Abstract
With advances in prevention, screening, and treatment, cancer patients are living longer; hence, non–cancer-related health status will likely play a larger role in determining their life expectancy. In this study, we present a novel method for characterizing non–cancer-related health status of cancer patients using population-based cancer registry data. We assessed non–cancer-related health status in the context of survival from other causes of death and prevalence of comorbidities. Data from the Surveillance, Epidemiology, and End Results program (2000–2006) were used to analyze cancer patients’ survival probabilities by cause of death. Other-cause survival was estimated using a left-truncated survival method with the hazard of death due to other causes characterized as a function of age. Surveillance, Epidemiology, and End Results data linked to Medicare claims (1992–2005) were used to quantify comorbidity prevalence. Relative to the US population, survival from a non–cancer-related death was higher for patients diagnosed with early stage breast and prostate cancer but lower for lung cancer patients at all stages. Lung cancer patients had worse comorbidity status than did other cancer patients. The present study represents the first attempt to evaluate the non–cancer-related health status of US cancer patients by cancer site (breast, prostate, colorectal, and lung) and stage. The findings provide insight into non–cancer-related health issues among cancer patients and their risk of dying from other causes.
Keywords: cancer survival, cancer survivorship, comorbidity, health status, left-truncated survival, non-cancer-related survival, other-cause mortality, SEER
Survival of cancer patients is of paramount interest. Broadly speaking, there are 2 competing causes of death for individuals diagnosed with cancer: cancer and other causes. In the past, population-based cancer registry data have been used to study the survival of cancer patients; these studies have focused on excess mortality attributable to cancer (1, 2). However, little consideration has been given to mortality (or survival) associated with other causes of death. Advances in early diagnosis and treatment have resulted in a higher proportion of cancer patients either being cured of their cancer or living longer with the cancer, making other-cause survival in cancer patients a highly relevant issue from a public health perspective. In the United States, there are approximately 13.7 million cancer survivors, and that number is expected to increase as the population ages and cancer survival rates improve (3, 4). Meanwhile, both the acute and long-term toxicity of cancer treatments may increase the risk of patients developing secondary cancers or other diseases. Also, comorbidities in cancer survivors impact their risk of death. Thus, it is critical for cancer survivorship studies to incorporate population-level assessments of survival from other causes of death to understand health status and life expectancy with respect to other causes of death in the US cancer patient population.
Other-cause survival refers to survival from causes of death other than the diagnosed cancer (hereafter referred to as “non-cancer survival”). The availability of cause of death (COD) information in cancer registry data provides an opportunity to investigate non-cancer survival. The net non-cancer survival probability can be estimated using a cause-specific analysis (5) in which patients who die of their diagnosed cancer are censored at the time of death. The word “net” indicates a measure independent of mortality due to competing causes of death (in this case, cancer). This net measure of non-cancer survival is useful for comparing the life expectancies of different cohorts of cancer patients while controlling for differential risks of cancer death. Net non-cancer survival differs from its counterpart net cancer survival; the latter refers to cancer-specific survival that is not influenced by the risk of death from other causes.
The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute is a geographic area–based cancer registry in the United States (http://seer.cancer.gov/). SEER collects and publishes cancer incidence, prevalence, and survival data. Until recently, COD information reported to population-based registries, including SEER, lacked sufficient accuracy to be used in cause-specific survival analyses (6, 7). Typically, COD is obtained from the death certificate provided by the National Center for Health Statistics; however, COD information in death certificates is prone to misclassification errors (6). For this reason, relative survival (8), a method that does not rely on COD information in estimating the excess mortality associated with cancer, has been used extensively to estimate net cancer survival from cancer registry data. In contrast, estimating non-cancer survival is challenging, and US life tables have been used instead. However, recent studies (9, 10) have suggested that US life tables may not accurately represent other-cause mortality for some cohorts of cancer patients.
A new algorithm that reclassifies COD to correct possible misattribution has recently been developed, allowing SEER data to be used to estimate cause-specific survival probabilities (10). To capture deaths due to specific cancers, the algorithm considers COD in conjunction with tumor sequence, the site of the original cancer diagnosis, and other diseases related to the cancer diagnosis (e.g., acquired immunodeficiency syndrome and/or site-related diseases). On the basis of this algorithm, a new COD variable (SEER cause-specific death classification variable) was implemented in SEER and extensively validated for accuracy. The variable facilitates the estimation of net cancer survival as well as net non-cancer survival using cause-specific analysis.
Two important features differentiate the analysis of non-cancer survival from that of cancer survival. First, cancer survival is typically estimated based on time since diagnosis, whereas non-cancer survival uses age as the time scale, similar to the US life tables. Second, data collected from cancer registries have a left-truncated feature in non-cancer survival time because individuals are enrolled in cancer registries after being diagnosed with cancer. Therefore, the analysis should account for left-truncated survival time with age as a time scale.
The main objectives of the present study were to 1) demonstrate how to estimate net non-cancer survival from cancer registry data; 2) estimate non-cancer survival for different cohorts of cancer patients in the US; and 3) compare the results to US life tables to provide insight into the non-cancer life expectancies of the US cancer patient population compared with the general population.
