Abstract
Presensitization to HLA antigens limits the success of organ transplantation. The achievement of donor-specific tolerance via mixed chimerism could improve outcomes of transplantation in presensitized patients. In presensitized B-cell-deficient μMT B6 mice, we developed nonmyeloablative bone marrow transplantation (BMT) regimens that successfully tolerized presensitized T cells, achieving long-term (LT) multilineage chimerism and tolerance to donor-type skin. To apply these regimens in wild-type (WT) animals while avoiding antibody-mediated destruction of donor bone marrow cells, presensitized WT B6 mice were rested >2 years to allow alloantibody clearance. However, chimerism and tolerance were not reliably achieved in LT presensitized WT B6 mice in which alloantibody had declined to minimal or undetectable levels before BMT. Strong antidonor memory T-cell responses were detected in LT presensitized WT B6 mice after rejection of donor bone marrow (BM) occurred, whereas levels of alloantibody remained consistently low. In contrast, presensitized μMT B6 mice had diminished memory T-cell responses compared to WT B6 mice. These data implicate T-cell memory, but not alloantibody, in rejection of donor BM in LT presensitized WT mice.
Keywords: Alloantibodies, memory, mixed chimerism, presensitization, T-cell memory, tolerance
Introduction
Allosensitized patients endure extended time on waiting lists, inferior graft function and poorer survival and outcomes compared to cross match-negative transplant recipients (1), even with the use of desensitization protocols (2). Tolerance is therefore a desirable goal in presensitized recipients.
Mixed chimerism induces tolerance in naive animals and this approach has recently been successfully applied in kidney allograft recipients (3). However, in this study, a patient with high panel-reactive antibodies pretransplant underwent acute humoral rejection, illustrating the difficulty of tolerizing presensitized patients. Several groups have reported chimerism and tolerance induction in presensitized mice. A myeloablative regimen involved anti-CD8, anti-CD40L, 9.5 Gy total body irradiation (TBI) and high-dose bone marrow transplantation (BMT; Ref. 4). However, we were unable to reproduce these results in our own laboratory (M. Sykes, C. Chester, D.H. Sachs, unpublished data October 1985–1987, Bethesda, MD). Engraftment was reported in sensitized, B-cell deficient mice receiving anti-mouse IgG, anti-CD4 and CD8 mAbs, 6 Gy TBI and high-dose BMT (5), but high-dose TBI is associated with significant toxicity in humans (6). We describe here the development of nonmyeloablative conditioning regimens that permit chimerism and tolerance in presensitized B-cell-deficient μMT B6 mice using only 3 Gy TBI. Our data implicate memory T cells as the dominant barrier to engraftment in presensitized wild-type (WT) mice.
Materials and Methods
Animals
WT C57BL/6 (B6) were obtained from the Frederick Cancer Research Facilities (Frederick, MD, USA), B-cell-deficient μMT B6 and B10.RIII from Jackson Laboratories (Bar Harbor, ME, USA) and B10.A mice from Taconic (Germantown, NY, USA). Animals were maintained in a specific pathogen-free microisolator environment in accordance with the AVMA Guide for The Guidelines and Care and Use of Laboratory Animals.
Nonmyeloablative conditioning and BMT
Recipient WT or μMT B6 mice were conditioned with intraperitoneal (IP) mAb injections including anti-CD4 (GK1.5, 1.8 mg), anti-CD8 (2.43, 1.4 mg) and anti-NK1.1 (PK136, 0.6 mg) on Day 5; anti-OX40L (RM134L, 2 mg) on Day 1; and anti-CD40L (MR1, 2 mg) on Day 0 with respect to the BMT. 3 Gy TBI were given about 6 h before injection of 80–100 × 106 bone marrow cells (BMC) into the lateral tail veins.
Flow cytometric analysis for multilineage chimerism
Peripheral blood chimerism was analyzed by four-color flow cytometry as described (7), using donor-specific anti-H2Dd mAb 34-2-12 with fluorochrome-labeled anti-B220, anti-MAC1, anti-CD4 and anti-CD8 APC (allophycocyanin; BD Pharmingen, San Diego, CA, USA) mAbs. Animals were considered chimeric when at least 5% of WBCs in any of these lineages were donor derived.
Indirect flow cytometric analysis for antidonor alloantibody
To block Fc receptors, 10 μL of 2.4G2 was added per 106 donor-derived splenocytes. Ten microliters of diluted sera (1, 2) from donor-sensitized mice were then incubated at 4°C for 30 min, followed by two washes and incubation with FITC-conjugated secondary rat anti-mouse IgM, IgG1, IgG2a, IgG2b or IgG3 mAbs, washing and flow cytometric (FCM) analysis. Staining is represented as median fluorescence intensity (MFI). Sera from hyperimmunized mice were used as positive controls. WT B6 mice were skin grafted with B10.A tail skin, and on Days 21, 28 and 35 after skin grafting boosted with 20 × 106 B10.A lymphocytes IP. Sera collected on Days 21, 28, 35 and 42 after skin grafting were used as positive controls.
Skin grafting
Full thickness tail skin grafting on the lateral thorax was performed as described (8), then scored daily from Day 7 until rejection for the first 2 weeks, weekly for the next 4 weeks and bimonthly until 100 days. Grafts were considered rejected when <10% remained viable.
IFN-γ memory T-cell ELISpot assay
Ninety-six-well ELISpot plates (Millipore, Billerica, MA, USA) were coated with a capture mAb in sterile ELISpot coating buffer (eBioscience) and incubated at 4°C overnight. Anti-IFN-γ capture mAb stock solution (eBioscience) was used at a 1:250 dilution. On the day of the experiment, the plates were washed twice with ELISpot coating buffer (eBioscience, Inc., San Diego, CA, USA), and blocked for 1 h with RPMI-1640 (GIBCO, Life Technologies, Carlsbad, CA, USA). Lymph node cells or splenocytes (5 × 105) were placed in each well with or without donor-type B10.A stimulator cells (5 × 105), medium (200 μL) or phytohemagglutinin (PHA) (1 μg per well) and cultured for 6 h at 37°C in 5% CO2. Cells capable of mounting an IFN-γ response to alloantigen after 6 h have been shown to represent memory T cells in monkeys (9), and the 6 h memory T-cell response in mice was confirmed in our laboratory (Figure S1). The plates were washed three times with phosphate buffered saline (PBS)/0.05% Tween-20. Biotinylated anticytokine detection stock solution (eBioscience; 1:250 dilution, eBioscience, Inc. San Diego, CA, USA) was added and incubated overnight at 4°C. After four washes with 0.05% Tween-20, avidin–horseradish peroxidase was added for 45 min. Three washes with 0.05% Tween-20, then two washes with PBS were performed before the spots were revealed with 800 μL of developing solution (3-amino 9-ethylcarbazole [Sigma, 10 mg dissolved in 1 mL dimethylformamide] in 24 mL of 0.1M sodium acetate, pH 5), catalyzed by 12 μL H2O2. Spots were counted and analyzed on a computer-assisted ELISpot image analyzer (CTL, Cleveland, OH, USA).
