Abstract
BACKGROUND
Although hypertension contributes to kidney dysfunction in the general population, the contributions of elevated systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP) to kidney function decline in community-dwelling older adults are unknown.
METHODS
We used linear and logistic regression to examine the separate and combined associations of SBP, DBP, and PP at baseline with kidney function decline among 4,365 older adults in the Cardiovascular Health Study. We used cystatin C to estimate glomerular filtration rate on 3 occasions over 7 years of follow-up. We defined rapid decline ≥ 3ml/min/year.
RESULTS
Average age was 72.2 and mean (standard deviation) SBP, DBP, and PP were 135 (21), 71 (11), and 65 (18) mm Hg, respectively. SBP and PP, rather than DBP, were most significantly associated with kidney function decline. In adjusted linear models, each 10-mm Hg increment in SBP and PP was associated with 0.13ml/min/year (–0.19, –0.08, P < 0.001) and 0.15-ml/min/year faster decline (–0.21, –0.09, P < 0.001), respectively. Each 10-mm Hg increment in DBP was associated with a nonsignificant 0.10-ml/min/year faster decline (95% confidence interval, –0.20, 0.01). In adjusted logistic models, SBP had the strongest associations with rapid decline, with 14% increased hazard of rapid decline (95% confidence interval, 10% to 17%, P < 0.01) per 10mm Hg. In models combining BP components, only SBP consistently had independent associations with rapid decline.
CONCLUSIONS
Our findings suggest that elevated BP, particularly SBP, contributes to declining kidney function in older adults.
Keywords: blood pressure, cystatin C, diastolic blood pressure, elderly, hypertension, kidney function, systolic blood pressure.
Chronic kidney disease is very common in the US population, and it is present in >30% of adults over age 65.1 High blood pressure (BP) is a well-established risk factor for chronic kidney disease and end stage renal disease in youth and middle age.2,3 Elevated pressure in the renal vasculature and consequent elevated intraglomerular pressure lead to failure of autoregulation and endothelial dysfunction and eventually to progressive glomerular and interstitial fibrosis. In contrast, data on the association of high BP with kidney function decline in the elderly, in whom hypertension is highly prevalent,4 are less clear.5,6 In particular, neither randomized trial data nor genome-wide association study data provide clear supporting data for associations between lower BP and reduced risk for kidney function decline. For example, in the placebo arm of the Systolic Hypertension in the Elderly Program, elevated systolic pressure was associated with kidney function decline. Because this arm only included individuals with systolic hypertension, it cannot be easily generalized to a community-dwelling older population.7 The Hypertension in the Very Elderly Trial has not reported kidney function outcomes. In the large International Consortium for Blood Pressure Genome-Wide Association studies, which provide analysis of associations between BP variants and cardiovascular disease, no association was seen between these variants and chronic kidney disease.8
In addition, the relative associations of systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse pressure (PP) with kidney function decline in the elderly are uncertain. This is of particular clinical importance because hypertension in the elderly is primarily characterized by high SBP and normal to low DBP (isolated systolic hypertension [ISH]).9 In the Cardiovascular Health Study, SBP was the best predictor of cardiovascular events, not PP or DBP.10 In the Framingham Heart Study, however, the association of each BP component with cardiovascular risk varied by age; contrary to associations in young and middle age, PP was the strongest predictor of coronary heart disease in the elderly and DBP was negatively associated with risk.11 The associations of each BP component with kidney function decline are not well known.
Recently, the American Heart Association consensus statement on treatment of hypertension in older adults highlighted this large knowledge gap.12 Therefore, we designed this study to examine the associations of SBP, DBP, and PP with kidney function decline using data from the Cardiovascular Health Study.
METHODS
Study population
The Cardiovascular Health Study recruited individuals aged ≤ 65 years from Medicare eligibility lists in 4 US communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania).13 The study recruited an initial cohort of 5,201 primarily white individuals in 1989–1990 (baseline). Subsequently, in 1992–1993 (study year 3), an additional 687 African American participants were recruited. Blood samples that were collected in 1989–1990, 1992–1993, and 1996–1997 (baseline, year 3, and year 7) were assayed for kidney function. We included all individuals with baseline measures of BP and at least 2 available measures of kidney function. In total, 4,365 of the 5,888 participants in the Cardiovascular Health Study met criteria for inclusion in this analysis.
