Abstract
Leptin, an adipose-secreted hormone, links metabolism and immunity. Our aim was to determine whether leptin affects the alloimmune response. We used an allogeneic skin transplant model as a means to analyze the allograft immune response in Lepob/ob and wild-type mice. Leptin deficiency results in an increased frequency of Treg and Th2 cells and a prolonged graft survival. These effects of leptin deficiency indicate the importance of leptin and obesity in modulating the allograft immune responses. Our data suggest a possible explanation for the increased susceptibility of hyperleptinemic obese patients to acute and chronic graft rejection.
Keywords: Alloimmunity, leptin, regulatory T cell, tolerance
Introduction
Obesity is a common and menacing public health problem that influences the outcome of several immune-mediated diseases (1,2). Out of the genes that have been linked to obesity, leptin has been found to play a central role. Leptin, a 16-kDa adipose tissue-derived hormone, identified for its capacity to control appetite, also influences immune function (3). Leptin may act as an endogenous-sensing factor that provides a critical link between the environment, metabolism and immune function (3). Leptin-deficient (Lepob/ob) and leptin receptor-deficient mice (Lepdb/db) manifest reduced thymic size and cellularity, and impaired cellular and humoral immune responses. As a consequence, Lepob/ob mice are protected from several immune-mediated diseases, such as experimental colitis, diabetes, experimental autoimmune encephalomyelitis (EAE) and ConA-induced hepatitis (4–8). In addition, leptin also possesses proinflammatory activities that potentially enhance T helper 1 (Th1) immune responses and constrain the proliferation of regulatory T cells (Tregs). Moreover, Lepdb/db mice have been reported to display increased Treg numbers and enhanced Treg suppressive potential (9). De Rosa and coworkers observed that the blockade of the leptin receptor during TCR activation reversed Treg hypore-sponsiveness to TCR- induced proliferation (10). Although leptin has been implicated in the alteration of a variety of immune responses, the mechanisms by which these sometimes inconsistent effects are mediated remain uncertain. Hence, we have now tested the hypothesis that the effect of leptin upon the allograft response, which is a T cell dependent process, may be partially mediated by the modulation of the CD4 T cell function.
Few studies have addressed the influence of adipose tissue-derived products, such as leptin, on the allograft response. Graft survival is significantly decreased in obese patients, in particular due to leptin resistance and hyperleptinemia (11). Therefore, we hypothesized that leptin deficiency may shift the immune response toward a more regulatory type of response, which directly affects the graft outcome. We performed skin allograft transplantation in both Lepob/ob and wild-type mice and observed that leptin deficiency resulted in prolonged graft survival, increased Treg frequency and a shift toward a Th2 profile.
Material and Methods
Mice
Ten-week-old male wild-type (WT) C57BL/6J (B6), C57BL/6J Lepob/ob, C57BL/6J Lepdb/db, C57BL/6J Rag−/− and CBA mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The mice were age matched for individual experiments. The animals were maintained under specific pathogen-free conditions in groups of five in a room with a 12 h/12 h light/dark cycle at 22° C at the University of Sao Paulo and received water and food ad libitum. All experiments were conducted in adherence with the COBEA (Brazilian Committee for Experimental Animals) and were approved by the Institutional Ethics Committee on Animal Use of the University of São Paulo, São Paulo, Brazil.
Skin graft survival, CD4+ T cell purification and adoptive transfer
Wild-type, and Lepob/ob B6 and Rag−/− B6 mice were transplanted with skin grafts, in their back, from B6 or CBA mice, as previously described (12). The CD4+ T cells were purified from wild-type, Lepob/ob and Lepdb/db B6 mice using a Mouse T Cell CD4+ Subset Column Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Flow cytometry was used to determine that the purity of the CD4+ T cells populations was >95%, and the frequency of contaminating CD8+ T cells was <2%.
The Rag−/− skin-grafted mice were reconstituted with 1.106 purified CD4+ T cells. The survival of the skin grafts were observed daily and defined as rejected when complete loss of the intact epithelia occurred. At the time of rejection, splenocytes were harvested and analyzed by flow cytometry to confirm the CD4+ T cell reconstitution. For the competitive homeostatic proliferation experiments, CD4+ T cells were purified from WT, WTgfp, Lepob/ob and Lepdb/db B6 mice. The purity of the CD4+ T cells was >92%. Rag−/− grafted mice were reconstituted with a mixture of 1.106 purified CD4+ T cells from WTgfp mice and 1.106 CD4+ T cells from WT, Lepob/ob or Lepdb/db B6 mice, and the homeostatic proliferation was evaluated after 10 days by labeling the cells with anti-CD4 antibody and evaluating the frequency of CD4+GFP+ and CD4+GFP− cells through flow cytometry.
Histology
The histology of the skin grafts was performed by staining 5 µm paraffin-embedded sections with hematoxylin and eosin (HE).
Cell sorting and T cell differentiation
CD4+ T cells (isolated by negative selection using the Miltenyi Biotec T Cell Isolation kit) from donor (WT) B6 and B6Lepob/ob mice were stained with fluorescein-labeled antibodies specific for CD4, CD44, CD25 and CD62L and sorted at high-speed (BD FACSAria II) to obtain the naive T cell population (CD4+CD44−CD62L+CD25−). After sorting, the purity of the cells was >98%. The naive T cells were then plated at a density of 2.0.105 cells/flat-bottom well and stimulated with anti-CD3 (1 µg/mL) and anti-CD28 (1 µg/mL) antibodies (BioLegend) for antigen-free T cell polarization. The polarization conditions used are the following: TGF-β1 (5 ng/mL for Tregs and 1 ng/mL for Th17 cells), IL-6 (50 ng/mL) and recombinant leptin (500 ng/mL) (all obtained from R&D Systems, USA). For induction of Foxp3+ Tregs, the cultures were supplemented with TGF-β1, whereas the cultures were supplemented with TGF-β1 and IL-6 for Th17 cell induction.
