Abstract
BACKGROUND
Strategies for initial drug therapy of hypertension are a thiazide diuretic for all or drug selection based on age/race criteria or on plasma renin activity (PRA). It is uncertain which of these strategies will achieve the highest control rate among patients with stage 1 essential hypertension. We sought to compare control rates among 3 drug selection strategies: (i) thiazide diuretic for all, (ii) thiazide diuretic for all black subjects and white subjects aged ≥50 years and a renin-angiotensin system blocker for white subjects aged <50 years, or (iii) thiazide diuretic for PRA < 0.6ng/ml/h (suppressed PRA) and a renin-angiotensin system blocker for PRA ≥ 0.6ng/ml/h (nonsuppressed PRA).
METHODS
Blood pressure responses from the Genetic Epidemiology of Responses to Antihypertensives (GERA) study were used to determine control rates for each of the 3 strategies. In GERA, hypertensive adults were treated with hydrochlorothiazide (n = 286 black subjects and 284 white subjects) or with candesartan (n = 248 black subjects and 278 white subjects).
RESULTS
In the overall sample, the PRA strategy was associated with the highest control rate of 69.4% vs. 61.3% with the age/race strategy (P < 0.001) and 53.8% with the thiazide for all strategy (P < 0.001). This was also true in each racial subgroup (in black subjects: 62.1% vs. 55.2% for the other 2 strategies, P = 0.02; in white subjects: 76.3% vs. 67.1% with the age/race strategy (P < 0.001) and 52.4% with the thiazide for all strategy (P < 0.001)).
CONCLUSIONS
This exploratory analysis suggests that choice of initial therapy for hypertension using a PRA strategy may be associated with higher control rates than alternative strategies recommended in current guidelines.
Keywords: blood pressure, control rate, hypertension, pharmacotherapy.
Current treatment guidelines in the United States suggest a thiazide diuretic (TD) as the initial agent of choice for most persons with uncomplicated hypertension (TD strategy).1 This is despite recognition that blood pressure (BP) response to any single antihypertensive drug is characterized by marked interindividual variation.2,3 Causes of variation in response are incompletely understood but are hypothesized to reflect the heterogeneity of pathophysiologic mechanisms under lying hypertension as well as variation in the counterregulatory physiological responses to BP reduction.4,5 It is recognized that the renin-angiotensin system (RAS) plays an important role in BP regulation and that hypertensives are characterized by interindividual variation in the activity of this system.6,7 Laragh and colleagues have long advocated initial drug selection based on an estimation of the activity of the RAS (plasma renin activity (PRA) strategy).8,9 Persons with low (suppressed) PRA are hypothesized to have sodium volume–dependent hypertension, directing initial therapy to a diuretic, whereas those with higher (nonsuppressed) PRA are hypothesized to have vasoconstriction-dependent hypertension mediated by angiotensin II, directing initial therapy to drugs that directly block renin release or the formation or action of angiotensin II (RAS blockers).9
The clinical usefulness of this approach has been questioned.7 Moreover, a PRA strategy of predicting drug response in a large sample of men with diastolic hypertension was shown to be no more effective than a method based on age and race, two more easily determined characteristics that are correlated with PRA.10 In this strategy, older age and black race are used as markers for low PRA, whereas younger age and white race are markers for higher PRA (age/race strategy).11 Which of the 3 competing strategies (TD, age/race, PRA) leads to the highest rate of control is uncertain. To address this question we took advantage of BP response information from 2 large studies in biracial samples of adults with uncomplicated essential hypertension treated with a TD or an angiotensin receptor blocker to determine BP control rates for each of the 3 drug selection strategies.
METHODS
Study population
This study represents a retrospective analysis of data previously collected in the Genetic Epidemiology of Responses to Antihypertensives (GERA) study.12,13 The GERA study was designed to determine the role of genetic variation in explaining interindividual differences in response to a TD in 286 black subjects and 284 white subjects (protocol 1) and to an angiotensin II receptor blocker in 248 black subjects and 278 white subjects (protocol 2). Independent patient samples were recruited for each protocol.