Statistical analysis
Days until rejection of primary sensitizing skin grafts versus rejection of secondary skin grafts were compared by paired T-test. Comparisons of chimerism and alloantibody levels (MFI) between groups were performed by T-test for comparison of means.
ELISpot data were analyzed in a generalized linear model with a log link function assuming the Poisson distribution. Comparisons were based on hypothesis tests of the between-mice contrasts under different experimental conditions, using the F-test for inference. Data analysis was conducted using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Second-set skin graft rejection in presensitized μMT B6 mice
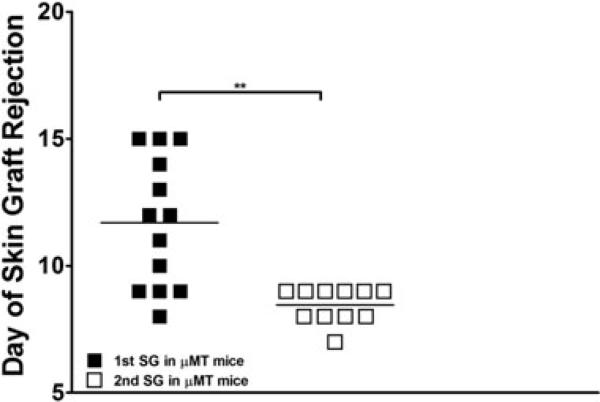
Previous serum transfer studies from presensitized mice demonstrated that alloantibodies prevent the engraftment of allogeneic bone marrow (BM), even in lethally irradiated recipients (C. Chester, M. Sykes and D.H. Sachs, 1985–1987, unpublished data, Bethesda, MD). Therefore, to focus on T-cell memory, we used μ heavy chain-deficient (μMT B6) mice, which lack B cells and therefore cannot produce alloantibodies (10). To assess second-set rejection responses, we compared the times for μMT B6 mice to reject primary and secondary skin grafts grafted 10 weeks later from the same allogeneic strain, B10.A. μMT mice rejected secondary grafts significantly more quickly than primary grafts (median 8 and 11 days, respectively). Thus, μMT B6 mice are capable of second-set graft rejection (Figure 1).
Figure 1. Rejection of primary and secondary allogeneic skin grafts in μMT B6 mice.
Comparison of the number of days until rejection of primary sensitizing skin grafts versus rejection of secondary skin grafts. μMT B6 (n = 11–13) mice were skin grafted with fully MHC-mismatched B10.A tail skin and followed daily for rejection. Ten weeks following rejection of the first graft, the mice were then regrafted and followed daily for second-set responses. The skin graft data from two separate experiments were compiled and subjected to T-test (**p < 0.005).
Development of a costimulatory blockade conditioning regimen for mixed chimerism induction in presensitized μMT mice
In presensitized WT and μMT B6 mice, we tested a nonmyeloablative, T-cell depleting, conditioning regimen that reliably achieved durable mixed chimerism and tolerance in naïve mice (11). μMT or WT B6 mice were presensitized with BALB/c (H-2d) skin 12 weeks before conditioning with anti-CD4 and CD8 mAbs, thymic irradiation (TI [7 Gy]) and TBI (3 Gy), and received high dose (80 × 106 compared to 15 × 106 used in naïve mice with this regimen) BALB/c BMCs. Chimerism was not achieved in WT B6 recipients, which contained antidonor IgG1, 2a and 2b antibodies in their sera, but 3 of 10 presensitized μMT B6 recipients achieved durable mixed chimerism. Achievement of chimerism was markedly more frequent in naive μMT B6 mice receiving conventional doses of BM (9 of 13 mice).
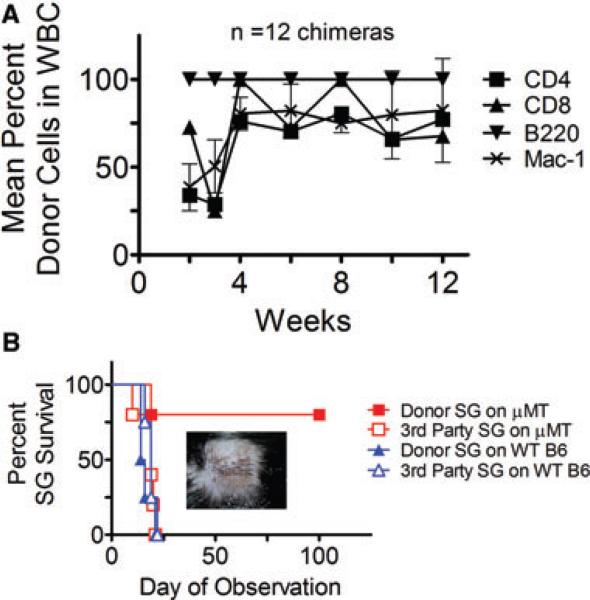
These results indicated that this nonmyeloablative conditioning regimen failed to completely overcome the heightened immune barrier to allogeneic marrow engraftment in most presensitized μMT B6 recipients. We therefore added costimulatory-blocking (and NK-cell depleting) agents to overcome resistant alloreactivity in these mice. WT and μMT B6 mice were presensitized using B10.A skin grafts. Three months later, they received T- and NK-cell depleting anti-CD4, CD8 and NK1.1 mAbs, anti-CD40L and anti-OX40L costimulatory blocking mAbs, along with 3 Gy TBI and high-dose (80 × 106) B10.A BMCs. TI was excluded because anti-CD40L obviates the need for TI in naïve BMT recipients (12). None of the WT B6 mice successfully engrafted, as expected in the presence of antidonor alloantibodies. However, B10.A donor BM engrafted in 12 of 15 presensitized μMT mice (Figure 2A), achieving durable chimerism with high levels (>60%) of donor T cell, monocyte and granulocyte cell repopulation (Figure 2A). As expected, all B cells were donor-derived in recipient μMT B6 mice.