Intracellular cytokine analysis and Foxp3 staining
Cells were obtained from the draining lymph nodes (dLNs) and analyzed by flow cytometry. To determine the frequency of putative Tregs, 1.106 cells were stained for the surface markers CD4 (Pacific Blue) and CD25 (FITC) and for the intracellular transcription factor Foxp3 using an APC-conjugated antimouse/rat Foxp3 antibody (eBioscience, USA). For the intracellular cytokine staining, 1.106 cells were stimulated in vitro for 4 h at 37° C in 5% CO2 with phorbol-12-myristate-13-acetate (PMA; 100 ng/mL), ionomycin (1 µg/mL) and brefeldin A (1 µg/mL) (all purchased from Sigma-Aldrich, St. Louis, MO, USA). The cells were then washed and stained with Pacific Blue-conjugated anti-CD4 (eBioscience, San Diego, CA, USA) and permeabilized using the BD Cytofix/Cytoperm Fixation/Permeabilization solution kit (BD Biosciences, San Jose, CA, USA). The intracellular staining was performed with APC-conjugated anti-TNF (Biolegend, San Diego, CA, USA), anti-IL-4, anti-IL-10 and anti-IL-17 antibodies and with FITC-conjugated IFN-γ antibody (all were purchased from BioLegend). Isotype antibodies were used as controls.
Flow cytometry
The cells were resuspended in PBS supplemented with 2% FCS and stained with saturating amounts of the following mAbs: Pacific Blue-conjugated anti-CD4, FITC-conjugated anti-CD8, and PE-conjugated anti-CD25 (BD Biosciences). The cells were then acquired with an LSR II flow cytometer (BD) and analyzed by FlowJo software.
RT–PCR and PCR-based array
The total RNA was extracted from the cells using an RNeasy Mini Kit (Qiagen, USA) according to the manufacturer’s instructions. All samples were digested with DNase prior to the assays. The cDNA was synthesized using an RT2 First Strand Kit (SABioscience, Qiagen, Venlo, the Netherlands). The GAPDH mRNA levels were measured (Applied Biosystems, Foster City, CA, USA) as an internal control. The relative expression levels of foxp3, rorc, tbx21, gata-3 are presented as the mean ± SEM of triplicate samples, and CT is the cycle threshold.
Mixed lymphocyte reaction and CD4+ T cell proliferation
The bone-marrow-derived dendritic cells (BMDC) obtained from CBA mice as previously described (13) were irradiated and used as the stimulators in the mixed lymphocyte reactions (MLRs). The cells in the draining lymph nodes (dLN) of WT or Lepob/ob B6 CBA skin grafted mice were used as the allogeneic responder cells. Seven days after WT or Lepob/ob B6 mice were transplanted with skin from CBA mice as described above, the dLN were obtained and the cells used as responder cells. The responder cells (WT or Lepob/ob B6, 3.105 cells/well) were pretreated with CFSE in PBS 1% BSA for 10 min at 37° C. These cells were then plated in 96-well plates and cultured with stimulator cells (BMDC) (CBA, 0.5105 cells/well) in triplicate. The cells were cultured at 37° C in 5% CO2 for 5 days in RPMI 1640 supplemented with 10% FBS, penicillin and streptomycin. The cell proliferation was quantified by flow cytometry (FACSCanto II, BD). The division cell index and the percentage of divided cells were calculated using the FlowJo software (Treestar, Ashland, OR, USA).
Serum and culture supernatant cytokine levels
The blood samples were obtained by intracardiac puncture of anesthetized mice immediately before euthanasia on day 7 posttransplant. The cytokine levels in the sera and in the culture supernatants were quantified using the Bio-Plex cytokine assay kit (Bio-Rad Laboratories, Hercules, CA, USA), as recommended by the manufacturer. The levels of IL-2, IFN-γ, IL-12 (p40), IL-17, MCP-1, IL-4 and TNF were determined. The beads were probed with the Bio-Plex suspension array system, and the data were analyzed using the Bio-Plex Manager software version 4.0.
Statistical analysis
The data were presented as the mean ± SEM. The differences among the groups were assessed using ANOVA (Tukey posttest), and the differences between the groups were evaluated with Student t-test. The log-rank test was used to analyze the skin graft survival. All the statistical analyses were performed using the GraphPad PRISM® 4 software, and the differences were considered significant when *p < 0.05; **p < 0.01; ***p < 0.001.
