Abstract
In the course of performing electrical stimulation functional mapping (ESFM) in neurosurgery patients we identified three subjects who experienced a hearing suppression effect following stimulation of sites within the superior temporal gyrus (STG). One of these patients has long standing tinnitus that affects both ears. In all subjects, auditory event related potentials (ERPs) were recorded from chronically implanted intracranial electrodes and the results were used to localize auditory cortex fields within the STG. Hearing suppression sites were identified within anterior lateral Heschl’s gyrus (HG) and posterior lateral STG, in what are likely belt and parabelt fields. Cortical stimulation suppressed hearing in both ears, and persisted beyond the period of electrical stimulation. Subjects experienced other stimulation evoked perceptions at some of these same sites, including symptoms of vestibular system activation and alteration of audio-visual speech processing. In contrast, stimulation of presumed core auditory cortex within posterior medial HG evoked sound perceptions, or in one case an increase in perceived tinnitus intensity, that affected the contralateral ear and did not persist beyond the period of stimulation. The current results provide confirmation of a rarely reported experimental observation, and for the first time correlate the brain sites associated with hearing suppression with anatomically and physiologically identified auditory cortex fields.
Keywords: Human, Auditory Cortex, Electrical Stimulation, Hearing Suppression, Tinnitus
Introduction
More than half a century ago, Penfield and Rasmussen (1950) first reported that electrical pulses applied to the superior temporal gyrus (STG) of neurosurgical patients produced alterations in auditory perception. In this and subsequent reports (Penfield and Jasper, 1954; Mullan and Penfield, 1959; Penfield, 1958; Penfield and Perot, 1963), Penfield and his colleagues observed that one form of auditory perceptual alteration (referred to as ‘auditory illusions’) was suppression of hearing. This hearing suppression effect was reported in only a small number of the more than 1100 patients studied (Mullan and Penfield, 1959; Penfield, 1958), and the effective stimulation sites were located mainly on the posterolateral aspect of the STG and the anterior portion of Heschl’s gyrus (HG). Years later Sinha et al. (2005) observed, in a single chronically implanted patient, that electrical stimulation of the surface of the left posterolateral STG resulted in reversible, moderate hearing loss in the right ear. In what may be a related phenomenon, it has also been reported that tinnitus is suppressed by electrical stimulation of posterolateral STG (de Ridder et al., 2004) and by repetitive transcranial magnetic stimulation (rTMS) directed toward temporal-parietal cortex (Plewnia et al., 2003). These observations raise the possibility that temporal lobe cortical stimulation might be an effective treatment for patients with intractable tinnitus.
Currently, little is known about this rarely reported hearing suppression phenomenon, and there have been no previous reports relating directly the effective cortical stimulation sites to physiologically mapped auditory fields. Human auditory cortex is made up of multiple fields on the STG (Galaburda and Sanides, 1980; Hackett et al., 2001; Wessinger et al., 2001; Wallace et al., 2002; Morosan et al., 2001; Rademacher et al., 1993) and using intracortical recording methods in humans we have electrophysiologically identified auditory fields on HG and on the posterolateral aspect of the STG (Howard et al., 1996a, 2000). In the course of these studies we encountered, somewhat serendipitously, three patients that experienced suppression of hearing during electrical stimulation functional mapping (ESFM) of sites along the posteromedial portion of HG and posterolateral STG. One of these patients experienced a suppression of his long-standing tinnitus. By combining ESFM with electrophysiological mapping of auditory event related potential (ERPs) we were able to localized effective stimulus sites to physiologically-identified auditory fields.
Results
The results described below were obtained during ESFM and electrophysiological recording in three neurosurgery patients with multi-contact intracranial electrodes chronically implanted on the posterolateral superior temporal gyrus and/or in Heschl’s gyrus (Table 1). Although our attention was first drawn to their reports of hearing suppression, a study of the video-taped sessions revealed that the sensations evoked by electrical stimulation were often more complex than this. Thus, we present the ESFM results as verbatim quotes that we consider representative of each subjects’ experiences. These are then related to maps derived from ERP recordings.
Table 1.
Patient Data and Characteristics.
| Patient Data and Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Patient | Age | Sex | Hand | Seizure Type | EEG | MRI findings | PET findings | Site of implantation |
| 10R | 29 | F | R | Simple and Complex Partial | Indeterminate; intracranial EEG revealed R posterior temporal localization |
Normal | Normal | R Heschl’s Gyrus |
| 18L | 25 | M | R | Simple Partial | L temporal spiking | Subependymoma in atrium of L Lateral Ventricle |
N/a | L posterolateral STG |
| 32R | 32 | M | R | Complex Partial | Suggestive of R focus; intracranial EEG revealed R mesial temporal focus |
Heterotopic grey matter adjacent to occipital horn of R Lateral Ventricle |
R mesial temporal lobe hypometabolism |
R Heschl’s Gyrus, R posterolateral STG |
Subject 10R
Subject 10R was a 29-year-old, right-handed female with a history of epilepsy that began 11 years prior to our study. The post-implantation MRI results confirmed placement of a hybrid depth electrode (HDE, Howard et al., 1996b) within HG. A grid electrode implanted over the perisylvian cortex was used for clinical ECoG recording only. The trajectory of the HDE and the location of each electrode contact along the shaft are shown in the scaled line drawing of the supratemporal plane in Figure 1. ERPs to repeated clicks are shown connected by dashed lines to the cortical sites from which they were derived. High amplitude ERPs were recorded from posteromesial HG. Their waveforms and the latency of major peaks are illustrated. Although we do not have a more accurate reconstruction of the electrode trajectory, we attribute the change in the shape of ERP waveforms in this region of HG to the recording contacts lying in different spatial relationships to the generating dipole. We interpret the most mesial ERPs as being derived from the auditory core (see also Celesia and Puletti, 1969; Liegeois-Chauvel et al., 1991). The fact that there is a very small ERP between two large ERPs with quite different latency characteristics suggests that the electrode crossed a functional boundary with the large lateral ERP being derived from an auditory belt field. The ESFM results further suggest that this may, indeed, have been the case.