Previous studies have focused on risk factors for competing causes of death in patients with specific cancers (9, 11–15). Recently, Dignam et al. (9) estimated non–cancer-related life expectancy for breast cancer patients in clinical trials and SEER to evaluate whether these estimates were representative of the overall population with respect to non-cancer mortality. The present study extends the approach outlined in the article by Dignam et al. by conducting a population-level characterization of the non–cancer-related health status of US cancer patients in the context of non-cancer survival and comorbidities and providing an in-depth explanation of these methods. In our study, we considered 4 types of invasive cancers that are common in the US population: female breast, prostate, colon and rectum, and lung and bronchus. For each cancer site, the net non-cancer survival probability was estimated by sex, race, and stage. In addition, SEER data linked to Medicare claims were used to provide an overview of the prevalence of comorbidities among cancer patients. The results were interpreted in light of previous evidence for the effects of screening and risk factors.
MATERIALS AND METHODS
SEER data used to estimate non-cancer survival probabilities
To evaluate non-cancer survival, we used data from 17 SEER registries (16). The study population consisted of patients diagnosed with malignant cancers between 2000 and 2006, and the data included follow-up information through 2007. We excluded patients diagnosed through death certificate or autopsy and patients whose age was not available (<2%). Cases with unknown or missing COD information (<1%) were also excluded from data analysis. The overall study cohort consisted of 1,603,666 cancer patients. Invasive cancers are considered. Details of the study cohorts by cancer site are shown in Table 1. “All sites” denotes all malignant first primary tumors combined. For the purposes of this analysis, we used SEER historical stage information, which classifies tumors as localized, regional, or distant depending on the spread of cancer from the primary site. Race was classified as white, black, or other.
Table 1.
Characteristics of Patients Diagnosed With Malignant Cancers at 50 Years of Age or Older in 17 Surveillance, Epidemiology, and End Results Registries, 2000–2006
| Variable | Cancer Sitea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Sitesb (n = 1,603,666) |
Breast (n = 217,660) |
Prostate (n = 315,155) |
Colorectal (n = 179,259) |
Lung (n = 229,108) |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Race/ethnicity | ||||||||||
| White | 1,340,816 | 84 | 185,131 | 85 | 252,103 | 80 | 147,367 | 82 | 193,171 | 84 |
| Black | 147,252 | 9 | 17,663 | 8 | 38,398 | 12 | 17,656 | 10 | 22,505 | 10 |
| Other | 96,562 | 6 | 13,589 | 6 | 15,575 | 5 | 13,068 | 7 | 12,937 | 6 |
| Unspecified/unknown | 19,036 | 1 | 1,277 | 1 | 9,079 | 3 | 1,168 | 1 | 495 | 0 |
| Sex | ||||||||||
| Male | 869,608 | 54 | 0 | 0 | 315,155 | 100 | 89,930 | 50 | 124,007 | 54 |
| Female | 734,058 | 46 | 217,660 | 100 | 0 | 0 | 89,329 | 50 | 105,101 | 46 |
| Stagec | ||||||||||
| Localized | 136,219 | 63 | 291,014 | 92 | 73,290 | 41 | 37,424 | 16 | ||
| Regional | 63,231 | 29 | 63,043 | 35 | 55,560 | 24 | ||||
| Distant | 13,713 | 6 | 13,269 | 4 | 34,059 | 19 | 120,056 | 52 | ||
| Unstaged | 4,497 | 2 | 10,872 | 3 | 8,867 | 5 | 16,068 | 7 | ||
| Vital status | ||||||||||
| Alive | 897,501 | 56 | 175,820 | 81 | 264,601 | 84 | 100,006 | 56 | 37,689 | 16 |
| Dead | 706,165 | 44 | 41,840 | 19 | 50,554 | 16 | 79,253 | 44 | 191,419 | 84 |
| Cause of death | ||||||||||
| Cancer of interest | 541,087 | 77 | 23,630 | 56 | 18,132 | 36 | 55,971 | 71 | 171,308 | 89 |
| Other | 165,078 | 23 | 18,210 | 44 | 32,422 | 64 | 23,282 | 29 | 20,111 | 11 |
a Definitions of cancer site are based on the primary site and histology. For details, see reference 41.
b “All sites” denotes all malignant first primary tumors in Surveillance, Epidemiology, and End Results combined.
c For prostate cancer, “localized” and “regional” are combined into one stage.
Estimating non-cancer survival: left-truncated survival data with age as the time scale
To estimate non-cancer survival probabilities, we considered death due to other causes as the event of interest and death due to the diagnosed cancer as the censoring event. Because aging is associated with a higher risk of comorbid conditions and mortality due to other causes, age, rather than time since diagnosis, represents a natural time scale for characterizing the hazard of death due to other causes. Furthermore, using age as a time scale directly accounts for the impact of age on mortality, thereby adjusting for its confounding effect (17, 18).
