuroticism scores between these twins and those who provided data on age.
References
- Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in Schizophrenia. J Abnorm Psychol. 1978;87(4):399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Venables PH, Bailes K. The structure of schizotypy, its relation to subdiagnoses of schizophrenia and to sex and age. Br J Clin Psychol. 1994:33277–33294. doi: 10.1111/j.2044-8260.1994.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Vollema MG, Vandenbosch RJ. The multidimensionality of schizotypy. Schizophr Bull. 1995;21(1):19–31. doi: 10.1093/schbul/21.1.19. [DOI] [PubMed] [Google Scholar]
- Rado S. Dynamic and classification of disordered behavior. Am J Psych. 1953;110(6):406–416. doi: 10.1176/ajp.110.6.406. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia revisited. Arch Gen Psychiatry. 1989;46(10):935–944. doi: 10.1001/archpsyc.1989.01810100077015. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Barrantes-Vidal N, Silvia PJ. The dimensional structure of the Wisconsin Schizotypy Scales: factor identification and construct validity. Schizophr Bull. 2008;34(3):444–457. doi: 10.1093/schbul/sbm098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. J Consult Clin Psychol. 1983;51(2):215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976:85374–85382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Linney YM, Murray RM, Peters ER, MacDonald AM, Rijsdijk F, Sham PC. A quantitative genetic analysis of schizotypal personality traits. Psychol Med. 2003;33(5):803–816. doi: 10.1017/s0033291703007906. [DOI] [PubMed] [Google Scholar]
- Hay DA, Martin NG, Foley D, Treloar SA, Kirk KM, Heath AC. Phenotypic and genetic analyses of a short measure of psychosis-proneness in a large-scale Australian twin study. Twin Research and Human Genetics. 2001;4(1):30–40. doi: 10.1375/1369052012128. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Pogue-Geile MF, Debski TT, Manuck S. Genetic and environmental influences on schizotypy: A community based twin study. Schizophr Bull. 2001;27(1):47–58. doi: 10.1093/oxfordjournals.schbul.a006859. [DOI] [PubMed] [Google Scholar]
- Claridge G, Hewitt JK. A biometrical study of schizotypy in a normal population. PAID. 1987;8(3):303–312. [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study. III. Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50(10):781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ, Kwapil TR. Does the Eysenck Psychoticism Scale predict psychosis - A 10-year-longitudinal-study. Personality and Individual Differences. 1994;17(3):369–375. [Google Scholar]
- Kendler KS, Walsh D. Schizotypal personality disorder in parents and the risk for schizophrenia in siblings. Schizophr Bull. 1995;21(1):47–52. doi: 10.1093/schbul/21.1.47. [DOI] [PubMed] [Google Scholar]
- Tienari P, Wynne LC, Laksy K, Moring J, Nieminen P, Sorri A, Lahti I, Wahlberg KE. Genetic boundaries of the schizophrenia spectrum: evidence from the Finnish Adoptive Family Study of Schizophrenia. Am J Psychiatry. 2003;160(9):1587–1594. doi: 10.1176/appi.ajp.160.9.1587. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ. Genes, environment and personality. Science. 1994;264(5166):1700–1701. doi: 10.1126/science.8209250. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ, Barrett P. A revised version of the psychoticism scale. Personality and Individual Differences. 1985;6(1):21–29. [Google Scholar]
- Lynn R, Martin T. Gender differences in extraversion, neuroticism, and psychoticism in 37 nations. J Soc Psychol. 1997;137(3):369–373. doi: 10.1080/00224549709595447. [DOI] [PubMed] [Google Scholar]
- Barrantes-Vidal N, Ros-Morente A, Kwapil TR. An examination of neuroticism as a moderating factor in the association of positive and negative schizotypy with psychopathology in a nonclinical sample. Schizophr Res. 2009;115(2–3):303–309. doi: 10.1016/j.schres.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Flak V, Sharma T, Davis RE, Corr PJ. Saccadic eye movements, schizotypy, and the role of neuroticism. Biol Psychol. 2005;68(1):61–78. doi: 10.1016/j.biopsycho.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Freeman D, Garety PA. Worry, worry processes and dimensions of delusions: An exploratory investigation of a role for anxiety processes in the maintenance of delusional distress. Behavioural and Cognitive Psychotherapy. 1999;27(01):47–62. [Google Scholar]