Figure 2. Achievement of chimerism and tolerance with an extensive costimulatory blockade regimen in presensitized μMT B6 mice.
(A) Mean percent repopulation of donor cells in each WBC lineage in presensitized μMT B6 mice over time as measured by flow cytometry. μMT B6 mice presensitized with B10.A skin received anti-CD8, anti-CD4, anti-NK1.1, anti-CD40L and anti-OX40L mAbs along with 3 Gy TBI and 80 × 106 B10.A BMCs. Twelve of 15 mice receiving the extensive costimulatory blockade regimen achieved chimerism. Nonchimeras are excluded from mean values. (B) Donor and third-party skin graft (SG) survival in B10.A→μMT B6 chimeras. Four of five chimeric mice accepted donor-type SGs for 100 days. Also shown are donor and third-party SG survival of B10.A→wild-type B6 failed chimeras prepared with the same regimen. Skin was grafted 8 weeks after BMT.
To assess tolerance to donor antigens, B10.A tail skin was grafted 8 weeks after BMT. B10.A donor skin was accepted in perfect condition >100 days by presensitized μMT B6 chimeras, whereas third-party grafts were rapidly rejected (MST = 19 days; Figure 2B). In contrast, presensitized WT B6 mice that did not accept donor BMCs, rejected both donor (MST = 15 days) and third-party (MST = 19 days) skin grafts (Figure 2B). The mild prolongation of third-party skin graft survival in both groups and of donor skin graft survival in WT nonchimeras probably reflects the persistence of anti-CD40L, which we have found to persist in blood >8 weeks (J. Kurtz and M. Sykes, 1997, unpublished data, Boston, MA). Thus, we established a nonmyeloablative conditioning regimen that quite reliably permits chimerism and tolerance induction in presensitized μMT B6 mice.
Further minimization of the regimen for achieving mixed chimerism and tolerance in presensitized μMT mice
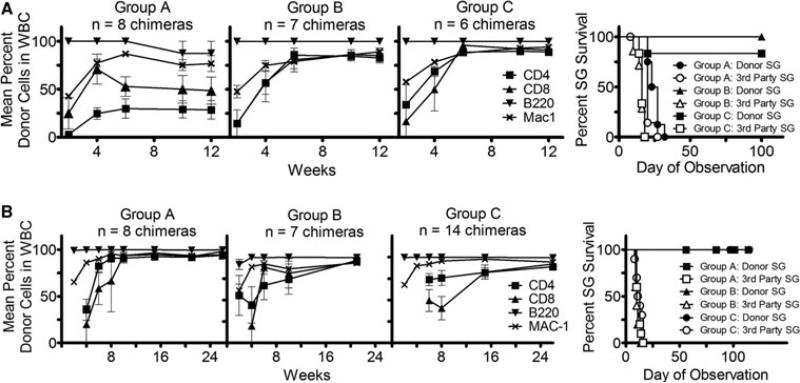
In naïve WT B6 mice, anti-CD40L is sufficient to overcome CD4 T-cell resistance to allogeneic BMT, so that 3 Gy TBI, anti-CD8 and anti-CD40L mAbs permit engraftment of fully allogeneic BMCs (13). To investigate whether exclusion of anti-OX40L, anti-NK1.1 and anti-CD4 mAbs from the conditioning regimen would still permit durable chimerism and tolerance in presensitized μMT B6 mice, we compared three regimens (Figure 3A). Group A received the above regimen that establishes chimerism and tolerance in naïve WT mice (anti-CD8 [1.44 mg] and anti-CD40L [2.0 mg] mAbs), Group B received the same mAbs as Group A plus anti-CD4 mAb (1.76 mg) and Group C received anti-CD4, anti-CD8 and anti-CD40L mAbs plus anti-OX40L mAb (2.0 mg). All mice received 3 Gy TBI and high-dose B10.A donor BMCs. All mice achieved durable mixed chimerism, although at lower levels in Group A compared to Groups B and C. The difference in chimerism was especially marked for T cells (Figure 3A).
Figure 3. Development of minimal costimulatory regimen.
Mean percent repopulation of donor cells in each WBC lineage in presensitized μMT B6 mice over time as measured by flow cytometry. μMT B6 mice presensitized with B10.A skin received the following conditioning regimens along with 3 Gy TBI and 80 × 106 B10.A BMCs. (A) Group A: anti-CD8 and anti-CD40L; Group B: anti-CD8, anti-CD4 and anti-CD40L; Group C: anti-CD8, anti-CD4, anti-CD40L and anti-OX40L. The incidence of chimerism was 100% in all groups. The right-hand panel demonstrates donor and third-party skin graft (SG) survival in B10.A→μMT B6 chimeras. Skin was grafted 8 weeks after allogeneic BMT. (B) Group A: anti-CD4 (1.76 mg), anti-CD8 (1.44 mg) and anti-CD40L (2.0 mg); Group B: anti-CD4, anti-CD8 and 0.5 mg of anti-CD40L; Group C: Two doses of anti-CD4 (1.76 mg each) and anti-CD8 (1.44 mg each) without anti-CD40L. All mice in Groups A and B and 14 of 15 mice in Group C achieved chimerism. The right-hand panel demonstrates donor and third-party SG survival in B10.A→μMT B6 chimeras. All grafted chimeric mice accepted donor-type SGs long term. Skin was grafted at least 9 weeks after allogeneic BMT.
Donor-specific skin was grafted 8 weeks after BMT. Groups B and C demonstrated LT acceptance of B10.A donor grafts (MST>100 days) and rejection of third-party grafts (MST = 16 days), whereas mice in Group A rejected both donor (MST = 25 days) and third-party (MST = 20 days) grafts (Figure 3A). Thus, while anti-CD40L mAb tolerizes CD4 T cells in naive mice receiving BMT (13), the addition of anti-CD4 was necessary to avoid skin, but not marrow graft rejection by memory CD4 T cells of presensitized μMT B6 mice. Furthermore, the similar levels of donor chimerism and skin graft survival observed in Groups B and C demonstrate that anti-OX40L was not necessary to achieve mixed chimerism or tolerance. Exclusion of anti-NK1.1 also did not impair achievement of chimerism or tolerance. Hence, anti-CD4, CD8 and CD40L with 3 Gy TBI provided a simplified regimen for establishing chimerism and tolerance in presensitized μMT mice.