Using age as the time scale introduced left-truncation into the data. Left-truncation arises when individuals come to observation sometime after the actual origin of time, which in this case is birth. Because data from cancer patients were collected after their diagnosis, only patients who were alive at the time of cancer diagnosis could be included in the calculation of non-cancer survival. As a result, the estimated curve represents non-cancer survival probability conditional on being alive at the youngest entry age (e.g., age at diagnosis for the youngest patient in the cohort). This conditional survival probability can be estimated using standard survival methods, such as the Kaplan-Meier or actuarial method, by defining the risk set to accommodate left truncation; patients enter the risk set at the age at which they are diagnosed and leave the risk set when they die or are censored (18). Kaplan-Meier estimates for left-truncated and right-censored survival data can be computed using commonly available statistical packages. We used PROC PHREG in SAS (19). For details and demonstration of the method, see the Web Appendix (available at http://aje.oxfordjournals.org/).
The present study included patients diagnosed at 50 years of age or older because the cancer types examined in this study are more common in older adults (2). Sensitivity analyses were performed that included patients diagnosed at 40 years of age or older and 60 years of age or older, and the results were similar. For studying cancers common in younger ages (e.g., childhood cancers), the lower age limit would need to be revised. For rare cancers, caution is advised, as the estimates could be extremely unstable because of the small risk set at all ages.
US life tables for comparisons with non-cancer survival
We used the US decennial life tables for 1999–2001 developed by National Center for Health Statistics (20) to represent the overall survival of the US population and compare it with our non-cancer survival estimates in cancer patients. When the proportion of deaths due to a specific cancer type in the general population is small, the all-cause survival probability provides reasonable estimates for other-cause survival in the US population (8). US life tables that excluded deaths due to each of the cancer types independently (colorectal, prostate, breast, and lung) were similar to the US all-cause life tables. However, the proportion of deaths due to cancer overall (all sites) represented a large proportion of all causes of death; for the population who were 50 years of age or older, it was 27% for males and 22% for females. Life tables excluding any type of cancer as a COD were estimated and used in comparisons with non-cancer survival for all sites. For details on the methods used to estimate life tables excluding a specific COD, see Elandt–Johnson and Johnson (21). Because the non-cancer survival estimates were conditional on cohort members being alive at 50 years of age, we estimated the comparable conditional survival at 50 years of age for the general US population.
SEER data linked to Medicare claims used to describe comorbidity
SEER data linked to Medicare claims (22) were used to quantify comorbidity. The cohort consisted of Medicare beneficiaries 66 years of age or older residing in the SEER 11 cancer registry areas who had received a cancer diagnosis between 1992 and 2005 (patient cohort), plus a random 5% sample of the beneficiaries in the area who had not been diagnosed with cancer (non-cancer cohort). For the cancer patients, their comorbid conditions were identified in the year before the date of cancer diagnosis. For individuals without cancer, their comorbid conditions in the year before the birthday of the calendar year were identified from 1992 to 2005. The cohort of cancer patients included 1,060,752 individuals (all cancer sites combined, excluding individuals with in situ cancers), with 212,527 patients with prostate cancer, 123,558 with female breast cancer, 137,107 with colorectal cancer, and 165,239 with lung cancer. The 5% cohort of cancer-free Medicare beneficiaries consisted of 3,099,833 records, with multiple records per individual.
Describing comorbidity prevalence
International Classification of Diseases, Ninth Revision, Clinical Modification and fourth edition of Common Procedural Terminology codes recorded in the claims were used to identify 16 common comorbid conditions described by Charlson et al. (23) and used in other studies (24–26). Similar to what was done by Klabunde et al. (24, 25), we calculated comorbidity scores based on weights indicating the effect of the comorbid conditions on non–cancer-related death. We used weights that were estimated using the Cox proportional hazards model adjusted for age, sex, and race (27). For data analysis, individuals were assigned to 1 of 3 comorbidity categories based on their comorbidity score: healthy, low to medium comorbidity, and high comorbidity. Each comorbidity category was characterized based on the severity of clinical outcomes associated with the specific condition. Healthy refers to individuals with no comorbid conditions. Low comorbidity refers to conditions that usually do not require physicians to adjust cancer treatment, such as history of myocardial infarction, ulcer, or rheumatologic disease. Medium comorbidity refers to conditions that may sometimes require modification of cancer treatment, including vascular disease, diabetes, and paralysis. High comorbidity refers to severe illnesses that frequently lead to organ failure or systemic dysfunction and always require adjustment of cancer treatment; these conditions include chronic obstructive pulmonary disease, liver dysfunction, chronic renal failure, dementia, congestive heart failure, and acquired immunodeficiency syndrome. Because of the small sample sizes, the low and medium comorbidity categories were combined. The prevalence of each comorbidity category was computed for individuals 66 to 90 years of age in both the cancer and non-cancer cohorts in the SEER data linked to Medicare claims.
