Abstract
Some evidence suggests that adolescents are more sensitive than adults to ethanol-induced cognitive deficits and that these effects may be long-lasting. The purpose of Exp 1 was to determine if early-mid adolescent [Postnatal day (P) 28-48] intermittent ethanol exposure would affect later learning and memory in a Pavlovian fear conditioning paradigm differently than comparable exposures in adulthood (P70-90). In Exp 2 animals were exposed to ethanol during mid-late adolescence (P35-55) to assess whether age of initiation within the adolescent period would influence learning and memory differentially. Male Sprague-Dawley rats were given 4 g/kg i.g. ethanol (25%) or water every 48 hours for a total of 11 exposures. After a 22 day non-ethanol period, animals were fear conditioned to a context (relatively hippocampal-dependent task) or tone (amygdala-dependent task), followed by retention tests and extinction (mPFC-dependent) of this conditioning. Despite similar acquisition, a deficit in context fear retention was evident in animals exposed to ethanol in early adolescence, an effect not observed after a comparable ethanol exposure in mid-late adolescence or adulthood. In contrast, animals that were exposed to ethanol in mid-late adolescence or adulthood showed enhanced resistance to context extinction. Together these findings suggest that repeated ethanol imparts long-lasting consequences on learning and memory, with outcomes that differ depending on age of exposure. These results may reflect differential influence of ethanol on the brain as it changes throughout ontogeny and may have implications for alcohol use not only throughout the developmental period of adolescence, but also in adulthood.
Keywords: Ethanol exposure, Adolescence, Adulthood, Lasting Consequences, Sprague-Dawley rat
Introduction
The adolescent brain is in a dynamic state of remodeling (see [1, 2] for review), which may make adolescence an especially vulnerable period for exposure to negative environmental agents [3, 4, 5, 6]. This is particularly concerning given that initiation of alcohol consumption is common during adolescence, and alcohol is often consumed by adolescents in a binge-like fashion (i.e., 4-5 drinks within 2 hours, achieving BECs ≥ 80 mg/dl) [7]. Indeed, preclinical and clinical evidence is mounting to suggest that exposure to alcohol during adolescence may be particularly detrimental. For instance, human adolescents that met criteria for an alcohol use disorder (AUD) showed impairments in cognitive tasks such as problem solving, verbal and nonverbal retrieval, visuospatial skills and working memory along with significant brain alterations, such as reduced hippocampal volumes and white matter abnormalities relative to their nonabusing agemates (see [8] for review). Causality cannot be concluded from such investigations, but preclinical studies have also found repeated alcohol (ethanol) exposure during adolescence results in greater impairment in memory [9] as well as greater brain damage in certain brain regions [10] when compared with adult exposed animals when all groups were tested shortly after exposure. An important question is whether enhanced neurobehavioral deficits after adolescent ethanol exposure would persist into adulthood.
Although only a limited number of studies to date have focused on long-term consequences of adolescent alcohol use, these studies generally support the hypothesis that adolescent ethanol exposure results in persistent behavioral and neural changes. For instance, Sircar & Sircar [11] found learning deficits in a Morris water maze task 25 days after exposure in animals exposed to ethanol during adolescence, but not adulthood. Pascual and colleagues [12] reported deficits on both conditional discrimination and novel object recognition tasks 21 days after repeated ethanol exposure in adolescence. Other recent studies found reversal learning deficits using the Barnes maze [13] and Morris water maze [14] tasks 15 and 35 days, respectively, after adolescent ethanol exposure. In conjunction with the behavioral deficits observed in these studies, adolescent ethanol exposure also produced long-term brain changes, with reports of upregulation of TLR4, TLR3 and HMGB1 expression in the PFC [13] and decreased basal forebrain volume and numbers of cholinergic neurons [14]. These data support the hypothesis that ethanol exposure during adolescence, a period of time during which the brain is still undergoing significant maturational changes, results in long-lasting alterations in neurobehavioral development (see [4,6] for discussion).
Given that adolescent ethanol exposure previously has been found to influence learning in adulthood on various cognitive tasks, the purpose of this series of experiments was to determine if adolescent or adult ethanol exposure would affect later learning and memory during contextual and auditory Pavlovian fear conditioning and extinction. Pavlovian fear conditioning is an ideal model to assess consequences of adolescent ethanol exposure on learning and memory because the neural mechanisms involved have been examined extensively, with key brain regions that include the amygdala, hippocampus, and medial prefrontal cortex (mPFC) playing a critical role in tone conditioning, context conditioning, and extinction of these tasks, respectively (see [15, 16, 17, 18, 19] for reviews). These structures and connectivity among these regions continue to develop during adolescence (see [1,2] for review), and therefore may be particularly vulnerable to ethanol exposure at that time, perhaps changing their developmental trajectory. Thus, Pavlovian fear conditioning and extinction may provide useful tools to assess potential structure-relevant cognitive alterations following adolescent ethanol exposure.
One study to date has examined long-term consequences of adolescent ethanol exposure on fear conditioning in adulthood. Bergstrom et al. [20] found a deficit in tone fear conditioning in adult Long Evans rats 30 days after repeated voluntary access to ethanol during adolescence (P22-45), but not adulthood (P80-97). Interpretation of these results, however, is complicated by the fact that adults consumed significantly less ethanol than the adolescents, thereby confounding amount of ethanol exposure across age. Thus, although that study suggests that adolescent ethanol exposure may result in long-term deficits in tone fear memory, it is unclear if comparable exposure in adults would produce similar deficits. Furthermore, no studies to date have examined context conditioning or extinction after either tone or context conditioning in adolescent ethanol-exposed animals. Given that context conditioning is relatively hippocampal-reliant [21, 22, 23] and the medial prefrontal cortex (mPFC) has been implicated in extinction learning (see [18,19] for review), assessing these particular learning and memory processes after adolescent ethanol exposure may add valuable information in terms of potential structure-specific changes after ethanol exposure.
Two experiments were conducted to assess tone and context fear acquisition, retention and extinction 22 days after repeated intermittent ethanol exposure consisting of 4 g/kg intragastric (i.g.) ethanol or water every 48 hrs for a total of 11 exposures. In the first experiment, exposure started in early adolescence [Postnatal day (P) 28-48] or adulthood (P70-90), with tone and context conditioning and extinction assessed 22 days later. In a follow-up to the first experiment, a comparable study was conducted in a second adolescent exposure group where the exposure began in mid-adolescence, continuing through late adolescence (P35-55).