We evaluated the ability of a reduced dose of anti-CD40L mAb to promote chimerism induction in presensitized μMT B6 mice receiving anti-CD4 and anti-CD8 mAbs. The previous doses of anti-CD4 and anti-CD8 mAbs with a lower dose of anti-CD40L (0.5 mg) and 3 Gy TBI permitted establishment of durable mixed chimerism in seven of seven mice (Figure 3B; Group B) and donor-specific skin graft acceptance in four of four chimeras (Figure 3B; Group B), similar to recipients of the same regimen with 2 mg anti-CD40L (Figure 3B; Group A). Thus, this regimen further reduced the amount of treatment necessary to achieve mixed chimerism and T-cell tolerance in presensitized μMT B6 mice.
Replacement of anti-CD40L with more exhaustive T-cell depletion in μMT B6 mice
Because anti-CD40L has been associated with thrombotic complications (14) in humans, it is not considered to be clinically applicable. We therefore tested whether a more exhaustive T-cell-depleting regimen could bypass the need for this reagent in presensitized μMT mice. We administered two full depleting doses of anti-CD4 and anti-CD8 mAbs, along with 3 Gy TBI, and high-dose allogeneic BMT to presensitized μMT B6 mice. Fourteen of 15 presensitized mice achieved durable mixed chimerism (Figure 3B; Group C). Eleven chimeras were skin grafted and all maintained their donor-type skin grafts for more than 100 days while showing normal rejection of third-party skin grafts (MST = 12 days; Figure 3B; Group C).
Failure to reliably achieve durable chimerism in LT presensitized WT B6 mice with nonmyeloablative conditioning after clearance of alloantibody
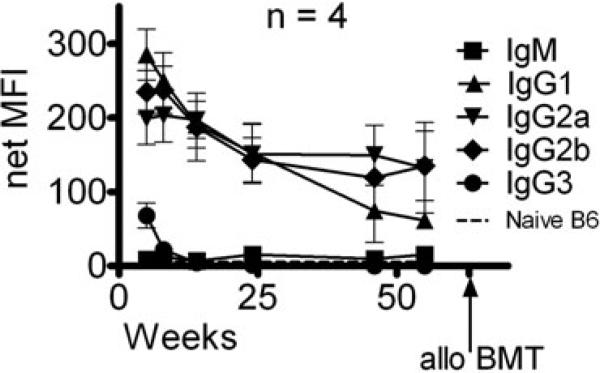
We next tested the ability of nonmyeloabative regimens that overcame the memory T-cell response in presensitized μMT B6 mice lacking B cells to permit chimerism in presensitized WT B6 mice. In WT B6 mice, the level of alloantibody had diminished but was still detectable by indirect FCM more than a year after primary skin graft rejection (Figure 4). We conditioned the two LT, presensitized mice surviving this extended period of time with the regimen described above (anti-CD4 [1.76 mg], anti-CD8 [1.44 mg], anti-CD40L [2 mg] mAbs and 3 Gy TBI). Both mice rejected the high dose of BM (108 BMCs), with no detectable donor cells in the blood 3 weeks after BMT.
Figure 4. Detection of alloantibody in presensitized wild-type B6 mice by indirect flow cytometry.
Sera from wild-type (WT) B6 mice presensitized with B10.A skin grafts were added to splenocytes of the sensitizing strain. FITC-conjugated secondary antibodies directed against various IgG subclasses were then added to detect antibody in the sera specific for B10.A alloantigens. Shown is the net median fluorescence intensity (MFI) of alloantibody in the sera of presensitized WT B6 mice over time as measured by indirect flow cytometry. The net MFI was calculated by subtracting nonspecific background staining of splenocytes incubated with the respective secondary antibodies alone. Positive control MFIs for this assay were IgG1 479, IgG2a 341 and IgG2b 438. Naïve B6 sera did not give staining above background values. Staining for the IgM class and IgG3 subclass was not above background values (not shown). Arrow indicates the time of allogeneic (allo) BMT. Presensitized WT B6 mice received 100 × 106 BMCs from the sensitizing B10.A strain.
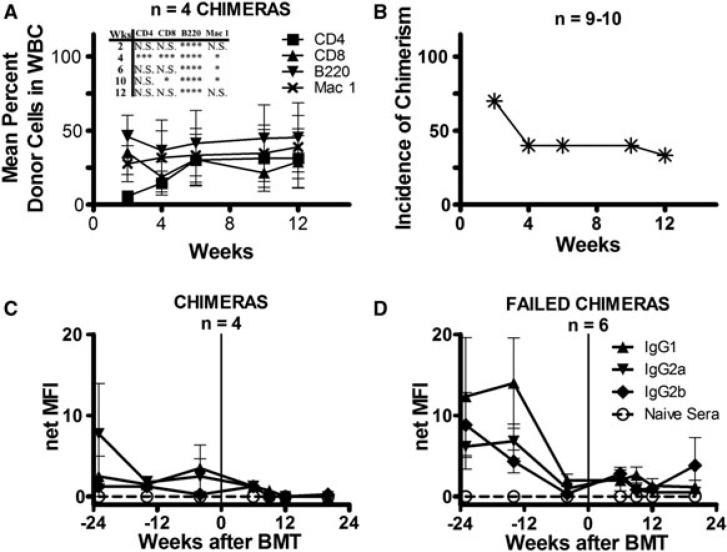
We next tested a more extensive conditioning regimen in LT presensitized WT B6 mice. Approximately 1 year after skin graft rejection, only low levels of alloantibody were detectable in the sera of a group of LT presensitized WT B6 mice, which then received anti-CD4/CD8/NK1.1/OX40L/CD40L/3 Gy TBI as described above with BMT from the sensitizing B10.A donor strain. In contrast to the high incidence of chimerism in similarly treated μMT B6 mice, only 40% (4 of 10) of the LT presensitized WT B6 mice retained substantial levels of multilineage donor chimerism up to 12 weeks (Figure 5A and B), and levels of donor chimerism in all cell lineages of chimeric mice were significantly lower than those in presensitized μMT B6 chimeras. In repeat experiments, donor chimerism was short-term (lasting up to 2 weeks) or not achieved at all.
Figure 5. Chimerism and detection of alloantibody in presensitized wild-type B6 mice.
