Abstract
Investigators in the Chicago Healthy Aging Study (CHAS) reexamined 1,395 surviving participants aged 65–84 years (28% women) from the Chicago Heart Association Detection Project in Industry (CHA) 1967–1973 cohort whose cardiovascular disease (CVD) risk profiles were originally ascertained at ages 25–44 years. CHAS investigators reexamined 421 participants who were low-risk (LR) at baseline and 974 participants who were non-LR at baseline. LR was defined as having favorable levels of 4 major CVD risk factors: serum total cholesterol level <200 mg/dL and no use of cholesterol-lowering medication; blood pressure 120/≤80 mm Hg and no use of antihypertensive medication; no current smoking; and no history of diabetes or heart attack. While the potential of LR status in overcoming the CVD epidemic is being recognized, the long-term association of LR with objectively measured health in older age has not been examined. It is hypothesized that persons who were LR in 1967–1973 and have survived to older age will have less clinical and subclinical CVD, lower levels of inflammatory markers, and better physical performance/functioning and sleep quality. Here we describe the rationale, objectives, design, and implementation of this longitudinal epidemiologic study, compare baseline and follow-up characteristics of participants and nonparticipants, and highlight the feasibility of reexamining study participants after an extended period postbaseline with minimal interim contact.
Keywords: cardiovascular disease, epidemiologic studies, follow-up examination, risk factors
While clinical cardiovascular disease (CVD) does not usually manifest until later in life, substantial evidence suggests that cardiovascular health is defined beginning at a young age. Persons with favorable levels of all major CVD risk factors (i.e., at low cardiovascular risk) in young adulthood and middle age experience markedly lower age-specific CVD and total mortality and have increased life expectancy, lower morbidity, lower health-care costs, a higher self-reported quality of life, and—as suggested by preliminary data—less extensive subclinical coronary atherosclerosis at older ages compared with others (1–6).
Despite increasing recognition of the critical importance of the low-risk concept in combating the CVD epidemic (7), to date there has been a dearth of large-scale studies on the association of low-risk status in younger age with objectively measured long-term consequences, including both clinical CVD and maintenance of favorable overall health in older age (i.e., freedom in older age from subclinical CVD, inflammatory markers of CVD risk, and impaired physical performance and functioning). Moreover, sleep quality and quantity have been linked with cardiovascular health, and conversely, poor health status may increase the prevalence of sleep disorders; however, the long-term association of low-risk status with sleep quality has not been examined. To address these gaps, investigators in the Chicago Healthy Aging Study (CHAS) reexamined a sample of participants from the Chicago Heart Association Detection Project in Industry (CHA) who underwent clinical examination in 1967–1973. The hypotheses of CHAS are that persons who are low-risk at baseline experience less clinical and subclinical CVD, lower levels of inflammatory markers, higher levels of physical performance, and better sleep quality in older age than other persons.
In this paper, we describe the rationale, objectives, design, and implementation of CHAS. It is anticipated that these data can inform strategies for achieving critical national health goals by documenting long-term objectively measured benefits of progressive sustained increases in the proportion of the population at low risk. In addition, this study highlights the feasibility of (and addresses potential issues in) reexamining research participants after an extended period postbaseline with minimal interim contact. This would allow cost-effective use of existing cohorts in obtaining data relevant for strategies to address the burgeoning chronic disease burden of the aging US population. Finally, in this paper we compare baseline characteristics of study participants and nonparticipants at reexamination and describe the characteristics of low-risk (LR) and non-LR participants at the baseline and follow-up examinations.
MATERIALS AND METHODS
Study objectives
The CHAS study aimed to generate objectively measured data on long-term consequences of LR status in young adulthood/early middle age for both men and women, across socioeconomic strata and ethnic groups. LR was defined as having favorable levels of all 4 major CVD risk factors: serum total cholesterol level <200 mg/dL and no use of cholesterol-lowering medication; blood pressure ≤120/≤80 mm Hg and no use of antihypertensive medication; no current smoking; and no history of diabetes or myocardial infarction.
CHA study: design and methods
Details on the design and methods of the original CHA study have been published elsewhere (8, 9). Briefly, the baseline CHA examination (1967–1973) was a cross-sectional survey of 39,522 young adult, middle-aged, and older men and women (mainly non-Hispanic white, about 10% African-American, and with small numbers of Asians, Hispanics, and others) employed at 84 cooperating companies and organizations in the Chicago, Illinois, area. All employees in the labor force at participating companies, totaling approximately 75,000 people, were invited to participate regardless of job classification; arrangements were made so that persons from every shift could be screened. Information was collected on demographic characteristics, smoking history, and medical treatments. Height, weight, serum total cholesterol, and supine blood pressure were measured by trained personnel, and resting electrocardiograms (ECGs) were administered.
Through 1979, the vital status of participants was assessed using multiple methods, including routine serial mailings to people at their last known address, submission of the data file to the Social Security Administration, mailings to employers, telephone inquiries, and neighborhood contacts. Since 1979, vital status has been ascertained by information obtained from the National Death Index and Equifax, Inc. (Atlanta, Georgia). With these methods, through 2003 only 86 persons (0.23% of all CHA participants) had never been traced beyond baseline.