Figure 1.
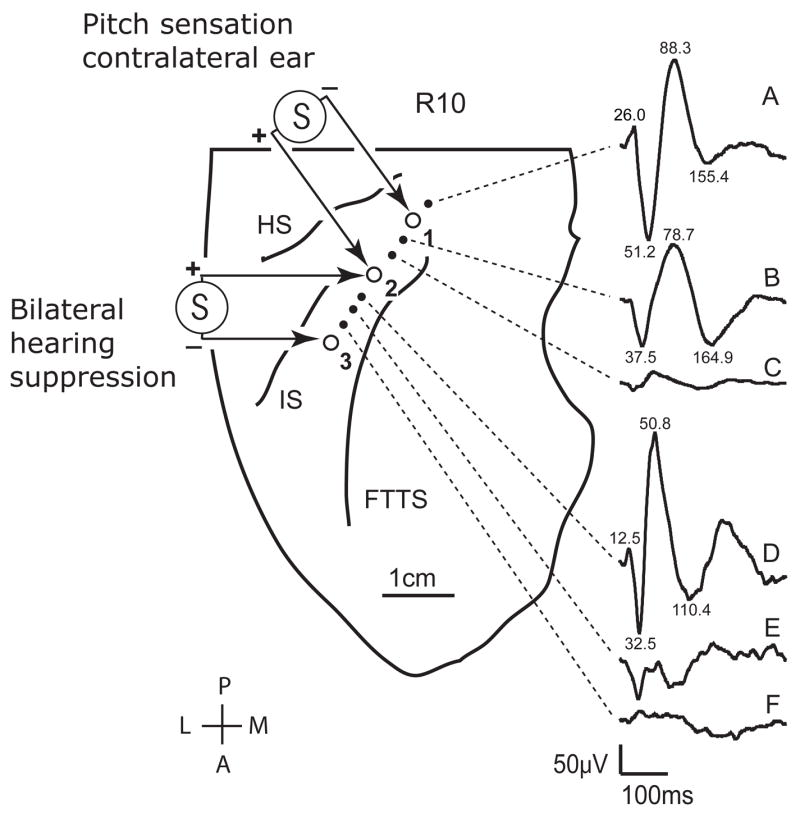
Scaled anatomical line drawing of the right supratemporal plane of subject R10, based on post-implantation volumetric MRI imaging data. The locations of high impedance (small filled circles) and low impedance (large open circles) electrode contacts within Heschl’s gyrus are shown. The low impedance contacts are numbered from the most posterior medial (#1), to the most anterior lateral (#3). Averaged auditory evoked potentials to click train stimuli recorded from the high impedance contacts are shown to the right of the figure. Latencies in milliseconds are provided for the most prominent peaks of waveforms A, B, and D; waveforms C, E and F do not have readily discernable peaks in their tracings. Hearing suppression effects were observed with bipolar electrical stimulation of low impedance contacts #2 (positive) and #3 (negative), whereas stimulation at contacts #1 (negative) and #2 (positive) elicited a perception of a tone lateralized to the contralateral ear. Abbreviations: HS- Heschl’s sulcus, FTTS- first transverse temporal sulcus, IS- intermediate sulcus.
During electrical stimulation of the posterior medial electrode sites (sites 1(+) 2(−)), which were in close proximity to the sites where high amplitude ERPs were recorded, the subject reported that she “perceived a tone” and that it was heard “mainly in the left ear.” She further reported that the intensity of the tonal sensation increased with increased stimulus level. Changing stimulus polarity led to a perceived change in pitch. This is consistent with the stimulus having activated core cortex, as changing the polarity of stimulation likely altered the spatial distribution of cortical activation around the electrode pair which then may have shifted the locus of excitation along the tonotopic axis. No other sensations were reported when these posterior medial sites were stimulated. Electrical stimulation of the more anterior lateral sites on HG (sites 2(+) 3(−)), where the ERP was also of high amplitude but of a waveform demonstrably different from that recorded medially, resulted in suppression of hearing that was embedded in other sensations. Hearing suppression outlasted electrical stimulation, although this time was not measured in this subject as it was in subject 32R. The hearing suppression effects were observed during each of four stimulation sessions on one day, and during each of three stimulation sessions on a second day. The following are quotes taken from the experimental transcript that characterize her hearing suppression when she was exposed only to ambient room sound:
“tuned my hearing out”
“once again is drowning out my hearing”
“it overpowers my hearing”
“my ability to hear was kind of dead”
“what it does is deadens, muffs my own hearing. So it kind of makes you feel like you’ve been in a loud room for a long time and walking out. My ears didn’t buzz or hum or anything but it deadened my hearing. What ever this was over-powered my hearing or the ability to hear.”
After listening to the examiner speaking while electrical stimuli were delivered to these same HG sites the subject reported:
“That was weird. It kind of muffed your voice a bit.”
But, after listening again to the examiner counting aloud the subject reported:
“it kind of made your voice … uhm … I want to say echo, kind of, almost like my hearing echoed your voice. Your numbers were clear… you weren’t as loud.”
Hearing suppression was not lateralized, unlike the perception in the left (contralateral) ear only of sound having pitch quality resulting from more mesial stimulation. When the subject listened to a 1 kHz tone delivered binaurally through headphones, she reported similar hearing suppression. When asked by the experimenter if it seemed like the suppression of the experimenter’s speech and the 1 kHz tone was occurring in one ear or the other, she replied that for both sounds it came “straight across…. It kind of overpowered both.”
From the transcript it appears that the auditory sensations elicited by anterior lateral stimulation were more complex than simple hearing suppression. On several occasions the subject reported that electrical stimulation “makes me feel a little strange”, that it is an “odd feeling”.
Also during such electrical stimulation, when there was no acoustic stimulus deliberately presented, the subject sometimes reported hearing a sound that was quite different from the one perceived as having a constant pitch when the stimulus was applied to posteromesial HG:
“It’s not a continuous sound.”
“(It sounds like) loud chopping. Like a rope in the air.”