RESULTS
Non-cancer survival
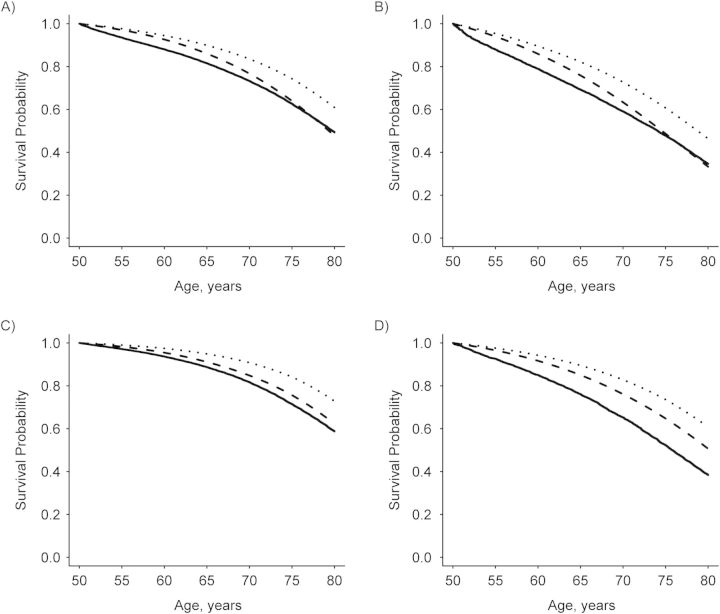
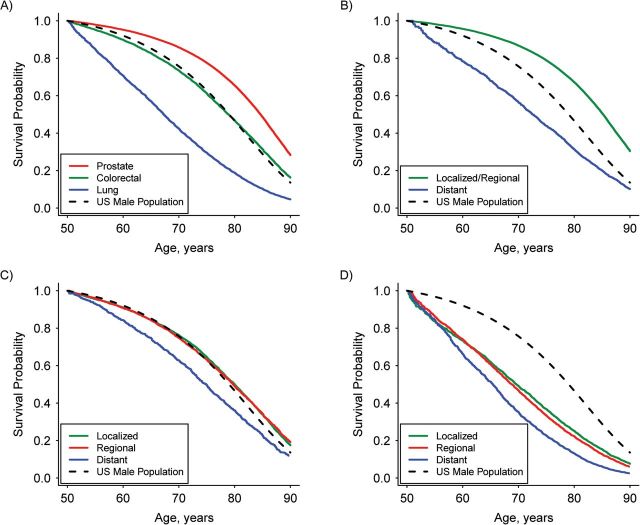
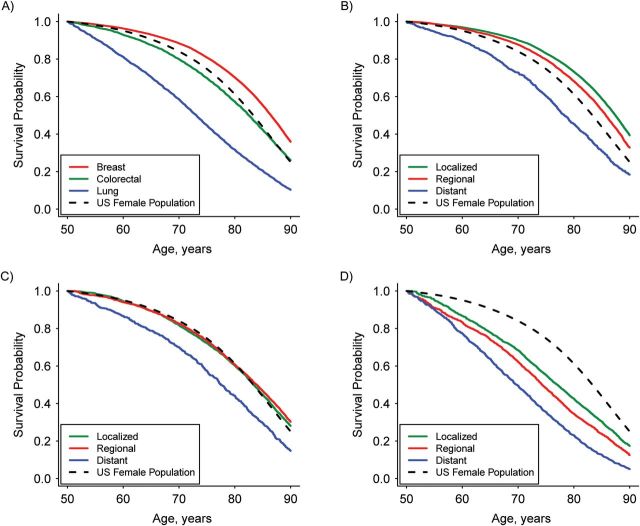
The overall non-cancer survival probability of SEER cancer patients (all sites) was lower than that of the general US population (Figure 1). Relative to the US population, non-cancer survival probabilities were higher for prostate and breast cancer patients, similar for colorectal cancer patients, and lower for lung cancer patients (Figures 2A and 3A). Results stratified by stage at diagnosis varied by cancer site (Figures 2B–D and 3B–D). For the 4 cancers studied, there was no substantial differential effect based on race in non-cancer survival trends or directions when the race-stratified survival probabilities in each cancer cohort were compared with those of the general US population for the same race (Web Figure 1).
Figure 1.
Estimated non-cancer survival probability for cancer patients conditional on surviving to 50 years of age, Surveillance, Epidemiology, and End Results (SEER), 2000–2006. All malignant first primary tumors in (A) white male, (B) black male, (C) white female, and (D) black female participants are included. The solid lines denote non-cancer survival probability for cancer patients in SEER; the dashed lines denote survival probabilities for the US populations estimated from the US life tables including all causes of death; and the dotted lines denote survival probabilities for the US population excluding all types of cancer death. The estimated survival probabilities from the life tables excluding all types of cancer death were approximately 10% higher than those from the US life tables that included all causes of death.
Figure 2.
Estimated non-cancer survival probability for male cancer patients conditional on surviving to 50 years of age, Surveillance, Epidemiology, and End Results, 2000–2006. A) Cancer by type for all stages combined; B) prostate cancer by stage; C) colorectal cancer by stage; and D) lung cancer by stage. Survival probability for the US male population was estimated from the US life table.
Figure 3.
Estimated non-cancer survival probability for female cancer patients conditional on surviving to 50 years of age, Surveillance, Epidemiology, and End Results, 2000–2006. A) Cancer by type for all stages combined; B) breast cancer by stage; C) colorectal cancer by stage; and D) lung cancer by stage. Survival probability for the US female population was estimated from the US life table.