CHAS study design and recruitment
The current study, CHAS, was a reexamination conducted in 2007–2010 of a sample of 1,395 surviving CHA participants (ages 65–84 years at reexamination; 28% women; 421 originally LR and 974 originally non-LR) with CVD risk profiles ascertained at ages 25–44 years, who were free of major ECG abnormalities or myocardial infarction at baseline (1967–1973). Baseline LR participants were oversampled (compared with their proportion in the CHA cohort, i.e., 10%) to obtain adequate numbers for between-group comparisons. The sample consisted primarily of non-Hispanic white Americans; approximately 9% were African Americans and approximately 3% were Asian or Hispanic Americans, reflecting the composition of the original cohort.
Updated addresses of CHA participants were obtained from the Centers for Medicare and Medicaid Services (CMS), based on submitted names and Social Security numbers. Surviving participants who would have been aged 65–84 years in 2007–2010 and were presumed to be alive based on the last received vital status information from National Death Index and CMS records were identified (n = 12,910). Based on power calculations, originally a recruitment target of 600 LR participants and 900 non-LR participants was set. A letter from CMS was first mailed to participants informing them of the upcoming study; it specified that their decision to participate was entirely optional and would not affect their Medicare benefits. The CMS database does not include telephone numbers; however, many participants had provided updated phone numbers on previous mailed questionnaires. Participant names and updated addresses supplied by CMS were also submitted to www.555-1212.com, a commercial Internet-based service that provides listed telephone numbers and change-of-address information from public files updated daily from telephone company records.
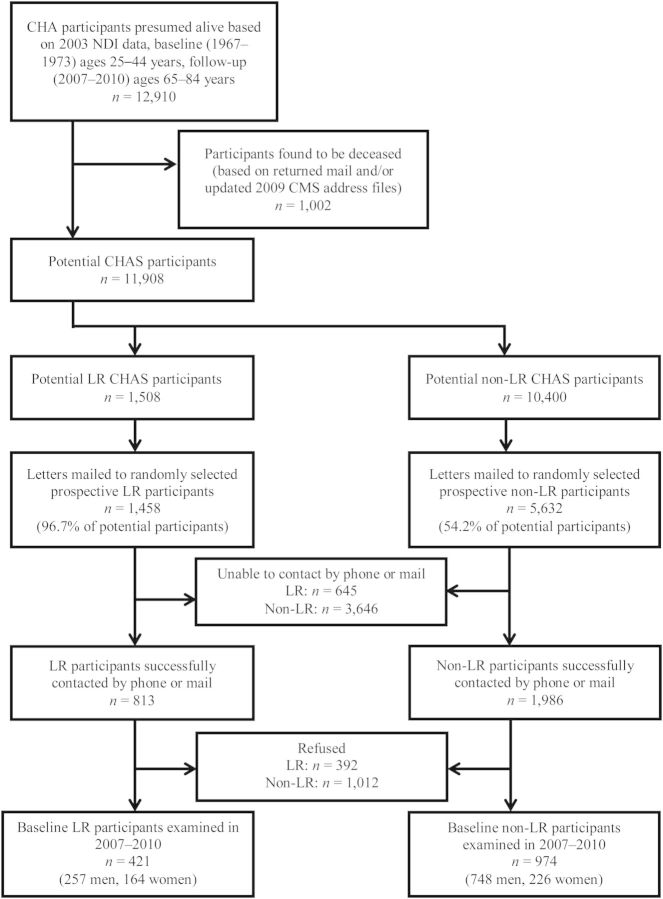
Using a computerized tracking system, recruiters generated randomly selected lists of potential participants to be contacted. Batches of names were selected to maintain a balance by risk status, sex, 5-year age group, and listed/unlisted telephone number; for this purpose, 3 matching non-LR participants were randomly identified for each LR participant selected. To keep study costs low, participants still residing in Chicago were targeted for recruitment first; later, recruitment was expanded to include participants outside the Greater Chicago area. Of 12,910 participants identified, 1,002 were found to be deceased (through returned mail and updated CMS address files received in 2009). In all, recruitment letters were mailed to 59.5% (n = 7,090) of the remaining 11,908 potential participants through the end of the study period; recruitment efforts were to be stopped when the target for non-LR participants was achieved or the list of potentially available LR participants was exhausted (Figure 1). Although the target number of LR participants could not be achieved, preliminary data analyses showed that the number of participants recruited was sufficient to demonstrate significant differences in key outcomes between LR and non-LR participants (data not shown).
Figure 1.
Recruitment for follow-up of participants in the Chicago Heart Association Detection Project in Industry (CHA) who were either low-risk (LR) or not low-risk (non-LR) for cardiovascular disease in 1967–1973, Chicago Healthy Aging Study (CHAS), 2007–2010. CMS, Centers for Medicare and Medicaid Services; NDI, National Death Index.
Persons with listed telephone numbers were mailed an introductory letter and brochure from the CHAS principal investigators describing the study and notifying them that they would receive a call from Northwestern University (Chicago, Illinois) staff in the next few days. Participants with unlisted telephone numbers were sent a letter providing more details and asking them to complete a response card or call the recruitment center at the toll-free phone number provided (answered by recruitment staff during business hours and by an answering machine at other times). A second letter followed if no response was received within 2 weeks. Letters returned with a forwarding address were remailed to the new address. Letters returned without a forwarding address were manually checked using websites such as www.555-1212.com, www.privateeye.com, and www.whitepages.com to locate a valid mailing address or phone number. When updated contact information was found, letters were remailed.