All participants provided written informed consent. The institutional review boards of the University of Washington and the affiliated clinical centers approved the study.
Predictor variables: Systolic, diastolic, and pulse pressures
BP was assessed at the initial clinic visit. Participants were asked to fast for at least 12 hours and to refrain from smoking for 30 minutes before the clinic visit. Trained study personnel obtained 3-seated BP readings with a random zero sphygmomanometer; the average of the last 2 readings was recorded. Patients were also asked about a history of hypertension, and the use of antihypertensive medications was noted using a medication inventory interview.14 PP was calculated as the difference between the average SBPs and DBPs.
Outcome variables: Change in estimated glomerular filtration rate using cystatin C
Cystatin C levels were measured from frozen samples using a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring [now Siemens Healthcare Diagnostics Inc], Deerfield, IL) with a nephelometer (BNII, Siemens Healthcare Diagnostics Inc). Previous work has shown this assay to be stable through several freeze–thaw cycles.15 Intraassay coefficients of variation ranged from 2.0% to 2.8%, and inter coefficients of variation ranged from 2.3% to 3.1%.
To calculate change in kidney function over time, we first used estimating equations to transform each cystatin C measurement into a glomerular filtration rate (GFR) estimate. This transformation was derived from a pooling of cohorts that used iothalamate clearance as the criterion standard; we used the equation eGFRcys = 76.7 × cystatin C−1.19.16 Rates of change were calculated using the 2 or 3 available eGFRcys measurements by calculating a least-squares regression slope. As in previous work, we defined an annual loss of 3ml/min/1.73 m2 as rapid decline; this change is known to be associated with clinically deleterious outcomes.17,18
Covariates
We examined a number of covariates that might plausibly confound the associations between systolic, diastolic, and pulse pressures and kidney function changes. We included demographic variables (age, gender, race); cardiovascular risk factors including body mass index, smoking, diabetes (use of insulin or an oral hypoglycemic agent or a fasting blood sugar >7 mmol/L [126mg/dl]), low-density lipoprotein and high-density lipoprotein; and prevalent cardiovascular disease (transient ischemic attack, stroke, coronary artery disease, myocardial infarction, or heart failure). Demographic variables that were not different across groups (income, education) were not included as covariates.
Analytical methods
We first examined the distribution of baseline characteristics according to categories of SBP (by 10-mm Hg increments) using analysis of variance or χ2 where appropriate. We modeled SBP and DBP as continuous variables in graphical form, using generalized additive models. After confirming linear relationships with kidney function decline, we categorized BP values in 10-mm Hg increments.
We proceeded to examine the associations of systolic, diastolic, and pulse pressures separately with change in kidney function. For these analyses, we used a series of nested linear models. Initially we examined unadjusted associations, then we used models adjusted for demographic factors and risk factors for cardiovascular disease.
We then used logistic regression to evaluate associations between each BP component and rapid decline in kidney function as a dichotomized outcome as above, using the lowest category of BP as the referent. Finally, we evaluated potential joint effects of the 3 BP components by adding them in pair-wise combinations to a model of rapid decline in eGFRcys.
Due to the high prevalence of ISH in the elderly, we also performed a separate analysis to examine the association of each BP component with kidney function decline among persons with SBP ≥ 140mm Hg and DBP < 90mm Hg. In a secondary a priori analysis, we stratified by use of antihypertensive medications in both the entire cohort and in those with ISH to determine whether associations between BP components and kidney function decline would differ among users and nonusers of antihypertensive medications. We assessed P values for interaction between BP components and medications with kidney function decline. We performed sensitivity analyses using only those with 3 measures of kidney function (58% of cohort) and using generalized estimating equations instead of linear models. We also assessed whether results were robust using combined cystatin C and creatinine-based GFR estimating equations.
Analyses were performed using S-Plus (release 8.0; Insightful Inc, Seattle, Washington) and SPSS statistical software (release 16.0.2; SPSS Inc, Chicago, IL).