- Van Os J, Jones PB. Neuroticism as a risk factor for schizophrenia. Psychol Med. 2001;31(6):1129–1134. doi: 10.1017/s0033291701004044. [DOI] [PubMed] [Google Scholar]
- Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophr Bull. 2008;34(5):856–874. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I, Krabbendam L, van Os J. Continuity of psychotic symptoms in the community. Current Opinion in Psychiatry. 2003;16(4):443–449. [Google Scholar]
- Maier W, Minges J, Lichtermann D, Heun R, Franke P. Personality variations in healthy relatives of schizophrenics. Schizophr Res. 1994;12(1):81–88. doi: 10.1016/0920-9964(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Baron M, Gruen RS. Schizophrenia and affective disorder: are they genetically linked? Br J Psychiatry. 1991;159(2):267–270. doi: 10.1192/bjp.159.2.267. [DOI] [PubMed] [Google Scholar]
- Verdoux H, van Os J, Maurice-Tison S, Gay B, Salamon R, Bourgeois ML. Increased occurrence of depression in psychosis-prone subjects: a follow-up study in primary care settings. Compr Psychiatry. 1999;40(6):462–468. doi: 10.1016/s0010-440x(99)90091-3. [DOI] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ. Psychosis Proneness. In: Albert M, editor. Controversies in Schizophrenia. New York: Guilford Press; 1985. pp. 152–174. [Google Scholar]
- Eckblad M, Chapman LJ. Development and validation of a scale for hypomanic personality. J Abnorm Psychol. 1986;95(3):214–222. doi: 10.1037//0021-843x.95.3.214. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ, Chapman JP, Mishlove M. The Revised Social Anhedonia Scale. Madison: University of Wisconsin; 1982. [Google Scholar]
- Cornes BK, Medland SE, Ferreira MA, Morley KI, Duffy DL, Heijmans BT, Montgomery GW, Martin NG. Sex-limited genome-wide linkage scan for body mass index in an unselected sample of 933 Australian twin families. Twin Research and Human Genetics. 2005;8(6):616–632. [PubMed] [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behav Genet. 1984;14(4):325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76(2):306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R-Development-Core-Team. R Foundation for Statistical Computing. Vienna, Austria: 2010. R: A language and environment for statistical computing. [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987:5242–5248. [Google Scholar]
- Neale MC, Maes HM. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer Academic Publishers BV; 2002. [Google Scholar]
- Kendler KS, Hewitt J. The structure of self-report schizotypy in twins. J Pers Disorders. 1992;6(1):1–17. [Google Scholar]
- Loehlin J. The Cholesky approach: A cautionary note. Behav Genet. 1996;26(1):65–69. [Google Scholar]
- Clementz BA, Grove WM, Katsanis J, Iacono WG. Psychometric detection of the schizotypy perceptual aberration and physical anhedonia in relatives of schizophrenics. J Abnorm Psychol. 1991;100(4):607–612. doi: 10.1037//0021-843x.100.4.607. [DOI] [PubMed] [Google Scholar]
- Franke P, Maier W, Hardt J, Hain C. Cognitive-functioning and anhedonia in subjects at risk for schizophrenia. Schizophr Res. 1993;10(1):77–84. doi: 10.1016/0920-9964(93)90079-x. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Kendler KS. The genetic relationship of personality to major depression and schizophrenia. Neurotoxicity Research. 2004;6(1):43–50. doi: 10.1007/BF03033295. [DOI] [PubMed] [Google Scholar]
- Siris SG. Depression in schizophrenia: Perspective in the era of"atypical" antipsychotic agents. Am J Psychiatry. 2000;157(9):1379–1389. doi: 10.1176/appi.ajp.157.9.1379. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2–3):131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J. The genetic and environmental relationship between major depression and the five-factor model of personality. Psychol Med. 2010;40(5):801–806. doi: 10.1017/S0033291709991140. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Wilkins JM, Ferreira MA, Moran J, Chambert K, Ruderfer DM, Lee PH, Purcell SM, Sklar P. Int Schizophrenia C Large-scale sequencing of DISC 1 in schizophrenia and bipolar disease yields novel variants. Schizophr Bull. :3769–3769. [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4(2):163–172. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]