Results
Leptin-deficient mice display enhanced skin graft Survival
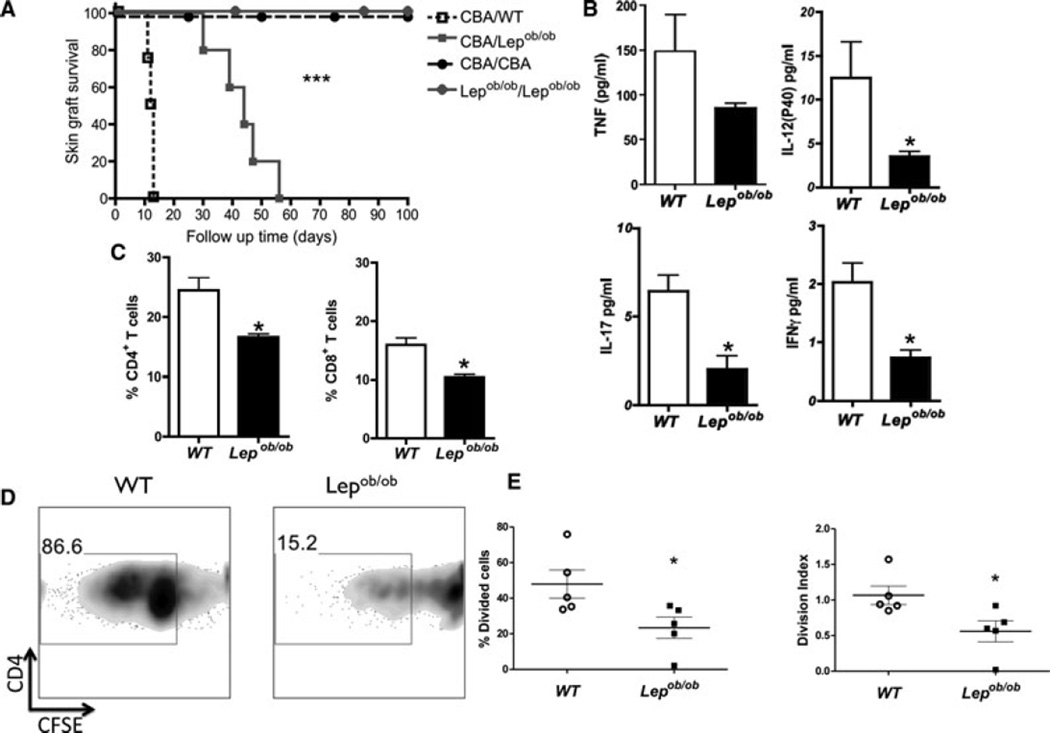
To evaluate the effect of leptin on the alloimmune response, we transplanted fully MHC-mismatched skin grafts onto Lepob/ob and WT mice. The graft survival was enhanced in Lepob/ob mice compared to WT mice (p < 0.05; Figure 1A). The histological analysis, which was performed within a new group of grafted animals, demonstrated diminished infiltration of mononuclear leukocytes into the grafted skin and lower graft fibrosis in Lepob/ob recipients than in the WT control (data not shown). The circulating levels of proinflammatory cytokines, such as TNF, IFN-γ, IL-12 and IL-17, were lower in Lepob/ob mice than in the control (p < 0.05), as shown in Figure 1B. Furthermore, the percentage of CD4+ and CD8+ T cells in the draining lymph nodes (dLNs) of Lepob/ob skin-transplanted mice was lower than that observed in WT skin-transplanted mice (p < 0.05; Figure 1C). This decreased frequency of T cells was not related to leptin deficiency itself because the age-matched nontransplanted Lepob/ob mice displayed normal frequencies of CD4+ and CD8+ T cells in the dLN (Supporting Figure S1). One possible cause for this decreased frequency of CD4+ and CD8+ T cells is a decreased alloreactivity in Lepob/ob mice. To determine whether the absence of leptin is indeed related to reduced alloreactivity, we performed an MLR by mixing irradiated CBA dendritic cells with cells from the Lepob/ob or WT draining lymph node and analyzed the proliferation on the gated CD4+ T cells. As shown in Figure 1D, the Lepob/ob CD4+ T cells exhibited reduced alloreactivity compared to the WT CD4+ T cells (p < 0.05). The percentage of divided cells and the division index demonstrated that fewer CD4+ T cells divided in Lepob/ob mice compared with their control littermates (p < 0.05; Figure 1E). These results indicate that leptin deficiency reduced allograft reactivity, thereby contributing to the increased allograft survival observed in leptin-deficient mice.
Figure 1. Wild-type but not Lepob/ob mice rapidly reject skin allografts.
(A) CBA skin was transplanted onto normal B6 or leptin-deficient (Lepob/ob) B6 mice. Signs of allograft rejection were monitored daily, and survival is reported for individual animals as well as the time of graft rejection. Isotransplants were used as transplantation controls. (B) Skin allograft survival was accompanied by decreased serum concentrations of proinflammatory cytokines. The sera were harvested, and the concentrations of cytokines (IFN-γ, IL-12p40, IL-17 and TNF-α) were evaluated on day 10 posttransplant by a Bio-Plex assay. (C) The percentage of CD4+ and CD8+ T cells in the draining lymph nodes of skin-grafted mice were evaluated by flow cytometry 7 days after transplantation. (D) In vitro mixed lymphocyte reactions (MLR) of CBA splenocytes with and wild-type B6 or Lepob/ob B6 splenocytes. The proliferation was evaluated by measuring the CFSE dilution by flow cytometry after 5 days of cell culture in CD4+ gated T cells. (E) The percentage of CD4+ T cells dividing cells and CD4+ T cells division index were calculated using Flow Jo software. The results are shown as the mean ± SEM. *SD. *p < 0.05, ***p < 0.001. n = 6 mice /group.