During electrical stimulation of HG sites this subject exhibited no speech abnormalities nor were there observable abnormal movements of the face, mouth, tongue or arms.
Subject 18L
Subject 18L was a 25-year-old, right-handed male with symptoms and EEG findings consistent with simple partial seizures involving visual cortical regions. An MRI revealed a tumor located within the atrium of the left lateral ventricle. The post-implantation MRI results confirmed placement of a 20-contact subdural recording grid over the left perisylvian cortex; no depth electrode was implanted in this patient. ERPs to clicks were recorded on the posterior lateral aspect of the STG, which corresponds to area PLST. Electrical stimuli were applied to those electrode sites at or near the site of maximal ERP amplitude while the subject listened to his own voice, to ambient room sound and to the voice of the examiner. Hearing suppression effects were observed repeatedly during four ESFM sessions on a single day. When the patient was counting aloud, electrical stimulation at these two adjacent contacts caused the patient to report that
“my voice gets lower, while I was talking…to me it sounded lower than the rest.”
The examiners noticed no difference in the pitch or volume of the subject’s voice.
Electrical stimulation at the same two contacts while the examiner was counting aloud led the patient to report:
“To me it sounded the same, but in the middle it made my ears feel a little different…almost plugged ears…a weird feeling…can’t describe it, just for a little time.”
While listening to ambient room sound, stimulation of the same two contacts caused the patient to report:
“a weird feeling…no pain…it just kind of played something in my ears.”
No abnormal movements of the face, mouth, tongue or arms were exhibited by the patient during electrical stimulation. Having either the patient or the examiner count during control trials, in which no electrical stimulation was applied, had no demonstrable effect on the patient’s hearing.
Subject 32R
Subject 32R was a 32-year-old, right-handed male who had experienced complex partial seizures from the age 14. In addition, this subject suffered from chronic bilateral tinnitus.
Placements of the HDE within the HG and of the electrode grid over posterior lateral STG were confirmed. The trajectory of the HDE and the location of each electrode contact on it are shown in the scaled line drawing of the supratemporal plane in Figure 2. As with subject 10R, a high amplitude ERP was evoked by a click stimulus at the most posteromesial contact within HG (Figure 2A). ERP amplitude decreased abruptly at more lateral recording sites. The low amplitude evoked activity gradually changed from a predominantly positive-going to a small but predominantly negative-going waveform at progressively more lateral recording sites. As with subject 10R, the ERP findings are consistent with the most mesial electrode having been within, or in close proximity to, core auditory cortex, with more anterior lateral sites making a transition to the auditory belt.
Figure 2.
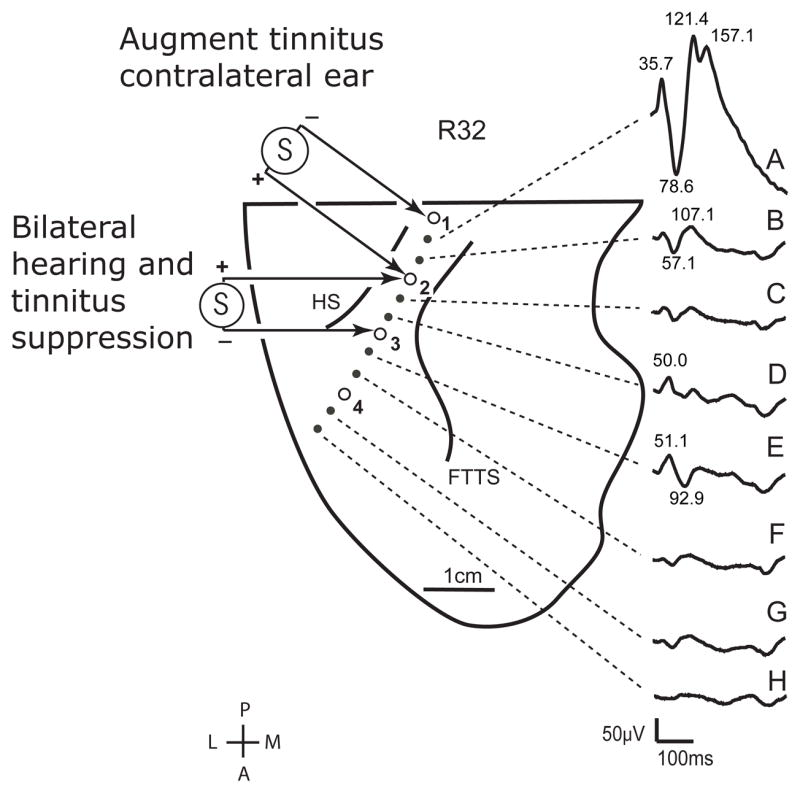
Scaled anatomical line drawing of the right supratemporal plane of subject R32. The locations of low impedance contacts (open circles) within Heschl’s gyrus are shown from the most posterior medial (#1) to the most anterior lateral (#4) sites. Averaged auditory evoked potentials recorded from these contacts are displayed to the right of the figure. Latencies in milliseconds are provided for the most prominent peaks of waveforms A, B, D and E. Bipolar electrical stimulation of sites #2 and #3 consistently produced a tinnitus and hearing suppression effect, as well as a subjective sense of falling and other sensations, as described in the text. Stimulation at contacts #1 (negative) and #2 (positive) elicited a subjective increase in tinnitus in the contralateral ear.
The findings from HG stimulation in subject 32R were similar in several ways to those from subject 10R in that electrical stimulation of HG resulted in suppression of hearing along with complex mix of other sensations. In this subject this also included a change in the intensity of his long-standing tinnitus. During electrical stimulation of the most posterior medial sites of HG (sites 1(−) 2(+)), which bracketed the site from which the high amplitude ERP was recorded, subject 32R reported an increase in the intensity of his tinnitus, which affected mostly his left (contralateral) ear, and which returned to baseline intensity shortly after the end of stimulation. No other sensation was reported. These observations suggest that the augmentation of tinnitus was the result of evoking a tonal sensation, similar to that evoked in subject 10R from posterior medial HG, which simply added to the existing tinnitus.