Prostate and breast cancer patients diagnosed with localized or regional cancer stages have higher non-cancer survival probabilities than the general US population. However, the non-cancer survival of patients diagnosed with distant stage cancer is lower than that of the US population (Figures 2B and 3B). Although the optimal age at which to begin mammography screening for breast cancer has been a subject of debate, the American Cancer Society (and previously the US Preventive Service Tasks Force) recommends that women begin this screening procedure at 40 years of age. Therefore, we also investigated non-cancer survival of breast cancer patients conditional on surviving to 40 years of age, and the non-cancer survival results were similar to the findings conditional on surviving to 50 years of age.
Individuals diagnosed with localized or regional stage colorectal cancer had non-cancer survival probabilities similar to those of the general US population, whereas individuals diagnosed with distant stage cancer had a lower probability of surviving from other causes of death (Figures 2C and 3C). Patients diagnosed with lung cancer had a lower probability of non-cancer survival than did the general US population, even in the early stages (Figures 2D and 3D).
Median survival age for non–cancer-related death
For patients diagnosed with early stage prostate or breast cancer, the median survival age was older than that of the general US population. However, the median survival age was younger for patients diagnosed with distant stage breast or prostate cancer. For patients with lung cancer, the median survival age was younger than that of the general US population at all stages. For example, the median survival age for men diagnosed with lung cancer, even those diagnosed with localized stage cancer, at age 50 years was 9 years earlier than that of the general population. See Tables 2 and 3 for detailed results by sex and race at ages 50, 60, and 70 years.
Table 2.
Difference Between Median Survival Agesa of Male Cancer Patients and a Male US Population of Same Age and Race for Non–Cancer-Related Death, Surveillance, Epidemiology, and End Results, 2000–2006
| Race and Participant Age, years | Median Survival Age for US Male Population, yearsb | Type of Cancerc |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colorectal Cancer |
Lung Cancer |
Prostate Cancerd |
||||||||||
| All | Localized | Regional | Distant | All | Localized | Regional | Distant | All | Localized/ Regional | Distant | ||
| White | ||||||||||||
| 50 | 79 | 0 | 1 | 0 | −4 | −12 | −9 | −10 | −13 | 6 | 6 | −6 |
| 60 | 80 | 0 | 1 | 1 | −3 | −7 | −5 | −7 | −9 | 5 | 6 | −3 |
| 70 | 83 | 1 | 1 | 1 | −1 | −4 | −2 | −3 | −5 | 4 | 4 | −1 |
| Black | ||||||||||||
| 50 | 75 | 0 | 1 | 1 | −4 | −11 | −9 | −10 | −13 | 5 | 6 | −5 |
| 60 | 77 | 0 | 1 | 2 | −2 | −7 | −5 | −5 | −9 | 5 | 5 | −1 |
| 70 | 81 | 0 | 0 | 2 | 0 | −3 | −2 | −2 | −3 | 3 | 3 | −1 |
a Median survival age for cancer patients is defined as the age at which non-cancer survival probability conditional on surviving at age a is 0.5, where a = 50, 60, and 70 years.
b Results are conditional on surviving at 50, 60, and 70 years of age.
c Negative values represent median survival ages lower than those of the general US population.
d For prostate cancer, “localized” and “regional” are combined into one stage.
Table 3.
Difference Between Median Survival Agesa of Female Cancer Patients and a Female US Population of Same Age and Race for Non–Cancer-Related Death, Surveillance, Epidemiology, and End Results, 2000–2006
| Race and Participant Age, years | Median Survival Age for US Female Population, yearsb | Type of Cancerc |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colorectal Cancer |
Lung Cancer |
Breast Cancer |
|||||||||||
| All | Localized | Regional | Distant | All | Localized | Regional | Distant | All | Localized | Regional | Distant | ||
| White | |||||||||||||
| 50 | 84 | −1 | 0 | 1 | −5 | −10 | −6 | −9 | −13 | 3 | 4 | 2 | −4 |
| 60 | 84 | 0 | 0 | 1 | −3 | −8 | −4 | −7 | −10 | 3 | 4 | 2 | −4 |
| 70 | 86 | 0 | 1 | 1 | −2 | −5 | −2 | −4 | −6 | 2 | 3 | 2 | −2 |
| Black | |||||||||||||
| 50 | 80 | −2 | 0 | −1 | −9 | −12 | −9 | −10 | −16 | 2 | 3 | 2 | −6 |
| 60 | 82 | −2 | 0 | −1 | −7 | −9 | −7 | −6 | −12 | 1 | 2 | 1 | −4 |
| 70 | 84 | −1 | 0 | −1 | −4 | −5 | −3 | −4 | −7 | 1 | 2 | 1 | −2 |
a Median survival age for cancer patients is defined as the age at which non-cancer survival probability conditional on surviving at age a is 0.5, where a = 50, 60, and 70 years.
b Results are conditional on surviving at 50, 60, and 70 years of age.
c Negative values represent median survival ages lower than those of the general US population.