Increased graft survival reflects Treg and Th2 immune response deviation
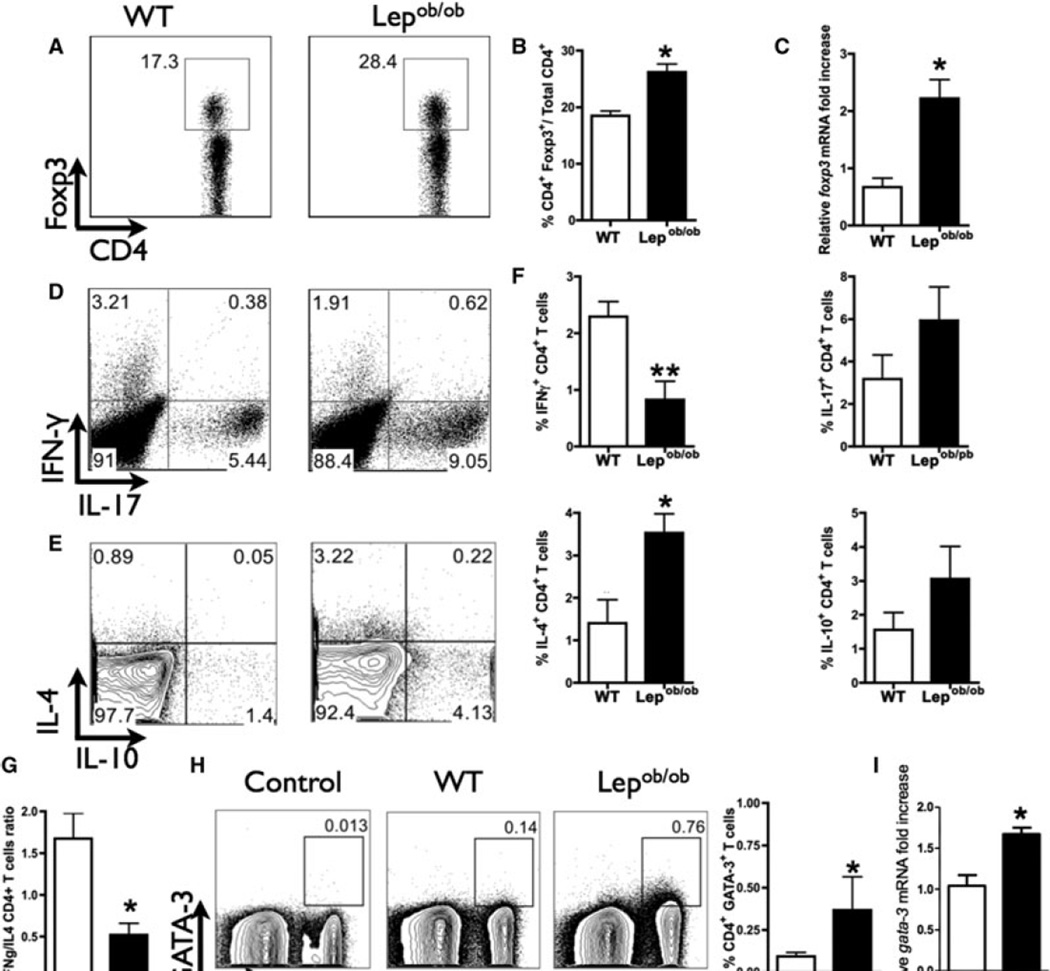
Regulatory T cells can also influence the initiation and maintenance of the alloimmune response (22). Although Tregs exert their allo-immunoregulatory activity in the lymph node, Tregs are activated at the site of inflammation prior to their migration to the lymph node (14). Therefore, based on the observation that Lepob/ob skin-grafted mice display an increased frequency of these cells in the dLN (Figure 2A, B), we evaluated the mRNA expression of Foxp3 in the transplanted skin to confirm that Treg cells are also present in the transplanted tissue. We observed that Lepob/ob skin-grafted mice expressed higher levels of Foxp3 mRNA levels in the transplanted skin than did WT grafted mice (Figure 2C). Based on the importance of Tregs in transplantation tolerance, it can be argued that this increased frequency of Tregs in the dLN could impair the development of proinflammatory CD4+ T cells. Therefore, we analyzed the frequency of CD4+ T cells expressing IFN-γ, IL-17, IL-4 and IL-10. Lepob/ob skin-transplanted mice displayed a decreased frequency of CD4+IFN-γ+ T cells (Figure 2D–F), and no difference was observed in the frequency of CD4+IL-10+ T cells. Interestingly, although Lepob/ob mice displayed enhanced graft survival, these animals also exhibited an increased frequency of CD4+IL-17+ T cells (Figure 2D and F). To address the participation of IL-17 on transplantation rejection, we performed fully mismatched skin transplantation in B6 IL-17KO and WT mice. We did not observe any difference in the graft survival between IL-17KO and WT mice, which indicates that IL-17 does not modulate the graft survival in our skin graft model (Supporting Figure S2).
Figure 2. Treg accumulation and Th1/Th2 balance is important for allograft survival.
The draining lymph nodes (dLNs) were harvested on day 7 posttransplantation, and the T cells were evaluated by flow cytometry. (A) Dot plot of CD4+Foxp3+ T cells from one representative grafted mouse from each group (wild-type or Lepob/ob B6 mice). (B) The frequency of Tregs in the dLN is represented by column bar graph. (C) Real-time PCR for the evaluation of Foxp3 expression in the skin graft on day 10 posttransplantation. The production of IFN-γ, IL-17, IL-4 and IL-10 was evaluated in the CD4+ T cells of the dLN on day 7 posttransplantation. The leukocytes were harvested, stimulated in vitro for 4 hours with PMA/ionomycin and evaluated by flow cytometry. (D) Represents the expression of IL-17 and IFN-γ and (E) the expression of IL-4 and IL-10, both gated in CD4+ T cells. (F) The percentages of IFN-γ+, IL17+, IL-4+ and IL-10+ CD4+ T cells; these data are the quantification of the data in (E). (G) The ratio between IL4+ CD4+ and IFN-γ+ CD4+ T cells was calculated to assess any possible imbalances between the Th1 and Th2 populations in wild-type or Lepob/ob B6 cells. (H) CD4+ T cells in the dLN of Lepob/ob mice express higher levels of intracellular GATA-3. The GATA-3 expression levels in the CD4+ T cells were analyzed in the dLN by flow cytometry 7 days after transplantation. (I) The dLN of Lepob/ob mice express higher levels of GATA-3 mRNA compared to the dLN of with WT control. The GATA-3 mRNA levels in the dLN were analyzed in the dLN 7 days after transplantation by real time PCR. *p < 0.05, **p < 0.01. Isotypic antibodies were used as the internal experimental controls. n = 5 animals /group. The results are shown as the mean ± SEM.