Electrical stimulation of sites anterior lateral to the presumed core auditory field (sites 2(+) 3(−)) resulted in a suppression of hearing both air-borne sound and of tinnitus. When the subject listened to a 1 kHz tone delivered binaurally through headphones the resulting hearing suppression was reported as follows:
32R: “It lowered it a bit, then brought it back up when you turned it off.”
Examiner: “Was it the sound intensity that was changed?”
32R: “Yeah.”
Using the same stimulus paradigm, the effect on his tinnitus was reported as follows:
Examiner: “What did this electrical stimulation do to that (tinnitus)?”
32R: “Actually, it quieted it.”
Examiner: “Was it noticeable?”
32R: “It was noticeable, but I could still hear it… but it wasn’t as intense.”
Examiner: “Was it an uncomfortable sensation?”
32R: “No. I wouldn’t say…call it uncomfortable.”
Examiner: “Did it change in pitch or change in intensity?”
32R: “Change in intensity.”
32R: “I can still hear it but it’s not as intense.”
The subject was instructed to judge how long the hearing and tinnitus suppression lasted beyond the period of electrical stimulation by observing the sweep-second hand of a clock. The experimenter provided a hand signal to the subject indicating when the electrical stimulation was started and stopped. Watching the clock, the subject estimated that suppression of hearing the 1 kHz test tone persisted for 20 sec beyond the period of electrical stimulation. This test on tinnitus suppression was then carried out on two other occasions resulting in estimates of 12 and 15 sec.
There was no lateralizing effect to the cortical stimulation induced suppression of hearing in this subject. When the subject was asked whether the tinnitus suppression affected one ear more than the other, he answered that the tinnitus was suppressed in “both ears.” Similarly, suppression of the intensity of the test tone also affected both ears. Changing stimulus polarity had no reported effect on the suppression of hearing or of tinnitus. Hearing suppression effects were observed during each of the 28 stimulation sessions carried out during six experimental sessions over a three-day period.
In addition to the reports of hearing suppression, the subject also reported other sensations when these sites were stimulated:
Examiner: “Did it (stimulation) change your perception of my voice?”
32R: “Yeah it did.”
Examiner: “In what way?”
32R: “Your voice got much deeper.”
Examiner: “My voice sounded lower?”
32R: “Yeah.”
Changing polarity of stimulation (sites 2(+) 3(−)) the following sensation was reported.
32R: “I’m having trouble putting your words with your mouth movements.”
Examiner: “Your perception of my speaking is…?”
32R: “…slower than how fast I’m hearing your words.”
Examiner: “When the stimulator was on, does my voice sound the same to you pretty much? Or different?”
32R: “It’s not matched up with your mouth moving.”
Examiner: “The timing is not quite right?”
32R: “The timing is off. I hear your voice… then I see your mouth.”
During HG stimulation of sites that resulted in suppression of the subject’s tinnitus, the subject’s facial movements and non-articulatory tongue movements appeared normal, as did fine finger and hand dexterity bilaterally. He verbally identified pictures of objects without difficulty and followed verbal commands without errors. However, when he was instructed to count aloud, or repeat phrases, such as “around the rugged rock, the ragged rascal ran,” his speech patterns were abnormal. When counting, his vocalizations were abnormally high pitched and the cadence of the number sequence was abnormally slow and irregular. When attempting to repeat a phrase spoken by the examiner, however, he had more pronounced difficulty articulating each word, his progress through the sentence was labored with obvious stuttering, and his intonation was abnormally high pitched and wavering. Within approximately 20 seconds of cessation of the electrical stimulation, the effect on the subject’s expressive speech function resolved.
In addition to the hearing related sensations experienced by this subject upon stimulation of anterior lateral HG sites, he also experienced a dropping or falling sensation suggesting a disruption of normal vestibular functions. He described it as a
“Dropping feeling…felt like the elevator dropped…. Slow dropping effect.”
In this same subject we recorded sound evoked ERPs at each of the 60 grid sites on posterior lateral STG. This is an auditory area we had identified earlier as field PLST (Howard et al., 2000). We then tested all sites using ESFM. Figure 3A shows the area on posterior lateral STG covered by the portion of the grid, containing 27 contacts, from which we obtained ERPs and where electrical stimulation effects were observed. Below are shown the grid sites from which click evoked ERPs were recorded (B) and sites where electrical stimulation resulted in a complex mix of sensations similar to those observed upon electrical stimulation of anterior lateral HG (C). The most robust click-evoked responses were recorded along the top row of the grid. A focus of maximal responsiveness is seen, with ERP amplitude decreasing with distance from this recording site (see Howard et al., 2000 for further details). Suppression of tinnitus was observed after stimulation through twelve electrode contacts (filled circles). Stimulation through four of these resulted in tinnitus suppression alone (Fig. 3B filled gray circles). The most effective sites were those that included the focus of maximal responsiveness to a click stimulus. Stimulation through eight contacts resulted in both suppression of tinnitus and a sensation of falling (Fig. 3C filled black circles). The sensation of falling, without tinnitus suppression, was obtained by stimulation of nine sites (Fig. 3C vertical hatch). Open circles depict sites from which no response was reported by the subject.
Figure 3.