Trained recruiters attempted to reach participants by telephone up to 15 times over a 2-week period, calling in the evenings and on weekends if necessary. Using predetermined scripts, recruiters described the study, reminded people of their involvement in the original study, and solicited participation in the follow-up examination. Uninterested participants were requested to complete a brief questionnaire on interim health status, disease events, and reasons for their lack of interest. Only a few participants provided reasons for nonparticipation, which included poor health and mobility or transportation issues. Interested participants were scheduled for a clinic examination. Of 2,799 participants who were successfully contacted by telephone or mail (55.8% and 35.3% of LR and non-LR individuals to whom recruitment letters were mailed, respectively), 1,395 (49.8%; 51.8% and 49.0% of LR and non-LR individuals, respectively) agreed to participate and attended the clinic examination (Figure 1). After verification of their current address, a confirmation letter with information, a map and directions, and self-administered questionnaires were mailed to participants with instructions to bring completed questionnaires to the examination. Staff members were available both by phone and in the clinic for assistance in completing questionnaires. Recruiters called participants the day before their scheduled visit to remind them to bring all completed forms and medications and to begin fasting 12 hours prior to their appointment.
In addition, ancillary studies involving sleep and magnetic resonance angiography were conducted. Questionnaire data and clinical measurements obtained in the main study and ancillary study measurements obtained for all participants are described below. These studies were approved by the institutional review board of Northwestern University.
Mailed questionnaires
The questionnaires, which were mailed to participants in advance and reviewed for clarity and completeness by staff during the examination, are described in Table 1. They included: 1) the Medical Outcomes Study 36-item Short Form Health Survey, for assessment of functional health status (10, 11); 2) the Index of ADL (as defined by Katz and Akpom (12)) and the Lawton IADL Scale, to determine self-care capacity and skills for independent living (13); 3) the National Institutes of Health Dietary History Questionnaire (14); 4) a medical history questionnaire; and 5) a personal history questionnaire designed to collect information on lifestyle risk factors. Additional questionnaires that were part of an ancillary study and were administered to all participants included: 1) the Pittsburgh Sleep Quality Index, for assessment of sleep habits, insomnia, and use of hypnotic medications (15); 2) the Epworth Sleepiness Scale, a measure of a person's general level of daytime sleep propensity (16, 17); and 3) the Berlin Questionnaire, for identification of adults likely to have sleep apnea (18).
Table 1.
Questionnaires Mailed to Chicago Heart Association Detection Project in Industry Participants for the Follow-up Examination, Chicago Healthy Aging Study, 2007–2010
| Questionnaire | Estimated Time Needed for Completion, minutes | Description |
|---|---|---|
| SF-36 | 15 | 36-item questionnaire that assesses 8 health domains: physical functioning, role limitations due to physical health problems, bodily pain, general health, vitality (energy/fatigue), social functioning, role limitations due to emotional problems, and mental health (psychological distress and psychological well-being) |
| ADL and IADL | 5 | ADL: 6 basic functions—bathing, dressing, toileting, transfer, continence, and feeding. IADL: homemaking skills necessary for independent living, including housework, meal preparation, taking medications, managing money, shopping, and telephone use |
| NIH Dietary History Questionnaire | 60 | Food frequency questionnaire assessing frequency of consumption of 124 common food items during the previous year; includes questions on both portion size and dietary supplements |
| Medical history | 10 | Questions on current and previous medical problems and treatments, hospitalizations, and outpatient visits, including occurrence and treatment of cardiovascular disease (angina, congestive heart failure, prior myocardial infarction, coronary occlusion, coronary thrombosis, stroke, transient ischemic attack), hypertension, hypercholesterolemia, diabetes mellitus, cancer (type and location), chronic lung disease, arthritis, hearing and visual impairments, other medical problems, and operations |
| Personal history | 10 | Questions on lifestyle risk factors, including current and past smoking status, coffee and alcohol consumption, and level of physical activity and exercise |
| Pittsburgh Sleep Quality Indexa | 21-item questionnaire on individual sleep habits (bedtime, morning rising time, sleep-onset latency, and night sleep duration), insomnia, and use of hypnotic medications over a 1-month time interval | |
| Epworth Sleepiness Scalea | Questions on the likelihood of dozing off or falling asleep in 8 different sedentary situations | |
| Berlin Questionnairea | Questions on snoring, daytime sleepiness or fatigue, and presence of obesity or hypertension |
Abbreviations: ADL, Activities of Daily Living; IADL, Instrumental Activities of Daily Living; NIH, National Institutes of Health; SF-36, 36-item Short Form Health Survey.
a Additional questionnaire mailed to all participants as part of an ancillary study on sleep.
Examination components
Risk/lifestyle factors and objective performance-based measures of functional capacity, subclinical atherosclerosis, cardiac function, and sleep are described in Web Table 1, available at http://aje.oxfordjournals.org/. Examinations, including a 15-minute snack period and multidetector row helical computed tomography, took approximately 4 hours; coronary magnetic resonance imaging, performed on eligible participants at a time convenient to them, lasted 1 hour. Participants (and optionally their physicians) were sent a detailed report approximately 1 month after their visit.
