Abstract
Background/Objective
HIV infection has a devastating impact on individual and public health, and affects populations disproportionately. Treatment with antiretroviral therapy (ART) saves lives, but long-term adherence to ART is critical to its success. We performed an observational cohort study to determine the influence of race, sex and other sociodemographic factors on early ART discontinuations among HIV-infected persons.
Methods
TennCare-enrolled adults of black or white non-Hispanic race beginning ART with either a non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI) between 1996–2003 (N=3,654) were assessed for early discontinuation. A subgroup of discontinuations was validated using the primary medical record.
Results
Blacks were more likely than whites to discontinue NNRTIs (37 vs. 28%; P=0.003) and PIs (36 vs. 25%; P≤0.001). In multivariable models adjusting for race, sex, age, early HIV-related medical encounter, urban residence and TennCare enrollment category, black race, female sex and younger age were independent predictors of discontinuation among those starting PIs. Among persons starting NNRTIs, black race, younger age and a disability-based enrollment category predicted early drug discontinuation, but female sex did not.
Conclusions
Our results suggest that sociodemographic factors were associated with early NNRTI and PI discontinuation in this population, and some factors were ART class specific.
Keywords: HIV/AIDS, treatment, health insurance
INTRODUCTION
Improvements in human immunodeficiency virus (HIV) therapy have led to decreases in HIV morbidity and mortality.1,2 Current treatment guidelines recommend that initial antiretroviral therapy (ART) for HIV infection include 2 nucleoside reverse transcriptase inhibitors (NRTIs) in combination with either a protease inhibitor (PI) or non-NRTI (NNRTI).3,4 Successful long-term treatment of HIV requires meticulous adherence to ART drugs, but ART (and thus, successful treatment of HIV) can be limited by early adverse effects. For example, PIs can cause gastrointestinal symptoms such as nausea, vomiting and diarrhea early after treatment initiation, and NNRTIs are associated with rash and drug-induced hepatitis, which can also occur early in therapy.5 The NNRTI efavirenz causes central nervous system symptoms, including insomnia, somnolence and intense dreams that are greatest early after initiation of therapy.6 These and other early adverse effects can affect adherence, long-term tolerability, and ultimately, success of treatment.7,8 Cohort studies have reported discontinuation rates of first ART regimens of 30–60% within the first year, with the majority of discontinuations due to toxicity.8–10 Studies have also suggested that women have higher rates of adverse drug events11,12 and drug discontinuation9,13,14 than males.
Importantly, genetic differences may also influence the incidence and/or severity of side effects of ART and, thus, contribute to the observed variations in toxicity and discontinuation by race.15 An example is a polymorphism in the hepatic cytochrome P450 2B6 enzyme (CYP2B6), the primary metabolic pathway of efavirenz. Increased plasma levels of efavirenz and central nervous system side effects have been reported shortly after treatment initiation in persons with a CYP2B6 polymorphism that is more common in African Americans than Caucasians.16–19 Increased drug levels are associated with an increased risk of adverse effects,6,20 which may contribute to poor adherence and drug discontinuation. Nevirapine is an NNRTI metabolized by both CYP2B6 and CYP3A4,21 and increased plasma levels have been associated with a CYP2B6 polymorphism.18 Data also suggest that variation in the multidrug resistance gene-1 (MDR1 or ABCB1) is associated with an increased risk of nevirapine-associated hepatotoxicity.22,23
Population-based studies could be useful for developing strategies to predict drug toxicity, monitor adverse events and optimize treatment regimens in HIV-infected populations. Our hypothesis was that demographic factors that reflect the populations with the greatest recent increases in HIV infection rates and disparities in HIV outcomes (e.g., black race and female sex) would also be risk factors for early discontinuation of ART drugs. The objective of this study was to use data from a demographically diverse, statewide population to determine the influence of race, sex and other sociodemographic factors on discontinuation of NNRTI or PI early after treatment initiation, which could be an important surrogate for longer-term treatment success.
METHODS
TennCare Cohort Identification
This was an observational cohort study of persons in TennCare who initiated ART with an NNRTI or PI between January 1996 and June 2003. TennCare is Tennessee’s managed care program for Medicaid enrollees and uninsured individuals. During the study period, all persons in Tennessee diagnosed with HIV infection were eligible for TennCare regardless of disease status. Data files, including enrollment, encounter and pharmacy information, were used to identify self-reported non-Hispanic white or black persons who initiated their first NNRTI or PI during the study period. Eligible persons were also required to have available data on urban or nonurban residence according to Standard Metropolitan Statistical Area (SMSA) and TennCare enrollment category (disability, aid to families of dependent children, uninsurable, qualified Medicare beneficiary or aged). The TennCare pharmacy file contains extensive data on outpatient prescriptions, including drug dose and days’ supply dispensed.