Comorbidity status
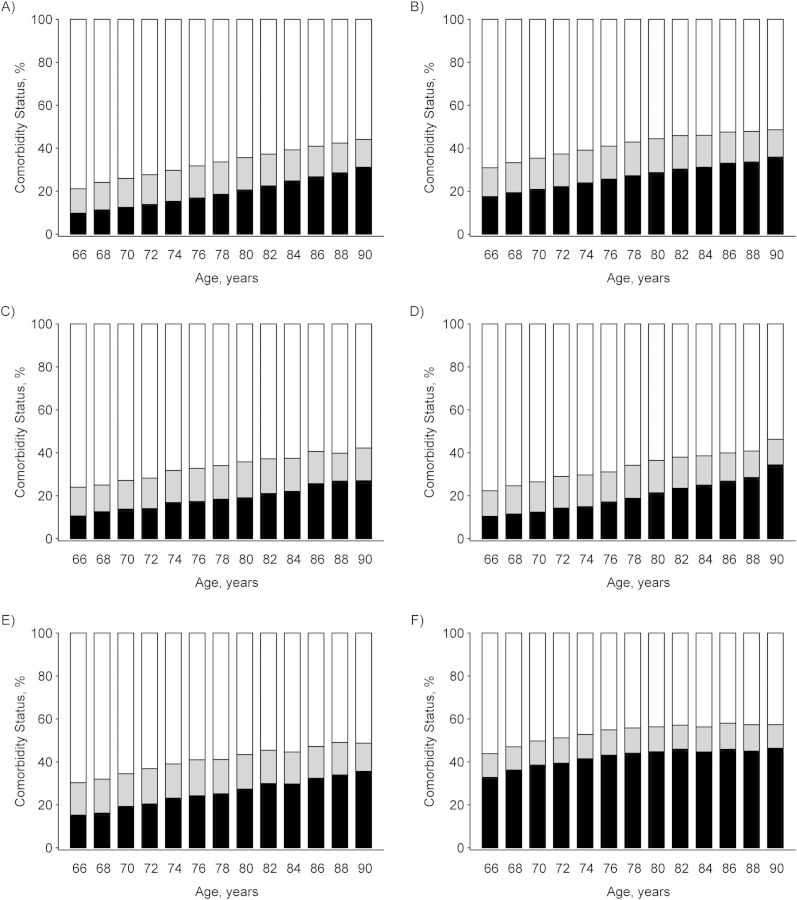
In general, for both the cancer and non-cancer cohorts, comorbidities became more frequent as age increased and the proportion of individuals with no comorbid conditions decreased. Comorbidity prevalence in the breast and prostate cancer cohorts was similar to that in the non-cancer cohort. In contrast, the lung and colorectal cancer cohorts had a higher comorbidity prevalence compared with those in the prostate or breast cancer cohorts. For example, at 66 years of age, the proportions of men with comorbid conditions were 43.2% (95% confidence interval (CI): 41.7, 44.7) for lung cancer, 22.3% (95% CI: 21.6, 23.1) for prostate cancer, and 29.5% (95% CI: 27.7, 31.2) for colorectal cancer. The corresponding proportions for women were 44.6% (95% CI: 42.9, 46.3) for lung cancer, 24% (95% CI: 22.9, 25.1) for breast cancer, and 31.5% (95% CI: 29.5, 33.5) for colorectal cancer. In particular, high comorbidity was more prevalent in the lung cancer cohort. Strikingly, the prevalence of high comorbidity was approximately 2-fold to 3-fold higher in the lung cancer cohort and 1.5-fold higher in the colorectal cancer cohort compared with the breast or prostate cancer cohorts (Figure 4).
Figure 4.
Comorbidity status from ages 66 years to 90 years in 2-year increments from Surveillance, Epidemiology, and End Results data linked to Medicare claims, 1992–2005. A) Non-cancer patients; B) cancer patients, all sites combined; C) female breast cancer patients; D) prostate cancer patients; E) colorectal cancer patients; and F) lung cancer patients. White bars denote the percentage of individuals with no comorbid conditions; gray bars denote the percentage of individuals in the low or medium comorbidity category; and black bars denote the percentage of individuals in the high comorbidity category.
DISCUSSION
In this study, we present a novel means of assessing the non–cancer-related health status of the cancer patient population using cancer registry data. We characterized the non-cancer survival experiences and comorbidity status of US patients with breast, prostate, colorectal, and lung cancers. We addressed estimation of net non-cancer survival, which is the counterpart of net cancer survival. In the estimation, we used a left-truncated survival method that is being implemented in SEER*Stat software (http://www.seer.cancer.gov). The software will soon be available to the public and will facilitate estimation of non-cancer survival by a broad range of researchers.
Because the goal was to quantify the non-cancer survival experience of a population of cancer patients irrespective of changes in the risk of dying from cancer, the current study obtained a net measure of survival. The estimated net non-cancer survival quantifies survival probability that is not affected by the risk of cancer death and represents survival at the population-level of analysis. Typically, net survival is used in comparing racial/ethnic groups or between registries and in tracking survival longitudinally. Thus, this estimate has utility for cancer control and public health purposes. Estimates calculated using competing risks methods may offer a better measure for patient prognosis because death from the diagnosed cancer plays a key role in assessing the risk of dying from other causes at an individual level. The crude probability of death (28), which is the probability of dying in the presence of competing risks, can be calculated using population-based cancer registry data. It is a personalized measure of survival and may be more useful for patients or clinicians. However, providing a personalized measure was not within the scope of this study.