General Methods
Subjects
Male Sprague-Dawley rats bred and reared in our colony at Binghamton University were used in this experiment. On the day after birth, postnatal day (P) 1, litters were culled to 8-10 pups, with a sex ratio of 6 males and 4 females retained whenever possible. Pups were housed with their mother in a standard clear plastic tub with shavings until pair-housed with same-sexed littermates at the time of weaning (P21). Animals were maintained in a temperature-controlled vivarium on a 12:12-h light: dark cycle (lights on 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. All animals were maintained and treated in accordance with the Guide for the Care and Use of Laboratory Animals established by the National Institutes of Health (8th Ed), using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Exposure
In order to reduce the impact of litter effects, no more than one animal from a given litter was assigned to any experimental group [24]. Littermates housed as pairs were randomly assigned to the same exposure group, with 1 animal of the pair assigned to tone conditioning and the other assigned to context conditioning. Animals at each age were given 4 g/kg (25% v/v) EtOH or an equivalent volume of tap water (H20) intragastrically (i.g.) every other day throughout a 20 day exposure period for a total of 11intubations. All intubations were given between 10 am and 12 pm. To assess tolerance development across the exposure period, each animal was observed for 15 sec in their home cage for degree of impairment 15 minutes after each intubation and assigned a score using an intoxication scale. The intoxication scale ranged from 1-5, with 1= normal (no sign of intoxication), 2= slightly intoxicated (minimal signs of intoxication such as slight motor impairment when walking, but otherwise appears normal), 3= moderately intoxicated (obvious signs of impaired motor activity but can still walk on its own), 4= highly intoxicated (severe motor impairment: may have trouble standing, may crawl instead of walking, dragging abdomen), and 5= extremely intoxicated (unconscious and difficult to arouse, loss of righting reflex). A score of 5 was never given to an animal in these experiments. After the exposure period, animals were not disturbed aside from routine animal care (i.e., cage changing, etc.) for 22 days. In these experiments, post-exposure period was held constant between the age groups rather than testing age in adulthood, an approach often used when assessing long-lasting consequences of ethanol exposure, given that the length of the drug-free period following the exposure period may impart the nature of the adaptations observed.
Fear Conditioning Methods
Apparatus
All behavioral assessments were conducted in 8 identical fear conditioning chambers (32 × 25 × 25 cm, Med Associates). Each conditioning chamber was made of clear polycarbonate (top, front walls), white acrylic (back wall), and stainless steel (sides, shock grids, drop pan) material, and equipped with a speaker in the side wall. The grid floors consisted of 19 parallel 4.8 mm diameter rods situated 1 cm apart. At the time of the test for tone retention/extinction (Day 3), the context was modified by the addition of a smooth floor covering made of white plastic and an A-frame made of black acrylic that fit tightly in the chamber (height:17.5cm, side length: 23.5cm). Chambers and inserts were cleaned with 6% hydrogen peroxide after each trial. Each chamber was located within a sound-attenuated wood box (63.5 cm wide, 35.5 cm high, 76 cm deep) affixed with a ventilation exhaust fan that provided background noise (65 dB) and an overhead LED-based light source (Med Associates NIR-100). All behavioral sessions were video recorded by a camera in each conditioning chamber that was connected to a computer in the room. Percent time spent freezing was calculated at 30 frames per second by the Med Associates VideoFreeze system, a validated method for automated assessment of Pavlovian conditioned freezing behavior [25].
Procedure
All manipulations throughout the fear conditioning procedure took place between the hours of 11 am and 2 pm. For 3 days prior to conditioning, animals were transported to a room adjacent to the conditioning room to be weighed and handled once daily.
Conditioning (DAY 1)
Eight animals at a time were transported in their homecages to a room adjacent to the conditioning room to be weighed. Animals were placed back in their homecages after weighing and, after 10 minutes, were transported to the conditioning room. The conditioning context was the same for both tone and context conditioning, and consisted of a grid floor delivering footshock, and white light illuminating the chambers. All animals were given a 2 minute habituation period in the conditioning chambers followed by a ~6 minute conditioning period, and a 2 minute interval following the final footshock. One animal of the littermate pair received tone conditioning and the other received context conditioning. Animals that were assigned to tone conditioning received 3 CS-US pairings of a 10 second (s) tone (80 dB, 2000 Hz) coterminated with a 1 s footshock (0.5 mA) presented on a 110 s variable ITI. Animals in the context conditioning group received 3 presentations of 1s footshock (0.5mA) at the same time intervals as for tone conditioning, but without any tone presentations. After conditioning, animals were immediately placed in their home cage and returned to the colony room.
Context Fear Retention Test and/or Extinction (Day 2)
Previous data from our lab indicated that context extinction prior to tone testing was necessary to reduce pre-CS freezing during the tone fear retention test [26]. Thus, approximately 24 hrs after tone and context conditioning, all animals were placed in their original conditioning context for a 12 minute extinction session, with identical pre- and post-test procedures as on conditioning day.
Tone Fear Retention Test /Extinction (Day 3)
Following the identical pre-test procedures as on Day 2, tone conditioned animals were placed in a novel context created by the addition of a smooth floor and an A-frame to the original conditioning chamber. After a 2 min acclimation period, 30 presentations of the 10s tone alone, with an ITI of 10s were delivered (total time= 12 min). Context conditioned animals went through a second context extinction session identical to the previous day; these data are not shown since the results obtained were similar to the first context extinction session. Similarly, data from a second tone extinction session conducted on Day 4 are not shown since the results obtained were similar to the first tone retention/extinction day.
Data Analysis
Statistics
The percentage of time spent freezing on the conditioning and two extinction days were separated into time bins for each type of conditioning. For the tone conditioning day, percent freezing was calculated for the 2 minutes prior to the first tone, the 9 second duration of each of the three tones prior to footshock and the 2 minutes following the final CS-US pairing (total of 5 bins). For context conditioning, percent freezing was calculated for the 2 minutes prior to the first footshock, the two time periods between footshock exposures (1st-2nd and 2nd-3rd), as well as the 2 minutes following the final footshock (total of 4 bins). For analysis of data during tone extinction sessions, the 30 tone presentations were divided into 5 bins, each consisting of 6 tones and their associated 10s interstimulus intervals [ISI]. Context extinction sessions were separated into 2 minute time bins (total of 6 bins). The first bin from each extinction session was used to analyze fear retention. Factorial ANOVAs with age and exposure solution (i.e., H20 or EtOH) included as independent variables were used to analyze pre-CS baseline freezing and fear retention tests (bin 1) for both context and tone conditioned animals, and Tukey’s HSD post hocs were used to assess the locus of significant effects. Repeated measures ANOVAs were used to analyze acquisition and extinction separately at each age with, with Fisher’s LSD planned comparisons used to investigate significant effects involving time bin.
