Abstract
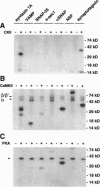
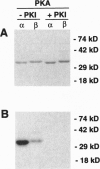
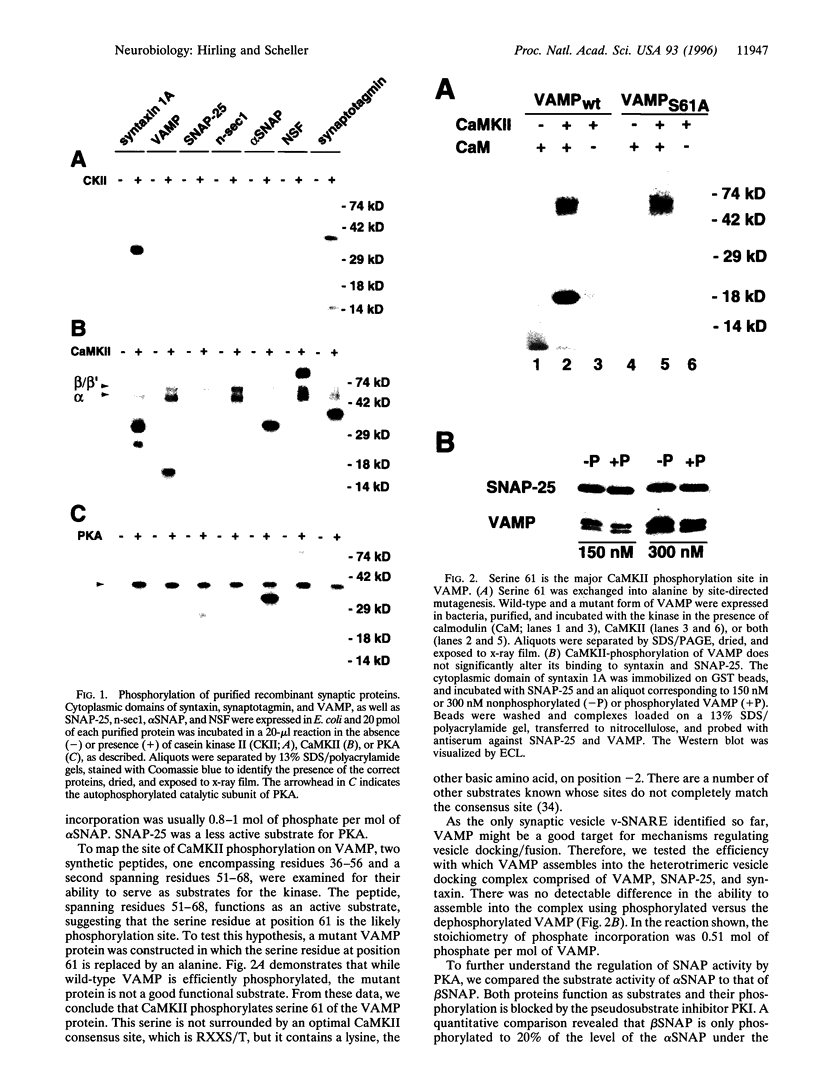
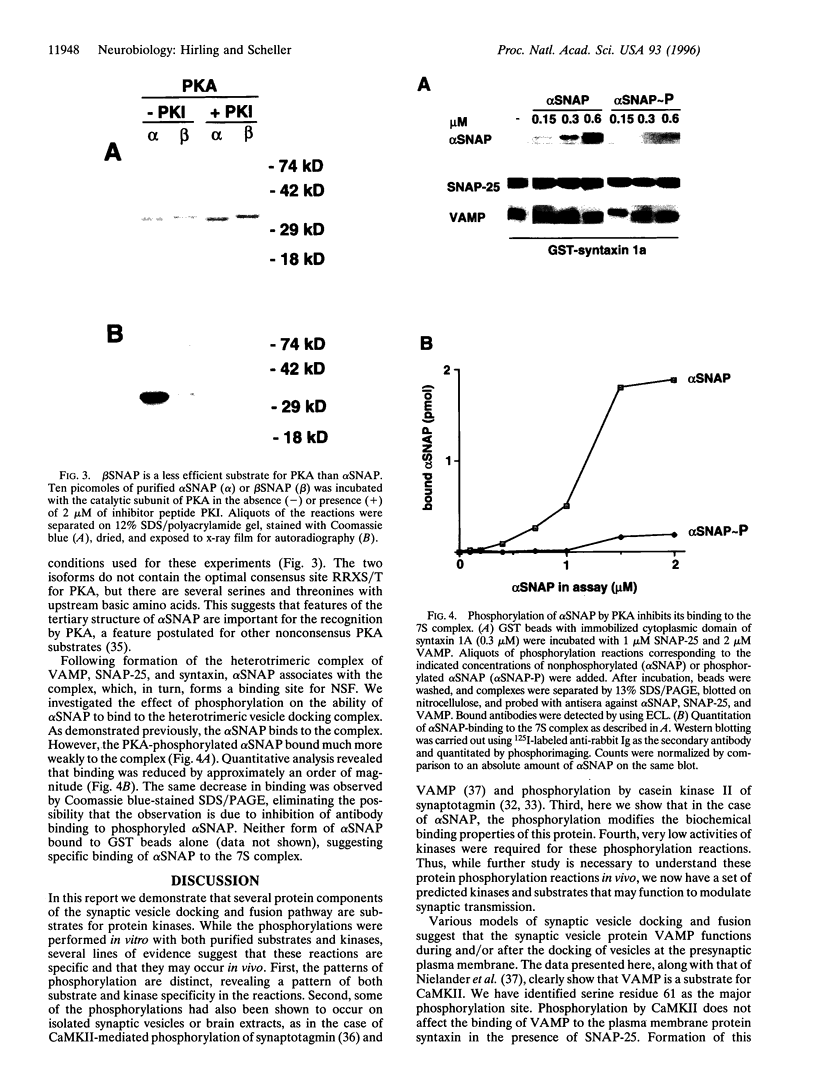
We analyzed whether synaptic membrane trafficking proteins are substrates for casein kinase II, calcium/calmodulin-dependent protein kinase II, and cAMP-dependent protein kinase (PKA), three kinases implicated in the modulation of synaptic transmission. Each kinase phosphorylates a specific set of the vesicle proteins syntaxin 1A, N-ethylmaleimide-sensitive factor (NSF), vesicle-associated membrane protein (VAMP), synaptosome-associated 25-kDa protein (SNAP-25), n-sec1, alpha soluble NSF attachment protein (alpha SNAP), and synaptotagmin. VAMP is phosphorylated by calcium/calmodulin-dependent protein kinase II on serine 61. alpha SNAP is phosphorylated by PKA; however, the beta SNAP isoform is phosphorylated only 20% as efficiently. alpha SNAP phosphorylated by PKA binds to the core docking and fusion complex 10 times weaker than the dephosphorylated form. These studies provide a first glimpse at regulatory events that may be important in modulating neurotransmitter release during learning and memory.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeliovich A., Chen C., Goda Y., Silva A. J., Stevens C. F., Tonegawa S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993 Dec 31;75(7):1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- Alberini C. M., Ghirardi M., Huang Y. Y., Nguyen P. V., Kandel E. R. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann N Y Acad Sci. 1995 Jun 30;758:261–286. doi: 10.1111/j.1749-6632.1995.tb24833.x. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Kandel E. R. Structural changes accompanying memory storage. Annu Rev Physiol. 1993;55:397–426. doi: 10.1146/annurev.ph.55.030193.002145. [DOI] [PubMed] [Google Scholar]
- Baxter D. A., Byrne J. H. Serotonergic modulation of two potassium currents in the pleural sensory neurons of Aplysia. J Neurophysiol. 1989 Sep;62(3):665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Aniksztejn L., Bregestovski P. Protein kinase C modulation of NMDA currents: an important link for LTP induction. Trends Neurosci. 1992 Sep;15(9):333–339. doi: 10.1016/0166-2236(92)90049-e. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Miller K. G., Scheller R. H. Casein kinase II phosphorylates the synaptic vesicle protein p65. J Neurosci. 1993 Apr;13(4):1701–1707. doi: 10.1523/JNEUROSCI.13-04-01701.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Chapman P. F., Frenguelli B. G., Smith A., Chen C. M., Silva A. J. The alpha-Ca2+/calmodulin kinase II: a bidirectional modulator of presynaptic plasticity. Neuron. 1995 Mar;14(3):591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]
- Colbran R. J., Soderling T. R. Calcium/calmodulin-dependent protein kinase II. Curr Top Cell Regul. 1990;31:181–221. doi: 10.1016/b978-0-12-152831-7.50007-x. [DOI] [PubMed] [Google Scholar]