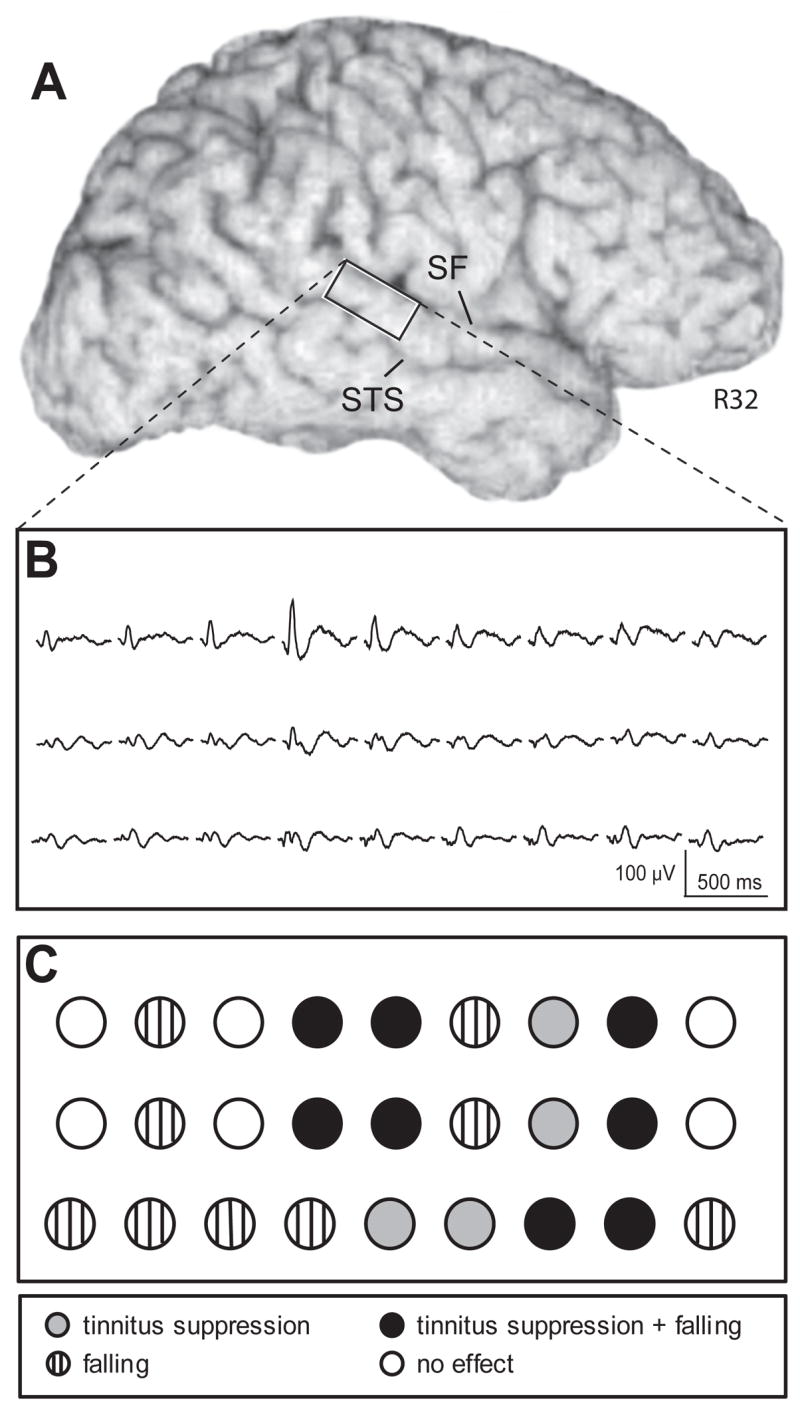
Anatomical and evoked potential data from the right lateral brain surface of subject 32R. A) volume rendered MRI showing the position of the posterior superior temporal gyrus recording array between the Sylvian fissure (SF) and superior temporal sulcus (STS). B) averaged auditory evoked potentials to click train stimuli recorded from posterior superior temporal gyrus contacts 1 through 27. C) graphic depiction of stimulation effects reported by the subject at each array contact.
Discussion
The current observation that electrical stimulation of the posterior lateral STG causes hearing suppression is in close agreement with previous reports by Penfield and colleagues (Penfield and Jasper, 1954; Mullan and Penfield, 1957; Penfield, 1958; Penfield and Perot, 1963). They reported that their patients experienced a ‘a sense of deafness’, ‘deafness instead of noise’, ‘feeling as though wearing a bathing cap’. The patients used other terms as well, such as ‘hard to hear’, ‘can’t hear’ and ‘deafness’, to describe their altered perceptions of ambient sound. These descriptors are very similar to those used by our patients when electrical stimuli were applied to physiologically-identified auditory cortex of posterior lateral STG. The results are also consistent with the recent well-documented case study by Sinha et al. (2005), which showed that electrical stimulation of a site on posterior lateral STG raised the acoustic threshold by 25–40 dB at frequencies between 200 and 2000 Hz at the ear contralateral to the cortical hemisphere being stimulated. Based on anatomical criteria alone, the effective stimulus sites identified by Sinha et al. appear to lie within or very close to our area PLST. The latter studies were carried out on the left cerebral hemisphere, whereas two of our subjects were right hemisphere cases, thus providing evidence that the hearing suppression effect may be observed in either hemisphere.
Nearly all of the anatomically identified human auditory cortical fields lie within the Sylvian fissure on the superior temporal plane, and hence are more difficult to study with ESFM than those on the lateral surface of the STG. Nonetheless, there have been reports of sensations or perceptual alterations resulting from electrical stimulation of HG. Penfield and Jasper (1954) reported that of the four documented stimulation sites on anterior HG, three resulted in ‘crude auditory sensations…usually a tone, a buzzing or knocking sound’. When the fourth site was stimulated the patient reported ‘can’t hear’. Two of our subjects described a similar experience of hearing suppression with electrical stimulation of lateral HG.
Plewnia et al. (2002) reported that rTMS of the left temporoparietal cortex significantly reduced chronic tinnitus in 8 of 14 patients studied. De Ridder et al. (2004) also found, in a single patient, that epidural electrical stimulation over posterior lateral STG suppressed tinnitus. Although the precise sites of stimulation are not known (see Howard, 2004), it appears that they may have been within area PLST, where we also found tinnitus suppression sites. De Ridder et al. (2004) also showed in this same patient that rTMS directed at auditory cortex suppressed the patient’s tinnitus. Again, the precise site of stimulation is not known, but it may correspond to the sites on anterior lateral HG from which we obtained tinnitus suppression.