The comparison with the matched general US population showed that non-cancer survival was higher for people diagnosed with early stage cancers for which screening is known to aid in early detection, for example, breast and prostate cancers. Patients diagnosed with early stage cancer had a lower risk of death from other causes than did patients diagnosed with cancer at an advanced stage. Moreover, patients diagnosed with cancer at an advanced stage had a lower non-cancer survival probability than did the general population. The higher non-cancer survival probability for patients diagnosed with early stage cancer might be related to higher socioeconomic status, healthier behaviors, more routine doctor visits to treat existing comorbid conditions, or better access to health care (29, 30). Conversely, the lower non-cancer survival probability for patients diagnosed with advanced stage cancer might be related to unhealthy behaviors or lifestyles (e.g., ignoring early symptoms, not treating existing comorbidities) or limited access to health care (29, 30). These results also suggest a screener effect in which cancer patients diagnosed at an early stage through screening may have better access to health care, leading to a lower risk of death than that in the general population. Similarly, the results may reflect an unhealthy non-screener effect in that patients who do not engage in cancer screening, ignore early symptoms, or have poor access to health care experience higher risks of death.
The non-cancer survival probability for individuals diagnosed with early stage colorectal cancer was similar to that of the general population. Although effective colorectal cancer screening is available, this did not appear to convey a survival advantage, in contrast to our findings for prostate and breast cancers. However, many colorectal cancer risk factors are related to unhealthy lifestyle factors (e.g., obesity, lack of physical activity, smoking, heavy alcohol use, and red meat consumption) (31–35) that are also associated with other diseases. Indeed, our findings indicated that individuals diagnosed with colorectal cancer had more comorbid conditions than did individuals diagnosed with breast or prostate cancer. In addition, previous studies (36, 37) have suggested that although screening for colorectal cancer is slowly increasing, it lags behind mammography use. Moreover, because colorectal cancer can be prevented by early detection and removal of adenomatous polyps (38), patients in whom screening prevented development of colorectal cancer do not appear in our cancer cohort.
For individuals who were diagnosed with lung cancer, the risk of dying due to other causes of death was higher than that of the general population. Lung cancer is a well-known smoking-related cancer that shares risk factors with other potentially life-threatening diseases, such as chronic obstructive pulmonary disease (39, 40).
Our comorbidity measure provides insight into the health status of cancer patients and the prevalence of risk factors. For instance, lung cancer patients had a higher prevalence of comorbid conditions in the year before cancer diagnosis, indicating the presence of risk factors (e.g., smoking) related to both lung cancer and other diseases before the diagnosis. The non-cancer cohort that we used included a 5% sample of individuals residing in the SEER area who had not been diagnosed with cancer; hence, this cancer-free cohort may have fewer risk factors and better comorbidity status than the general US population. Therefore, the ability to generalize the findings in the non-cancer cohort to the general US population may be limited.
Overall, the present study provides an overview of the non–cancer-related health status of US patients with common types of cancer. Because people diagnosed with certain cancers at specific stages are not necessarily representative of the general population (9, 10), their health profiles at the time of diagnosis differ from those of the US population. Therefore, these findings offer insights for policy makers, clinicians, and public health practitioners into the risk of cancer patients dying from other causes, as well as perspective on the non–cancer-related health issues among cancer patients. This information may also be useful for informing cancer control and intervention policies (e.g., early screening, tobacco control policies) by taking into account their impact on both cancer and non-cancer outcomes and for assessing health disparities in cancer populations. Future studies may entail investigating the impact of common risk factors, modes of detection (i.e., screening or symptoms), treatment, and access to health care on non-cancer survival.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Data Modeling Branch, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, Maryland (Hyunsoon Cho, Angela B. Mariotto); Clinical Investigations Branch, Cancer Therapy Evaluation Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, Maryland (Bhupinder S. Mann); Health Services and Economics Branch, Applied Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, Maryland (Carrie N. Klabunde); and Statistical Methodology and Applications Branch, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Bethesda, Maryland (Eric J. Feuer).