- Dash P. K., Karl K. A., Colicos M. A., Prywes R., Kandel E. R. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. P., Squire L. R. Protein synthesis and memory: a review. Psychol Bull. 1984 Nov;96(3):518–559. [PubMed] [Google Scholar]
- Davletov B., Sontag J. M., Hata Y., Petrenko A. G., Fykse E. M., Jahn R., Südhof T. C. Phosphorylation of synaptotagmin I by casein kinase II. J Biol Chem. 1993 Mar 25;268(9):6816–6822. [PubMed] [Google Scholar]
- Deisseroth K., Bito H., Tsien R. W. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996 Jan;16(1):89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Sasaki T., Fukui K., Kotani H., Kimura T., Hata Y., Südhof T. C., Scheller R. H., Takai Y. Phosphorylation of Munc-18/n-Sec1/rbSec1 by protein kinase C: its implication in regulating the interaction of Munc-18/n-Sec1/rbSec1 with syntaxin. J Biol Chem. 1996 Mar 29;271(13):7265–7268. doi: 10.1074/jbc.271.13.7265. [DOI] [PubMed] [Google Scholar]
- Fykse E. M., Li C., Südhof T. C. Phosphorylation of rabphilin-3A by Ca2+/calmodulin- and cAMP-dependent protein kinases in vitro. J Neurosci. 1995 Mar;15(3 Pt 2):2385–2395. doi: 10.1523/JNEUROSCI.15-03-02385.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S. G., O'Dell T. J., Karl K. A., Stein P. L., Soriano P., Kandel E. R. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992 Dec 18;258(5090):1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Greengard P., Valtorta F., Czernik A. J., Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993 Feb 5;259(5096):780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Hanson P. I., Otto H., Barton N., Jahn R. The N-ethylmaleimide-sensitive fusion protein and alpha-SNAP induce a conformational change in syntaxin. J Biol Chem. 1995 Jul 14;270(28):16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- Hochner B., Kandel E. R. Modulation of a transient K+ current in the pleural sensory neurons of Aplysia by serotonin and cAMP: implications for spike broadening. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11476–11480. doi: 10.1073/pnas.89.23.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Y., Li X. C., Kandel E. R. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994 Oct 7;79(1):69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Kee Y., Lin R. C., Hsu S. C., Scheller R. H. Distinct domains of syntaxin are required for synaptic vesicle fusion complex formation and dissociation. Neuron. 1995 May;14(5):991–998. doi: 10.1016/0896-6273(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Montarolo P. G., Goelet P., Castellucci V. F., Morgan J., Kandel E. R., Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986 Dec 5;234(4781):1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- Morgan A., Wilkinson M., Burgoyne R. D. Identification of Exo2 as the catalytic subunit of protein kinase A reveals a role for cyclic AMP in Ca(2+)-dependent exocytosis in chromaffin cells. EMBO J. 1993 Oct;12(10):3747–3752. doi: 10.1002/j.1460-2075.1993.tb06052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielander H. B., Onofri F., Valtorta F., Schiavo G., Montecucco C., Greengard P., Benfenati F. Phosphorylation of VAMP/synaptobrevin in synaptic vesicles by endogenous protein kinases. J Neurochem. 1995 Oct;65(4):1712–1720. doi: 10.1046/j.1471-4159.1995.65041712.x. [DOI] [PubMed] [Google Scholar]