Electrical stimulation of HG resulted in altered auditory sensations in a site-specific manner. In one subject, stimulation of posterior medial HG resulted in a pitch sensation lateralized to the contralateral ear. In a second subject, stimulation of posterior medial HG augmented his long-standing tinnitus. We may interpret this latter observation to mean that electrical stimulation of this site elicited a pitch sensation that was added to the ongoing tinnitus. No sensations, other than these, were reported by either subject when posterior medial HG sites were stimulated. These observations, coupled with the high amplitude ERPs recorded in close proximity to the stimulation sites, suggest that in both cases the contacts were within the core auditory area. The anterior lateral HG, where suppression of hearing and tinnitus were obtained, exhibited quite a different ERP response, and thus likely represents a portion of the auditory belt complex. The variability and site specificity of the stimulation effects on this patient’s tinnitus suggest that the proposed use of cortical stimulation to treat tinnitus may be complex and challenging to implement clinically (see Dobell, 1973). Area PLST, which is physiologically differentiated from HG auditory fields but functionally connected with them, may be a parabelt field. Thus, although the identities of these fields are still somewhat tentative (see Hackett et al., 2001; Wessinger et al., 2001; Wallace et al., 2002; Morosan et al., 2001; Rademacher et al., 1993) they are consistent with their acoustic response properties and functional connectivity (Howard et al., 2000; Brugge et al., 2003, 2005).
The incidence of stimulus-induced altered auditory percepts reported by Penfield and his colleagues cannot be determined precisely from their papers, but judging from their published quotes from patient transcripts hearing suppression may have been observed in fewer than 10 of the more than 1000 patients studied. Although the findings for our three subjects were robust they were also rare. We have carried out ESFM of HG in eight subjects, three of which are reported here. We have performed ESFM on the posterior lateral STG of more than 60 patients for the purpose of identifying language related cortex prior to resection surgery, but none except those reported here volunteered that their hearing was suppressed. These observations seem consistent with those of Penfield and his colleagues as well as later ESFM studies of the human temporal lobe that systematically scrutinized the effects of electrical stimulation on speech and language comprehension (e.g. Boatman et al., 1995; Boatman, 2004; Ojemann and Engel, 1986; Ojemann et al., 1989; Schaffler et al., 1996) without reporting hearing suppression.
The relative paucity of data on hearing suppression invites the question of why this phenomenon is observed so infrequently during ESFM of lateral STG if every human undergoing this procedure possesses some variant of the same auditory cortical systems. It may due in part to lack of attention to this phenomenon during ESFM. In the Penfield studies perhaps it was overshadowed by the more dramatic experiential hallucinations that were more frequently evoked by temporal lobe stimulation. On the other hand, the three subjects in this paper reported to us, without prompting, their electrically-induced hearing suppression; during clinical ESFM testing of other subjects we did not routinely ask if they experienced suppression of hearing nor did we perform audiometric testing. The 25–40 dB hearing loss associated with posterior lateral STG observed by Sinha et al. (2005) would almost certainly have been noticed by the patient. It is quite possible that during ESFM many subjects fail to note transient hearing suppression, particularly if the suppressive effects are of modest magnitude. More systematic audiometric testing along with ESFM may reveal a greater incidence of hearing and tinnitus suppression than has heretofore been recognized. It is also possible that posterior lateral STG is functionally organized such that hearing suppression sites occupy small and sparsely distributed modules that were rarely in close enough proximity to our grid electrodes. The effects of cortical stimulation were highly circumscribed and could disappear or change when the stimulation was applied to a contact not more than 5 mm away. A well circumscribed, modular pattern of speech arrest sites has also been demonstrated during ESFM of the language dominant hemisphere (Ojemann and Engel, 1986; Ojemann et al., 1989, 1991; Boatman et al., 1995; Boatman, 2004; Schaffler et al., 1996).
Sinha et al. (2005) used objective audiometric testing to demonstrate that their subject experienced an increase in threshold that selectively affected hearing in the ear contralateral to the site of cortical stimulation. In the current report, and in the early studies by Penfield and colleagues, audiometric testing was not used to quantify objectively the magnitude and laterality of the stimulation-induced hearing suppression effects. Subjectively, both of the patients that we studied in detail described a hearing suppression effect that involved both ears. The third subject (18L) was not asked what ear, or ears, were affected by the stimulation-induced “plugged ears” he reported. In the Penfield series, it appears that some subjects may have experienced contralateral effects alone, while others experienced bilateral effects, as reflected in the statement; “when lateralized at all, the sound or the deafness is usually referred to the contralateral ear”(Penfield, 1958). Whether the laterality effects are site specific and/or more biased toward one ear or the other is yet to be determined.
Subjects 10R and 32R both described a persistent hearing suppression effect. One of the subjects estimated that it outlasted the stimulus by 12 to 20 seconds. This agrees with the report of de Ridder et al. (2004) that rTMS of auditory cortex resulted in tinnitus suppression that persisted about 20 seconds beyond the period of stimulation. In the earlier reports by Sinha et al. and Penfield and colleagues, the duration of hearing suppression effects was not discussed.
During electrical stimulation of some, but not all, HG and STG sites, subjects also reported experiencing perceptual changes that differed from the hearing-suppression effect. When listening to the examiner counting aloud, two of our subjects perceived an altered timing of the series of acoustic stimuli. One subject thought it created an “echoing” effect. The other described a loss of temporal synchrony between the speaker’s articulatory lip movements and the speech sounds produced. The STG is now known to play some role in audiovisual interactions (Calvert et al., 1997, 2000; Giard and Peronnet, 1999; Sams et al., 1991; Wright et al., 2003) and it may be that our electrical stimulation measurably desynchronized the two modalities.
One subject reported that electrical stimulation affected the hearing of his own voice. Primate studies of single unit activity in auditory cortices have revealed reduction of neural activity both when animals are electrically stimulated to vocalize (Muller-Preuss and Ploog, 1981) or during spontaneous vocalization (Elaides and Wang, 2003, 2005). Eliades and Wang (2005) observed vocalization-induced suppression of single neuronal activity in the awake marmoset that was more pronounced in upper cortical layers and hypothesized that the primary target for both long- and short-range inhibitory cortico-cortical connections may be largely through local GABAergic interneurons.