RESULTS
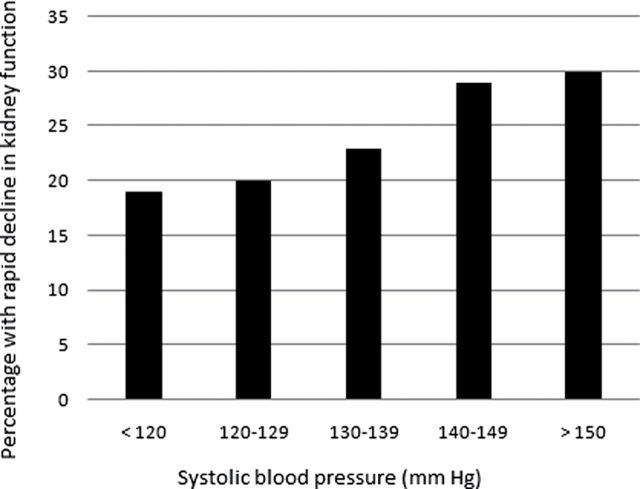
Of the original 5,888 individuals recruited for the Cardiovascular Health Study, 1,523 did not have repeated measures of cystatin C and thus were excluded from this study. These individuals were older and had more comorbidities than those who had repeated measures of cystatin C, as reported previously.18 Of the 4,365 participants included in this study, mean (standard deviation) systolic, diastolic, and pulse pressures were 135 (21), 71 (11), and 65 (18) mm Hg, respectively; 1,994 (46%) reported antihypertensive use. In general, those with higher SBP were older, had higher BMI and wider PP, and were more likely to have diabetes, subclinical and prevalent cardiovascular disease (Table 1). Isolated systolic hypertension was present in 1,499, representing 34% of the cohort but 61% of those with hypertension at the study visit. Median (interquartile range) annual decline in eGFRcys was –1.58 [–3.00, –0.33]. Rapid decline in eGFRcys was seen in 1,075 (25%) of the cohort overall, with increasing rates among those with higher SBP (Figure 1).
Table 1.
Participant characteristics at baseline by systolic blood pressure categories
| Characteristic | Systolic blood pressure (mm Hg) | |||||
|---|---|---|---|---|---|---|
| < 120 | 120–129 | 130–139 | 140–149 | 150–159 | ≥ 160 | |
| N | 1,022 | 835 | 828 | 677 | 442 | 561 |
| Age, years | 71 (5) | 72 (5) | 72 (5) | 73 (5) | 73 (5) | 74 (5) |
| Male | 409 (40%) | 364 (44%) | 357 (43%) | 260 (38%) | 159 (36%) | 219 (39%) |
| African American | 86 (8%) | 103 (12%) | 107 (13%) | 91 (13%) | 86 (20%) | 109 (19%) |
| Diabetes | 93 (9%) | 103 (12%) | 131 (16%) | 114 (17%) | 73 (17%) | 102 (18%) |
| Former smoker | 45 (45%) | 357 (43%) | 354 (43%) | 276 (41%) | 184 (42%) | 238 (43%) |
| Current smoker | 143 (14%) | 99 (12%) | 83 (10%) | 53 (8%) | 38 (9%) | 49 (9%) |
| Body mass index, kg/m2 | 25.8 (4.2) | 26.5 (4.6) | 27.2 (4.5) | 27.3 (4.6) | 27.1 (4.7) | 26.9 (4.8) |
| Diastolic blood pressure, mm Hg | 63 (8) | 68 (9) | 71 (10) | 74 (10) | 77 (10) | 80 (12) |
| Pulse pressure, mm Hg | 47 (9) | 57 (9) | 63 (10) | 71 (10) | 77 (10) | 94 (16) |
| Low-density lipoprotein, mg/dl | 129 (36) | 130 (34) | 130 (35) | 131 (34) | 128 (35) | 132 (35) |
| High-density lipoprotein, mg/dl | 55 (16) | 54 (16) | 54 (15) | 54 (15) | 56 (17) | 55 (15) |
| Triglycerides, mg/dl | 113 [88, 153] | 122 [92,1.64] | 124 [96,169] | 130 [94,178] | 122 [89,163] | 120 [94,166] |
| Hemoglobin, g/dl | 14.0 (1.3) | 14.1 (1.4) | 14.0 (1.3) | 14.0 (1.3) | 13.9 (1.2) | 14.0 (1.7) |
| Prevalent cardiovascular disease | 218 (21%) | 202 (24%) | 183 (22%) | 139 (21%) | 92 (21%) | 144 (26%) |
| Prevalent heart failure | 29 (3%) | 29 (4%) | 30 (4%) | 13 (2%) | 13 (3%) | 22 (4%) |
| eGFRcys, ml/min/1.73 m2 | 82 (43) | 79 (18) | 79 (17) | 78 (18) | 79 (18) | 77 (20) |
| Taking blood pressure medications | 362 (35%) | 347 (42%) | 387 (47%) | 330 (49%) | 216 (49%) | 352 (63%) |
Figure 1.