(A) Mean percent repopulation of donor cells in each WBC lineage in presensitized wild-type (WT) B6 mice over time as measured by flow cytometry. All mice received anti-CD8, anti-CD4, anti-NK1.1, anti-CD40L and anti-OX40L along with 3 Gy TBI and 80 × 106 B10.A BMCs. Only mice that maintained chimerism are shown. Also shown are statistics comparing the percent chimerism for the CD4, CD8, B220 and Mac-1 cell lineages between the presensitized WT B6 and μMT B6 chimeras (Figure 2A) receiving the same conditioning (*0.01 ≤ p < 0.05; ** 0.001 ≤ p < 0.01; ***p < 0.001; ****p < 0.0001). (B) Incidence of chimerism over time in 10 presensitized WT B6 mice receiving the above regimen. At week 12, the incidence of chimerism among nine mice is shown because of the death of one mouse in this experiment. (C, D) Sera from WT B6 mice presensitized with B10.A skin grafts were added to splenocytes of the sensitizing strain. FITC-conjugated secondary antibodies directed against various IgG subclasses were then added to detect alloantibody recognizing B10.A alloantigens. Shown is the net median fluorescence intensity (MFI) of alloantibody in the sera of presensitized WT B6 chimeras (C), and failed chimeras (D) over time as measured by indirect flow cytometry. The net MFI was calculated by subtracting nonspecific background staining of splenocytes incubated with the respective secondary antibodies alone. Positive control MFIs for this assay were IgG1 140, IgG2a 189 and IgG2b 331. Vertical line indicates the time of allogeneic (allo) BMT.
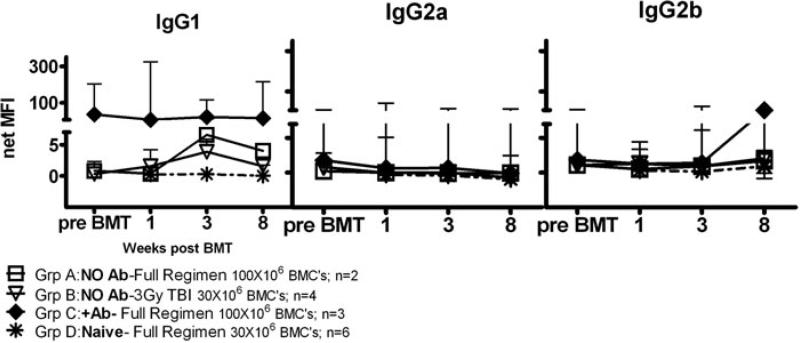
Analysis of alloantibody levels in LT presensitized WT B6 mice before and after BMT did not reveal significant differences between animals that did or did not achieve durable chimerism (Figure 5C and D). However, the timing of the posttransplant analysis could have missed a rapid alloantibody response following BMT that caused marrow rejection. We therefore compared levels of alloantibody before and after BMT in additional groups of presensitized B6 mice (Figure 6). Mice in Groups A and B had no remaining alloantibody detectable at the time of BMT. Group A mice received a costimulatory blockade regimen that had allowed engraftment of donor BMCs in presensitized μMT B6 mice (Figure 6; Group A: anti-CD4/CD8/NK1.1/OX40L/CD40L/3 Gy TBI/108 BMCs), whereas Group B mice received a regimen insufficient for engraftment of donor BMCs in naïve or presensitized mice (Figure 6; Group B: 3 Gy TBI/30 × 106 BMCs). Group B was expected to mount a rapid secondary alloantibody response following BMT.
Figure 6. Alloantibody in LT presensitized B6 mice after conditioning and BMT.
LT presensitized B6 mice with (+Ab) or without (no Ab) measurable alloantibody or naïve WT B6 control animals received the following conditioning before BMT: Group A (LT presensitized B6 mice, no Ab): anti-CD8, anti-CD4, anti-NK1.1, anti-CD40L and anti-OX40L mAbs along with 3 Gy TBI and 100 × 106 B10.A BMCs (full regimen; n = 2). Group B (LT presensitized B6 mice, no Ab; n = 4): 3 Gy TBI and 30 × 106 B10.A BMCs. Group C (LT presensitized B6 mice, +Ab; n = 3): Full Regimen. Group D (naïve WT B6 controls; n = 6): Full Regimen with 30 × 106 B10.A BMCs. Sera from LT presensitized B6 mice (2 years after rejection of B10.A skin grafts) and naïve WT B6 controls were collected before and after BMT, and added to splenocytes of the sensitizing strain. FITC-conjugated secondary antibodies directed against various IgG subclasses were then added to detect antibody in the sera specific for B10.A alloantigens. Shown is the net median fluorescence intensity (MFI) of alloantibody in the sera of LT presensitized B6 mice or naïve WT B6 control animals over time as measured by indirect flow cytometry. The net MFI was calculated by subtracting nonspecific background staining of splenocytes incubated with the respective secondary antibodies alone. Positive control MFIs for this assay were IgG1 16.5, IgG2a 14.2 and IgG2b 13.6. Sera from untreated, naïve B6 sera did not give staining above background values.
Both groups rejected donor BMCs within 2 weeks. Surprisingly, neither group recovered significant alloantibody levels compared to naïve mice receiving adequate conditioning and BMT (Figure 6; Group D). A group of conditioned, presensitized LT WT B6 mice with persistent alloantibodies before BMT was also prepared. These animals also failed to show an increase in alloantibody levels in association with rejection of donor marrow (Figure 6; Group C). These results suggest that persistent alloantibody or rapid antibody responses could not explain the rejection of donor BMCs in conditioned, LT presensitized WT B6 mice.
Memory responses in presensitized LT WT and μMT B6 mice
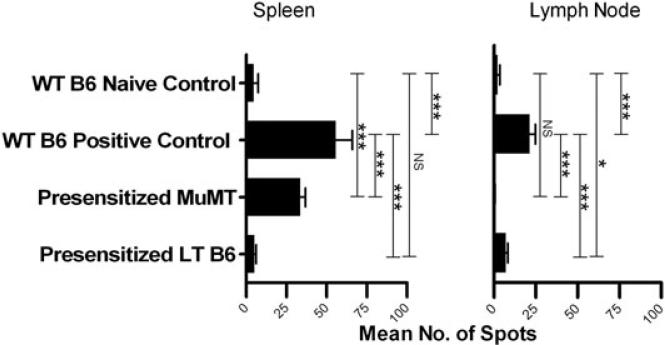
Because the preceding experiment did not point to a role for alloantibodies in rejecting donor marrow in LT presensitized mice, we considered the possibility that memory T cells might be responsible for rejection in LT WT mice. Antidonor memory cytokine-producing cell responses were therefore compared between presensitized μMT and WT B6 mice in a 6 h memory ELISpot assay measuring antidonor IFN-γ responses of spleen and lymph node cells 15–18 weeks after skin grafting. This assay measures primarily memory T-cell responses, as whole splenocyte responses are accounted for by purified memory T cells (Figure S1). Increased numbers of donor-reactive IFN-γ-secreting cells were detected in spleens of presensitized μMT and WT B6 positive control mice and in lymph nodes of WT B6 positive control mice compared to naïve control animals. However, there were significantly more donor-reactive IFN-γ-producing cells in WT positive control mice compared to μMT B6 mice in both the spleen and lymph nodes (Figure 7), whereas similar numbers were detected in BM (Table S1). These data demonstrate stronger T-cell effector memory responses in the spleen and lymph nodes of WT compared to μMT B6 mice.