Study procedures for GERA have been previously described.12,13 Briefly, healthy men and women aged 30–59 years with essential hypertension were recruited at Emory University in Atlanta, Georgia (black subjects), and the Mayo Clinic in Rochester, Minnesota (white subjects). The study protocol was approved by the institutional review boards of both institutions. All subjects provided written informed consent. In Atlanta, black subjects were recruited through outpatient medical clinics at Grady Memorial Hospital, the Hypertension and Renal Diseases Research Center at Emory University, advertisements in public media, and mailing lists of registered voters. In Rochester, white subjects were recruited from a list of all residents of Olmsted County with the diagnosis of essential hypertension who had been seen by a health-care provider within 3 years of recruitment. Essential hypertension was defined as a BP >140/90mm Hg or a previous diagnosis of essential hypertension and current treatment with antihypertensive medication. Exclusions of participation included a known secondary cause of hypertension. Women using oral contraceptive medications were disqualified; however, those receiving postmenopausal hormone replacement were allowed.
At enrollment, subjects were instructed by trained dieticians to maintain a standard sodium intake of 2 mmol/kg/day throughout the study periods. Adherence was monitored weekly by 24-hour urine collections alternating with recall diaries. Antihypertensive medications were discontinued, and subjects were evaluated every other week during a 4–6 week washout phase. Once stable elevation of BP (>90mm Hg diastolic but <180/110mm Hg) was achieved, antihypertensive therapy was administered for 4–6 weeks. Subjects were treated with 25mg of hydrochlorothiazide (HCTZ) orally once daily for 4 weeks (protocol 1) or 16mg of candesartan orally once daily for 2 weeks with a subsequent increase to 32mg orally once daily for 4 additional weeks (protocol 2). BP was measured in triplicate 3 minutes apart in the seated position after 5 minutes of rest, as previously described.12,13 The average of the second and third readings was used for all analyses. BPs were measured at the end of the washout period and at the end of the treatment period. All readings were made in the morning at the end of the dosing interval. Compliance with drug therapy was assessed by pill counts at each study visit.
Laboratory analyses
At the end of the washout period, blood was drawn in the morning after 30 minutes in the seated position. PRA was determined by radioimmunoassay of angiotensin I in the presence of reagents that inhibit angiotensin I–converting enzyme and angiotensinases (Dupont Company, Boston MA, for protocol 1; DiaSorin, Stillwater MN, for protocol 2). PRA determinations by both assays were performed according to the manufacturers’ recommendations. The standard incubation time for the Dupont assay is 1 hour, whereas the standard incubation time for the DiaSorin assay is 3 hours.
Statistical analyses
Data were summarized by calculating means and SDs or medians and upper and lower quartiles for quantitative variables and percentages for categorical variables. P values for differences in quantitative variables were calculated using analysis of variance for variables that were normally distributed and the Mann–Whitney rank sum test for quantitative variables that were not normally distributed. In all analyses, statistical significance was inferred when P < 0.05. BP was considered controlled if it was <140mm systolic and <90mm Hg diastolic at the end of drug therapy. Control rates were calculated for the following 3 strategies: (i) HCTZ for all subjects (TD strategy); (ii) HCTZ for all black subjects and for white subjects aged ≥50 years, with candesartan for white subjects aged <50 years (age/race strategy); and (iii) HCTZ if PRA < 0.6ng/ml/h (suppressed PRA) and candesartan if PRA ≥ 0.6ng/ml/h (nonsuppressed PRA) (PRA strategy). A formal test of the null hypothesis of equality of control rates between 2 strategies was based on the fact that each strategy involves a choice between 1 of 2 antihypertensive drugs within each of 8 age/race/PRA strata. Within each of these 8 strata, the difference in control rates was calculated as the difference between 2 binomial proportions with associated SEs. The overall difference in control rates between strategies is a weighted average of the 8 stratum-specific differences in control rates. If both strategies dictated the same choice of drug in a given stratum, then the rate difference for that stratum was zero, and its SE was zero. For stratum weights, we used the number of subjects in the stratum as a proportion of the total sample. The usual formulae for binomial proportions and linear combinations of independent random variables were used to derive a single z statistic to test the null hypothesis that the overall difference in control rates was zero, as well as to derive confidence intervals for the difference in control rates.
RESULTS
Sample description
The overall sample consisted of 534 (49%) black subjects and 562 (51%) white subjects; 555 (51%) were women (Table 1). On average, subjects were obese (body mass index = 30.6±5.3kg/m2) and had stage 1 hypertension (147±14/96±5mm Hg) after washout of previous therapy. In black subjects, BP response was greater to HCTZ than to candesartan (P < 0.001 for systolic BP and P = 0.005 for diastolic BP), and in white subjects, the reverse was true (P < 0.001 for both systolic BP and diastolic BP).
Table 1.