Demographic data were updated and confirmed to verify information obtained in 1967–1973 and to obtain information on any interim changes. Health interviewers reviewed all prescription and nonprescription medications brought by participants and recorded the names and dosages of all medications taken. Medications were later coded into broad groups. Height, weight, and waist circumference were measured using protocols and equipment adapted from the Multi-Ethnic Study of Atherosclerosis (MESA) and the Coronary Artery Risk Development in Young Adults (CARDIA) Study (19, 20). All measurements were obtained with participants wearing light clothing and no shoes. Neck circumference was measured in a standardized manner horizontally above the cricothyroid cartilage (21). Three seated blood pressure measurements were obtained according to the CARDIA protocol.
During the initial part of the visit, approximately 40 mL of blood were drawn from an anticubital vein and analyzed for fasting glucose, lipids, high-sensitivity C-reactive protein, and white blood cell count. Participants received a snack immediately after blood drawing. Approximately 15 mL of blood was stored for future studies.
A standard 12-lead ECG was performed with the participant in a supine or semirecumbent position. Tracings were electronically transmitted to the ECG Reading Center at Wake Forest University (Winston-Salem, North Carolina) and read using criteria for diagnostic classification from the University of Minnesota ECG Coding Center (Minneapolis, Minnesota) (22). Protocols developed for MESA were used to determine ankle-brachial index, calculated as the ratio of Doppler-recorded systolic pressures in the lower and upper extremities (19).
The repeated chair-rise test was used to measure exercise tolerance, leg strength, and balance; the timed 4-m walk was used to measure walking velocity (23–26). Lower-extremity balance and strength were tested using tandem stands, that is, 3 increasingly difficult standing positions held for 10 seconds each (25, 26). Based on the participant's performance on the above tests, a summary performance score (the Short Physical Performance Battery) was calculated for assessment of lower extremity function (24, 26). Performance on each of these tasks was scored from 0 to 4; zero indicated inability to complete the task, and scores of 1–4 were based on normative data from over 5,000 community-dwelling men and women from the Established Populations for Epidemiologic Studies of the Elderly. Individual scores were summed to obtain the total score, ranging from 0 (worst) to 12 (best) (24, 26). Additional physical performance measures obtained were the 6-minute walk (a validated test of walking endurance) (27, 28) and grip strength.
Coronary computed tomography was done on all eligible participants. Persons who weighed over 350 pounds (159 kg) or who had undergone computed tomographic scanning or radiation therapy in the previous year were ineligible. The following additional measures were obtained from all participants as part of an ancillary study. Carotid intima-media thickness was measured to assess the degree of atherosclerosis in different arteries, using protocols similar to those in the CARDIA Study (29). Eligible participants also underwent coronary magnetic resonance imaging. Participants with a history of myocardial infarction, coronary heart disease, or atrial fibrillation; those who had undergone coronary artery stenting or bypass surgery; those with a permanent pacemaker or cardioverter-defibrillator; and those with claustrophobia, ferromagnetic implants, or excessive abdominal girth were excluded. The Mini-Mental State Examination was used to screen for cognitive impairment, and the Digit Symbol Substitution Test was used to determine cognitive functioning and to measure sustained attention, response speed, and visual-motor coordination (30). The Center for Epidemiologic Studies Depression Scale (31) and the State-Trait Anxiety Inventory questionnaire (32) were used to assess mood and anxiety, respectively. The Perceived Stress Scale was used to determine levels of stress during the previous month (33). Finally, participants were provided with a wrist actigraphy monitor to wear for 7 consecutive days, to determine duration and timing of major sleep periods and naps, phases of the rest/activity cycle, amplitude of rest/activity rhythm, and activity levels during the day and during sleep. Participants were also asked to maintain a daily sleep and activity log for those 7 days using the Karolinska Sleep Diary (34).
Ascertainment of comorbid conditions
Self-reported medical history will be verified and supplemented using Medicare claims data from the CMS (cross-referenced by Social Security number, sex, and birth date) and current medications brought to the clinic by participants. CMS data contain 100% of the claims submitted to Medicare for covered health-care services. Records include—for each medical service billed to Medicare—date(s) of service, total charges and reimbursements, procedure codes, Diagnosis-Related Group code, the primary diagnosis, and up to 9 other diagnoses coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). CMS data files were obtained for the CHA cohort for the years 1984–2002, and additional CMS data for 2003–2010 have now been acquired. Final verification of self-reported medical conditions will be performed by comparing self-reports with primary and other diagnoses specified by ICD-9-CM discharge codes.
Quality assurance and control
Data collection procedures were standardized prior to the start of the study and maintained throughout the data collection period. Initial and ongoing training of study personnel was conducted. A “trial run” was conducted on 10 persons to identify and resolve potential complexities in data collection and clinic examination flow. Protocols were continually monitored and periodically reviewed; deviations were documented and corrected. Repeat measurements of a random subsample of participants were built into the schedule for ongoing assessment of intra- and interobserver variation. To monitor and evaluate laboratory technical error, a 5%–10% random, blinded sample of blood specimens was reanalyzed. A blood pool was established at the beginning, and specimens from the pool were periodically re-sent to detect possible laboratory drift. Similar protocols were used to monitor inter- and intrareader variation for ECG, ankle-brachial index, and coronary computed tomography measurements and to assess comparability between readings over time.
Statistical analyses
All analyses were conducted separately for LR and non-LR participants. Baseline characteristics were compared between participants and nonparticipants (i.e., those to whom recruitment letters were mailed but who either could not be contacted by telephone or explicitly refused participation) to assess the presence of any follow-up bias. Baseline and follow-up characteristics of participants were also examined by sex and baseline risk status, to assess changes in risk status over 39 years and identify risk factors responsible for loss of LR status. Differences between groups were tested using χ2 tests for categorical variables and F tests for continuous variables. Analyses were conducted using SAS software, version 9.2 (SAS Institute Inc., Cary, North Carolina).