Lepob/ob skin-grafted mice showed an increased frequency of CD4+IL-4+ T cells when compared to WT skin-grafted mice (Figures 2E and F). Thus, we can assume that in the absence of leptin, a shift toward a Th2 alloimmune response might occur. This shift is better visualized by analyzing the ratio of IFN-γ+ and IL-4+ CD4+ T cells in transplanted mice, which, as shown in Figure 2G, was lower in Lepob/ob mice than in control mice. Furthermore, the analysis of the Th2 transcription factor GATA-3 in CD4+ T cells demonstrated that Lepob/ob skin-grafted mice had an increased percentage of GATA-3-expressing cells and increased gata-3 mRNA expression compared to control mice (Figure 2H and I). Taken together, these results indicate that the enhanced allograft survival observed in the absence of leptin may be the result of a predominantly regulatory and Th2 T cell response.
Leptin affects allograft responses through the modulation of CD4+ T cells and the differentiation of Treg cells
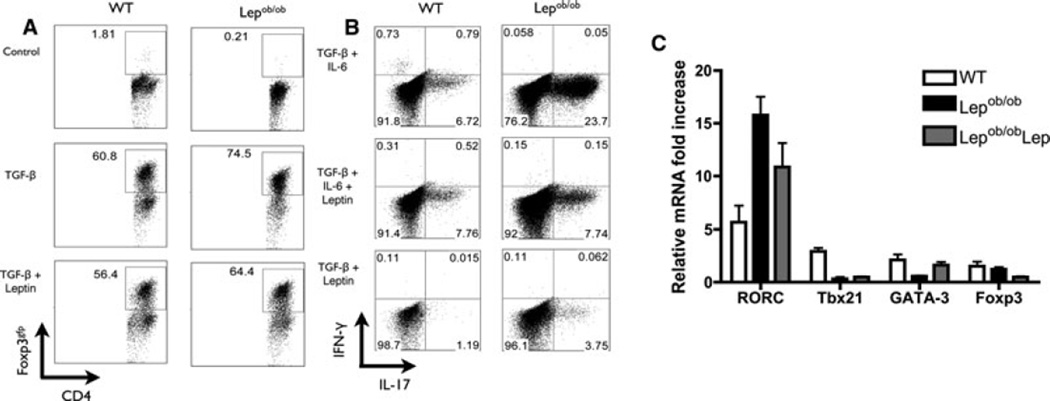
The increased frequency of Tregs cells in the Lepob/ob skin-grafted mice prompted us to investigate the possible mechanisms that drive this phenotype in the absence of leptin. First, because leptin mainly signals through STAT3, we confirmed the ability of leptin to induce the phosphorylation of STAT3 in leukocytes (data not shown), as previously described (10,15). Once we observed the ability of leukocytes to respond to leptin, we differentiated sorted naïve (CD4+CD62L+CD44−CD25−) T cells from Lepob/ob and WT mice into Tregs. We observed that the addition of TGF-β led to the induction of Tregs from both naïve Lepob/ob and WT CD4+ naïve T cells (Figure 3A). Moreover, Lepob/ob CD4+ T cells demonstrated an increased capacity to generate Tregs compared to WT CD4+ T cells (Figure 3A, Supporting Figure S3). The addition of recombinant leptin to the differentiation assay had a minor effect on both Lepob/ob Lepob/obob Lep) and WT Tregs (Figure 3A, Supporting Figure S3).We then analyzed the effect of leptin on the generation of Th17 cells. We incubated sorted naive CD4+ T cells from Lepob/ob and WT mice under different conditions (IL-6 + TGF-β, IL-6 + TGF-β + leptin and leptin + TGF-β). The naive cells from Lepob/ob mice cultured with IL-6 + TGF-β produced a higher frequency of Th17 cells than the WT naive cells cultured under the same conditions (Figure 3B, Supporting Figure S3). Moreover, whereas the recombinant leptin had no effect on the Th17 differentiation of naive WT CD4+ T cells, the addition of leptin to Lepob/ob Lepob/ob Lep) naïve CD4+ T cells cultured under Th17 conditions inhibited the generation of Th17 cells (Figure 3B, Supporting Figure S3). The differentiation of Th17 cells was confirmed by increased RORC expression and decreased expression of Tb×21, GATA-3 and Foxp3 (Figure 3C). These results indicate that leptin deficiency affects CD4+ T cell fate by inducing both Tregs and Th17.
Figure 3. Tregs and Th17 cells from Lepob/ob naïve T cells are more effectively induced than those from WT naïve Lepob/ob T cells.
Naive CD4+CD62L+CD44−CD25− T cells from wild-type or Lepob/ob B6 mice were cultured for 5 days in the presence or absence of TGF-β, IL-6 and/or leptin. (A) Treg differentiation of naïve CD4+ T cells from WT and Lepob/ob mice. The induction of Foxp3+ CD4+ T cells was evaluated through the intracellular staining of Foxp3, which was then quantified in CD4+ T cells by flow cytometry. The data are representative of three independent experiments. (B) Th17 differentiation of naive CD4+ T cells from WT and Lepob/ob mice. The induction of IL17+ CD4+ T cells was evaluated through the intracellular staining of IL-17 and IFN-γ, which was then quantified by flow cytometry. The data are representative of three independent experiments. (C) The mRNA expression of RORC, Tbx21, GATA-3 and Foxp3 were analyzed after 5 days of in vitro Th17 differentiation of naïve CD4+ T cells. The total mRNA was isolated and used to prepare the complementary DNA. The mRNA levels were normalized to the GAPDH levels. Lepob/ob: CD4+ T cells from Lepob/ob cultured in the absence of leptin; Lepob/obLep: CD4+ T cells from Lepob/ob cultured in the presence of recombinant leptin; WT: CD4+ T cells from wild-type mice. The data are representative of two independent experiments. The results are shown as the mean ± SEM.