Exclusion Criteria
For tone conditioning, animals were excluded if they showed baseline freezing of >50% during the 2 min period prior to CS presentation on the tone fear retention/extinction session, given that high baseline freezing can make interpretation of CS freezing difficult [27]. Percent freezing during the tone and context fear retention test was checked for outliers at each age, with scores > 2 standard deviations from the mean of each experimental condition excluded from analysis. Exclusion numbers and final group n’s are indicated for each experiment.
Experiment 1
Subjects & Design
A total of 96 male Sprague-Dawley rats were used in this 2 exposure age (adolescent: P28-48; adult: P70-90) × 2 exposure (H20; EtOH) × 2 conditioning stimulus (tone; context) factorial study, with an n=12/group.
Animal Exclusion
A total of 2 adolescent and 3 adult EtOH-exposed, tone conditioned animals were excluded due to elevated baseline (pre-CS) freezing on the tone test day. Also, 2 adolescent-exposed animals from the H20-exposure, TONE conditioned group were excluded, with one excluded as a statistical outlier during the retention test and the other excluded due to an equipment problem during fear conditioning. Final n’s for the fear conditioning groups ranged 9-12/group.
Results
Tone Conditioning
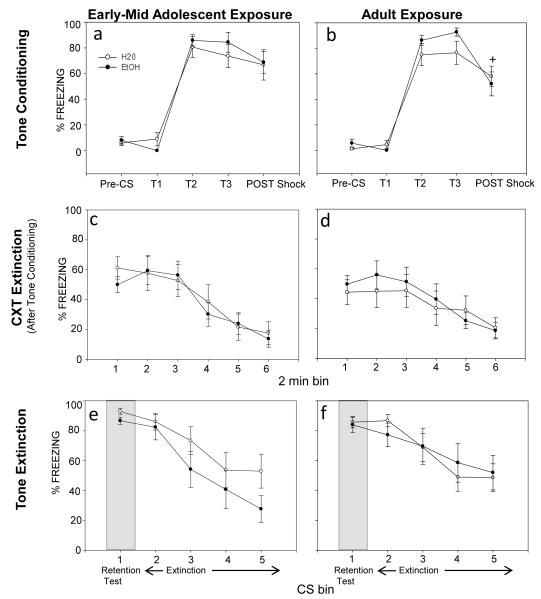
No effects of prior ethanol exposure emerged during tone conditioning, retention or extinction at either age (see Fig. 1a-1b and 1e-1f). Also, no effects of prior exposure emerged during context extinction after tone conditioning (see Fig. 1c-1d). As detailed below, significant effects of bin emerged in the analysis of freezing behavior during tone conditioning and extinction, indicating acquisition and extinction of conditioned fear to the tone CS, but again these effects did not differ as a function of prior exposure at either age.
Figure 1.
Tone conditioning (a-b), context extinction after tone conditioning (c-d) and tone extinction (e-f) in adult animals that were exposed to H20 or EtOH as adolescents (left panel) or adults (right panel). (a) Animals showed acquisition of tone fear during conditioning, but freezing did not differ as a function of adolescent exposure. (b) The adult exposure groups showed acquisition of tone fear, along with a significant decline in freezing during the post-shock period compared to freezing during tones 2 and 3 (T2 and T3), regardless of prior exposure (see +). (c d)Both exposure age groups showed a significant decline in context freezing, indicating extinction, but no effects of prior exposure were detected at either age. (e-f) Similarly, all animals showed tone extinction as indexed by a significant decline in freezing, but no effects of exposure were evident at either age.
Day 1
Conditioning
Separate 2 (exposure: H20; EtOH) × 5 (time bin) repeated measure ANOVAs of the adolescent-exposed and adult-exposed tone acquisition data revealed only significant main effects of bin [F(4,72)= 65.23, p<.05; F(4,76)= 84.60, p<.05, respectively]. As expected, freezing during tones 2 & 3 was significantly higher than pre-CS and tone 1 freezing, indicating acquisition of tone fear at both exposure ages (see Fig 1a and 1b). Adult animals also showed a significant decline in freezing during the 2 min. post shock period from freezing observed during the 2nd and 3rd tone (see Fig 1b).
Day 2
Context Extinction after tone conditioning
Separate 2 (exposure: H20; EtOH) × 6 (time bin) repeated measure ANOVAs conducted on the context extinction data in animals that were tone conditioned from each exposure age revealed only significant main effects of bin [F(5,90)= 19.94, p<.05; F(5,95)= 9.78, p<.05, respectively], with both ages showing extinction to the conditioning context (sees Fig 1c and 1d).
Day 3
Baseline (pre-CS) freezing
The 2 (exposure age: adolescent; adult) × 2 (exposure: H20; EtOH) factorial ANOVA of the baseline (pre-CS) freezing data revealed no differences in freezing at either age [F(1,37)= 0.01, p=.91], suggesting that freezing to the novel context did not differ as a function of prior exposure or exposure age (see Table 1).
Table 1.
Baseline freezing (%) prior to tone testing in Exp 1 & 2
| Adolescent Exposure | Adult Exposure | |||
|---|---|---|---|---|
|
|
||||
| H20 | EtOH | H20 | EtOH | |
| Exp 1 | 17 ± 4.2 | 21 ± 7.8 | 17 ± 4.6 | 20 ± 5.8 |
| Exp 2 | 14 ± 5.6 | 15 ± 4.2 | -- | -- |
Tone fear retention test
The 2 (exposure age: adolescent; adult) × 2 (exposure: H20; EtOH) factorial ANOVA examining tone fear retention revealed no effects of prior ethanol exposure, regardless of exposure age [F(1,37)= 0.40, p=.53] (see bin 1 of Fig. 1e and 1f).
Tone Extinction
Separate 2 (exposure: H20; EtOH) × 5 (CS bin) repeated measure ANOVAs of the tone extinction data from each exposure age only revealed significant main effects of bin [F(4,72)= 17.77, p<.05; F(4,76)= 14.54, p<.05, respectively], reflecting a significant decline in freezing across bin associated with the extinction of tone fear (see Fig. 1e and 1f).