Enrollment and Study Definitions
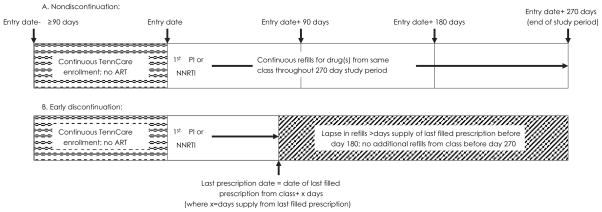
A schema of the overall study cohort is shown in Figure 1. Persons eligible for the cohort had to be enrolled in TennCare for ≥90 days prior to the entry date, have no other ART prescriptions during this 90-day period, have ≥1 additional medication refill and/or 1 HIV-related medical encounter claim during the 90-day period after the entry date, and have maintained continuous TennCare enrollment or died during the 270-day study period after entry. Other study definitions are listed below and also shown in Figure 1.
Figure 1. Schematic representation of study definitions for qualifying drug initiation and discontinuation using the TennCare pharmacy data file.
To qualify for the overall study cohort, an individual was required to have been non-Hispanic white or black and have ≥1 additional prescription medication refill (of any type) and/or 1 HIV-related medical encounter during the first 90 days after entry. Entry date could have been from January 1, 1996 to June 30, 2003. Schema A represents a nondiscontinuer; Schema B represents an individual defined as an early discontinuer, including the subgroup included in the validation analysis.
ART: Antiretroviral therapy; PI: Protease inhibitor; NNRTI: Non-nucleoside reverse transcriptase inhibitor
Entry date
Date of the first NNRTI or PI prescription was considered the date of cohort entry.
Qualifying drug
The study-qualifying drug was the NNRTI or PI prescribed at entry date. Persons prescribed a PI and NNRTI concomitantly, and those initiating the NNRTI delavirdine were excluded.
Early discontinuation
Early discontinuation was defined as a lapse in filled prescription days for the qualifying drug that exceeded the last dispensed prescription amount within 180 days after entry date, with no additional filled prescriptions for that drug during the remainder of the 270-day study period.
Validation Subgroup Identification
To validate the accuracy of the TennCare data files regarding drug exposure and discontinuation, a subgroup of persons from the cohort who received care at the Comprehensive Care Center (CCC) in Nashville, TN, were identified for detailed medical record review (Figure 2). The CCC is the largest HIV care provider in the middle Tennessee region and 1 of 8 designated AIDS Centers of Excellence in Tennessee. Persons included in this group had to have ≥1 TennCare medical claim from a CCC provider during their 270-day study period. Records for the subgroup of persons identified as early discontinuers underwent a standardized review by one of the authors (SA) to ascertain correct PI and NNRTI exposure and discontinuations, define the reason(s) for discontinuation, and to characterize any disagreement between the medical records and TennCare data files. Any uncertainty regarding these categories was resolved by consensus decision of a group of coauthors (TH, SPR, TRS). The State of Tennessee and Vanderbilt institutional review boards, and the Bureau of TennCare approved these studies.
Figure 2. Flow diagram of disposition of individuals included in the overall TennCare cohort and validation analyses.
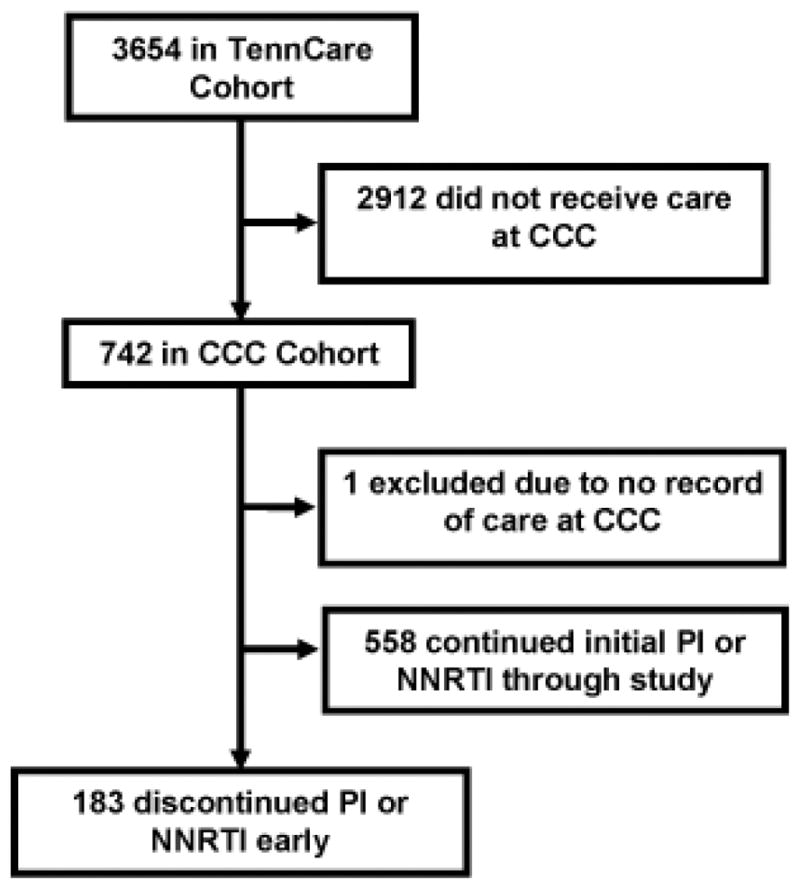
CCC: Comprehensive care center; PI: Protease inhibitor; NNRTI: Non-nucleoside reverse transcriptase inhibitor
Statistical Analyses
Demographic data and treatment and discontinuation categories are presented as proportions. Univariate comparisons were made using Fisher’s exact, chi-squared or Wilcoxon rank-sum tests. In order to better examine early PI and NNRTI discontinuation among persons most likely to have good adherence to treatment and follow-up, a predetermined subgroup analysis included only persons meeting eligibility criteria who also had ≥1 outpatient medical claim that included an HIV-related diagnosis within 90 days of study entry. Multivariable logistic regression models were used to adjust for race, sex, age, TennCare enrollment category (disabled versus other), early medical encounters, qualifying drug (PI versus NNRTI) and residence (urban versus nonurban). Because reasons for discontinuing different ART classes may differ, separate models limited to persons initiating a PI or NNRTI were used as well. Analyses were performed using Stata SE (Stata Corp., College Station, TX).