RESULTS
LR participants and nonparticipants differed significantly in terms of racial background (P = 0.0003) and educational attainment (P < 0.0001) (Table 2). For example, a higher proportion of LR participants versus LR nonparticipants were white (89.8% vs. 81.5%) and had attained a college degree or more (55.8% vs. 35.8%). Mean baseline body mass index, serum cholesterol, and systolic and diastolic blood pressure levels did not differ between LR participants and LR nonparticipants. Similarly, non-LR participants and non-LR nonparticipants differed significantly in terms of racial background and educational attainment, as well as smoking status (P < 0.0001 for all) (Table 3). For example, compared with non-LR nonparticipants, a higher proportion of non-LR participants were white (87.5% vs. 80.6%) and had a college degree or more (50.1% vs. 26.7%), and a lower proportion were current smokers at baseline (34.3% vs. 49.8%). Mean baseline body mass index, systolic and diastolic blood pressure, and serum cholesterol levels and the baseline prevalence of diabetes did not differ significantly between non-LR participants and nonparticipants (P's = 0.06–0.99).
Table 2.
Baseline (1967–1973) Characteristics of Baseline Low-Risk Participantsa as Compared With Low-Risk Nonparticipants, Chicago Healthy Aging Study, 2007–2010
| Characteristic | LR Participants (n = 421) |
LR Nonparticipantsb (n = 1037) |
P Valuec | ||
|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | ||
| Age, years | 32.6 (4.9) | 32.9 (5.7) | 0.29 | ||
| Sex | <0.0001 | ||||
| Men | 61.1 | 43.0 | |||
| Women | 39.0 | 57.0 | |||
| Race | 0.0003 | ||||
| White | 89.8 | 81.5 | |||
| African-American | 6.9 | 10.6 | |||
| Other | 3.3 | 7.9 | |||
| Years of education | 15.0 (2.4) | 13.8 (2.6) | <0.0001 | ||
| Educational attainment | <0.0001 | ||||
| Less than high school | 3.8 | 10.1 | |||
| High school graduation | 22.1 | 33.5 | |||
| Some college | 18.3 | 20.6 | |||
| College degree or higher | 55.8 | 35.8 | |||
| Cigarette smoking status | 0.49 | ||||
| Never smoker | 60.6 | 62.5 | |||
| Former smoker | 39.4 | 37.5 | |||
| Current smoker | 0 | 0 | |||
| No. of cigarettes per dayd | 0 | 0 | 0 | ||
| Blood pressure, mm Hge | |||||
| Systolic pressure | 114.5 (6.1) | 114.8 (6.1) | 0.46 | ||
| Diastolic pressure | 69.7 (7.3) | 69.9 (7.6) | 0.66 | ||
| Cholesterol level, mg/dL | 167.3 (20.1) | 169.2 (19.5) | 0.12 | ||
| Body mass indexf | 23.9 (3.3) | 23.6 (3.3) | 0.06 | ||
| Diabetes | 0 | 0 | |||
Abbreviations: CHA, Chicago Heart Association Detection Project in Industry; CHAS, Chicago Healthy Aging Study; LR, low-risk; SD, standard deviation.
a CHA participants who were at low risk of cardiovascular disease at baseline.
b All surviving LR CHA participants aged 65–84 years in 2007–2010 who were mailed a letter but did not participate in CHAS (n = 1,458 LR participants to whom letters were mailed minus 421 examined).
c Differences between CHAS participants and nonparticipants were tested with F tests for continuous variables and χ2 tests for categorical variables.
d LR participants were by definition not current smokers; thus, the number of cigarettes per day was zero.
e Includes persons using antihypertensive medication.
f Body mass index was calculated as weight in kilograms divided by the square of height in meters.
Table 3.
Baseline (1967–1973) Characteristics of Baseline Non-Low-Riska Participants as Compared With Non-Low-Risk Nonparticipants, Chicago Healthy Aging Study, 2007–2010
| Characteristic | Non-LR Participants (n = 974) |
Non-LR Nonparticipantsb
(n = 4,658) |
P Valuec | ||
|---|---|---|---|---|---|
| % | Mean (SD) | % | Mean (SD) | ||
| Age, years | 32.9 (4.5) | 32.9 (5.1) | 0.93 | ||
| Sex | <0.0001 | ||||
| Men | 76.8 | 62.5 | |||
| Women | 23.2 | 37.4 | |||
| Race | <0.0001 | ||||
| White | 87.5 | 80.6 | |||
| African-American | 10.4 | 14.3 | |||
| Other | 2.2 | 5.1 | |||
| Education, years | 14.7 (2.3) | 13.3 (2.5) | <0.0001 | ||
| Educational attainment | <0.0001 | ||||
| Less than high school | 4.3 | 14.7 | |||
| High school graduation | 23.1 | 39.4 | |||
| Some college | 22.5 | 19.2 | |||
| College degree or higher | 50.1 | 26.7 | |||
| Cigarette smoking status | <0.0001 | ||||
| Never smoker | 38.8 | 29.1 | |||
| Former smoker | 26.9 | 21.1 | |||
| Current smoker | 34.3 | 49.8 | |||
| No. of cigarettes per dayd | 6.8 (11.3) | 9.8 (12.3) | <0.0001 | ||
| Blood pressure, mm Hge | |||||
| Systolic pressure | 132.6 (14.6) | 133.2 (15.4) | 0.23 | ||
| Diastolic pressure | 77.9 (9.7) | 78.6 (10.3) | 0.06 | ||
| Cholesterol level, mg/dL | 196.3 (37.1) | 196.3 (35.0) | 0.99 | ||
| Body mass indexf | 25.3 (3.4) | 25.5 (4.1) | 0.21 | ||
| Diabetes | 1.0 | 1.2 | 0.72 | ||
Abbreviations: CHA, Chicago Heart Association Detection Project in Industry; CHAS, Chicago Healthy Aging Study; LR, low-risk; SD, standard deviation.