Rates of rapid decline of kidney function across systolic blood pressure categories.
Among the entire cohort, we found that higher SBP was associated with faster kidney function decline. In multivariate linear models, for each 10-mm Hg increment in SBP, there was a 0.13-ml/min/year faster decline in eGFRcys (95% confidence interval, 0.08, 0.19, P < 0.001); higher PP was also associated with faster decline, whereas the association of DBP with decline was not significant (Table 2).
Table 2.
Blood pressure as a predictor of change in eGFRcys in ml/min/1.73 m2/year (linear regression)
| Continuous blood pressure measures | ΔeGFRcys | |||||
|---|---|---|---|---|---|---|
| Full cohort | Stratified by hypertensive medication use at baseline | |||||
| No (n = 2,371) | Yes (n = 1,994) | |||||
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| Systolic blood pressure (per 10mm Hg)† | ||||||
| Unadjusted | –0.15 (–0.20, –0.10) | <0.001 | –0.17 (–0.25, –0.08) | <0.001 | –0.15 (–0.20, –0.09) | <0.001 |
| Demo adjusteda | –0.14 (–0.19, –0.09) | <0.001 | –0.16 (–0.24, –0.07) | 0.001 | –0.13 (–0.19, –0.08) | <0.001 |
| Fully adjustedb | –0.13 (–0.19, –0.08) | <0.001 | –0.13 (–0.22, –0.04) | 0.004 | –0.13 (–0.19, –0.08) | <0.001 |
| Diastolic blood pressure (per 10mm Hg)‡ | ||||||
| Unadjusted | –0.09 (–0.19, 0.01) | 0.080 | –0.16 (–0.32, –0.004) | 0.045 | –0.01 (–0.12, 0.10) | 0.865 |
| Demo adjusteda | –0.10 (–0.20, 0.03) | 0.056 | –0.18 (–0.34, –0.017) | 0.031 | –0.01 (–0.12, 0.10) | 0.801 |
| Fully adjustedb | –0.10 (–0.20, 0.01) | 0.051 | –0.17 (–0.33, 0.001) | 0.051 | –0.03 (–0.14, 0.08) | 0.631 |
| Pulse pressure (per 10mm Hg)§ | ||||||
| Unadjusted | –0.17 (–0.23, –0.11) | <0.001 | –0.16 (–0.26, –0.06) | 0.002 | –0.19 (–0.25, –0.13) | <0.001 |
| Demo adjusteda | –0.16 (–0.22, –0.10) | <0.001 | –0.15 (–0.25, –0.04) | 0.007 | –0.18 (–0.25, –0.12) | <0.001 |
| Fully adjustedb | –0.15 (–0.21, –0.09) | <0.001 | –0.12 (–0.23, –0.01) | 0.029 | –0.18 (–0.24, –0.11) | <0.001 |
Abbreviation: CI, confidence interval.
aAdjusted for age, gender, race.
bAdjusted for age, gender, race, body mass index, smoking, diabetes mellitus, prevalent cardiovascular disease, low-density lipoprotein, and high-density lipoprotein .
†P value for interaction between systolic blood pressure and hypertensive medications = 0.802.