Figure 7. Memory T-cell ELISpot in LT presensitized B6 and MuMT B6 mice.
The IFN-γ responses of LT presensitized B6 and presensitized MuMT B6 mice were compared to those of presensitized WT B6 positive control animals, and naïve WT B6 negative control animals (n = 1 per group). The number of IFN-γ-secreting spleen and lymph node cells was enumerated in an ELISpot assay 15–18 weeks after donor skin rejection in WT B6 positive control and presensitized MuMT mice, and 2 years after donor skin rejection in presensitized LT B6 mice. Three wells were averaged to determine the mean number of spots.
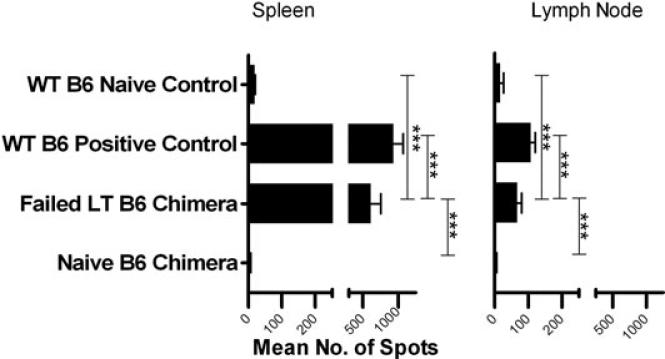
We next asked whether persistent memory was present in LT skin-grafted WT mice that reject donor marrow. An effector memory T-cell ELISpot assay performed >2 years after rejection of donor-type skin in LT presensitized WT B6 mice revealed negligible to minimal IFN-γ responses to the donor in spleen, lymph node (Figure 7) and BM (Table S1). However, following nonmyeloablative conditioning and rejection of donor BMCs, LT presensitized WT B6 mice recovered strong IFN-γ effector memory responses in the spleen and lymph nodes (Figure 8). A measurable response was detected in BM in one of two assays (Table S1). Spleen and lymph node responses were almost as strong as those in WT B6 positive control animals grafted just 20 weeks earlier. Because IFN-γ production in presensitized mice is predominantly T-cell-derived (Figure S1), these data suggest the existence of a quiescent, alloreactive T-cell population that rapidly reactivates following BMT in LT mice. However, our data do not rule out a contribution from naive T-cell clones sensitized during the marrow rejection process. As expected, naïve WT B6 chimeras receiving a similar regimen did not develop alloreactive memory T-cell responses (Figure 8).
Figure 8. Memory T-cell ELISpot in LT presensitized B6 mice after conditioning and BMT.
LT B6 mice were grafted with fully MHC-mismatched B10.A tail skin and followed. Two years after rejection they were conditioned with anti-CD8, anti-CD4, anti-NK1.1, anti-CD40L and anti-OX40L mAbs along with 3 Gy TBI and 100 × 106 B10.A BMCs. These conditioned LT presensitized B6 mice rejected donor BMCs (failed LT B6 chimera). Naïve WT B6 control animals that did achieve chimerism (naïve B6 chimera) with the same conditioning and 30 × 106 B10.A BMCs were included as controls. The illustrated ELISpot assay is representative of two separate assays performed (n = 1 per group). The number of IFN-γ-secreting spleen and lymph node cells was enumerated in an ELISpot assay 6 weeks after BMT in the naïve or failed LT B6 chimeras and 20 weeks after donor skin rejection in WT B6 positive control mice. Three wells were averaged to determine the mean number of spots.
Discussion
Using B-cell-deficient μMT mice to assure the absence of alloantibody, we assessed the ability of BMT with nonmyeloablative conditioning regimens to tolerize memory T cells. We expected the B-cell-deficient μMT B6 mouse model to model sensitized humans following plasmapheresis and B-cell-depleting therapy. Sublethal TBI has been reported to help overcome the memory T-cell response in sensitized μMT mice (4), and lethal irradiation was reported to permit BM engraftment in sensitized WT mice (5). In a nonsensitized monkey model, high levels of donor-reactive memory T cells were associated with failure to achieve mixed chimerism with a nonmyeloablative regimen that was successful in animals with low numbers of donor-specific memory T cells (15). To our knowledge, the data here provide the first demonstration that presensitized T-cell responses in μMT mice can be overcome with minimally myelosuppressive conditioning and BMT. However, extension of this regimen to presensitized WT mice whose alloantibody responses had extinguished was unreliable. Rather than a rapid resurgence of alloantibody responses, our data instead suggest that persistent T-cell memory prevents marrow engraftment in WT mice. The absence of such a barrier in μMT mice suggests that this late memory T-cell response is dependent on B cells.
Although CD4 T cells from μMT mice are reportedly less capable than WT cells of allograft rejection (16), our data do not suggest that this defect interferes with skin graft rejection in μMT mice. CD8-depleted μMT mice failed to reject cardiac allografts (17) and splenic CD4 T cells had normal IFN-γ production, but at lower frequencies than those in spleens of WT mice in one report. However, in our study, μMT mice were presensitized through rejection of donor-type skin, in which case alloantigen is first presented to CD4 T cells in the lymph nodes (17), where only a 30% decrease in CD4 T cells was detected in μMT compared to WT control mice (17). μMT mice that rejected allogeneic skin grafts rejected previously-accepted cardiac allografts (17). CD4 T-cell responses to antigens delivered into skin were relatively normal in μMT B6 mice compared to responses to intravenous antigens (18, 19). We demonstrate that μMT T cells are primed by allogeneic skin grafts, because second-set skin graft rejection occurred and memory IFN-γ responses, presumably from T cells, were detected in the spleen 15 weeks after skin graft rejection. Moreover, despite the use of several-fold greater marrow doses, the regimens required to achieve durable mixed chimerism and tolerance in presensitized μMT mice were more extensive than those required in naïve WT mice. Therefore, the successful achievement of chimerism and tolerance in presensitized μMT mice demonstrates that the nonmyeloablative conditioning regimens described here successfully overcome alloreactive T-cell memory.