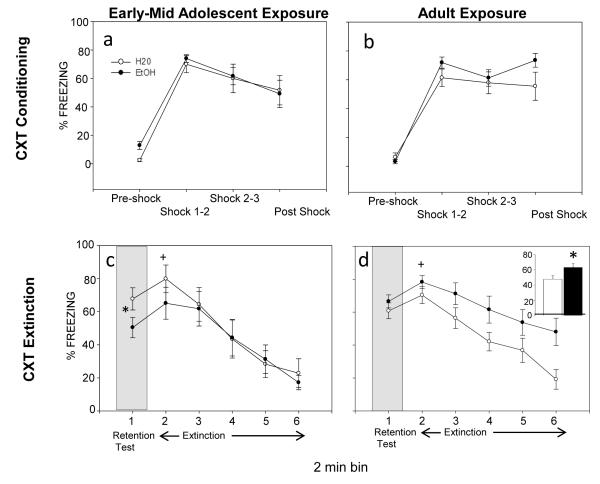
Context Conditioning
Ethanol exposure during adolescence (but not adulthood) produced a deficit in context fear retention (see Fig. 2c) whereas, ethanol exposure in adulthood resulted in impairment in context extinction (see Fig. 2d). Like tone conditioning, significant bin effects emerged in the analyses of context conditioning and extinction, indicating acquisition (see Fig 2a-2b) and extinction (see Fig. 2c-2d) of conditioned fear to the training context. Acquisition of context fear did not differ as a function of prior exposure at either age, and context extinction was not influenced by ethanol exposure during adolescence.
Figure 2.
Context conditioning (a-b), context fear retention (c) and context extinction in adult animals that were exposed to H20 or EtOH as adolescents or adults. (a-b) All animals showed acquisition of context fear, but freezing did not differ as a function of prior exposure in either exposure age group. (c) During the context retention test, adults that were exposed to ETOH as adolescents showed significantly less freezing than adolescent H20-exposed animals, indicating a deficit in context fear memory (see *). During extinction, freezing significantly increased from bin 1 to bin 2 (see +), and then declined during context extinction regardless of exposure group. (d) Exposure in adulthood did not influence context fear retention. Adults with a history of EtOH exposure, however, showed significantly more freezing overall than H20-exposed adults (see insert). Also, freezing significantly increased from bin 1 to bin 2 regardless of exposure group (see +), with freezing declining thereafter, indicating extinction.
Day 1
Conditioning
Separate 2 (exposure: H20; EtOH) × 4 (time bin) repeated measure ANOVAs conducted on the context acquisition data from each exposure age revealed only significant main effects of bin [F(3,57)= 47.54, p<.05; F(3,66)= 55.51, p<.05, respectively]. Freezing significantly increased between the first and second shock and remained elevated, indicating conditioning to the context (see Fig 2a and 2b).
Day 2
Context Retention Test
A 2 (exposure age: adolescent; adult) × 2 (exposure: H20; EtOH) factorial ANOVA on the context fear retention data revealed only a significant exposure × age interaction [F(1,42)= 4.52, p<.05], with animals exposed to ethanol as adolescents showing significantly less freezing to the context than their H20-exposed age-mates or than animals exposed to ethanol in adulthood (see Fig 2c).
Context Extinction after context conditioning
The 2 (exposure: H20; EtOH) × 6 (time bin) analysis of the context extinction data in context conditioned animals from the adolescent exposure groups only revealed a main effect of bin [F(5,100)= 25.84, p<.05], with a significant increase in freezing from bin 1 to bin 2 and a significant decline in freezing by bin 4 relative to bin 2 (see Fig 2d), regardless of exposure condition.
In the analysis of the adult context extinction data in context conditioned animals, significant main effects of exposure [F(1,22)= 4.52, p<.05] and bin[F(5,110)= 1.96, p<.05] emerged. Overall, animals with a history of EtOH during adulthood showed significantly more freezing than H20-exposed adults, although this effect tended to be more pronounced towards the end of the extinction session (see Fig. 2d). Similar to the adolescent exposure data, a significant increase in freezing was observed from bin 1 to bin 2 regardless of exposure history, followed by a significant decline in freezing by bin 3 (see Fig 2d). Increased freezing during bin 2 may be related to animals associating that time interval with the footshock that occurred on conditioning day given that bin 2 during extinction occurred at 2 minutes, the same time that the 1st footshock occurred on conditioning day.
Experiment 2
Introduction
Adolescence in the rat extends roughly from P28-55 in males [28, 29], thus the adolescent exposure period used in the previous experiment started in early adolescence. An important question is whether timing of exposure within the adolescent period would influence later behavioral effects. Indeed, there is some evidence to suggest that there may be differences in the lasting consequences of ethanol [30, 31], nicotine [32, 33, 34] and stress [35] exposure between pre-pubertal (~P21-34) and mid-(~P35-42) adolescents. The purpose of this experiment was to examine long-term effects of ethanol exposure starting in mid-adolescence (P35-55) on fear conditioning in adulthood to compare to the results obtained after early adolescent ethanol exposure in Exp 1.
Subjects & Design
A total of 48 male Sprague-Dawley rats were used in this 2 exposure (H20, EtOH) × 2 conditioning stimulus (tone or context) factorial study, with an n=12/group. Animals were exposed, conditioned and tested identically as in Exp 1, with the same 22 day post-exposure to test interval.
Exclusion
A total of 2 tone conditioned, H20-exposed animals were excluded due to elevated baseline (pre-CS) freezing during the tone retention test. 2 mid-adolescent exposed animals were excluded as outliers during the fear retention test (1 H20-exposed, CXT conditioned and 1 EtOH-exposed, TONE conditioned). Also, 1 animal was excluded due to equipment errors during fear conditioning (EtOH-exposed, CXT). Final n’s were 10-11/group.
Results
Significant bin effects were evident in the analyses of conditioning and extinction for both tone and context conditioning, reflecting acquisition and extinction on both tasks as expected from Exp 1 (data not shown). An effect of exposure emerged during context extinction after tone conditioning, with animals exposed to EtOH as mid-late adolescents showing significantly more freezing overall [F(1,21)= 4.53, p<.05] (see Fig. 3). This effect is reminiscent of findings from the adult ethanol exposure group in Exp 1, although the effect of prior ethanol exposure on context extinction was evident after context (not tone) conditioning in adults. No other significant exposure effects emerged during tone or context conditioning, retention or extinction. Thus, ethanol exposure starting in mid-adolescence did not produce the later context retention deficits that were evident after early adolescent ethanol exposure in Exp 1.