Other effects included a generalized feeling of “strangeness”, and a falling sensation, as though being in elevator that was dropping rapidly. Such ‘labyrtinthine sensations’ or ‘equilibratory responses’ were similarly described by subjects in Penfield’s series as ‘queer’ or feelings as though they were ‘standing up and dropping over toward the floor’. As with the hearing suppression, these effects were rarely observed (fewer than 10 of the more than 100 subjects studied by Penfield and Rasmussen (1950) and Penfield and Jasper (1954)). In a more recent study, Kahane et al. (2003) reported vestibular sensations elicited upon electrical stimulation within neocortex of the STG in 28 of 260 epilepsy patients undergoing chronic seizure monitoring.
The neural mechanisms that result in suppression of hearing during cortical stimulation are unknown. It is possible that disruption of local cortical activity alone could suppress hearing, although it is generally agreed that in humans (Tramo et al., 2002; Kaga et al., 2000; Hausler and Levine, 2000; Penfield and Perot, 1963) and in laboratory animals (Heffner and Heffner, 1986; Heffner, 1997; Colombo et al.,1996; Whitfield et al., 1978) that unilateral destruction of auditory cortex of the STG does not result in deafness. More likely, hearing suppression is the result of activating corticofugal efferent pathways that project to auditory thalamic, midbrain and brainstem structures, and possibly the cochlea (Hazama et al., 2004; He, 2003; Jacomme et al., 2003, Suga and Ma, 2003; Pandya et al., 1994; Weedman and Ryugo, 1996a,b). The corticofugal pathways, which originate in different auditory cortical fields, may exert different functional influences on auditory processing, including suppression of hearing. If such specific pathways exist in humans, this might account for the site specificity of the stimulation effects observed in our patients (Khalfa et al., 2001).
Experimental Procedure
The subjects reported here are part of a larger study of the functional organization of human auditory cortex. As part of the surgical treatment plan for intractable epilepsy, or in the case of 18L intraventricular tumor resection, an electrode array was positioned over the perisylvian region of the left (18L) or right (10R, 32R) lateral STG. In subjects 10R and 32R a modified hybrid depth electrode (HDE) was stereotactically implanted along the long axis of HG. Acoustically responsive cortex was identified using single clicks (0.1 ms) or click trains (5 clicks, 100 Hz) delivered (1 per 2 sec) through insert earphones at a comfortable sound level. Recorded field potentials were amplified, filtered, digitized and averaged (n=100). Additional details of the electrophysiological recording methods employed are found in Howard et al. (1996a,b; 2000). ESFM was carried out according to accepted neurosurgical practice (Boatman, 2004). A Grass SD9 constant-voltage stimulator or a custom designed constant current stimulator was used to deliver trains of charge-balanced biphasic electrical pulses (50–100 Hz, 2–5 sec duration) through adjacent electrode contacts. The stimulus strength was gradually increased until a perception was evoked or after-discharge threshold was reached. Perception threshold varied somewhat from contact to contact, but was typically in the range of 1.5 to 2.0 mA. Research recording and stimulation sessions were conducted in a quiet room on the epilepsy monitoring ward where typical ambient nose levels are in the range of 40–43 dB A (68–72 dB SPL). During recording and ESFM sessions the subjects were awake, alert and resting comfortably in a hospital bed. Two investigators were present during each experiment. One interacted directly with the patient, asking directed questions and prompting the patient to describe his/her stimulation-evoked experiences. The second experimenter selected the electrode contacts to be stimulated and delivered the electrical stimuli. The patient was unaware of what brain sites were being stimulated. The validity and consistency of the findings were assessed by stimulating multiple cortical sites during repeated, randomized stimulation sequences. ESFM sessions were recorded on videotape and, later, verbatim transcripts were created. Each ESFM session lasted no more than 30–45 minutes; when multiple sessions occurred on the same day, they were divided into morning and afternoon sessions, separated by lunch and adequate rest time. Participation in the research protocol did not disrupt clinical electrocorticographic (ECoG) monitoring or increase the surgical treatment risk for the subjects. All protocols were approved by the University of Iowa Institutional Review Board.
Acknowledgments
We thank our patients who generously agreed to participate in these studies and who gave unselfishly of their time and effort to help advance scientific knowledge. Supported by the Hoover Fund, the Carver Fund, and NIDCD R01 DC04290 (MAH, JFB)
Abbreviations
- ESFM
Electrical stimulation functional mapping
- STG
Superior temporal gyrus
- HG
Heschl’s gyrus
- ERP
Event related potential
- ECoG
Electrocorticography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boatman D, Lesser RP, Gordon B. Auditory speech processing in the left temporal lobe, and electrical interference study. Brain and Language. 1995;51:269–290. doi: 10.1006/brln.1995.1061. [DOI] [PubMed] [Google Scholar]
- 2.Boatman D. Cortical basis of speech perception: evidence from functional lesion studies. Cognition. 2004;92:47–65. doi: 10.1016/j.cognition.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Brugge JF, Volkov IO, Garell PC, Reale RA, Howard MA. Functional connections between auditory cortex on Heschl’s gyrus and on the lateral superior temporal gyrus in humans. J Neurophysiol. 2003;90:3750–3763. doi: 10.1152/jn.00500.2003. [DOI] [PubMed] [Google Scholar]