Subject characteristics
| Black subjects (n = 534) | White subjects (n = 562) | ||||||
|---|---|---|---|---|---|---|---|
| Trait | Overall sample | HCTZ protocol | Candesartan protocol | P value | HCTZ protocol | Candesartan protocol | P value |
| No. (%) | 1,096 (100%) | 286 (26%) | 248 (23%) | 284 (26%) | 278 (25%) | ||
| Age, y | 48.7 (6.7) | 47.8 (6.1) | 48.8 (6.37) | 0.06 | 48.6 (7.5) | 49.7 (6.6) | 0.08 |
| Female No. (%) | 555 (50.6%) | 147 (51) | 144 (58) | 0.12 | 121 (43) | 143 (51) | 0.04 |
| BMI, kg/m2 | 30.6 (5.3) | 31.5 (6.4) | 30.3 (4.5) | 0.02 | 30.9 (5.4) | 29.6 (4.1) | 0.001 |
| Heart rate, bpm (end of washout) | 73 (9) | 72 (9) | 72 (8) | 0.53 | 73 (9) | 75 (10) | 0.01 |
| 24-hour urine sodium, mmol/24h (end of washout) | 153 (107–201) | 161 (118–210) | 117 (83–168) | <0.001 | 154 (112–192) | 163 (122–214) | 0.03 |
| SBP, mm Hg (end of washout) | 147 (14) | 150 (15) | 148 (12) | 0.08 | 142 (12) | 148 (13) | <0.001 |
| SBP, mm Hg (end of treatment) | 133 (14) | 132(16) | 138(15) | <0.001 | 131 (12) | 129 (14) | 0.04 |
| Δ SBP, mm Hg | 15 (14) | 18 (13) | 10 (14) | <0.001 | 11 (12) | 19 (14) | <0.001 |
| DBP, mm Hg (end of washout) | 96 (5) | 97 (5) | 96 (5) | 0.09 | 96 (5) | 95 (5) | 0.04 |
| DBP, mm Hg (end of treatment) | 87 (9) | 87 (9) | 89 (9) | 0.12 | 89 (8) | 81 (9) | <0.001 |
| Δ DBP, mm Hg | 9.3 (8.6) | 9.4 (8.5) | 7.3 (8.3) | 0.005 | 6.6 (7.3) | 13.6 (8.6) | <0.001 |
| PRA, ng/ml/h (end of washout) | 0.7 (0.3–1.3) | 0.6 (0.3–1.1) | 0.2 (0.1–0.6) | <0.001 | 1.2 (0.7–2.1) | 0.7 (0.3–1.4) | <0.001 |
Unless otherwise noted, all values are expressed as mean (SD) except plasma renin activity (PRA) and 24-hour urine sodium, which are expressed as median (upper and lower quartiles). P values are for contrasts of hydrochlorothiazide (HCTZ) protocol vs. candesartan protocol. P values were calculated using analysis of variance or Mann–Whitney rank-sum test.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Within each age stratum, the proportion in each PRA stratum differed significantly by race (P < 0.001 for all comparisons) (Table 2). A majority of black subjects in each age stratum were in the suppressed PRA stratum (PRA < 0.6ng/ml/h) with no difference in the proportion within each PRA stratum across age strata (P = 0.95 for differences in the proportion within each PRA stratum across age strata). In contrast, the majority of white subjects in each age stratum were in the nonsuppressed PRA stratum (PRA ≥ 0.6ng/ml/h) with an increase in the proportion of suppressed PRA subjects in the aged ≥50 years stratum (P = 0.01 for differences in the proportion within each PRA stratum across age strata).
Table 2.
Effect of race and age on PRA classification
| Aged <50 years | Aged ≥ 50 years | |||||
|---|---|---|---|---|---|---|
| PRA Classification | Black subjects (n = 300) | White subjects (n = 264) | P value | Black subjects (n = 234) | White subjects (n = 298) | P value |
| Suppressed PRA, No. (%) | 184 (61) | 72 (27) | <0.001 | 143(61) | 111 (37) | <0.001 |
| Nonsuppressed PRA, No. (%) | 116 (39) | 192 (73) | <0.001 | 91(39) | 187 (63) | <0.001 |
Abbreviation: PRA, plasma renin activity.
As expected in both racial subgroups, baseline systolic BP was higher in the aged ≥50 years strata than in the aged <50 years strata independent of PRA strata, whereas baseline diastolic BP did not differ across the age/PRA strata (Supplementary Table S1).