RESULTS
Overall TennCare Cohort Results
There were 3,654 HIV-infected TennCare enrollees who met eligibility criteria (Table 1). The median age of enrollees at entry date was 36 years, 1,070 (29%) were female and 1,948 (53%) were black. Most persons (75%) were prescribed a PI as part of their initial regimen. Cohort characteristics and differences in sociodemographic factors according to race and sex are also shown in Table 1.
Table 1.
Characteristics of TennCare Study cohort, total and by race and sex
| Total Cohort | Black, Non-Hispanic | White, Non-Hispanic | P valueb | Female | Male | P valueb | |
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| N=3,654 | N=1,948 | N=1,706 | N=1,070 | N=2,584 | |||
| Age at Entry Date in Years, Median (Range) | 36 (18–98) | 36 (18–87) | 36 (18–98) | 0.01 | 33 (18–87) | 37 (18–98) | <0.001 |
| Female Sex | 1,070 (29) | 776 (40) | 294 (17) | <0.001 | – | – | |
| Race | |||||||
| Non-Hispanic, black | 1,948 (53) | – | – | 776 (73) | 1,172 (45) | <0.001 | |
| Non-Hispanic, white | 1,706 (46) | – | – | 294 (27) | 1,412 (55) | ||
| Urban Residencea | 3,178 (87) | 1835 (94) | 1343 (79) | <0.001 | 944 (88) | 2,234 (87) | 0.16 |
| Enrollment Category | <0.001 | <0.001 | |||||
| Disabled | 1,976 (54) | 1,007 (52) | 969 (57) | 416 (39) | 1,560 (60) | ||
| Uninsured | 1,280 (35) | 637 (33) | 643 (38) | 333 (31) | 947 (37) | ||
| Aid to families of dependent children | 355 (10) | 284 (15) | 71 (4) | 317 (30) | 38 (1) | ||
| Qualified Medicare beneficiary | 37 (1) | 14 (<1) | 23 (1) | 2 (<1) | 35 (1) | ||
| Aged | 6 (<1) | 6 (<1) | – | 2 (<1) | 4 (<1) | ||
| Cohort qualifying Medication | |||||||
| Protease inhibitor | 2,757 (75) | 1,427 (73) | 1,330 (78) | 0.001 | 753 (70) | 2,004 (78) | <0.001 |
| Nelfinavir | 1,293 (47) | 800 (56) | 493 (37) | 430 (57) | 863 (43) | ||
| Indinavir | 1,105 (40) | 482 (34) | 623 (47) | 245 (33) | 860 (43) | ||
| Saquinavir | 155 (6) | 53 (4) | 102 (8) | 32 (4) | 123 (6) | ||
| Lopinavir/ritonavir | 142 (5) | 69 (5) | 73 (5) | 36 (5) | 106 (5) | ||
| Ritonavir | 39 (<1) | 12 (<1) | 27 (2) | 7 (<1) | 32 (2) | ||
| Amprenavir | 23 (<1) | 11 (<1) | 12 (<1) | 3 (<1) | 20 (1) | ||
| Non-nucleoside reverse transcriptase inhibitor | 897 (25) | 521 (27) | 376 (22) | 317 (30) | 580 (22) | ||
| Efavirenz | 407 (45) | 245 (47) | 162 (43) | 126 (40) | 281 (48) | ||
| Nevirapine | 490 (55) | 276 (53) | 214 (57) | 191 (60) | 299 (52) | ||
| Early HIV-Related Medical Encounters | 2,896 (79) | 1,469 (75) | 1,427 (84) | <0.001 | 825 (77) | 2,071 (80) | 0.04 |
| Early Drug Discontinuations | 1,153 (32) | 713 (37) | 440 (26) | <0.001 | 425 (40) | 728 (28) | <0.001 |
Data shown are n (%) except where noted otherwise.
Urban residence defined as primary residence in a county classified as a standard metropolitan statistical area;
P values for race and sex comparisons obtained from chi-squared, Fisher’s exact, and Wilcoxon rank-sum tests, as appropriate
Thirty-two percent of the cohort met criteria for early discontinuation. There was no overall difference in early discontinuations by drug class (33% of NNRTI group versus 31% of PI group; P=0.22), but blacks were more likely than whites to discontinue NNRTIs (37 vs. 28%; P=0.003) and PIs (36 vs. 25%; P<0.001), and females were more likely than males to discontinue NNRTIs (38% vs. 31%; P=0.03) and PIs (41 vs. 27%; P<0.001). These differences persisted when the analysis was limited to persons with an HIV-related outpatient medical claim (n=2,896; 79% of total; data not shown).