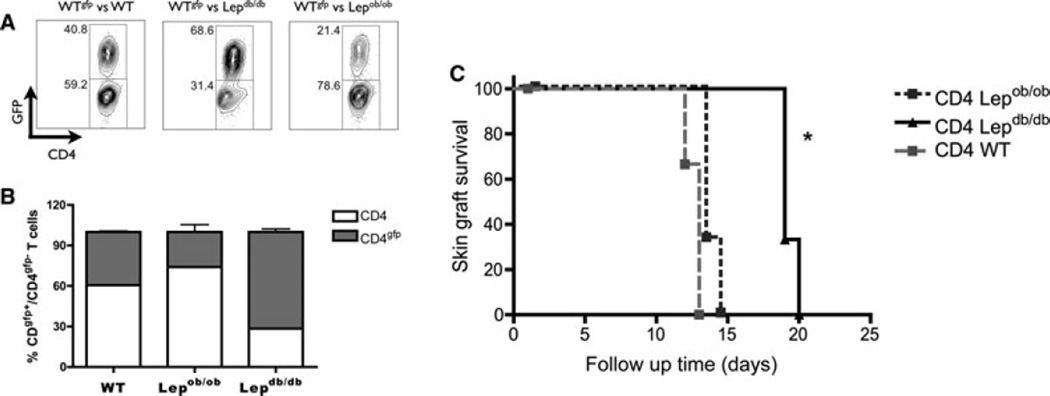
Leptin deficiency in skin-transplanted mice resulted in decreased alloreactivity, which can be attributed to either decreased allostimulation or a decreased capacity of CD4+ T cells to respond to a stimulus. Thus, we investigated the mechanisms by which leptin modulates the in vivo proliferation of CD4+ T cells. We performed an in vivo competitive homeostatic proliferation experiment to assess CD4+ T cells. Equal numbers of CD4+ T cells from WTgfp mice and CD4+ T cells from WT, Lepob/ob or Lepdb/db mice were transferred into a Rag−/− host. The competitive homeostatic proliferation was evaluated by measuring the ratio of CD4+GFP+ T cells to CD4+GFP− T cells 10 days posttransfer. Lepdb/db CD4+ CD4+GFP−) T cells displayed decreased competitive proliferation compared to WT and Lepob/ob CD4+ T cells (CD4+GFP−) (Figure 4A). Interestingly, upon transfer of CD4+ T cells from Lepob/ob mice into a Rag−/− leptin-sufficient host, these cells displayed increased competitive proliferation compared to WT CD4+GFP+ T cells, which demonstrates that these cells are hypersensitive to normal levels of leptin (Figures 4A and B). Together, these data indicate that leptin directly affects CD4+ T cell fitness and proliferative responses in vivo.
Figure 4. Leptin receptor deficiency results in both reduced T cell proliferation capacity and enhanced allograft survival in RAG−/− mice.
(A) Analysis of competitive homeostatic proliferation. CD4+ T cells from wild-type B6, B6gfp, Lepob/ob B6 and Lepdb/db B6 mice were purified and 2.106 CD4+ T cells from wild-type, Lepob/ob or Lepdb/db mice were mixed with the same number of CD4+ T cells from B6gfp mice. A, and a total of 4.106 CD4+ T cells were transferred into RAG−/− mice to evaluate the competitive homeostatic proliferation. The frequency of CD4+ T cells in the pooled lymph nodes was analyzed 10 days later by flow cytometry. (B) Graphic representation of homeostatic proliferation of wild-type, Lepob/ob or Lepdb/db CD4+ T cells competing with wild-type CD4+ T cells from GFP mice (n = 3 mice/group). (C) 5.106 CD4+ T cells from wild-type, Lepob/ob or Lepdb/db mice were transferred into skin-grafted RAG−/− mice. Signs of allograft rejection were monitored daily, and survival is reported for individual animals as well as the time of graft rejection (n = 3 mice/group). *p < 0.05. The results are shown as the mean ± SEM.
The influence of leptin on allograft immune responses through CD4+ T cells mediates the maintenance of skin grafts
Leptin deficiency resulted in a modified CD4+ T cell response that is characterized by an increased induction of Tregs, a Th2 phenotype and a decreased ability of CD4+ T cells to proliferate in vivo. To directly confirm that the changes in the CD4+ T cells that are caused by leptin deficiency are one of the mechanisms responsible for the enhanced graft survival observed in leptin-deficient mice, we transferred purified splenic CD4+ T cells from WT, Lepob/ob or Lepdb/db mice into skin-grafted Rag−/− mice and evaluated the graft survival. Rag−/− mice reconstituted with Lepdb/db CD4+ T cells displayed enhanced skin graft survival compared to Rag−/− mice reconstituted with Lepob/ob or WT CD4+ T cells (Figure 4C). This enhanced graft survival was accompanied by a decreased frequency of CD4+ T cells in the lymph node of Rag−/− mice reconstituted with Lepdb/db CD4+ T cells compared to Rag−/− mice reconstituted with Lepob/ob or WT CD4+ T cells (Supplementary Figure 4). This result demonstrates that CD4+ T cells display decreased in vivo proliferative fitness in the absence of leptin signaling. Although the graft survival in Rag−/− mice reconstituted with Lepdb/db CD4+ T cells was higher than that observed in control mice, the graft survival in these mice was lower than that observed which indicates that other pathways, in addition to the effect of leptin on CD4+ T cells, account for the enhanced skin graft survival observed in Lepob/ob mice.
Discussion
In this study, we investigated the participation of leptin in alloimmune responses. The immunological changes associated with the increase in the weight of the recipient have become an important challenge in clinical transplantation; however, the amount of information on obesity-associated immune responses after organ transplantation remains scarce. Although obese and hyperleptinemic patients have been shown to have poor graft survival (15–17), the mechanisms underlying this effect are completely unknown. We observed that obese leptin-deficient (Lepob/ob) mice displayed enhanced skin graft survival, which suggests that the graft outcome observed in obese patients is not directly related to obesity but may be a result of hyperleptinemia. It was recently proposed that obesity affects graft function but not graft survival; however, the leptin levels were not investigated in this study (18). Thus, we investigated the immunologic mechanisms that result in increased graft survival. We found that CD4+ T cells are at least partially responsible for the enhanced graft survival that is observed in leptin-deficient mice. The CD4+ T cells from Lepob/ob mice differentiated more efficiently into Tregs cells and displayed impaired proliferation in vivo, which might account for the enhanced graft survival observed in Lepob/ob mice.