Control rate by treatment strategy
In the overall sample, the strategy of drug selection based on pretreatment PRA was associated with the highest control rate (69.4% with the PRA strategy vs. 61.3% with the age/race strategy (P < 0.001) and 53.8% with the TD strategy (P < 0.001)) (Table 3).
Table 3.
Blood pressure control rates by treatment strategy
| Blood pressure control rate, % | |||
|---|---|---|---|
| Treatment strategy | Overall | Black subjects | White subjects |
| Thiazide diuretic | 53.8 | 55.2 | 52.4 |
| Age/race | 61.3 | 55.2 | 67.1 |
| Plasma renin activity | 69.4* | 62.1** | 76.3* |
Blood pressure control rate defined as percentage of subjects with BP < 140/90mm Hg. Thiazide diuretic strategy was hydrochlorothiazide in all subjects. Age/race strategy was hydrochlorothiazide in all black subjects and in white subjects aged ≥50 years and candesartan in white subjects aged <50 years. Plasma renin activity (PRA) strategy was hydrochlorothiazide for pretreatment PRA < 0.6ng/ml/h and candesartan for pretreatment PRA ≥ 0.6ng/ml/h.
*P < 0.001 vs. other strategies.
**P = 0.02 vs. other strategies.
In black subjects, the strategy of drug selection based on pretreatment PRA was also associated with a higher control rate than drug selection based on the other 2 strategies, both of which directed the use of a TD for all subjects (control rate = 62.1% with the PRA strategy vs. 55.2% with the other 2 strategies; P = 0.02) (Table 3). In black subjects with suppressed PRA, the control rate was higher with HCTZ than with candesartan (57.9% vs. 36.9%; P < 0.001) (Table 4), whereas in black subjects with nonsuppressed PRA, the control rate was higher with candesartan than with HCTZ (70.5% vs. 52.7%; P = 0.02) (Table 4).
Table 4.
Blood pressure control rates for each drug by plasma renin activity (PRA) and age criteria
| Blood pressure control rate, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Black subjects | White subjects | |||||||||
| Drug | All | PRA < 0.6 ng/ml/h | PRA ≥ 0.6 ng/ml/h | Aged < 50 y | Aged ≥ 50 y | All | PRA < 0.6 ng/ml/h | PRA ≥ 0.6 ng/ml/h | Aged < 50 y | Aged ≥ 50 y |
| HCTZ | 55.2 | 57.9 | 52.7 | 43.2 | 47.2 | 52.4 | 49.2 | 53.8 | 49.7 | 55.9 |
| Candesartan | 45.2*** | 36.9** | 70.5*** | 57.7**** | 51.4 | 78.1** | 63.7* | 89.6** | 77.2** | 78.7** |
Blood pressure control rate defined as percentage of subjects with BP <140/90mm Hg.
Abbreviation: HCTZ, hydrochlorothiazide.
*P = 0.006 candesartan vs. HCTZ.
**P < 0.001 candesartan vs. HCTZ.
***P = 0.02 candesartan vs. HCTZ.
****P = 0.01 candesartan vs. HCTZ.
In white subjects, the strategy of drug selection based on pretreatment PRA was also associated with a higher control rate than drug selection based on either of the other 2 strategies (control rate = 76.3% with the PRA strategy vs. 67.1% with the age/race strategy (P < 0.001) and 52.4% with the TD strategy (P < 0.001)) (Table 3). Consistent with the findings in black subjects, control rates were higher with candesartan than with HCTZ in white subjects with nonsuppressed PRA (89.6% vs. 53.8%; P < 0.001) (Table 4). However, in contrast with the findings in black subjects, control rates were also higher with candesartan than HCTZ in white subjects with suppressed PRA (63.7% vs. 49.2%; P = 0.006). Moreover, control rates in white subjects were also higher with candesartan than with HCTZ in both age strata (aged <50 years: 77.2% vs. 49.7%, P < 0.001; aged ≥50 years: 78.7% vs. 55.9%, P < 0.001) (Table 4). Because of higher control rates with candesartan than with HCTZ in both PRA and age strata, an alternate “optional” strategy in white subjects of an RAS blocker for all was associated with the highest control rate of 78.1% (P < 0.06 compared with the PRA strategy).
Effects of race, PRA, and age classification on differences in BP response to HCTZ vs. candesartan
Differences in systolic and diastolic BP responses (mean, 95% confidence interval (CI)) with HCTZ vs. candesartan for each racial subgroup overall and by age/PRA strata within each racial subgroup are shown in Figure 1. All values were adjusted for baseline BP, age, sex, and baseline urinary sodium excretion.