‡P value for interaction between diastolic blood pressure and hypertensive medications = 0.191.
§P value for interaction between pulse pressure and hypertensive medications = 0.419.
Distribution of antihypertensive medication classes between those with or without rapid decline in kidney function were not significantly different. We stratified by use of antihypertensive medications as specified a priori; however, the P values for interaction were not statistically significant for the associations of SBP, DBP, or PP with kidney function decline. In addition, the association between DBP and kidney decline appeared qualitatively different between treated vs. untreated persons. Among those not using antihypertensive medications, higher DBP was associated with faster kidney function decline, whereas there was no association among treated persons (Table 2).
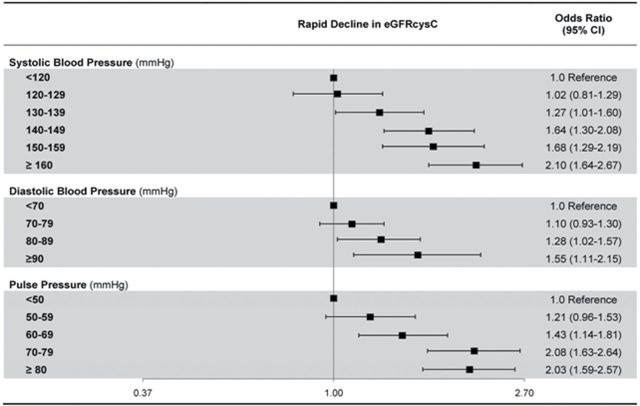
We then considered the association of clinically significant categories of systolic, diastolic, and pulse pressures with rapid decline in kidney function. Higher SBP categories were associated with faster rates of kidney function decline. Compared with persons with SBP < 120, persons with SBP 130 had only a 27% higher odds of rapid decline, while persons with SBP ≥ 140 had almost 2-fold higher odds of rapid decline in kidney function; similar associations were seen for PP, with approximately 2-fold risk seen with PP > 80mm Hg. The odds of rapid decline associated with elevated DBP were less pronounced, with a 50% increased risk in those with DBP > 90mm Hg (Figure 2).
Figure 2.
Adjusted association of blood pressure categories with rapid decline in eGFRcys.
When we studied the association of each BP component with kidney function decline among persons with ISH, we found that SBP was associated with kidney function decline only among those not taking antihypertensive medications (Table 3). In contrast, the association of SBP and kidney function decline was not significant among participants with treated ISH (P value for interaction, 0.079).
Table 3.
Blood pressure as a predictor of change in eGFRcys in participants with isolated systolic hypertension (linear regression)
| Continuous BP measures | ΔeGFRcys | |||||
|---|---|---|---|---|---|---|
| Isolated systolic hypertension (SBP ≥ 140 and DBP < 90) N = 1,499 | Stratified by hypertensive medication use at baseline | |||||
| No (n = 711) | Yes (n = 788) | |||||
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |
| SBP (per 10mm Hg) | ||||||
| Unadjusted | 0.06 (0.16, 0.04) | 0.231 | 0.17 (0.32, 0.03) | 0.019 | 0.01 (0.13, 0.14) | 0.990 |
| Demo adjusteda | 0.04 (0.14, 0.06) | 0.416 | 0.16 (0.31, 0.01) | 0.032 | 0.03 (0.11, 0.16) | 0.721 |
| Fully adjustedb | 0.04 (0.14, 0.06) | 0.457 | 0.15 (0.29, 0.01) | 0.049 | 0.03 (0.10, 0.17) | 0.639 |
| DBP (per 10mm Hg) | ||||||
| Unadjusted | 0.02 (0.12, 0.16) | 0.788 | 0.10 (0.30, 0.10) | 0.321 | 0.12 (0.09, 0.33) | 0.248 |
| Demo adjusteda | 0.001 (0.15, 0.15) | 0.996 | 0.14 (0.34, 0.07) | 0.189 | 0.11 (0.10, 0.33) | 0.300 |
| Fully adjustedb | 0.04 (0.19, 0.11) | 0.567 | 0.19 (0.39, 0.01) | 0.066 | 0.08 (0.14, 0.29) | 0.475 |
| PP (per 10mm Hg) | ||||||
| Unadjusted | 0.06 (0.15, 0.03) | 0.210 | 0.09 (0.22, 0.04) | 0.160 | 0.04 (0.17, 0.08) | 0.494 |
| Demo adjusteda | 0.04 (0.13, 0.06) | 0.447 | 0.07 (0.21, 0.06) | 0.287 | 0.02 (0.15, 0.11) | 0.770 |
| Fully adjustedb | 0.02 (0.11, 0.08) | 0.737 | 0.04 (0.17, 0.09) | 0.556 | 0.001 (0.13, 0.13) | 0.990 |
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; ISH, isolated systolic hypertension; PP, pulse pressure; SBP, systolic blood pressure.