Results of a multivariable logistic regression model, including sociodemographic variables, presence of an early medical encounter (yes versus no) and qualifying drug class (PI versus NNRTI), are shown in Table 2. In the entire cohort, both black race (OR=1.54; 95% CI: 1.32–1.79; P<0.001) and female sex (1.45; 1.23–1.70; P<0.001) were independent predictors of early discontinuation. In addition, younger age (0.98; 0.97–0.99) per year increase; P<0.001) and having enrolled in TennCare due to disability (1.26; 1.08–1.46; P=0.003) were associated with early discontinuation in this model, but qualifying drug class was not (0.94; 0.80–1.11; P=0.45). In a separate model that was limited to persons initiating PI-based ART (n=2,757), black race (1.55; 1.30–1.85; P<0.001), female sex (1.54; 1.28–1.86; P<0.001) and younger age (0.98; 0.97–0.99; P<0.001) remained predictors of early discontinuation, but TennCare enrollment category (1.14; 0.96–1.35; P=0.14) was no longer statistically associated with early discontinuation. Among persons initiating NNRTI-based ART (n=897), black race (1.46; 1.08–1.99; P=0.015), younger age (0.97; 0.95–0.98; P<0.001) and disability as a TennCare enrollment category (1.73; 1.28–2.34; P<0.001) were associated with early discontinuation, but sex was not (1.20; 0.87–1.64; P=0.26).
Table 2.
Multivariable logistic regression model results for the total study cohort and by qualifying drug
| Covariate | Total Cohort Model (n=3,654) | PI Model (n=2,757) | NNRTI Model (n=897) |
|---|---|---|---|
|
| |||
| OR (95% CI); P value | OR (95% CI); P value | OR (95% CI); P value | |
| Race (Black vs. White) | 1.54 (1.32–1.79); <0.001 | 1.55 (1.30–1.85); <0.001 | 1.46 (1.08–1.99); 0.015 |
| Sex (Female vs. Male) | 1.45 (1.23–1.70); <0.001 | 1.54 (1.28–1.86); <0.001 | 1.20 (0.87–1.64); 0.26 |
| Age (Per Year Increase) | 0.98 (0.97–0.99); <0.001 | 0.98 (0.97–0.99); <0.001 | 0.97 (0.95–0.98); <0.001 |
| TennCare Enrollment Criteria (Disabled vs. Othera) | 1.26 (1.08–1.46); 0.003 | 1.14 (0.96–1.35); 0.14 | 1.73 (1.28–2.34); <0.001 |
| Early Medical Encounterb (Yes vs. No) | 1.05 (0.88–1.25); 0.59 | 0.97 (0.79–1.19); 0.78 | 1.32 (0.94–1.86); 0.11 |
| Qualifying Drug (PI vs. NNRTI) | 0.94 (0.80–1.11); 0.45 | – | – |
| Urban Residencec (Yes vs. No) | 0.97 (0.78–1.22); 0.82 | 0.95 (0.73–1.23); 0.68 | 1.06 (0.70–1.60); 0.80 |
OR: Odds ratio; CI: Confidence interval; PI: Protease inhibitor; NNRTI: Non-nucleoside reverse transcriptase inhibitor;
Other includes uninsured status, aid to families of dependent children, qualified Medicare beneficiary and aged;
Defined as an outpatient visit with an HIV-related billing code within 90 days of qualifying drug prescription.
Validation Subgroup Analysis
Of the 741 persons in the subgroup who received care at the CCC during the study period, 183 (25%) met the definition for discontinuation using the TennCare data files and went on to have medical record review for validation (Table 3). One-hundred-forty-seven (80%) of these persons entered the study with a PI prescription, and the remaining 36 (20%) initiated an NNRTI. The qualifying drug identified in TennCare data files agreed with the medical record 96% of the time. One-hundred-forty-four persons [79% (95% CI: 72–84%)] were found to have discontinued their PI or NNRTI at a time that agreed with the TennCare data files. Of these, 118 [82% (95% CI: 75–88%); 64% (57–71%) of total] also had entry date agreement. Of note, when only cases that were able to be validated with the medical record are included, the rate of complete agreement between TennCare and the medical record was >80%. Reasons for ART discontinuations, and—where applicable—reasons for disagreement between data sources, are shown in Table 4.
Table 3.