Figure 1.
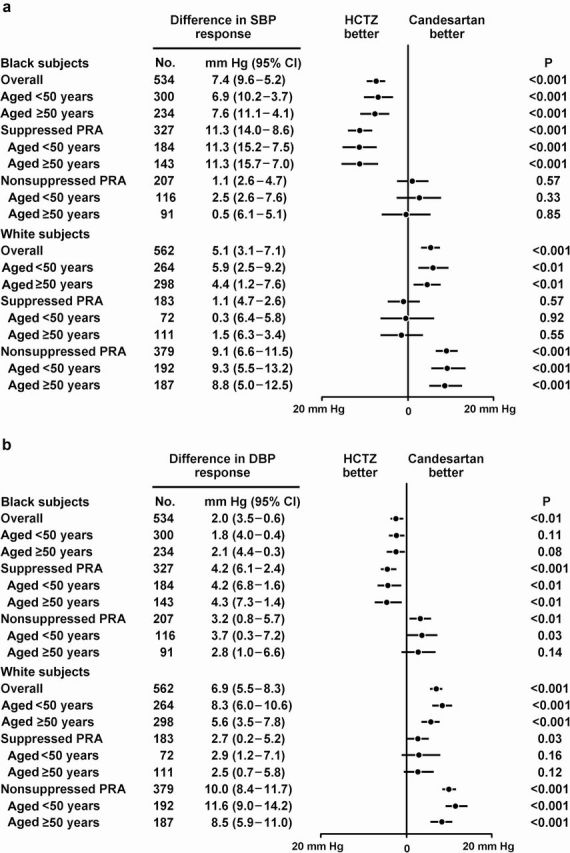
Differences in (a) systolic (SBP) and (b) diastolic blood pressure (DBP) response (mean, 95% confidence interval (CI)) with hydrochlorothiazide (HCTZ) vs. candesartan for each racial subgroup overall and by age/plasma renin activity (PRA) strata.
Overall in black subjects, compared with treatment with candesartan, treatment with HCTZ was associated with a greater decrease in mean systolic BP of −7mm Hg (95% CI = −5 to −10mm Hg; P < 0.001) and a greater decrease in mean diastolic BP of −2mm Hg (95% CI = −1 to −4mm Hg; P < 0.01). These differences in systolic and diastolic BP response favoring HCTZ did not differ across age strata but were greater in the suppressed PRA stratum. In the suppressed PRA stratum, these greater differences in systolic and diastolic BP response favoring HCTZ also did not differ across age strata. In the nonsuppressed PRA stratum, there was no difference in SBP response between HCTZ and candesartan (P = 0.57). This was also true across age strata. However, compared with treatment with HCTZ, treatment with candesartan was associated with a greater decrease in mean diastolic BP of −3mm Hg (95% CI = −1 to −6mm Hg; P < 0.01) This difference in the mean diastolic BP responses favoring candesartan did not differ across age strata.
Overall in white subjects, compared with treatment with HCTZ, treatment with candesartan was associated with a greater decrease in mean systolic BP of −5mm Hg (95% CI = −3 to −7mm Hg; P < 0.001) and a greater decrease in mean diastolic BP of −7mm Hg (95% CI = −6 to −8mm Hg; P < 0.001). These differences in mean systolic and diastolic BP response favoring candesartan decreased slightly across age strata and were greater in the nonsuppressed PRA stratum. Within the nonsuppressed PRA stratum, these greater differences in systolic and diastolic BP response favoring candesartan were similar across age strata for systolic BP but were less in the aged ≥50 year age stratum for diastolic BP. In the suppressed PRA stratum, there was no difference in mean systolic BP response between HCTZ and candesartan (P = 0.57). This was also true across age strata. However, compared with treatment with HCTZ, treatment with candesartan was associated with a greater decrease in mean diastolic BP of −3mm Hg (95% CI= −0.2 to −5mm Hg; P = 0.03). This difference in diastolic BP response favoring candesartan did not differ across age strata.
Differences in PRA values by method of determination
As noted in the Methods, PRA was determined using assays from Dupont for protocol 1 and from DiaSorin in protocol 2. PRA was determined in stored samples from 39 subjects using both assays (Supplementary Table S2). On average, PRA values were 0.44ng/ml/h (32%; P < 0.001) lower with the DiaSorin assay than with the Dupont assay. This resulted in disagreement regarding PRA classification in 6 of 39 subjects (15%). All of these instances of disagreement involved patients with relatively low PRA values (≤1.13ng/ml/h).