P value for interaction between SBP and hypertensive medications = 0.079. P value for interaction between DBP and hypertensive medications = 0.112. P value for interaction between PP and hypertensive medications = 0.608. ISH is defined as systolic blood pressure ≥ 140 and diastolic blood pressure < 90.
aAdjusted for age, gender, and race.
bAdjusted for age, gender, race, body mass index, smoking, diabetes mellitus, prevalent cardiovascular disease, low-density lipoprotein, and high-density lipoprotein.
When we added BP components sequentially to a model of rapid change in eGFRcys, we found that SBP attenuated the associations between either DBP or PP and rapid decline (Table 4), suggesting that findings with PP were driven mainly by SBP.
Table 4.
Comparison of BP measures with risk of rapid decline (decline in eGFRcys > 3ml/min/1.73 m2)
| Model (adjusted a) | Δ eGFRcys > 3 | ||
|---|---|---|---|
| SBP b | DBP b | PP b | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| SBP | 1.14 (1.10, 1.17) | -- | -- |
| DBP | -- | 1.09 (1.02, 1.17) | -- |
| PP | -- | -- | 1.15 (1.11, 1.20) |
| SBP + DBP | 1.15 (1.11, 1.20) | 0.94 (0.87, 1.02) | -- |
| SBP + PP | 1.09 (1.02, 1.16) | -- | 1.06 (0.98, 1.15) |
| DBP + PP | -- | 1.09 (1.02, 1.16) | 1.15 (1.11, 1.20) |
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; OR, odds ratio; PP, pulse pressure; SBP, systolic blood pressure.
aAdjusted for age, gender, race, hypertension medications, body mass index, smoking, diabetes mellitus, prevalent cardiovascular disease, low-density lipoprotein, and high-density lipoprotein.
bContinuous per 10mm Hg
In sensitivity analyses, results were robust in those with 3 vs. 2 measures of kidney function and were similar using the generalized estimating equation modeling technique. Use of combined cystatin C– and creatinine-based eGFR estimates did not substantially alter the results of the analysis.
DISCUSSION
We found that in this large cohort of community-dwelling elderly individuals, each component of BP was associated with kidney function decline, with SBP having the strongest association. Our findings were parallel to those observed by Psaty and colleagues in examining BP components and cardiovascular outcomes in the same cohort10; in both investigations SBP had stronger independent associations with the outcomes of interest in comparison with PP or DBP.
Our data provide additional support for the association of systolic hypertension with kidney function decline, even in an older population. We observed a statistically significant risk of rapid kidney function decline associated with SBP levels > 130mm Hg, values below those recommended as treatment targets for the general hypertensive population.
A number of studies have suggested increased risk of other outcomes, such as stroke and cardiovascular and all-cause mortality, at low DBP levels in older adults.19–21 In those with ISH in the Systolic Hypertension in the Elderly Program Study, DBP was not significantly associated with declining kidney function.22 We demonstrated an absence of significant associations between DBP and declining kidney function, both in the overall cohort and in those with ISH. This finding may add to the overall idea that it is systolic, rather than diastolic, hypertension that is of most importance in older adults, whereas in prior studies in younger populations, both diastolic and systolic hypertension were associated with progression to end-stage kidney failure.3
Of interest is the subgroup finding that those with treated ISH did not have the same association between SBP and decline as did those not taking antihypertensive agents. There are several potential explanations for this observation, including confounding by indication or by unmeasured comorbidities. It is possible that those not treated could potentially benefit from treatment in terms of preservation of kidney function; however, this cannot be inferred from our observational data.