- Oyler G. A., Higgins G. A., Hart R. A., Battenberg E., Billingsley M., Bloom F. E., Wilson M. C. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989 Dec;109(6 Pt 1):3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T. D., Coorssen J. R., Horstmann H., Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995 Nov;15(5):1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Hsu S. C., Scheller R. H. n-Sec1: a neural-specific syntaxin-binding protein. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1445–1449. doi: 10.1073/pnas.91.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M. Synaptotagmin is endogenously phosphorylated by Ca2+/calmodulin protein kinase II in synaptic vesicles. FEBS Lett. 1993 Feb 8;317(1-2):85–88. doi: 10.1016/0014-5793(93)81496-m. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Greengard P., Czernik A. J. Calcium-dependent serine phosphorylation of synaptophysin. Synapse. 1993 Feb;13(2):161–172. doi: 10.1002/syn.890130207. [DOI] [PubMed] [Google Scholar]
- Scheller R. H. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995 May;14(5):893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- Sheng M., Thompson M. A., Greenberg M. E. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991 Jun 7;252(5011):1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Paylor R., Wehner J. M., Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995 Jun 22;375(6533):645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Van Patten S. M. Multiple pathway signal transduction by the cAMP-dependent protein kinase. FASEB J. 1994 Dec;8(15):1227–1236. doi: 10.1096/fasebj.8.15.8001734. [DOI] [PubMed] [Google Scholar]
- Wang Y. T., Salter M. W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994 May 19;369(6477):233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Weisskopf M. G., Castillo P. E., Zalutsky R. A., Nicoll R. A. Mediation of hippocampal mossy fiber long-term potentiation by cyclic AMP. Science. 1994 Sep 23;265(5180):1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- Whiteheart S. W., Griff I. C., Brunner M., Clary D. O., Mayer T., Buhrow S. A., Rothman J. E. SNAP family of NSF attachment proteins includes a brain-specific isoform. Nature. 1993 Mar 25;362(6418):353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]
- Zhuo M., Hu Y., Schultz C., Kandel E. R., Hawkins R. D. Role of guanylyl cyclase and cGMP-dependent protein kinase in long-term potentiation. Nature. 1994 Apr 14;368(6472):635–639. doi: 10.1038/368635a0. [DOI] [PubMed] [Google Scholar]