DISCUSSION
Of the 3 strategies recommended to select a diuretic (HCTZ) or an RAS blocker (candesartan) for initial treatment of uncomplicated essential hypertension (TD, age/race, PRA), the PRA strategy was associated with a higher BP control rate than either alternative strategy. This was true for the sample overall and for each racial subgroup. Whereas use of pretreatment PRA was the optimum strategy for drug selection in black subjects, selection of candesartan for all subjects regardless of age or pretreatment PRA was the optimum strategy in white subjects.
Overall in black subjects, average BP response was greater to the TD than to the RAS blocking drug. This is consistent with previous studies14,15 and is usually explained by the well-known fact reaffirmed in our study that the majority of hypertensive black subjects have suppressed values of PRA. Also, consistent with previous studies, average BP response was greater in white subjects to the RAS-blocking drug than to the TD.14 This observation is also usually explained by the higher prevalence of nonsuppressed values of PRA in white subjects. However, our data suggest that not all of the racial difference in response to candesartan vs. HCTZ can be explained by differences in PRA status. Although in white subjects the BP control rate was higher with candesartan in the nonsuppressed PRA stratum than in the suppressed PRA stratum, it was also significantly higher with candesartan than with HCTZ in both the suppressed and nonsuppressed PRA strata. In contrast, in black subjects a suppressed PRA was associated with a greater systolic and diastolic BP response to HCTZ than to candesartan, and a nonsuppressed PRA was associated with a greater diastolic BP response to candesartan. Although a nonsuppressed PRA was associated with a better systolic and diastolic BP response to candesartan than to HCTZ in white subjects, a suppressed PRA was also associated with a better diastolic BP response to candesartan (Figure 1).What accounts for a higher BP control rate and a better BP response to candesartan than HCTZ in suppressed PRA white subjects is uncertain.
Laragh and colleagues were first to advocate measurement of PRA in all hypertensive patients to guide initial antihypertensive drug selection.8,9 This recommendation has been controversial and not widely advocated by guideline groups. Moreover, a large study by the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents (VACSG) involving a biracial sample of 1,031 hypertensive men randomized to 6 different monotherapies concluded that the combination of age (younger or older than 60 years) and race (black or white) was equally predictive compared with PRA and preferable because it did not require additional laboratory measurements.17 The results of our study demonstrated that in white subjects neither knowledge of PRA status nor age provided additional information for drug selection, whereas in black subjects, knowledge of PRA status did provide additional information. Thus, our data would suggest that a drug selection strategy limited to using PRA alone or age/race criteria as a surrogate for PRA alone might be less effective for drug selection than one that uses a combination of these criteria.
However, our conclusions are limited to persons aged <60 years and may not apply to persons aged ≥60 years. Results of the VACSG study provide information about the effect of age on antihypertensive drug response that can be incorporated with our findings to speculate on a treatment strategy that expands on the notion of combining the selective use of age, race, and PRA for optimum antihypertensive drug selection rather than using PRA or age and race as a surrogate for PRA-based drug selection in all patient subgroups. In the VACSG study, the response rate in black subjects to HCTZ was higher in those aged ≥60 years than in those aged <60 years, whereas response to captopril decreased across these age groups.18–20 These observations would be consistent with an age-associated increase in the proportion of black persons with suppressed PRA such that the value of measuring PRA in older black persons for drug selection might be questionable. Eighty percent of the black subjects in our study were classified as having suppressed PRA. If this percentage increased significantly from this level in black hypertensive persons aged >60 years, measurement of PRA for drug selection would likely be cost ineffective because the number needed to measure to influence drug selection might be prohibitively high. The VACSG also observed that white subjects aged <60 years of age responded better to RAS blocking drugs than to HCTZ, consistent with our results. However, among white subjects aged ≥60 years of age, response rates to RAS blocking drugs and HCTZ were similar. This observation suggests the possibility that with aging in white persons, the advantage of an RAS blocker over HCTZ regardless of PRA status is diminished and, thus, measurement of PRA in older white persons might be helpful in drug selection. The observations from the VACSG study taken together with the results of our study allows construction of a proposed strategy that combines age/race criteria and PRA criteria for drug selection rather than using one as a surrogate for the other (Figure 2). In this proposed strategy, white persons aged <60 years would preferentially be given an RAS blocker, whereas white persons aged ≥60 years would have drug selection based on PRA measurement. Drug selection in black persons aged <60 years would be guided by PRA, whereas black persons aged ≥60 years of age would preferentially be given a TD. Obviously, the effectiveness of such a strategy is unproven and would require confirmation in a future study.