Our finding that PP is associated with kidney function decline suggests that arterial stiffness is an important component when considering the associations of BP and kidney function decline in the elderly. In this cohort, as in other older cohorts, PP is mainly driven by elevations in SBP rather than decreases in DBP, explaining the attenuation of the association between PP and kidney function decline after adjusting for SBP. This phenomenon is likely explained by increased arterial stiffness associated with aging; in this age group, elevations in SBP are indicative of arterial stiffness.
In our study, overall findings were similar for SBP regardless of whether BP medication was in use to achieve any given BP level. This is in contrast to associations between BP components and cardiovascular outcomes, which were attenuated in those taking antihypertensive medications.14 Antihypertensive medications may have pleiotropic effects unrelated to BP control on cardiovascular outcomes,23 whereas in the case of kidney function, their effect may occur mainly through achieved BP control. Of note, the prevalence of angiotensin-converting-enzyme inhibitor use in this study was low at baseline (less than 7%), and we did not have power to detect an interaction of angiotensin-converting-enzyme inhibitors and BP with kidney function decline. We did detect somewhat diminished associations between diastolic hypertension and kidney function decline in those who reported the use of antihypertensive agents. Although our overall findings are that DBP does not have independent associations with kidney function decline, this could suggest some additional effect of medication use on kidney function decline in those with diastolic hypertension and can be explored in further studies.
The strengths of this study include the large sample, detailed measures of important potential confounders, repeated measures of kidney function, and long follow-up time. Whereas most of the available clinical trial data are from studies that excluded participants with comorbid diseases, this cohort study included a broad sample of older adults with multiple comorbid conditions and thus is more generalizable to older adults.
This study also has several limitations. The subset of participants we could study were able to return for at least 1 follow-up visit and thus are healthier than the original community-based sample. Since the data were collected in an observational cohort setting, use of antihypertensive medications was at the discretion of the participant and his or her physician, and thus we cannot infer a causal relationship between BP treatment and outcomes. Interestingly, interaction terms for treated vs. untreated BP were nonsignificant, suggesting that achieved SBP level rather than medication use itself was most important in driving kidney function outcomes in this cohort. We do not have information on proteinuria, which is known to have significant effects on risk of progressive kidney disease, nor do we have measured GFR by direct clearance. We did not observe an increased risk of kidney function decline in those with SBP < 120mm Hg or DBP < 70mm Hg, but our confidence limits do not allow us to exclude a J-shaped association at the extremes of low SBP or DBP.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NHLBI contracts HHSN268201200036C, N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grant HL080295, with additional contribution from NINDS. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. See also http://www.chs-nhlbi.org/pi.htm.