Figure 3.
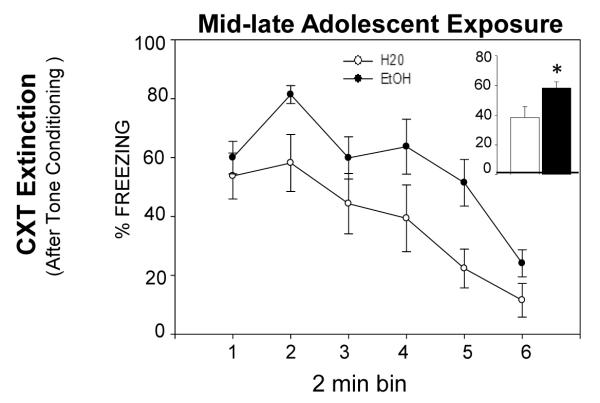
Freezing to the context after tone conditioning in animals that were exposed to H20 or EtOH starting in mid-adolescence (P35). Overall, significantly more freezing was observed in the EtOH exposure group than H20 group (see insert). No other effects of mid-adolescent EtOH exposure emerged during tone or context conditioning or extinction (data not shown).
Experiment 1 & 2: Exploratory Analyses
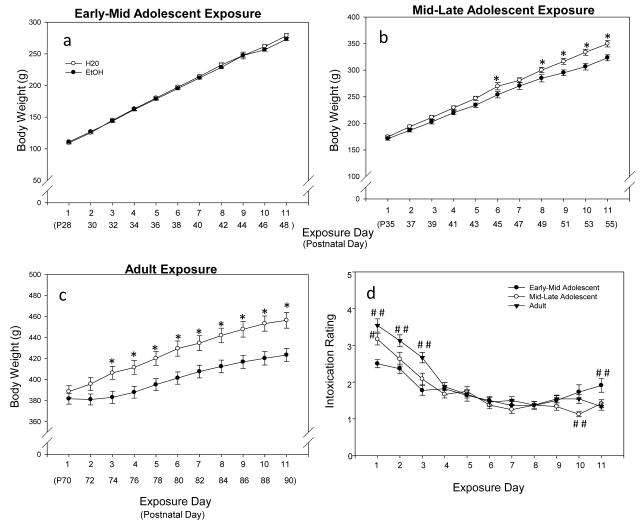
Given that the impact of repeated ethanol on fear retention and extinction varied with exposure age, potential age differences in the impact of ethanol on measures of body weight and intoxication collected during the exposure periods were explored. As described below, these exploratory analyses revealed that weight deficits emerging during the ethanol exposure period were particularly marked in adults, though also evident to some extent in animals exposed to ethanol in mid-late, but not early-mid adolescence. Intoxication ratings revealed greater intoxication in adults than both adolescent groups, and among mid-late adolescents than early adolescents early in the exposure period, with apparent tolerance to these effects emerging at all ages across days.
Results
Body Weight
Given the obvious initial weight difference between the age groups, exposure effects on body weight (g) across the 11 exposure days were analyzed using separate repeated measures ANOVAs for the early-mid adolescent, mid-late adolescent and adult data. In both the mid-late adolescent and adult analyses, significant main effects of exposure [F(1,46)= 5.11, p<.05; F(1,46)= 8.02, p<.05, respectively] and day [F(10,460)= 1127.62, p<.05; F(10,460)= 593.36, p<.05], and their interaction [F(10,460)= 5.89, p<.05; F(10,460)= 24.66, p<.05] emerged. EtOH-exposed animals weighed significantly less than H20 controls on Days 6 and 8-11 in mid-late adolescent animals, and on Days 3-11 in adults. No significant exposure effects emerged among the early-mid adolescent animals (see Fig. 4a-c).
Figure 4.
Early-mid adolescent, mid-late adolescent, and adult body weight and intoxication rating throughout the exposure period. (a) Weight deficits were not evident during the early adolescent exposure period. (b) Mid-late adolescents exposed to EtOH weighed significantly less than H20- exposed counterparts on exposure days 6 and 8-11 (see *’s). (c) EtOH-exposed adults showed weight deficits compared to H20-exposed adults starting on exposure day 3, which continued throughout the rest of the exposure period (see *’s). (d) During the first 3 days of the exposure period, adults showed significantly more signs of intoxication than both adolescent exposure groups (see ##) and mid-late adolescents showed significantly more intoxication than the early-mid adolescent exposure group (see #) on the first exposure day only. On exposure day 10, the mid-late adolescents showed significantly less signs of intoxication than early-mid adolescent and adult groups (see # #). By day 11, the early-mid adolescent exposure group had significantly higher intoxication scores than the mid-late adolescent and adult groups (see ##).
Intoxication Rating
A 3 (age) × 11 (day) repeated measures ANOVA revealed a significant main effect of day [F(10,670)= 81.21, p<.05] and a day × age interaction [F(20,670)= 5.61, p<.05]. Fifteen minutes after the first ethanol exposure, adults showed significantly more signs of intoxication than both adolescent groups, with the mid-late adolescent group also having significantly higher intoxication scores than early-mid adolescents. On Days 2 & 3, adults continued to show more signs of intoxication than both adolescent groups. Age effects also emerged at the end of the exposure, with the early-mid adolescents as well as adult animals showing significantly more intoxication on Day 10 than the mid-late adolescent group and the early adolescent group showing more intoxication signs than both mid-late adolescent and adult animals on Day 11, a finding that could potentially reflect an increase in ethanol sensitivity, perhaps related to emerging age and/or repeated ethanol exposure, by this day at the youngest exposure age, (see Fig. 4d).