a CHA participants who were not at low risk of cardiovascular disease at baseline.
b All surviving non-LR CHA participants aged 65–84 years in 2007–2010 who were mailed a letter but did not participate in CHAS (n = 5,632 non-LR participants to whom letters were mailed minus 974 examined).
c Differences between CHAS participants and nonparticipants were tested with F tests for continuous variables and χ2 tests for categorical variables.
d Smokers only.
e Includes persons using antihypertensive medication.
f Body mass index was calculated as weight in kilograms divided by the square of height in meters.
Among the 421 participants who were LR in 1967–1973, only 10.5% of men and 9.3% of women remained LR at follow-up. Hypertension and hypercholesterolemia were the most common reasons for loss of LR status: 54.5% and 60.4% of baseline LR men and women had hypertension at reexamination, and 51.4% and 51.2%, respectively, had hypercholesterolemia at reexamination. In addition, 14.0% of LR men and 7.9% of LR women developed diabetes. Among baseline non-LR participants, prevalences of hypertension, hypercholesterolemia, and diabetes increased over 39 years of follow-up for both men and women. However, a much lower proportion of non-LR men and women remained current smokers after 39 years of follow-up (data not shown).
DISCUSSION
Current trends of population aging, increasing life expectancy, and an accompanying rise in chronic disease conditions have made it increasingly important to address not only morbidity but also disability and the impaired quality of life that can accompany aging even in the absence of clinical disease. The ideal outcome would be increased years of survival free of disability and disease, a concept described by the compression-of-morbidity hypothesis, which proposes that while life span has its limits, the age of onset of morbidity can be postponed, reducing the number of years of disability and disease to a brief period before death (35, 36). Alternately, some contend that increased life expectancy leads to growing numbers of frail, disabled, and institutionalized older persons with poor quality of life and increased health-care costs (37–39). However, older persons with favorable levels of CVD risk factors during young adulthood or middle age have been shown to have greater longevity, markedly lower age-specific CVD and total mortality, and less morbidity, substantially lower health-care costs, and a better health-related quality of life (1–5). Findings from CHAS can add relevant information on key possible objectively measured long-term consequences of LR status in young adulthood, including a potentially lower long-term risk of subclinical atherosclerotic disease, more favorable levels of CVD-related inflammatory markers, and more favorable objectively measured physical performance, physical functioning, and sleep in older men and women of varied socioeconomic and ethnic backgrounds.
Moreover, this study relates to the feasibility of addressing new research questions by in-person reexaminations of surviving aging participants in existing cohorts after an extended period postbaseline with minimal interim contact (in this case, through sporadic mailed surveys); that is, it extends previously reported findings on the feasibility of conducting mailed follow-up surveys of participants after decades of little or no contact (40). Approximately half of the original CHA participants successfully contacted agreed to participate in this follow-up reexamination, conducted 4 decades after baseline. However, successful contact rates were substantially higher for LR individuals than for non-LR individuals, and participation rates among those successfully contacted were also somewhat higher among LR individuals. Among both LR and non-LR individuals, those who agreed to participate had higher educational levels than nonparticipants. However, this is unlikely to have biased findings, since our experience with both risk groups was similar. Among non-LR individuals, a significantly higher proportion of nonparticipants had been current smokers at baseline (1967–1973); thus, their lower participation may have resulted from poorer current health status; this could have biased results towards the null. CHA participants with 1 or more adverse CVD risk factors (i.e., non-LR) at baseline have been shown to have poorer physical functioning, greater role limitations due to physical health status, and a higher self-reported prevalence of CVD and other chronic conditions in older age compared with those who were LR (3). Moreover, Galea and Tracy (41) reported higher rates of morbidity and poorer health status among nonparticipants compared with study participants. In a report from the Cardiovascular Health Study, among adults aged ≥65 years, Tell et al. (42) found that, compared with participants, a higher proportion of nonparticipants (for whom self-reported information on health was available) perceived their health status as fair/poor and reported limitations in performing activities of daily living or instrumental activities of daily living.
Our follow-up participation rates were lower than those reported in studies such as the Framingham Heart Study and the Honolulu Heart Program, which followed participants for similar lengths of time but conducted regular follow-up examinations and thus had more frequent participant contact (43, 44). Interim follow-up of CHA participants was largely conducted without face-to-face contact, which may have affected retention rates (45). On the other hand, due to minimal interim participant contact, the health of CHAS participants is unlikely to have been modified by participation in a research study; thus, findings from CHAS are both representative of the natural history of disease and widely generalizable. Issues faced by CHAS investigators included difficulties in tracing the original CHA study participants despite the availability of addresses from CMS, an unwillingness of frail/ill participants to attend clinic examinations, and the fact that many participants could not recollect participating in the baseline examination almost 4 decades previously. Additionally, the participation rate would certainly have been higher if funds had been available to conduct in-home examinations, as has been the case in other studies (44). However, our experience demonstrates that existing cohorts with rich baseline data can be cost-effectively used to examine objective long-term outcomes—a strategy that is particularly relevant given the shrinking national research budget.