Characteristics of the CCC cohort and validation subgroup
| Characteristic | CCC Cohort
|
||
|---|---|---|---|
| Total | Nondiscontinuers | Discontinuers (validation Group) | |
|
| |||
| N=741 | N=558 | N=183 | |
| Age at Entry in Years, Median (Range) | 36 (18–72) | 36 (19–72) | 36 (18–56) |
| Female Sex | 168 (23)* | 109 (20) | 59 (32)# |
| Race | |||
| Black, non-Hispanic | 277 (37)* | 196 (35) | 81 (44)# |
| White, non-Hispanic | 464 (63) | 362 (65) | 102 (56) |
| Urban Residencea | 662 (89) | 496 (89) | 166 (91) |
| Enrollment Category | |||
| Disabled | 384 (52) | 293 (53) | 91 (50) |
| Uninsured | 305 (41) | 232 (42) | 73 (40) |
| Aid to families of dependent children | 52 (7) | 33 (6) | 19 (10) |
| Aged | – | – | – |
| Cohort qualifying Medication | |||
| Protease Inhibitor | 592 (80)* | 445 (80) | 147 (80) |
| Nelfinavir | 315 (53)** | 214 (48) | 101 (69)## |
| Indinavir | 217 (37) | 186 (42) | 31 (21) |
| Saquinavir | 11 (2) | 9 (2) | 2 (1) |
| Lopinavir/rito navir | 33 (6) | 23 (5) | 10 (7) |
| Ritonavir | 8 (1) | 7 (2) | 1 (1) |
| Amprenavir | 8 (1) | 6 (1) | 2 (1) |
| Non-nucleoside reverse transcriptase inhibitor | 149 (20)* | 113 (20) | 36 (20) |
| Efavirenz | 58 (39) | 51 (45) | 7 (19)## |
| Nevirapine | 91 (61) | 62 (55) | 29 (81) |
Data shown are n (%) except where noted otherwise.
Urban residence defined as primary residence in a county classified as a standard metropolitan statistical area;
p<0.01 for comparison of TennCare cohort versu.total CCC cohort (Fisher’s exact test);
p<0.01 for comparison of nelfinavir versus all other protease inhibitors among the protease inhibitor qualifying drug group;
p<0.01 for comparison of CCC discontinuers versus nondiscontinuers;
p<0.01 for comparisons of nelfinavir versus all other protease inhibitors among protease inhibitor qualifying drug group, and nevirapine versus efavirenz among non-nucleoside reverse transcriptase inhibitor qualifying drug group.
Table 4.
Results of limited validation medical record review (total n=183)
| Validation Category | n (%)a |
|---|---|
| Reasons for Disagreement of qualifying Drug Discontinuation | |
| Unable to Validate | 13 (7) |
| No qualifying drug documented | 11 (6) |
| Medical record not available for review | 1 (<1) |
| Person did not receive care at the CCC | 1 (<1) |
| Lost to follow-up | 11 (6) |
| Continued use of drug after last TennCare prescription date | 7 (4) |
| Subtotal (n, % of total discontinuation group) | 31 (17) |
| Reasons for Exclusion | |
| Concomitant use of PI/NNRTI | 7 (4) |
| Race incorrectly recorded | 1 (<1) |
| Subtotal | 8 (4) |
| Reasons for Disagreement in qualifying Drug Initiation | |
| Initial ART not documented in medical record | 12 (7) |
| AIDS drug assistance program | 5 (3) |
| Living in another state | 3 (2) |
| Clinical trial | 3 (2) |
| Incarcerated | 2 (1) |
| Private Insurance plan | 1 (<1) |
| Subtotal | 26 (14) |
| Reasons for Discontinuation among the Group with Agreement in Both qualifying Drug Initiation and Discontinuation, N (% of Group) | |
| Undocumented | 40 (34) |
| Toxicity | 34 (29) |
| Gastrointestinal intolerance | 15 (44) |
| Unspecified | 8 (24) |
| Rash | 4 (12) |
| Central nervous system/psychiatric toxicity | 4 (12) |
| Hepatitis | 3 (9) |
| Nonadherence | 29 (25) |
| Deathb | 11 (9) |
| Virologic failurec | 4 (3) |
| Subotal | 118 (65) |
Data are presented as mutually exclusive categories for the purposes of the table. Individuals with disagreement in both qualifying drug initiation/entry date and discontinuation are included in the latter category;
No deaths were believed to be due to ART or a direct result of ART discontinuation;
Virologic failure defined as an increase in plasma HIV-RNA to or above the pretreatment level on ≥1 measure prior to documented discontinuation; CCC: Comprehensive Care Center; ART: Antiretroviral therapy; PI: Protease inhibitor; NNRTI: Non-nucleoside reverse transcriptase inhibitor
DISCUSSION
In this study, TennCare data files were used to identify a large, demographically diverse cohort of HIV-infected individuals who initiated ART containing a PI or NNRTI. Approximately one-third of this cohort discontinued therapy within 180 days of initiation. Blacks were more likely than whites to discontinue drugs from either class. Females were more likely than males to discontinue PIs early, but not NNRTIs.
Our findings are consistent with previous studies reporting racial differences in efavirenz adherence and efficacy. A study from the Johns Hopkins HIV Cohort found that African Americans were more likely to discontinue efavirenz at 1 year than non-Hispanic whites.24 Similarly, a study of more than 400 U.S. military health care beneficiaries found significantly shorter time to failure among African Americans receiving efavirenz compared to Caucasians.25 In contrast, data from a multinational trial found no significant differences in time to treatment failure between blacks and Caucasians on an efavirenz-based regimen.26 A randomized, controlled trial of dual- or triple-NRTI therapy in combination with efavirenz in treatment-naïve subjects, AIDS Clinical Trials Group Study A5095, did not find a difference in outcomes between efavirenz-based treatment arms but did identify an increased risk of virologic failure and grade-3 or -4 adverse events among blacks compared to whites.27 Additional analyses of this clinical trial population have found a greater effect of nonadherence on risk of virologic failure in blacks on efavirenz-based regimens compared to whites.28 These results merit further study.