Figure 2.
Suggested scheme of initial drug selection for patients with uncomplicated, stage 1 essential hypertension based on race, age, and plasma rennin activity (PRA). Abbreviation: RAS, renin-angiotensin system.
This study has several limitations. This was not a single, prospective, clinical trial that exposed the same sample to both drug monotherapies. Response data from 2 separate studies, each with their own independently recruited sample, were retrospectively analyzed. Thus, the response to each drug in each racial subgroup in this analysis was inferred from the responses in a subset from each subgroup. The dose of HCTZ was fixed at 25mg daily for 4 weeks, whereas the dose of candesartan was fixed at 32mg for 4 weeks after 2 weeks of an interim dose of 16mg daily. Theoretically the differences in dose and duration of therapy between HCTZ and candesartan could pose a bias in favor of candesartan. However, it should be noted that the doses of drugs used in this analysis were chosen in the original protocols because they represent the maximum doses of each drug most commonly used as monotherapy for hypertension in clinical practice. Moreover, the additional decrease in mean BP from 2 weeks of candesartan 16mg daily to 6 weeks of therapy (2 weeks of 16mg daily and 4 weeks of 32mg daily) was only −2.6/−1.6mm Hg in the study sample. Thus, in our opinion, the differences in dose and duration of therapy with the drugs used in this analysis are unlikely to be a significant source of bias regarding the inferences of this study. The assessment of the predictive value of age on drug response is limited by the fact that our sample consisted of individuals who were aged <60 years. Additionally, assessment of BP control rates by selection strategy is limited to a relatively short period of observation. Long-term control with each drug was not assessed. It is also important to note that PRA measures were made under careful conditions that may not be easily reproduced in the usual clinical setting. Moreover, PRA measurements were made using 2 different assays, and an analysis suggests that up to 15% of participants would have been classified differently depending on the assay used. In all instances, the disagreements in PRA classification between the 2 assays involved participants with relatively low PRA. The lower values with the DiaSorin assay than with the Dupont assay likely resulted from the longer incubation time of the DiaSorin assay in samples with low renin. It is uncertain how these differences in PRA classification by assay might affect our results. However, we did repeat the analyses using a higher PRA cutpoint of 1.0ng/ml/h to distinguish suppressed from nonsuppressed PRA. The inferences of this study were unchanged, and with the higher PRA cutpoint, the percentage of subjects classified differently by the two assays was reduced from 15% to 5%. Lastly, whether the results of this study apply to RAS-blocking drugs other than candesartan is uncertain. Thus, the results of this analysis should be considered exploratory and in need of confirmation.
In conclusion, the results of our study suggest that in persons aged <60 years with uncomplicated essential hypertension, choice of initial antihypertensive drug therapy using a PRA strategy is associated with higher control rates than a strategy based on age/race criteria or a strategy of using a TD as the initial drug of choice for all. In black subjects, the optimal control rate was achieved when initial drug selection was based on PRA and in white subjects when an RAS blocker was used as the initial drug of choice in all. Consideration of our results with those of previous studies suggests that control rates in uncomplicated hypertension with drug monotherapy could be enhanced by use of a drug selection strategy that combines criteria of age, race, and PRA rather than a strategy that uses age/race criteria as a surrogate for PRA. The value of this suggested strategy needs to be tested in a prospective clinical trial.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLAIMER
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Center For Transitional Science Activities at Mayo Clinic Rochester, Minnesota (M01-RR00585), and Emory University, Atlanta, Georgia (M01-RR00039); U.S. Public Health Service grants R01-HL53330 from the Division of Research Resources, National Institutes of Health; and funds from the Mayo Foundation.