The need for such research is underscored by the limited success to date in counteracting the CVD epidemic as well as other chronic diseases, such as Alzheimer's disease, which become more prevalent with age. In recent years, there have been disturbing trends showing that mortality declines in the United States have slowed and that gains in life expectancy have lagged behind those in other developed countries (46). Unfortunately, despite earlier declines in national prevalence of most major CVD risk factors, LR status remains rare in the US population. A report based on National Health and Nutrition Examination Survey (NHANES) data showed that while the prevalence of LR status increased from 4.4% in 1971–1975 (NHANES I) to 10.5% in 1988–1994 (NHANES III), by 1999–2004 LR prevalence had decreased to 7.5% (47).
The crucial need to promote LR status in order to overcome the CVD epidemic has been recognized by a new developmental goal in Healthy People 2020—to increase overall cardiovascular health in the US population (48)—and by new American Heart Association metrics for optimal cardiovascular health based on maintaining favorable levels of all major risk factors and lifestyle factors, through nutritional hygienic means without the need for pharmacological intervention whenever possible (7). These recent guidelines highlight the continued relevance of the LR concept. If these goals are to be realized, the presently uncommon LR profile needs to become the population norm, underscoring the relevance of strategic priorities for research to expand knowledge on the benefits of LR status. We anticipate that CHAS findings—by documenting lasting, pervasive benefits of LR status—will provide an impetus for the development of comprehensive public health policy and health reform and will effectively motivate people to adopt and maintain healthy lifestyles starting early in life.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Amber Pirzada, Daniel B. Garside, Brandon Lu, Thanh-Huyen T. Vu, Donald M. Lloyd-Jones, Kiang Liu, Jeremiah Stamler, and Martha L. Daviglus); and Department of Neurology, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Kathryn Reid, Daniel Kim, Phyllis Zee).
This research was supported by the National Heart, Lung, and Blood Institute (grant R01 HL089695).
The research of the Chicago Heart Association Detection Project in Industry (CHA) was accomplished thanks to the invaluable cooperation of 84 Chicago-area companies and organizations and their officers, staff, and employees. Acknowledgement is also gratefully extended to all Chicago Heart Association staff and volunteers serving in the project; the CHA investigators and staff members (many of whom are listed in reference 8); and the staff of the Chicago Healthy Aging Study, including Sue Giovanazzi, Veronica Herzog, Karen Mancera-Cuevas, Claudia Pulido-Chambers, Frances Horn, Brenna Michael, Marry Harris, Xuan Cai, Man Yee (Ivy) Wong, Ka (Simon) Chung, Cheryl Westbrook, Katharine Secunda, Debbie Davis, Caroline Zee, Lavinia Sinitean, Jessica Luna-Ramos, Liliana Bolanos, Jillian Hirt, Carla Valdez, Angelica Espinoza, Elizabed Sotelo, Ingrid Guzman, Brigitte Salembier, Bonnie J. Kane, Shelley Sarson, and Brent M. Ardaugh.
Conflict of interest: none declared.
REFERENCES
- 1.Daviglus ML, Stamler J, Pirzada A, et al. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA. 2004;292(13):1588–1592. doi: 10.1001/jama.292.13.1588. [DOI] [PubMed] [Google Scholar]
- 2.Stamler J, Stamler R, Neaton JD, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282(21):2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 3.Daviglus ML, Liu K, Pirzada A, et al. Favorable cardiovascular risk profile in middle age and health-related quality of life in older age. Arch Intern Med. 2003;163(20):2460–2468. doi: 10.1001/archinte.163.20.2460. [DOI] [PubMed] [Google Scholar]
- 4.Daviglus ML, Liu K, Greenland P, et al. Benefit of a favorable cardiovascular risk-factor profile in middle age with respect to Medicare costs. N Engl J Med. 1998;339(16):1122–1129. doi: 10.1056/NEJM199810153391606. [DOI] [PubMed] [Google Scholar]
- 5.Daviglus ML, Liu K, Pirzada A, et al. Cardiovascular risk profile earlier in life and Medicare costs in the last year of life. Arch Intern Med. 2005;165(9):1028–1034. doi: 10.1001/archinte.165.9.1028. [DOI] [PubMed] [Google Scholar]
- 6.Daviglus ML, Pirzada A, Liu K, et al. Comparison of low risk and higher risk profiles in middle age to frequency and quantity of coronary artery calcium years later. Am J Cardiol. 2004;94(3):367–369. doi: 10.1016/j.amjcard.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 8.Stamler J, Dyer AR, Shekelle RB, et al. Relationship of baseline major risk factors to coronary and all-cause mortality, and to longevity: findings from long-term follow-up of Chicago cohorts. Cardiology. 1993;82(2-3):191–222. doi: 10.1159/000175868. [DOI] [PubMed] [Google Scholar]
- 9.Stamler J, Rhomberg P, Schoenberger JA, et al. Multivariate analysis of the relationship of seven variables to blood pressure: findings of the Chicago Heart Association Detection Project in Industry, 1967–1972. J Chronic Dis. 1975;28(10):527–548. doi: 10.1016/0021-9681(75)90060-0. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE, Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 suppl):AS264–AS279. [PubMed] [Google Scholar]