The rejection of fully MHC class II-mismatched skin grafts was significantly delayed in Lepob/ob recipients. First, Lepob/ob recipients displayed a decreased frequency of CD4+ T cells in the dLN. Second, Lepob/ob mice displayed decreased alloreactivity, as observed in the MLR experiments. Third, in the absence of leptin signaling, CD4+ T cells displayed decreased in vivo fitness and proliferation. Moreover, decreased Th1 serum cytokine concentrations were observed in Lepob/ob skin-transplanted mice. These findings are suggestive of a shift in the Th1/Th2 balance in the dLN because a decreased percentage of CD4+IFN-γ+ T cells and an increased percentage of both CD4+IL-4+ and CD4+GATA-3+ T cells were found in Lepob/ob skin-transplanted mice. In addition, grafted mice displayed an increased frequency of Tregs in the dLN and enhanced expression of Foxp3 in the grafted skin, which suggests Treg infiltration. These results are in accordance with a study published by Waaga et al. in which the authors treated kidney grafts with CTLA4-Ig and reported that the rejected tissue from untreated mice yielded T cell clones that produced IFN-γ, whereas the clones from tolerant mice produced IL-4 and IL-10. Furthermore, these researchers also demonstrated that although the Th1 clones from rejected subjects induced DTH responses, the Th2 clones from tolerant recipients suppressed the DTH responses that were mediated by the Th1 clones and blocked IFN-γ production in vitro (19). This shift toward a Th2 response is further supported by the decreased serum concentrations of Th1 cytokines in Lepob/ob mice compared to their WT skin-grafted littermates.
Furthermore, we observed an increased frequency of regulatory T cells in the dLN and enhanced expression of Foxp3 in the grafted skin of Lepob/ob mice. The importance of Tregs in inducing transplant acceptance and tolerance is well known (19–21); therefore, together with the shift in the Th1/Th2 balance, the increased frequency of Tregs can account for the enhanced graft survival observed in leptin-deficient mice.
To verify the role of leptin in Treg frequency, we investigated the generation of Tregs from naïve Lepob/ob CD4+ T cells. To ensure the absence of leptin, we used autologous serum from Lepob/ob mice because fetal bovine serum contains leptin, which has been shown to reverse the effects of leptin deficiency (10). We observed enhanced Treg induction from naïve Lepob/ob CD4+ T cells compared to WT CD4+ T cells. Moreover, the addition of recombinant leptin to these differentiation assays had a minor effect. The role of leptin in Treg homeostasis was previously investigated (10,23). Tregs from Lepob/ob mice have been reported to display similar suppressive capacities to WT Treg cells, and leptin neutralization has been shown to increase IL-2-dependent Treg proliferation both in humans and in mice (10,23). In this study, we found that in the absence of leptin, an increased frequency of Treg cells were generated from their naive precursors. This finding indicates that Lepob/ob naive cells can be converted into Tregs more easily than can WT naïve T cells.
The dichotomy that exists between Tregs and Th17 cells (24,25) and the increased frequencies of CD4+IL-17+ T cells in the dLNs of Lepob/ob skin-grafted mice may suggest an increased Treg/Th17 axis. We further investigated this result and found that naive T cells from Lepob/ob mice were more efficiently converted into Th17 cells than were WT naïve T cells. Increasing evidence has pointed to the involvement of Th17 cells in graft rejection (26). However, the role of Th17 cells in graft survival remains unclear. IL-17A appears not to be required for corneal allograft rejection, but it has been shown to contribute to the immune privilege of allografts (27).
The decreased alloreactivity observed in the absence of leptin may indicate that CD4+ T cells do not proliferate under allostimulus or that an intrinsic defect exists in CD4+ T cells in the absence of leptin signaling. To clarify this issue, we performed a homeostatic competitive proliferation assay and showed that Lepdb/db CD4+ T cells proliferate less in lymphopenic hosts than WT CD4+ cells. To exclude the possibility of an intrinsic defect in CD4+ T cell development in the absence of leptin, we also performed this assay with cells from Lepob/ob mice. Therefore, if the development of CD4+ T cell in the absence of leptin results in a defect, Lepob/ob T cells would not have a normal proliferative response even when transferred to leptin-sufficient mice. In contrast, the inhibition or absence of leptin has been reported to trigger a compensatory upregulation of the leptin receptor, which makes the cells more responsive to leptin (10). We observed that Lepob/ob CD4+ T cells transferred to Rag−/− mice, which normally produce leptin, proliferated more than the cotransferred WT CD4+ T cells, which is in agreement with the previous report. This finding indicates that there is no defect in the development of CD4+ T cells in the absence of leptin and that leptin is an important factor in T cell proliferative fitness.
To further confirm the role of leptin signaling in the ability of CD4+ T cells to respond to an allostimulus, we transferred CD4+ T cells from Lepdb/db, Lepob/ob or WT mice into skin-transplanted Rag−/− mice and observed a prolonged graft survival when CD4+ Lepdb/db T cells were transferred. This finding was accompanied by a decreased frequency of peripheral CD4+ T cells, which demonstrates that leptin modulates the CD4+ T cell alloimmune response in vivo and plays an important role in CD4+ T cell proliferative fitness.
These findings demonstrate that leptin deficiency affects T cell polarization. In leptin-deficient mice, these modifications resulted in enhanced graft survival indicating the importance of leptin, but not necessarily obesity, in modulating the allograft immune response. These findings suggest a possible explanation for the increased susceptibility of hyperleptinemic obese transplanted individuals to acute and chronic graft rejection.