Discussion
The purpose of these experiments was to assess potential long-lasting consequences of early-adolescent, mid-adolescent and adult ethanol exposure on tone and context fear conditioning retention and extinction. Early adolescent animals were relatively less sensitive than mid-adolescent and adult animals during the ethanol exposure period in terms of weight deficits and signs of intoxication. These results add to an established literature that adolescents are less sensitive than adults to the intoxicating effects of ethanol [36, 37, 38, 39, 40] and further suggests that age differences in ethanol sensitivity are evident across adolescence, with the sensitivity of mid-late adolescents intermediate between early-mid adolescents and adults. Despite the relative decreased ethanol sensitivity of the early-mid adolescent exposure group, repeated intermittent ethanol exposure starting in early adolescence attenuated context fear retention 22 days after the exposure period, an effect that was not observed after mid-late adolescent or adult ethanol exposure. Given that context fear retention is thought to be relatively hippocampal-dependent [21, 22, 23] these data suggest that early adolescence may be a vulnerable period for long-lasting disruption of hippocampal processing as a result of repeated EtOH exposure. Importantly, acquisition of context fear (or tone fear for that matter) was not influenced by exposure history at any exposure age, indicating that the context retention deficits observed after early adolescent ethanol exposure were not due to learning impairments, but rather were related to a deficit in memory retention of the context fear that was learned the prior day. The only exposure effect that emerged during either tone or context extinction was evidence of delayed context extinction in animals exposed repeatedly to ethanol in mid-adolescence and adulthood, but not in early-adolescence.
Two important points emerged regarding the effects of prior history of ethanol exposure on fear conditioning. First, the long-lasting effects of ethanol exposure were evident during context fear retention and extinction, but did not influence behavior of animals that were tone fear conditioned. These results are reminiscent of prior studies reporting that acute ethanol disrupts context, but not tone conditioning [26, 41, 42, 43], and further suggests that context fear is likewise vulnerable to long-lasting effects of repeated ethanol exposure. Second, the effects of ethanol exposure on context conditioning differed depending on the age of exposure, with early adolescent exposure resulting in deficits in context fear retention, whereas delayed context extinction was observed in the two older age groups. These results support the suggestion that the brain regions affected by ethanol exposure may vary with the developmental stage of exposure, with early adolescent ethanol exposure perhaps more likely to affect hippocampal processes [21, 22, 23], whereas ethanol exposure later in ontogeny may potentially influence extinction processes that are thought to be mediated by mPFC (see [18, 19] for review). Indeed, the mPFC is thought to be a late maturing structure, with the mPFC undergoing maturational changes during the transition from late adolescence into adulthood in terms of decreases in neuron numbers [44] and increases in fiber density from the basolateral nucleus of the amygdala [45] within this structure.
It was somewhat surprising that no effects of adolescent ethanol exposure emerged with tone conditioning, given that the only other study that assessed age differences in the long-term effects of repeated ethanol exposures found a deficit in tone fear retention in animals exposed to ethanol during early-mid adolescence (Bergstrom et al., 2006). Several experimental differences may account for the incongruent findings obtained in that study and the present study, such as differences in strain of rat (Long-Evans vs Sprague-Dawley), exposure (voluntary intake vs i.g.), and dependent measure (latency to movement vs percent freezing). Furthermore, it is not known whether context retention deficits analogous to those seen in the current work would have been observed under the circumstances of Bergstrom et al. [20] because that study did not examine context fear retention. However, another study assessing long-term effects of nicotine exposure found similar age-related vulnerability to drug-induced disruptions of context conditioning during adolescence as that seen in the present study. Portugal et al. [34] found that repeated nicotine exposure starting in early or mid-adolescence (but not adulthood) resulted in a deficit in context, but not tone fear retention in adulthood, with the early adolescent exposure group being particularly vulnerable, with these effects emerging at a lower exposure dose of nicotine in that group than the other exposure groups. Given evidence for a particularly important role of the hippocampus in context conditioning (as referenced above), these results suggest that early adolescent exposure to drugs may result in long-lasting alterations in hippocampal processes.
Previous studies examining the lasting consequences of adolescent ethanol exposure on other cognitive tasks in adulthood also tended to find impairments on tasks that are considered relatively hippocampal-dependent. For instance, the hippocampus has been implicated in conditional discrimination (Murray & Ridley, 1999) and novel object recognition (Ennaceur & Aggleton, 1997; Eichenbaum, 2000) tasks, both of which Pascual et al. [12] found to be affected by adolescent (but not adult) ethanol exposure. Learning deficits in the Morris water maze, a classic hippocampal-dependent task, were also found immediately after adolescent ethanol exposure, and this effect persisted into adulthood [11]. Other studies reported deficits during reversal learning (but not acquisition) in the Morris water maze[14] and Barnes maze [13] – two tasks thought to be hippocampally-dependent, although other brain regions, like the OFC [46] and basal forebrain [47], are thought to contribute to reversal learning per se. The results of this study contribute to the mounting evidence that adolescent ethanol exposure may lead to long-lasting deficits in cognition, particularly affecting tasks that are relatively hippocampal-dependent.
Ethanol exposure later in ontogeny, however, delayed extinction of context fear when EtOH-exposed animals were compared with their H20-exposed counterparts. It is not apparent why delayed context fear extinction after mid-adolescent ethanol exposure was only evident in animals that were tone conditioned, whereas in adult exposed animals, the effect emerged after context conditioning. While it is unknown whether processes differ between context extinction that occurs after cued vs. non-cued conditioning (i.e., when contextual cues are in the background versus foreground during conditioning), the results of this study suggest that there may be somewhat of a divergence in neural mechanisms underlying extinction after these two types of context conditioning, given that a delay in context extinction was not evident after both types of conditioning following ethanol exposure at the two older exposure ages. Indeed, Phillips & LeDoux [48] found that the neural mechanisms differed between background and foreground conditioning in terms of acquisition of context fear (although see [49]). However, given that the infralimbic (IL) portion of the mPFC has been implicated in both tone and context extinction [50, 51, 52], two types of conditioning that also differ in the neural mechanisms supporting acquisition, the mPFC likely contributes to context extinction after both cued and non-cued conditioning. Previous studies have reported that electrolytic lesions [53] and low frequency stimulation of the mPFC [54] delayed tone extinction and chemical inactivation of dopaminergic neurons in mPFC resulted in resistance to tone [52] and context [50] extinction. Thus, results of the current study suggest ethanol exposure at these later times results in delays in extinction reminiscent of those associated with disruption of the mPFC, although it is important to note that this impaired extinction was only evident in terms of extinction of context fear. In line with different mechanisms underlying cued and non-cued context fear extinction, it is also likely that the neural substrates critical for tone and context extinction differ to some extent, given the different sensory inputs and levels of integration involved. Indeed, some manipulations have been shown to dissociate these two forms of fear extinction, with for instance anisomycin, a protein synthesis inhibitor, reported to produce a deficit in tone fear extinction [55], while enhancing context extinction [56].