A regimen including anti-CD4, anti-CD8 and TI that is highly reliable in naïve mice (20) did not reliably permit chimerism induction in presensitized μMT mice receiving much higher marrow doses. We therefore added anti-CD40L and anti-OX40L, of which only anti-CD40L proved to be necessary. The increased chimerism from 30% to 94% with the replacement of TI with anti-CD40L in recipients of anti-CD4 and anti-CD8 plus TBI suggests that, unlike other models (20, 21), anti-CD40L helps to overcome memory T-cell responses to BMT. Anti-CD40L obviated the need for TI and more effectively overcame resistance to donor marrow engraftment than TI in presensitized mice. Although OX40-OX40L interactions have been implicated in recall responses (22–24), blockade of this interaction was not required to achieve tolerance in presensitized μMT mice.
Our results demonstrate the importance of CD4 depletion in fully overcoming presensitization in μMT mice. Although anti-CD8 and anti-CD40L alone permitted durable, multilineage chimerism, these chimeras rejected donor skin grafts, suggesting a failure to achieve complete tolerance in these presensitized mice. BM chimeras have been reported to reject donor skin grafts in some strain combinations (25), possibly because of a failure of BM to tolerize T cells recognizing skin-specific antigens not shared by hematopoietic stem cells. Thymic APCs would not express these skin-specific antigens and therefore fail to negatively select thymocytes reactive to skin antigens presented by donor MHC (major histocompatibility complex). Although few background genes differ between the B6 and B10.A strains, presentation of a common skin-specific gene product by the different MHC's could create skin-specific antigenic determinants. An alternative explanation for rejection of skin but not BMCs from the same donor is that the marrow is partially rejected, but its regenerative capacity or relative resistance to alloreactive T cells allows persistent chimerism despite persistence of low-level antidonor T-cell reactivity. Consistently, lower levels of chimerism developed in presensitized mice conditioned with this regimen compared to those receiving more extensive regimens.
Although μMT mice had effector/memory cell responses to alloantigens that impeded donor marrow engraftment, this response may be stronger in WT mice. Regimens that overcame T-cell memory in presensitized μMT B6 mice were insufficient in presensitized WT B6 mice, apparently because of persistent effector/memory T-cell responses in the latter. After clearance of antidonor antibody >2 years after skin grafting, donor BM was usually rejected by LT presensitized mice. The lack of recurrent alloantibody responses following marrow rejection suggests that BM is not highly conducive to alloantibody responses in recipients of 3 Gy TBI. However, we cannot rule out an unknown, antibody-independent role for B cells in promoting marrow rejection.
Following BMT in LT presensitized WT mice, robust memory/effector T-cell responses reappeared in association with rejection of donor BM, despite negligible responses pre-BMT. This rapid T-cell response in the absence of circulating alloantibody implicates memory T cells in rejection. These T-cell responses were significantly reduced in presensitized μMT mice compared to WT controls. CD4 T-cell memory for antiviral responses has been reported to depend on the antigen-presenting function of B cells (26), which help alloreactive memory T cells to differentiate, expand and/or survive (27). These results are consistent with achievement of chimerism in presensitized μMT mice with a nonmyeloablative conditioning regimen that fails in presensitized WT mice, even in the absence of circulating alloantibody. Thus, in addition to alloantibody, T-cell memory/effector responses in presensitized WT mice persist years after sensitization, presenting a formidable barrier to donor BM engraftment. Further studies are needed to develop methods of tolerizing these resistant cells in presensitized WT B6 mice using clinically applicable nonmyeloablative conditioning regimens.
Supplementary Material
Figure S1: BALB/c mice were transplanted with a fully allogeneic B6 skin allograft. One hundred days after transplantation, spleen T cells (CD3+) as well as memory T cells (CD3+CD44+) and naïve T cells (CD3+CD44−) were sorted by FCM from the spleens of recipients. To measure direct alloresponses, T cells were cultured in vitro for various amounts of time (X-axis) with donor irradiated APCs and the frequencies of IFN-γ-producing cells were measured by ELISpot. The results are expressed as IFN-γ spots per million T cells ± SD obtained from triplicate wells and are representative of 5–8 mice tested individually.
Table S1: Bone marrow memory responses in presensitized MuMT, LT and failed LT chimeric mice. The IFN-γ responses of presensitized MuMT, presensitized LT B6 and failed LT B6 chimeric mice were compared to those of presensitized WT B6 positive control mice, naïve WT B6 negative control mice and mixed chimeras prepared in naïve B6 mice (“naïve B6 Chimera”; n = 1 per group per assay). The number of IFN-γ-secreting bone marrow cells (BMCs) was enumerated in an ELISpot assay. Three wells were averaged to determine the mean number of spots (left). The right side of the table shows the number of weeks postskin grafting for each animal on the date of the assay, and the statistical comparison of the responses to the naïve WT B6 negative control mice in the same assay (*0.01 ≤ p < 0.05; ** 0.001 ≤ p < 0.01; ***p < 0.001; NS = not statistically significant). Chimeric animals were conditioned with anti-CD8, anti-CD4, anti-NK1.1, anti-CD40L and anti-OX40L mAbs along with 3 Gy TBI and either 100 × 106 B10.A BMCs for the failed LT B6 chimeric mice or 30 × 106 B10.A BMCs for the naïve chimeric mice. The assay was performed 6 weeks after BMT.
Acknowledgments
The authors thank Dr. Emmanuel Zorn for critical review of the manuscript, Mr. Orlando Moreno for outstanding animal husbandry and Ms. Guiling Zhao and Ms. Susan Shea for skilled technical assistance.