The relationship between sex and early discontinuation was studied in drug-class-specific multivariable models and was only seen with PI use, a finding consistent with prior reports of increased adverse drug events and toxicities in females.11,12 This phenomenon may be due to sex differences in plasma concentration of some PIs.29–31 A single small study reported higher nevirapine plasma concentrations in HIV-infected women versus men,32 but a correlation between plasma nevirapine levels and hepatotoxicity has not been established.33,34 Together with the results of our study, this finding would suggest that drug-class-specific factors that are not directly related to adherence, toxicity or access to care can influence early discontinuations. Interestingly, females in our study were not significantly more likely than males to discontinue NNRTIs when controlling for race and other sociodemographic factors. The sex difference seen in the PI model could be due in part to pharmacodynamic factors (body weight and composition, effects of sex hormones, etc.), the effects of which on more subtle clinical toxicities such as gastrointestinal adverse effects have not yet been well characterized.
The findings that disability enrollment status and younger age predicted a higher likelihood of early discontinuation are intriguing. Disability status may reflect more advanced HIV disease, less capacity to tolerate mild-to-moderate adverse effects (e.g., nausea, diarrhea) or an increased risk for more severe adverse effects during the early stages of ART. One might predict that older age would be associated with increased risk of drug toxicity and discontinuation due to decreases in drug metabolism with aging. We found the opposite association; one that was also reported in another cohort study of treatment discontinuation.35 This could reflect other unknown and/or unmeasured sociodemographic or clinical factors that contributed to early drug discontinuation. We cannot exclude the possibility that older age was associated with better adherence to drugs despite subtle early side effects that may have caused younger patients to discontinue their therapy.
Although pharmacy databases are considered relatively accurate36,37 and compare favorably to self-report,38 they cannot fully account for medication adherence. We were unable to include additional measures of adherence. We were also unable to control for other factors known to influence ART discontinuation (such as substance abuse/injection drug use and hepatitis C coinfection),39 or measures of HIV disease status and treatment efficacy (such as immunologic status, AIDS-defining illnesses or HIV suppression), nor did we characterize the role of specific ART combinations beyond the PI/ NNRTI used. Our data cannot yield information on why individual persons discontinued their therapy. This study was restricted to the highly active ART era (post-1996), but temporal changes in therapy have also impacted drug tolerability. Due to programmatic changes in TennCare that affected enrollment status of many HIV-infected persons in July 2003, we ended our study period at that time. As a result, the majority of PI use in this study was early-generation therapy (e.g., nelfinavir and indinavir) that did not include pharmacologic boosting with low-dose ritonavir. These regimens tended to have greater pill burdens than newer PIs (e.g., atazanavir, lopinavir/ ritonavir), which may have influenced early drug discontinuations to an extent that our data could not determine. Nonetheless, the fundamental relationships between sociodemographic factors and early discontinuation would not be expected to vary substantially between early- or later-generation therapies. Use of NNRTIs in this population was less frequent than PIs throughout the study period, limiting our ability to compare discontinuations in smaller subpopulations (e.g., black versus white females initiating efavirenz) and decreasing the sample size in drug-class-specific multivariable models.
The TennCare administrative data files have been used to answer important pharmacoepidemiologic questions for several years,40–42 but until recently no studies assessing ART utilization had been published.43 Our results are strengthened by the fact that TennCare enrollees made up a substantial proportion of HIV-infected persons in Tennessee during the study period, with >45% of HIV-infected persons in Tennessee enrolled in TennCare programs for ≥1 day.44,45 We performed a limited validation of drug exposure and discontinuation through a detailed medical record review of a subgroup of the TennCare cohort. Where records were complete, the accuracy of the TennCare data files was good.
Acknowledging the limitations of this study, we conclude that black TennCare enrollees were more likely than whites to discontinue their first NNRTI or PI within 180 days of starting therapy, and females were more likely than males to discontinue their first PI—after adjustment for other potentially important sociodemographic factors. Although our results cannot be used to determine the specific reasons for these differences, careful analysis of TennCare and other administrative databases could potentially be used to monitor drug discontinuation at the population level and could enhance our understanding of disparities in responses to treatment of HIV infection.
Acknowledgments
Financial support: Drs. Hulgan and Sterling received support from National Institutes of Health grants #K23 AT002508 and K24 AI065298, respectively. This study was supported in part by a grant (to Dr. Ray) from the Agency for Healthcare Research and quality, Centers for Education and Research on Therapeutics cooperative agreement (grant #HS1-0384).