- 11.Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 12.Katz S, Akpom CA. 12. Index of ADL. Med Care. 1976;14(5):116–118. doi: 10.1097/00005650-197605001-00018. [DOI] [PubMed] [Google Scholar]
- 13.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 14.National Cancer Institute. Diet History Questionnaire, Version 1.0. Bethesda, MD: National Cancer Institute; 2007. Alied Research Program. [Google Scholar]
- 15.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 18.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 20.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA—study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 21.Laakso M, Matilainen V, Keinanen-Kiukaanniemi S. Association of neck circumference with insulin resistance-related factors. Int J Obes Relat Metab Disord. 2002;26(6):873–875. doi: 10.1038/sj.ijo.0802002. [DOI] [PubMed] [Google Scholar]
- 22.Prineas R, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings. Littleton, MA: John Wright-PSG, Inc;; 1982. [Google Scholar]
- 23.Guralnik JM, Branch LG, Cummings SR, et al. Physical performance measures in aging research. J Gerontol. 1989;44(5):M141–M146. doi: 10.1093/geronj/44.5.m141. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guralnik JM. The Women's Health and Aging Study: Health and Social Characteristics of Older Women With Disability. Bethesda, MD: National Institute on Aging; 1995. [Google Scholar]
- 26.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 27.Lipkin DP, Scriven AJ, Crake T, et al. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J (Clin Res Ed) 1986;292(6521):653–655. doi: 10.1136/bmj.292.6521.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peeters P, Mets T. The 6-minute walk as an appropriate exercise test in elderly patients with chronic heart failure. J Gerontol A Biol Sci Med Sci. 1996;51A(4):M147–M151. doi: 10.1093/gerona/51a.4.m147. [DOI] [PubMed] [Google Scholar]
- 29.Polak JF, Person SD, Wei GS, et al. Segment-specific associations of carotid intima-media thickness with cardiovascular risk factors. Stroke. 2010;41(1):9–15. doi: 10.1161/STROKEAHA.109.566596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wechsler D. WAIS-III: Administration and Scoring Manual: Wechsler Adult Intelligence Scale—Third Edition. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 31.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 32.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory (Self-Evaluation Questionnaire) Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 33.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 34.Akerstedt T, Hume K, Minors D, et al. The subjective meaning of good sleep, an intraindividual approach using the Karolinska Sleep Diary. Percept Mot Skills. 1994;79(1):287–296. doi: 10.2466/pms.1994.79.1.287. [DOI] [PubMed] [Google Scholar]
- 35.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303(3):130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 36.Vita AJ, Terry RB, Hubert HB, et al. Aging, heath risks, and cumulative disability. N Engl J Med. 1998;338(15):1035–1041. doi: 10.1056/NEJM199804093381506. [DOI] [PubMed] [Google Scholar]
- 37.Verbrugge LM. Longer life but worsening health? Trends in health and mortality of middle-aged and older persons. Milbank Mem Fund Q Health Soc. 1984;62(3):475–519. [PubMed] [Google Scholar]
- 38.Schneider EL, Guralnik JM. The aging of America: impact on health care costs. JAMA. 1990;263(17):2335–2340. [PubMed] [Google Scholar]
- 39.Schneider EL, Brody JA. Aging, natural death and the compression of morbidity: another view. N Engl J Med. 1983;(309):854–856. doi: 10.1056/NEJM198310063091411. [DOI] [PubMed] [Google Scholar]
- 40.Pirzada A, Yan LL, Garside DB, et al. Response rates to a questionnaire 26 years after baseline examination with minimal interim participant contact and baseline differences between respondents and nonrespondents. Am J Epidemiol. 2004;159(1):94–101. doi: 10.1093/aje/kwh012. [DOI] [PubMed] [Google Scholar]
- 41.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17(9):643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 42.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3(4):358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 43.Anderson KM, Castelli WP, Levy D. Cholesterol and mortality: 30 years of follow-up from the Framingham Study. JAMA. 1987;257(16):2176–2180. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 44.Kagan A, editor. The Honolulu Heart Program: An Epidemiological Study of Coronary Heart Disease and Stroke. Amsterdam: Harwood Academic Publishers; 1996. [Google Scholar]
- 45.Hunt JR, White E. Retaining and tracking cohort study members. Epidemiol Rev. 1998;20(1):57–70. doi: 10.1093/oxfordjournals.epirev.a017972. [DOI] [PubMed] [Google Scholar]
- 46.Crimmins EM, Preston SH, Cohen B, editors. Explaining Divergent Levels of Longevity in High-Income Countries. Panel on Understanding Divergent Trends in Longevity in High-Income Countries. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 47.Ford E, Li C, Zhao G, et al. Trends in the prevalence of low risk factor burden for cardiovascular disease among United States adults. Circulation. 2009;120(13):1181–1188. doi: 10.1161/CIRCULATIONAHA.108.835728. [DOI] [PubMed] [Google Scholar]
- 48.Office of Disease Prevention and Health Promotion, Healthy People 2020. Washington, DC:: US Department of Health and Human Services. http://www.healthypeople.gov/2020/default.aspx. (Accessed May, 2011) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.