It is also possible that the specific disruption in extinction of context (but not tone) fear extinction observed in the present study after mid-adolescent and adult ethanol exposure could be related to alterations in anxiety levels in these animals. A dissociation of the evoked emotional response between tone and context conditioning has been proposed, with fear thought to be elicited by a discrete cue (e.g., tone), whereas anxiety is elicited by a more diffuse threat such as in context conditioning [57]. Consistent with this general notion, context fear conditioning has been proposed as a model for generalized anxiety disorder (GAD), whereas tone fear has been suggested to better model phobias [58]. Indeed, increased anxiety after protracted abstinence from repeated ethanol exposure has been reported previously in adults [59, 60]. The present data, therefore, extend previous findings to include resistance to context fear extinction, and suggest that repeated ethanol exposure later in ontogeny (during mid-late adolescence or adulthood) may result in a persistent anxiety-like profile.
It is important to note that the exposure-test interval of 22 days was held constant across exposure age, rather than age at testing. We decided to take this approach to ensure that the effects we observed were not due to potential differences in stage of withdrawal from ethanol exposure across group. Indeed, previous studies have indicated that effects of ethanol exposure on learning and memory, as well as associated neural changes, differ depending on the time between exposure and testing [61]. Furthermore, given that animals in this study were tested during protracted abstinence, the observed changes in behavior are presumably relatively long-lasting effects as a consequence of prior ethanol exposure.
While the human implications of these basic research findings of course remain most speculative, these data provide cues as to potential target outcomes that might be associated with different developmental histories of alcohol use and abuse in humans. Interestingly, human imaging studies have reported significantly smaller hippocampal volumes in adolescents who met criteria for AUD relative to comparison subjects [62, 63], although given the cross sectional nature of these data, it is not clear whether hippocampal volume deficits preceded or were a consequence of adolescent alcohol use. In our preclinical study, however, early adolescent ethanol exposure produced later attenuations in retention of context fear, consistent with the idea that adolescent alcohol exposure may persistently disrupt hippocampal processes. To the extent that the data collected using this animal model serve as a useful model of human alcohol exposure, these findings raise the possibility that later retention of memories, particularly those that are episodic in nature (i.e., the recollection of the time, place, and emotion of a particular event [64]), could potentially be affected in human adolescents who engage in alcohol use during early adolescence, even if alcohol use does not extend beyond the adolescent years. Moreover, to the extent that it is possible to extrapolate these findings to humans, it would additionally be expected that repeated alcohol use/abuse beginning later in adolescence or adulthood might have different, but also persisting consequences, with the resistance to context fear extinction in ethanol-exposed mid-adolescent and adult animals seen in the current study potentially implicating a profile of heightened anxiety. The differing behavioral outcomes observed depending on age of ethanol exposure are consistent with the speculation that the hippocampus may be particularly susceptible to lasting perturbations by ethanol during early adolescence, with the mPFC perhaps showing greater sensitivity beginning in mid-adolescence. Taken together, these data also emphasize the critical importance of the timing of ethanol exposure within the broad adolescent period. These results provide a basis for further investigation of age specific neural changes induced by ethanol, and ultimately their relationship to potentially persisting consequences of alcohol use and abuse that may vary with the timing of alcohol exposure among early adolescent and older youth.
Highlights.
Context fear retention was disrupted after early adolescent ethanol exposure
Mid-adolescent and adult ethanol-exposed animals showed delayed context extinction
Thus, long-term effects of ethanol exposure differ depending on age of exposure
Acknowledgments
The research presented in this paper was supported by NIAAA grants NIAAA grants R01AA018026 and U01AA019972-NADIA Project
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Spear L. The Behavioral Neuroscience of Adolescence. W.W. Norton & Company Inc.; New York, NY: 2010. [Google Scholar]
- [2].Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience & Biobehavioral Reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- [3].Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. International Journal Of Developmental Neuroscience: The Official Journal Of The International Society For Developmental Neuroscience. 2004;22:423–40. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [4].Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacology, Biochemistry, And Behavior. 2007;86:189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- [5].Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals Of The New York Academy Of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- [6].Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol (Fayetteville, NY) 2010;44:15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- [7].Johnston L, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2008 (NIH Publication No. 09-7401) National Institute on Drug Abuse; Bethesda, MD: 2009. [Google Scholar]
- [8].Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: basic to clinical studies. Annals Of The New York Academy Of Sciences. 2004;1021:234–44. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- [9].Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcoholism, Clinical And Experimental Research. 1998;22:416–21. [PubMed] [Google Scholar]
- [10].Crews FT, Braun CJ, Hoplight B, RCS, Knapp DJ. Binge Ethanol Consumption Causes Differential Brain Damage in Young Adolescent Rats Compared With Adult Rats. Alcoholism: Clinical and Experimental Research. 2000;24:1712–23. [PubMed] [Google Scholar]
- [11].Sircar R, Sircar D. Adolescent Rats Exposed to Repeated Ethanol Treatment Show Lingering Behavioral Impairments. Alcoholism: Clinical and Experimental Research. 2005;29:1402–10. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- [12].Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. The European Journal Of Neuroscience. 2007;25:541–50. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- [13].Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–88. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Coleman LG, Jr., He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism, Clinical And Experimental Research. 2011;35:671–88. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review Of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- [16].LeDoux JE. Emotion circuits in the brain. Annual Review Of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- [17].Sanders MJ, Wiltgen BJ, Fanselow MS. The place of the hippocampus in fear conditioning. European Journal Of Pharmacology. 2003;463:217–23. doi: 10.1016/s0014-2999(03)01283-4. [DOI] [PubMed] [Google Scholar]
- [18].Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–84. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- [19].Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology: Official Publication Of The American College Of Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bergstrom HC, McDonald CG, Smith RF. Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long-Evans rats. Physiology & Behavior. 2006;88:466–72. doi: 10.1016/j.physbeh.2006.04.021. [DOI] [PubMed] [Google Scholar]