The authors gratefully acknowledge the Vanderbilt Meharry Center for AIDS Research (NIH program P30 AI 54999) for providing infrastructure and academic support for collaborative efforts; Bryan Shepherd, PhD, and David Haas, MD, for helpful discussions; the Tennessee Bureau of TennCare and Department of Health, which provided study data; and the HIV-infected Tennesseans and their healthcare providers who contributed to this data.
References
- 1.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed 03/17/08];Guidelines for the Use of Antiretroviral Agents in HIV-1 Infected Adults and Adolescents. 2008 Jan 29; http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 4.Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296:827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 5.Bell C, Matthews GV, Nelson MR. Non-nucleoside reverse transcriptase inhibitors--an overview. Int J STD AIDS. 2003;14:71–77. doi: 10.1258/095646203321156827. [DOI] [PubMed] [Google Scholar]
- 6.Clifford DB, Evans S, Yang Y, et al. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005;143:714–721. doi: 10.7326/0003-4819-143-10-200511150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Dieleman JP, Jambroes M, Gyssens IC, et al. Determinants of recurrent toxicity-driven switches of highly active antiretroviral therapy. The ATHENA cohort. AIDS. 2002;16:737–745. doi: 10.1097/00002030-200203290-00009. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien ME, Clark RA, Besch CL, et al. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr. 2003;34:407–414. doi: 10.1097/00126334-200312010-00008. [DOI] [PubMed] [Google Scholar]
- 9.d’Arminio Monforte A, Lepri AC, Rezza G, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer E, Consiglio E, Podzamczer D, et al. Analysis of the discontinuation of protease inhibitor therapy in routine clinical practice. Scand J Infect Dis. 1999;31:495–499. doi: 10.1080/00365549950164030. [DOI] [PubMed] [Google Scholar]
- 11.Bonfanti P, Ricci E, Landonio S, et al. Predictors of protease inhibitor-associated adverse events. Biomed Pharmacother. 2001;55:321–323. doi: 10.1016/s0753-3322(01)00070-1. [DOI] [PubMed] [Google Scholar]
- 12.Bonfanti P, Valsecchi L, Parazzini F, et al. Incidence of adverse reactions in HIV patients treated with protease inhibitors: a cohort study. Coordinamento Italiano Studio Allergia e Infezione da HIV (CISAI) Group. J Acquir Immune Defic Syndr. 2000;23:236–245. doi: 10.1097/00126334-200003010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Murri R, Lepri AC, Phillips AN, et al. Access to antiretroviral treatment, incidence of sustained therapy interruptions, and risk of clinical events according to sex: evidence from the I.Co.N.A. Study. J Acquir Immune Defic Syndr. 2003;34:184–190. doi: 10.1097/00126334-200310010-00008. [DOI] [PubMed] [Google Scholar]
- 14.Spire B, Carrieri P, Garzot MA, et al. Factors associated with efavirenz discontinuation in a large community-based sample of patients. AIDS Care. 2004;16:558–564. doi: 10.1080/09540120410001716342. [DOI] [PubMed] [Google Scholar]
- 15.Quirk E, McLeod H, Powderly W. The pharmacogenetics of antiretroviral therapy: a review of studies to date. Clin Infect Dis. 2004;39:98–106. doi: 10.1086/421557. [DOI] [PubMed] [Google Scholar]
- 16.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–2400. [PubMed] [Google Scholar]
- 17.Rodriguez-Novoa S, Barreiro P, Rendon A, et al. Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clin Infect Dis. 2005;40:1358–1361. doi: 10.1086/429327. [DOI] [PubMed] [Google Scholar]
- 18.Rotger M, Colombo S, Furrer H, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Tsuchiya K, Gatanaga H, Tachikawa N, et al. Homozygous CYP2B6 *6 (q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004;319:1322–1326. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 20.Marzolini C, Telenti A, Decosterd LA, et al. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 21.Erickson DA, Mather G, Trager WF, et al. Characterization of the In Vitro Biotransformation of the HIV-1 Reverse Transcriptase Inhibitor Nevirapine by Human Hepatic Cytochromes P-450. Drug Metab Dispos. 1999;27:1488–1495. [PubMed] [Google Scholar]
- 22.Haas DW, Bartlett JA, Andersen JW, et al. Pharmacogenetics of nevirapine-associated hepatotoxicity: an Adult AIDS Clinical Trials Group collaboration. Clin Infect Dis. 2006;43:783–786. doi: 10.1086/507097. [DOI] [PubMed] [Google Scholar]
- 23.Ritchie MD, Haas DW, Motsinger AA, et al. Drug transporter and metabolizing enzyme gene variants and nonnucleoside reverse-transcriptase inhibitor hepatotoxicity. Clin Infect Dis. 2006;43:779–782. doi: 10.1086/507101. [DOI] [PubMed] [Google Scholar]
- 24.Moore RD, Keruly J, Gebo KA, et al. Racial Differences in Efavirenz Discontinuation in Clinical Practice. Paper presented at: 12th Conference on Retroviruses and Opportunistic Infections; February 22–25, 2005; Boston, MA. [Google Scholar]