REFERENCES
- 1. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ; National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42: 1206–1252 [DOI] [PubMed] [Google Scholar]
- 2. Bidivilli J, Nussberger J, Waeber G, Porchet M, Waeber B, Brunner HR. Individual responses to converting enzyme inhibitors and calcium antagonists. Hypertension 1988; 11: 166–173 [DOI] [PubMed] [Google Scholar]
- 3. Turner ST, Schwartz GL, Chapman AB, Hall WD, Boerwinkle E. Antihypertensive pharmacogenetics: getting the right drug into the right patient. J Hypertens 2001; 19: 1–11 [DOI] [PubMed] [Google Scholar]
- 4. van Brummelen P, Man in’t Veld AJ, Schalekamp MA. Hemodynamic changes during long-term thiazide treatment of essential hypertension in responders and nonresponders. Clin Pharmacol Ther 1980; 27: 328–336 [DOI] [PubMed] [Google Scholar]
- 5. Guyton AC, Hall JE, Lohmeier TE, Jackson TE, Kastner PR. Blood pressure regulation: basic concepts. Fed Proc 1981; 40: 2252–2256 [PubMed] [Google Scholar]
- 6. Duprez DA. Role of the renin-angiotensin-aldosterone system in vascular remodeling and inflammation. A clinical review. J Hypertens 2006; 24: 983–991 [DOI] [PubMed] [Google Scholar]
- 7. Kaplan NM. Renin profiles. The unfilled promises. JAMA 1977; 238: 611–613 [PubMed] [Google Scholar]
- 8. Laragh JH, Sealey JE. Relevance of the plasma renin hormonal control system that regulates blood pressure and sodium balance for correctly treating hypertension and for evaluating ALLHAT. Am J Hypertens 2003; 16: 407–415 [DOI] [PubMed] [Google Scholar]
- 9. Laragh JH, Sealey JE. The plasma renin test reveals the contribution of body sodium-volume (V) and renin-angiotensin (R) vasoconstriction to long-term blood pressure. Am J Hypertens 2011; 24: 1164–1180 [DOI] [PubMed] [Google Scholar]
- 10. Preston RA, Materson BJ, Reda DJ, Williams DW, Hamburger RJ, Cushman WC, Anderson RJ. Age-race subgroup compared with renin profile as predictors of blood pressure response to antihypertensive therapy. JAMA 1998; 280: 1168–1172 [DOI] [PubMed] [Google Scholar]
- 11. Williams B, Poulter NR, Brown MJ, Davis M, MicInnes GT, Potter JF, Sever PS, McG Thom S. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004; 328: 634–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turner ST, Schwartz GL, Chapman AB, Boerwinkle E. C825T polymorphism of the G protein β3-subunit and antihypertensive response to a thiazide diuretic. Hypertension 2001; 37: 739–743 [DOI] [PubMed] [Google Scholar]
- 13. Canzanello VJ, Baranco-Pryor E, Rahbari-Oskoui F, Schwartz GL, Boerwinkle E, Turner ST, Chapman AB. Predictors of blood pressure response to the angiotensin receptor blocker candesartan in essential hypertension. Am J Hypertens 2008; 21: 61–66 [DOI] [PubMed] [Google Scholar]
- 14. Veterans Administration Cooperative Study Group on Antihypertensive Agents Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. I. Results of short-term titration with emphasis on racial differences in response. JAMA 1982; 248: 1996–2003 [PubMed] [Google Scholar]
- 15. Moser M, Lunn J. Responses to captopril and hydrochlorothiazide in black patients with hypertension. Clin Pharmacol Ther 1982; 32: 307–312 [DOI] [PubMed] [Google Scholar]
- 16. Under T, Gohlke P. Tissue rennin-angiotensin systems in the heart and vasculature: possible involvement in the cardiovascular actions of converting enzyme inhibitors. J Am Coll Card 1990; 65: 3–10 [DOI] [PubMed] [Google Scholar]
- 17. Preston RA, Materson BJ, Reda DJ, Williams DW, Hamburger RJ, Cushman WC, Anderson RJ. Age-race subgroup compared with renin profile as predictors of blood pressure response to antihypertensive therapy. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. JAMA 1998; 280: 1168–1172 [DOI] [PubMed] [Google Scholar]
- 18. Materson BJ, Reda DJ, Cushman WC; for the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents Single-drug therapy for hypertension in men—a comparison of six antihypertensive agents with placebo. N Engl J Med 1993; 328: 914–921 [DOI] [PubMed] [Google Scholar]
- 19. Materson BJ, Reda DJ. Correction: single-drug therapy for hypertension in men. N Engl J Med 1994; 330: 1689 [DOI] [PubMed] [Google Scholar]
- 20. Materson BJ, Reda DJ, Cushman WC; for the Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents Department of Veterans Affairs single-drug therapy of hypertension study: revised figures and new data. Am J Hypertens 1995; 8: 189–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