Abbreviations
- AVMA
American Veterinary Medical Association
- BM
bone marrow
- BMT
bone marrow transplantation
- FCM
flow cytometry
- FITC
fluorescein isothiocyanate
- Gy
Gray
- HLA
Human Leukocyte Antigen
- IP
intraperitoneal
- LT
long-term
- MFI
median fluorescence intensity
- MHC
Majority Histocompatibility Complex
- MST
mean survival time
- PBS
phosphate buffered saline
- PHA
phytohemagglutinin
- SG
skin graft
- TBI
total body irradiation
- TI
thymic irradiation
- WBC
white blood cells
- WT
wild-type
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting Information
Additional information may be found in the online version of this article:
References
- 1.Jordan SC, Pescovitz MD. Presensitization: The problem and its management. Clin J Am Soc Nephrol. 2006;1:421–432. doi: 10.2215/CJN.01651105. [DOI] [PubMed] [Google Scholar]
- 2.Akalin E. Posttransplant immunosuppression in highly sensitized patients. Contrib Nephrol. 2009;162:27–34. doi: 10.1159/000170810. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. New Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Chilton PM, Tanner MK, Huang Y, Schanie CL, Dy-Liacco M, et al. Humoral immunity is the dominant barrier for allogeneic bone marrow engraftment in sensitized recipients. Blood. 2006;108:3611–3619. doi: 10.1182/blood-2006-04-017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor PA, Ehrhardt MJ, Roforth MM, Swedin JM, Panoskaltsis-Mortari A, Serody JS, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109:1307–1315. doi: 10.1182/blood-2006-05-022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehr T, Sykes M. Clinical experience with mixed chimerism to induce transplantation tolerance. Transpl Int. 2008;21:1118–1135. doi: 10.1111/j.1432-2277.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 7.Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112:2149–2155. doi: 10.1182/blood-2007-12-127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadazdin O, Boskovic S, Murakami T, O'Connor DH, Wiseman RW, Karl JA, et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. American Journal of Transplantation. 2010;10:1375–1384. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 11.Sharabi Y, Sachs DH. Engraftment of allogeneic bone marrow following administration of anti-T cell monoclonal antibodies and low-dose irradiation. Transplant Proc. 1989;21:233–235. [PubMed] [Google Scholar]
- 12.Wekerle T, Sayegh MH, Ito H, Hill J, Chandraker A, Pearson DA, et al. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non-myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Kurtz J, Shaffer J, Sykes M. CD4 T cell-mediated alloresistance to fully MHC-mismatched allogeneic bone marrow engraftment is dependent on CD40-CD40L interactions, and lasting T cell tolerance is induced by bone marrow transplantation with initial blockade of this pathway. J Immunol. 2001;166:2970–2981. doi: 10.4049/jimmunol.166.5.2970. [DOI] [PubMed] [Google Scholar]
- 14.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 15.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 16.Wasowska BA, Qian Z, Cangello DL, Behrens E, Khanh VT, Jodi L, et al. Passive transfer of alloantibodies restores acute cardiac rejection in IgKO mice. Transplantation. 2001;71:727–736. doi: 10.1097/00007890-200103270-00007. [DOI] [PubMed] [Google Scholar]
- 17.Nozaki T, Rosenblum JM, Ishii D, Tanabe K, Fairchild RL. CD4 T cell-mediated rejection of cardiac allografts in B cell-deficient mice. J Immunol. 2008;181:5257–5263. doi: 10.4049/jimmunol.181.8.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topham DJ, Tripp RA, Hamilton-Easton AM, Sarawar SR, Doherty PC. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J Immunol. 1996;157:2947–2952. [PubMed] [Google Scholar]
- 19.Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161:4480–4483. [PubMed] [Google Scholar]
- 20.Zhai Y, Meng L, Gao F, Busuttil RW, Kupiec-Weglinski JW. Allo-graft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of allore-active memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172:5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 22.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4–1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 23.Croft M. Co-stimulatory members of the TNFR family: Keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 24.Yuan X, Salama AD, Dong V, Schmitt I, Najafian N, Chandraker A, et al. The role of the CD134-CD134L costimulatory pathway in alloimmune responses in vivo. J Immunol. 2003;170:2949–2955. doi: 10.4049/jimmunol.170.6.2949. [DOI] [PubMed] [Google Scholar]
- 25.Umemura A, Morita H, Li XC, Tahan S, Monaco AP, Maki T. Dissociation of hemopoietic chimerism and allograft tolerance after allogeneic bone marrow transplantation. J Immunol. 2001;167:3043–3048. doi: 10.4049/jimmunol.167.6.3043. [DOI] [PubMed] [Google Scholar]
- 26.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, et al. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng YH, Oberbarnscheidt MH, Chandramoorthy HC, Hoffman R, Chalasani G. B cells help alloreactive T cells differentiate into memory T cells. Am. J. Transplant. 2010;10:1970–1980. doi: 10.1111/j.1600-6143.2010.03223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: BALB/c mice were transplanted with a fully allogeneic B6 skin allograft. One hundred days after transplantation, spleen T cells (CD3+) as well as memory T cells (CD3+CD44+) and naïve T cells (CD3+CD44−) were sorted by FCM from the spleens of recipients. To measure direct alloresponses, T cells were cultured in vitro for various amounts of time (X-axis) with donor irradiated APCs and the frequencies of IFN-γ-producing cells were measured by ELISpot. The results are expressed as IFN-γ spots per million T cells ± SD obtained from triplicate wells and are representative of 5–8 mice tested individually.
Table S1: Bone marrow memory responses in presensitized MuMT, LT and failed LT chimeric mice. The IFN-γ responses of presensitized MuMT, presensitized LT B6 and failed LT B6 chimeric mice were compared to those of presensitized WT B6 positive control mice, naïve WT B6 negative control mice and mixed chimeras prepared in naïve B6 mice (“naïve B6 Chimera”; n = 1 per group per assay). The number of IFN-γ-secreting bone marrow cells (BMCs) was enumerated in an ELISpot assay. Three wells were averaged to determine the mean number of spots (left). The right side of the table shows the number of weeks postskin grafting for each animal on the date of the assay, and the statistical comparison of the responses to the naïve WT B6 negative control mice in the same assay (*0.01 ≤ p < 0.05; ** 0.001 ≤ p < 0.01; ***p < 0.001; NS = not statistically significant). Chimeric animals were conditioned with anti-CD8, anti-CD4, anti-NK1.1, anti-CD40L and anti-OX40L mAbs along with 3 Gy TBI and either 100 × 106 B10.A BMCs for the failed LT B6 chimeric mice or 30 × 106 B10.A BMCs for the naïve chimeric mice. The assay was performed 6 weeks after BMT.