- 25.Wegner S, Vahey M, Dolan M, et al. Racial differences in clinical efficacy of efavirenz-based antiretroviral therapy. Paper presented at: 9th Conference on Retroviruses and Opportunistic Infections; February 24–28, 2002; Seattle, WA. [Google Scholar]
- 26.Lupo L, Maa J-F, Dezii C, et al. Efficacy of efavirenz in different racial groups. Paper presented at: 6th International Congress on Drug Therapy in HIV Infection; 2002; Glasgow, Scotland. [Google Scholar]
- 27.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Three- vs Four-Drug Antiretroviral Regimens for the Initial Treatment of HIV-1 Infection: A Randomized Controlled Trial. JAMA. 2006;296:769–781. doi: 10.1001/jama.296.7.769. [DOI] [PubMed] [Google Scholar]
- 28.Schackman BR, Ribaudo HJ, Krambrink A, et al. Racial differences in virologic failure associated with adherence and quality of life on efavirenz-containing regimens for initial HIV therapy: results of ACTG A5095. J Acquir Immune Defic Syndr. 2007;46:547–554. doi: 10.1097/qai.0b013e31815ac499. [DOI] [PubMed] [Google Scholar]
- 29.Burger DM, Siebers MC, Hugen PW, et al. Pharmacokinetic variability caused by gender: do women have higher indinavir exposure than men? J Acquir Immune Defic Syndr. 2002;29:101–102. doi: 10.1097/00126334-200201010-00014. [DOI] [PubMed] [Google Scholar]
- 30.Pai MP, Schriever CA, Diaz-Linares M, et al. Sex-related differences in the pharmacokinetics of once-daily saquinavir soft-gelatin capsules boosted with low-dose ritonavir in patients infected with human immunodeficiency virus type 1. Pharmacotherapy. 2004;24:592–599. doi: 10.1592/phco.24.6.592.34744. [DOI] [PubMed] [Google Scholar]
- 31.Ribera E, Lopez RM, Diaz M, et al. Steady-state pharmacokinetics of a double-boosting regimen of saquinavir soft gel plus lopinavir plus minidose ritonavir in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2004;48:4256–4262. doi: 10.1128/AAC.48.11.4256-4262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regazzi M, Villani P, Seminari E, et al. Sex differences in nevirapine disposition in HIV-infected patients. AIDS. 2003;17:2399–2400. doi: 10.1097/00002030-200311070-00018. [DOI] [PubMed] [Google Scholar]
- 33.Dailly E, Billaud E, Reliquet V, et al. No relationship between high nevirapine plasma concentration and hepatotoxicity in HIV-1-infected patients naive of antiretroviral treatment or switched from protease inhibitors. Eur J Clin Pharmacol. 2004;60:343–348. doi: 10.1007/s00228-004-0769-5. [DOI] [PubMed] [Google Scholar]
- 34.De Maat MM, Mathot RA, Veldkamp AI, et al. Hepatotoxicity following nevirapine containing regimens in HIV-1-infected individuals. Pharmacol Res. 2002;46:295–300. doi: 10.1016/s1043-6618(02)00146-9. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Margolick JB, Conover CS, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005;38:320–328. [PubMed] [Google Scholar]
- 36.Strom BL, Carson JL. Use of automated databases for pharmacoepidemiology research. Epidemiol Rev. 1990;12:87–107. doi: 10.1093/oxfordjournals.epirev.a036064. [DOI] [PubMed] [Google Scholar]
- 37.Federspiel CF, Ray WA, Schaffner W. Medicaid records as a valid data source: the Tennessee experience. Med Care. 1976;14:166–172. doi: 10.1097/00005650-197602000-00006. [DOI] [PubMed] [Google Scholar]
- 38.West SL, Savitz DA, Koch G, et al. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142:1103–1112. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 39.Bongiovanni M, Cicconi P, Landonio S, et al. Predictive factors of lopinavir/ritonavir discontinuation for drug-related toxicity: results from a cohort of 416 multi-experienced HIV-infected individuals. Int J Antimicrob Agents. 2005;26:88–91. doi: 10.1016/j.ijantimicag.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Griffin MR, Ray WA, Schaffner W. Nonsteroidal anti-inflammatory drug use and death from peptic ulcer in elderly persons. Ann Intern Med. 1988;109:359–363. doi: 10.7326/0003-4819-109-5-359. [DOI] [PubMed] [Google Scholar]
- 41.Ray WA, Murray KT, Meredith S, et al. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351:1089–1096. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 42.Ray WA, Stein CM, Daugherty JR, et al. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;360:1071–1073. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- 43.Hulgan T, Sterling TR, Daugherty J, et al. Prescribing of contraindicated protease inhibitor and statin combinations among HIV-infected persons. J Acquir Immune Defic Syndr. 2005;38:277–282. [PubMed] [Google Scholar]
- 44.Bailey J, Jenkins PH, Arheart KL, et al. Infectious Diseases Society of America. 2003. The Impact of the Ryan White CARE Act on quality and Outcomes of HIV/AIDS Care in Tennesee’s TennCare System 1998–2000. [Google Scholar]
- 45.Bailey JE, Van Brunt DL, Raffanti SP, et al. Improvements in access to care for HIV and AIDS in a statewide Medicaid managed care system. Am J Manag Care. 2003;9:595–602. [PubMed] [Google Scholar]