REFERENCES
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047 [DOI] [PubMed] [Google Scholar]
- 2. Hsu C, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 2005; 165:923–928 [DOI] [PubMed] [Google Scholar]
- 3. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med 1996; 334:13–18 [DOI] [PubMed] [Google Scholar]
- 4. Keenan NL, Rosendorf KA. Prevalence of hypertension and controlled hypertension - United States, 2005–2008. MMWR Surveill Summ 2011; 60(Suppl):94–97 [PubMed] [Google Scholar]
- 5. Shlipak MG, Katz R, Kestenbaum B, Fried LF, Newman AB, Siscovick DS, Stevens L, Sarnak MJ. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. Am J Nephrol 2009; 30:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Odden MC, Tager IB, Van der Laan MJ, Delaney JAC, Peralta CA, Katz R, Sarnak MJ, Psaty BM, Shlipak MG. Antihypertensive medication use and change in kidney function in elderly adults: a marginal structural model analysis. Int J Biostat 2011; 7:Article 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898 [DOI] [PubMed] [Google Scholar]
- 8. International Consortium for Blood Pressure Genome-Wide Association Studies, Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sõber S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjögren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimäki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND; CARDIoGRAM consortium; CKDGen Consortium; KidneyGen Consortium; EchoGen consortium; CHARGE-HF consortium, Aspelund T, Garcia M, Chang YP, O’Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kähönen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Köttgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grässler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stančáková A, Raffel LJ, Yao J, Kathiresan S, O’Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT, Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikäinen LP, Soininen P, Tukiainen T, Würtz P, Ong RT, Dörr M, Kroemer HK, Völker U, Völzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Järvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 2011; 478:103–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P. Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 2001; 37:869–874 [DOI] [PubMed] [Google Scholar]
- 10. Psaty BM, Furberg CD, Kuller LH, Cushman M, Savage PJ, Levine D, O’Leary DH, Bryan RN, Anderson M, Lumley T. Association between blood pressure level and the risk of myocardial infarction, stroke, and total mortality: the cardiovascular health study. Arch Intern Med 2001; 161:1183–1192 [DOI] [PubMed] [Google Scholar]
- 11. Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the relation of blood pressure to coronary heart disease risk change with aging? The Framingham Heart Study. Circulation 2001; 103:1245–1249 [DOI] [PubMed] [Google Scholar]
- 12. Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Ann Forciea M, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ, Harrington RA, Bates ER, Bhatt DL, Bridges CR, Eisenberg MJ, Ferrari VA, Fisher JD, Gardner TJ, Gentile F, Gilson MF, Hlatky MA, Jacobs AK, Kaul S, Moliterno DJ, Mukherjee D, Rosenson RS, Stein JH, Weitz HH, Wesley DJ. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Soc Hypertens. 2011 Jul-Aug;5(4)259–352. 10.1016/j.jash.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 13. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991; 1:263–276 [DOI] [PubMed] [Google Scholar]
- 14. Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol 1992; 45:683–692 [DOI] [PubMed] [Google Scholar]
- 15. Finney H, Newman DJ, Gruber W, Merle P, Price CP. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II). Clin Chem 1997; 43:1016–1022 [PubMed] [Google Scholar]
- 16. Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, Zhang Y, Greene T, Levey AS. Estimating GFR using Serum Cystatin C Alone and in Combination with Serum Creatinine: A Pooled Analysis of 3418 Individuals with CKD. Am J Kidney Dis 2008; 51:395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klein R, Klein BE, Moss SE, Cruickshanks KJ, Brazy PC. The 10-year incidence of renal insufficiency in people with type 1 diabetes. Diabetes Care 1999; 22:743–751 [DOI] [PubMed] [Google Scholar]
- 18. Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 2008; 168:2212–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pastor-Barriuso R, Banegas JR, Damián J, Appel LJ, Guallar E. Systolic blood pressure, diastolic blood pressure, and pulse pressure: an evaluation of their joint effect on mortality. Ann Intern Med 2003; 139:731–739 [DOI] [PubMed] [Google Scholar]
- 20. Somes GW, Pahor M, Shorr RI, Cushman WC, Applegate WB. The role of diastolic blood pressure when treating isolated systolic hypertension. Arch Intern Med 1999; 159:2004–2009 [DOI] [PubMed] [Google Scholar]
- 21. Staessen JA, Gasowski J, Wang JG, Thijs L, Den Hond E, Boissel JP, Coope J, Ekbom T, Gueyffier F, Liu L, Kerlikowske K, Pocock S, Fagard RH. Risks of untreated and treated isolated systolic hypertension in the elderly: meta-analysis of outcome trials. Lancet 2000;355:865–872 [DOI] [PubMed] [Google Scholar]
- 22. Young JH, Klag MJ, Muntner P, Whyte JL, Pahor M, Coresh J. Blood pressure and decline in kidney function: findings from the Systolic Hypertension in the Elderly Program (SHEP). J Am Soc Nephrol 2002; 13:2776–2782 [DOI] [PubMed] [Google Scholar]
- 23. Sever PS, Poulter NR, Elliott WJ, Jonsson MC, Black HR, Sever PS, Poulter NR, Elliott WJ, Jonsson MC, Black HR. Blood Pressure Reduction Is Not the Only Determinant of Outcome. Circulation 2006; 113:2754–2774 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.