- [21].Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behavioral Neuroscience. 1993;107:1093–8. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- [22].Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiology of Learning and Memory. 1997;67:142–9. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- [23].Antoniadis EA, McDonald RJ. Amygdala, hippocampus and discriminative fear conditioning to context. Behavioural Brain Research. 2000;108:1–19. doi: 10.1016/s0166-4328(99)00121-7. [DOI] [PubMed] [Google Scholar]
- [24].Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology And Teratology. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- [25].Anagnostaras SG, Wood SC, Shuman T, Cai DJ, Leduc AD, Zurn KR, et al. Automated assessment of pavlovian conditioned freezing and shock reactivity in mice using the video freeze system. Frontiers In Behavioral Neuroscience. 2010;4 doi: 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Broadwater MB, Spear L. Age differences in fear conditioning and extinction: Effects of ethanol challenge during conditioning. Behavioral Brain Research. doi: 10.1016/j.bbr.2013.06.029. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jacobs NS, Cushman JD, Fanselow MS. The accurate measurement of fear memory in Pavlovian conditioning: Resolving the baseline issue. Journal Of Neuroscience Methods. 2010;190:235–9. doi: 10.1016/j.jneumeth.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schneider M. Adolescence as a vulnerable period to alter rodent behavior. Cell And Tissue Research. 2013 doi: 10.1007/s00441-013-1581-2. [DOI] [PubMed] [Google Scholar]
- [29].Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Developmental Psychobiology. 2012;54:523–35. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, et al. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–31. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- [31].Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. International Journal of Developmental Neuroscience. 2009;27:805–15. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- [32].Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology: Official Publication Of The American College Of Neuropsychopharmacology. 2002;27:212–24. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- [33].Dao JM, McQuown SC, Loughlin SE, Belluzzi JD, Leslie FM. Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology: Official Publication Of The American College Of Neuropsychopharmacology. 2011;36:1319–31. doi: 10.1038/npp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiology of Learning and Memory. 2012;97:482–94. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wilkin MM, Waters P, McCormick CM, Menard JL. Intermittent physical stress during early- and mid-adolescence differentially alters rats’ anxiety- and depression-like behaviors in adulthood. Behavioral Neuroscience. 2012;126:344–60. doi: 10.1037/a0027258. [DOI] [PubMed] [Google Scholar]
- [36].Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcoholism, Clinical And Experimental Research. 1996;20:1346–51. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- [37].Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: Comparison to Zolpidem binding. Alcoholism: Clinical and Experimental Research. 1998;22:1485–92. [PubMed] [Google Scholar]
- [38].Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism-Clinical and Experimental Research. 1998;22:670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- [39].Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: Role of familiarity of the test situation. Alcoholism: Clinical and Experimental Research. 2002;26:1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- [40].White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, et al. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2002;73:673–7. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- [41].Hunt PS, Levillain ME, Spector BM, Kostelnik LA. Post-training ethanol disrupts trace conditioned fear in rats: effects of timing of ethanol, dose and trace interval duration. Neurobiologyof Learning and Memory. 2009;91:73–80. doi: 10.1016/j.nlm.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Melia KR, Ryabinin AE, Corodimas KP, Wilson MC, Ledoux JE. Hippocampal-dependent learning and experience-dependent activation of the hippocampus are preferentially disrupted by ethanol. Neuroscience. 1996;74:313–22. doi: 10.1016/0306-4522(96)00138-8. [DOI] [PubMed] [Google Scholar]
- [43].Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–15. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- [44].Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–8. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- [45].Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. The Journal Of Comparative Neurology. 2002;453:116–30. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- [46].Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK. A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nature Reviews. 2009;10:885–892. doi: 10.1038/nrn2753. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cabrera SM, Chavez CM, Corley SR, Kitto MR, Butt AE. Selective lesions of the nucleus basalis magnocellularis impair cognitive flexibility, in Behavioral Neuroscience. Vol. 120. American Psychological Association; 2006. pp. 298–306. [DOI] [PubMed] [Google Scholar]
- [48].Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- [49].Schenberg EE, Oliveira MGM. Effects of pre or posttraining dorsal hippocampus D-AP5 injection on fear conditioning to tone, background, and foreground context. Hippocampus. 2008;18:1089–93. doi: 10.1002/hipo.20475. [DOI] [PubMed] [Google Scholar]
- [50].Fernandez Espejo E. Prefrontocortical dopamine loss in rats delays long-term extinction of contextual conditioned fear, and reduces social interaction without affecting short-term social interaction memory. Neuropsychopharmacology: Official Publication Of The American College Of Neuropsychopharmacology. 2003;28:490–8. doi: 10.1038/sj.npp.1300066. [DOI] [PubMed] [Google Scholar]
- [51].Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109:681–8. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- [52].Morrow BA, Elsworth JD, Rasmusson AM, Roth RH. The role of mesoprefrontal dopamine neurons in the acquisition and expression of conditioned fear in the rat. Neuroscience. 1999;92:55364. doi: 10.1016/s0306-4522(99)00014-7. [DOI] [PubMed] [Google Scholar]
- [53].Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163:109–13. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- [54].Shehadi K, Maroun M. Different effects of low frequency stimulation to infralimbic prefrontal cortex on extinction of aversive memories. Brain Research. 2013;1490:111–6. doi: 10.1016/j.brainres.2012.10.026. [DOI] [PubMed] [Google Scholar]
- [55].Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. The Journal Of Neuroscience: The Official Journal Of The Society For Neuroscience. 2004;24:5704–10. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. The Journal Of Neuroscience: The Official Journal Of The Society For Neuroscience. 2004;24:1962–6. doi: 10.1523/JNEUROSCI.5112-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Seligman ME. Chronic fear produced by unpredictable electric shock. Journal Of Comparative And Physiological Psychology. 1968;66:402–11. doi: 10.1037/h0026355. [DOI] [PubMed] [Google Scholar]
- [58].Luyten L, Vansteenwegen D, van Kuyck K, Gabriels L, Nuttin B. Contextual conditioning in rats as an animal model for generalized anxiety disorder. Cognitive, Affective & Behavioral Neuroscience. 2011;11:228–44. doi: 10.3758/s13415-011-0021-6. [DOI] [PubMed] [Google Scholar]
- [59].Santucci AC, Cortes C, Bettica A, Cortes F. Chronic ethanol consumption in rats produces residual increases in anxiety 4 months after withdrawal. Behavioural Brain Research. 2008;188:24. doi: 10.1016/j.bbr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- [60].Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol (Fayetteville, NY) 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- [61].George O, Sanders C, Freiling J, Grigoryan E, Shayla V, Allen CD, Crawford E, Mandyam CD, Koob GF. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2012;109:18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. The American Journal Of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- [63].Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tulving E. Episodic memory: from mind to brain. Annual Review Of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]