We thank Dr. Kathleen A. Cronin at the Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, for her helpful comments on the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD) Lancet Oncol. 2008;9(8):730–756. doi: 10.1016/S1470-2045(08)70179-7. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. http://seer.cancer.gov/csr/1975_2008/ (Accessed February 1, 2012) [Google Scholar]
- 3.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 5.Marubini E, Valsecchi MG. Analyzing Survival Data from Clinical Trials and Observational Studies. New York; Chichester: John Wiley & Sons; 1995. [Google Scholar]
- 6.Begg CB, Schrag D. Attribution of deaths following cancer treatment. J Natl Cancer Inst. 2002;94(14):1044–1045. doi: 10.1093/jnci/94.14.1044. [DOI] [PubMed] [Google Scholar]
- 7.Percy C, Stanek E, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71(3):242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 9.Dignam JJ, Huang L, Ries L, et al. Estimating breast cancer-specific and other-cause mortality in clinical trial and population-based cancer registry cohorts. Cancer. 2009;115(22):5272–5283. doi: 10.1002/cncr.24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howlader N, Ries LAG, Mariotto AB, et al. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Argiris A, Brockstein BE, Haraf DJ, et al. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res. 2004;10(6):1956–1962. doi: 10.1158/1078-0432.ccr-03-1077. [DOI] [PubMed] [Google Scholar]
- 12.Du XL, Fox EE, Lai DJ. Competing causes of death for women with breast cancer and change over time from 1975 to 2003. Am J Clin Oncol. 2008;31(2):105–116. doi: 10.1097/COC.0b013e318142c865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollingsworth JM, Miller DC, Daignault S, et al. Five-year survival after surgical treatment for kidney cancer—a population-based competing risk analysis. Cancer. 2007;109(9):1763–1768. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]
- 14.Mell LK, Dignam JJ, Salama JK, et al. Predictors of competing mortality in advanced head and neck cancer. J Clin Oncol. 2010;28(1):15–20. doi: 10.1200/JCO.2008.20.9288. [DOI] [PubMed] [Google Scholar]
- 15.Ng AK, Bernardo MP, Weller E, et al. Long-term survival and competing causes of death in patients with early-stage Hodgkin's disease treated at age 50 or younger. J Clin Oncol. 2002;20(8):2101–2108. doi: 10.1200/JCO.2002.08.021. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. SEER Registry Groupings for Analyses. Bethesda, MD: National Cancer Institute; http://seer.cancer.gov/registries/terms.html. (Accessed June 4, 2013) [Google Scholar]
- 17.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 18.Lamarca R, Alonso J, Gomez G, et al. Left-truncated data with age as time scale: an alternative for survival analysis in the elderly population. J Gerontol A Biol Sci Med Sci. 1998;53(5):M337–M343. doi: 10.1093/gerona/53a.5.m337. [DOI] [PubMed] [Google Scholar]
- 19.SAS Institute, Inc. SAS/STAT 9.2 User's Guide. Cary, NC: SAS Institute, Inc.; 2008. [Google Scholar]
- 20.Arias E, Curtin LR, Wei R, et al. U.S. decennial life tables for 1999–2001, United States life tables. Natl Vital Stat Rep. 2008;57(1):1–36. [PubMed] [Google Scholar]
- 21.Elandt-Johnson RC, Johnson NL. Survival Models and Data Analysis. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- 22.National Cancer Institute. SEER-Medicare Linked Database. Bethesda, MD: National Cancer Institute; 2011. http://healthservices.cancer.gov/seermedicare/ Accessed June 2010. [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 25.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 suppl):IV-3–IV-8. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 27.Mariotto AB, Wang Z, Klabunde CN, et al. Health-adjusted age: a tool for assessing non-cancer survival of recently diagnosed cancer patients. J Clin Epidemiol. doi: 10.1016/j.jclinepi.2013.07.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cronin KA, Feuer EJ. Cumulative cause-specific mortality for cancer patients in the presence of other causes: a crude analogue of relative survival. Stat Med. 2000;19(13):1729–1740. doi: 10.1002/1097-0258(20000715)19:13<1729::aid-sim484>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Chen AY, Schrag NM, Halpern MT, et al. The impact of health insurance status on stage at diagnosis of oropharyngeal cancer. Cancer. 2007;110(2):395–402. doi: 10.1002/cncr.22788. [DOI] [PubMed] [Google Scholar]
- 30.Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 31.Cronin KA, Krebs-Smith SM, Feuer EJ, et al. Evaluating the impact of population changes in diet, physical activity, and weight status on population risk for colon cancer (United States) Cancer Causes Control. 2001;12(4):305–316. doi: 10.1023/a:1011244700531. [DOI] [PubMed] [Google Scholar]
- 32.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31(4):925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 33.Giovannucci E, Ascherio A, Rimm EB, et al. Physical-activity, obesity, and risk for colon-cancer and adenoma in men. Ann Intern Med. 1995;122(5):327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Giovannucci E, Colditz GA, Stampfer MJ, et al. Physical activity obesity and risk of colorectal adenoma in women (United States) Cancer Causes Control. 1996;7(2):253–263. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 35.Giovannucci E, Willett WC, Stubbs A. Dietary factors and risk of colon-cancer. Ann Med. 1994;26(6):443–452. doi: 10.3109/07853899409148367. [DOI] [PubMed] [Google Scholar]
- 36.Breen N, Wagener DK, Brown ML, et al. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93(22):1704–1713. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- 37.Swan J, Breen N, Graubard BI, et al. Data and trends in cancer screening in the United States. Cancer. 2010;116(20):4872–4881. doi: 10.1002/cncr.25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Iribarren C, Tekawa IS, Sidney S, et al. Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N Engl J Med. 1999;340(23):1773–1780. doi: 10.1056/NEJM199906103402301. [DOI] [PubMed] [Google Scholar]
- 40.Washington, DC: United States Department of Health, Education, and Welfare, Public Health Service; 1964. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Public Health Service Publication No. 1103. [Google Scholar]
- 41.National Cancer Institute. Site Recode ICD-O-3 (1/27/2003) Definition. Bethesda, MD: National Cancer Institute; 203; (http://seer.cancer.gov/siterecode/icdo3_d01272003/ ). (Accessed June 4, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.