Supplementary Material
Acknowledgments
We thank Rosana Rosa Miranda for her help with the histological analysis. This study was supported by grants 07/07139-3, 08/55447-1, 09/50450-7, 08/58564-9, 10/52180-4 and 12/02270-2 from the State of Sao Paulo Foundation for Research Support (FAPESP), the International Associated Laboratory in Renal Immune RegulationImmuneregulation (CNPq/Inserm), the Brazilian Council of Scientific and Technologic Development (470533/2007-2, CNPq/DECIT/MS) and Complex Fluids INCT.
Abbreviations
- dLN
draining lymph node
- EAE
experimental autoimmune encephalomyelitis
- Lepob/ob
Leptin-deficient mice
- Lepdb/db
leptin receptor-deficient mice
- TCR
T cell receptor
- Treg
regulatory T cell
- Th
helper T cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 235:1412–1424. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- 3.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 4.Bozkurt A, Cakir B, Ercan F, Yegen BC. Anti-inflammatory effects of leptin and cholecystokinin on acetic acid-induced colitis in rats: Role of capsaicin-sensitive vagal afferent fibers. Regul Pept. 2003;116:109–118. doi: 10.1016/s0167-0115(03)00194-0. [DOI] [PubMed] [Google Scholar]
- 5.Siegmund B, Lear-Kaul KC, Faggioni R, Fantuzzi G. Leptin deficiency, not obesity, protects mice from Con A-induced hepatitis. Eur J Immunol. 2002;32:552–560. doi: 10.1002/1521-4141(200202)32:2<552::AID-IMMU552>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 6.De Rosa V, Procaccini C, La Cava A, et al. Leptin neutralization interferes with pathogenic T cell autoreactivity in autoimmune encephalomyelitis. J Clin Invest. 2006;116:447–455. doi: 10.1172/JCI26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matarese G, Sanna V, Lechler RI, et al. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;51:1356–1361. doi: 10.2337/diabetes.51.5.1356. [DOI] [PubMed] [Google Scholar]
- 8.Matarese G, Di Giacomo A, Sanna V, et al. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol. 2001;166:5909–5916. doi: 10.4049/jimmunol.166.10.5909. [DOI] [PubMed] [Google Scholar]
- 9.Taleb S, Herbin O, Ait-Oufella H, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2691–2698. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 10.De Rosa V, Procaccini C, Cali G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 12.Markees TG, Phillips NE, Noelle RJ, et al. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64:329–335. doi: 10.1097/00007890-199707270-00026. [DOI] [PubMed] [Google Scholar]
- 13.Macia L, Delacre M, Abboud G, et al. Impairment of dendritic cell functionality and steady-state number in obese mice. J Immunol. 2006;177:5997–6006. doi: 10.4049/jimmunol.177.9.5997. [DOI] [PubMed] [Google Scholar]
- 14.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moraes-Vieira PM, Bassi EJ, Araujo RC, Camara NO. Leptin as a link between the immune system and kidney-related diseases: Leading actor or just a coadjuvant? Obes Rev. 2012;13:733–743. doi: 10.1111/j.1467-789X.2012.00997.x. [DOI] [PubMed] [Google Scholar]
- 16.Kalaitzidis RG, Siamopoulos KC. The role of obesity in kidney disease: Recent findings and potential mechanisms. Int Urol Nephrol. 2011;43:771–784. doi: 10.1007/s11255-011-9974-1. [DOI] [PubMed] [Google Scholar]
- 17.Papalia T, Greco R, Lofaro D, Maestripieri S, Mancuso D, Bonofiglio R. Impact of body mass index on graft loss in normal and overweight patients: Retrospective analysis of 206 renal transplants. Clin Transplant. 2010;24:E241–E246. doi: 10.1111/j.1399-0012.2010.01258.x. [DOI] [PubMed] [Google Scholar]
- 18.Ditonno P, Lucarelli G, Impedovo SV, et al. Obesity in kidney transplantation affects renal function but not graft and patient survival. Transplant Proc. 2011;43:367–372. doi: 10.1016/j.transproceed.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Waaga AM, Gasser M, Kist-van Holthe JE, et al. Regulatory functions of self-restricted MHC class II allopeptide-specific Th2 clones in vivo. J Clin Invest. 2001;107:909–916. doi: 10.1172/JCI11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechler RI, Ng WF, Camara NO. Infectious tolerance? Mechanisms and implications. Transplantation. 2001;728(Suppl):S29–S31. [PubMed] [Google Scholar]
- 21.Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y. Regulatory T cells and organ transplantation. Semin Immunol. 2004;16:119–126. doi: 10.1016/j.smim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Jarvinen LZ, Blazar BR, Adeyi OA, Strom TB, Noelle RJ. CD154 on the surface of CD4+CD25+ regulatory T cells contributes to skin transplant tolerance. Transplantation. 2003;76:1375–1379. doi: 10.1097/01.TP.0000093462.16309.73. [DOI] [PubMed] [Google Scholar]
- 23.Procaccini C, De Rosa V, Galgani M, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–941. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 25.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 26.Chadha R, Heidt S, Jones ND, Wood KJ. Th17: Contributors to allograft rejection and a barrier to the induction of transplantation tolerance? Transplantation. 91:939–945. doi: 10.1097/TP.0b013e3182126eeb. [DOI] [PubMed] [Google Scholar]
- 27.Cunnusamy K, Chen PW, Niederkorn JY. IL-17 promotes immune privilege of corneal allografts. J Immunol. 185:4651–4658. doi: 10.4049/jimmunol.1001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vokaer B, Van Rompaey N, Lemaitre PH, et al. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. J Immunol. 185:3417–3425. doi: 10.4049/jimmunol.0903961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.