- 4.Brugge JF, Volkov IO, Reale RA, Garell PC, Kawasaki H, Oya H, Li Q, Howard MA. The posteriolateral superior temporal auditory field in humans. Functional organization and connectivity. In: Konig R, Heil P, Budinger E, Scheich H, editors. The Auditory Cortex - Toward a Synthesis of Human and Animal Research. Erlbaum; Mahwah, NJ: 2005. pp. 145–162. [Google Scholar]
- 5.Calvert GA, Bullmore ET, Brammer MJ, Campbell R, Williams SC, McGuire PK, Woodruff PW, Iversen SD, David AS. Activation of auditory cortex during silent lipreading. Science. 1997;276:593–596. doi: 10.1126/science.276.5312.593. [DOI] [PubMed] [Google Scholar]
- 6.Calvert GA, Campbell R, Brammer MJ. Evidence from functional magnetic resonance imaging of crossmodal binding in the human heteromodal cortex. Curr Biol. 2000;10:649–657. doi: 10.1016/s0960-9822(00)00513-3. [DOI] [PubMed] [Google Scholar]
- 7.Celesia GG, Puletti F. Auditory cortical areas of man. Neurology. 1969;19:211–220. doi: 10.1212/wnl.19.3.211. [DOI] [PubMed] [Google Scholar]
- 8.Colombo M, Rodman HR, Gross CG. The effects of superior temporal cortex lesions on the processing and retention of auditory information in monkeys (Cebus apella) J Neuroscience. 1996;16:4501–17. doi: 10.1523/JNEUROSCI.16-14-04501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ridder D, De Mulder G, Walsh V, Muggleton N, Sunaert S, Moller A. Magnetic and electrical stimulation of the auditory cortex for intractable tinnitus. Case report. J Neurosurg. 2004;100:560–564. doi: 10.3171/jns.2004.100.3.0560. [DOI] [PubMed] [Google Scholar]
- 10.Dobelle WH, Stensaas SS, Mladejovsky MG, Smith JB. A prosthesis for the deaf based on cortical stimulation. Ann Otol Rhinol Laryngol. 1973;82:445–463. doi: 10.1177/000348947308200404. [DOI] [PubMed] [Google Scholar]
- 11.Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. J Neurophysiology. 2003;89:2194–2207. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- 12.Eliades SJ, Wang X. Dynamics of Auditory--Vocal Interaction in Monkey Auditory Cortex. Cerebral Cortex. 2005;15:1510–1523. doi: 10.1093/cercor/bhi030. [DOI] [PubMed] [Google Scholar]
- 13.Galaburda AM, Sanides F. Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol. 1980;190:597–610. doi: 10.1002/cne.901900312. [DOI] [PubMed] [Google Scholar]
- 14.Giard MH, Peronnet F. Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J Cognitive Neuroscience. 1999;11:473–490. doi: 10.1162/089892999563544. [DOI] [PubMed] [Google Scholar]
- 15.Hackett TA, Preuss TM, Kaas JH. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J Comp Neurol. 2001;441:197–222. doi: 10.1002/cne.1407. [DOI] [PubMed] [Google Scholar]
- 16.Hausler R, Levine RA. Auditory dysfunction in stroke. Acta Oto-Laryngologica. 2000;120:689–703. doi: 10.1080/000164800750000207. [DOI] [PubMed] [Google Scholar]
- 17.Hazama M, Kimura A, Donishi T, Sakoda T, Tamai Y. Topography of corticothalamic projections from auditory cortex of the rat. Neuroscience. 2004;124:655–67. doi: 10.1016/j.neuroscience.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 18.He J. Corticofugal modulation of the auditory thalamus. Exp Brain Res. 2003;153:579–590. doi: 10.1007/s00221-003-1680-5. [DOI] [PubMed] [Google Scholar]
- 19.Heffner HE, Heffner RS. Effect of unilateral and bilateral auditory cortex lesions on the discrimination of vocalizations by Japanese macaques. J Neurophysiology. 1986;56:683–701. doi: 10.1152/jn.1986.56.3.683. [DOI] [PubMed] [Google Scholar]
- 20.Heffner HE. The role of macaque auditory cortex in sound localization. Acta Oto-Laryngologica Sup A chronic microelectrode investigation of the tonotopic organization of human auditory cortex. plement. 1997;532:22–27. doi: 10.3109/00016489709126140. [DOI] [PubMed] [Google Scholar]
- 21.Howard MA, Volkov IO, Abbas PJ, Damasio H, Ollendieck MC, Granner MA. Brain Res. 1996a;724:260–264. doi: 10.1016/0006-8993(96)00315-0. [DOI] [PubMed] [Google Scholar]
- 22.Howard MA, Volkov IO, Granner MA, Damasio HM, Ollendieck MC, Bakken HE. A hybrid clinical-research depth electrode for acute and chronic in vivo microelectrode recording of human brain neurons. Technical note. J Neurosurg. 1996b;84:129–132. doi: 10.3171/jns.1996.84.1.0129. [DOI] [PubMed] [Google Scholar]
- 23.Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE, Brugge JF. Auditory cortex on the posterior superior temporal gyrus of human cerebral cortex. J Comp Neurol. 2000;416:76–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Howard MA. Tinnitus and auditory cortex. J Neurosurg (Letter) 2004;101:171. doi: 10.3171/jns.2004.101.1.0171. [DOI] [PubMed] [Google Scholar]
- 25.Jacomme AV, Nodal FR, Bajo VM, Manunta Y, Edeline JM, Babalian A, Rouiller EM. The projection from auditory cortex to cochlear nucleus in guinea pigs: an in vivo anatomical and in vitro electrophysiological study. Exp Brain Res. 2003;153:467–476. doi: 10.1007/s00221-003-1606-2. [DOI] [PubMed] [Google Scholar]
- 26.Kaas JH, Hackett TA. Subdivisions of auditory cortex and levels of processing in primates. Audiol Neurootol. 1998;3:73–85. doi: 10.1159/000013783. [DOI] [PubMed] [Google Scholar]
- 27.Kaga K, Shindo M, Tanaka Y, Haebara H. Neuropathology of auditory agnosia following bilateral temporal lobe lesions: a case study. Acta Oto-Laryngologica. 2000;120:259–62. doi: 10.1080/000164800750001053. [DOI] [PubMed] [Google Scholar]
- 28.Kahane P, Hoffmann D, Minotti L, Berthoz A. Reappraisal of the Human Vestibular Cortex by Cortical Electrical Stimulation Study. Ann Neurol. 2003;54:615–624. doi: 10.1002/ana.10726. [DOI] [PubMed] [Google Scholar]
- 29.Khalfa S, Bougeard R, Morand N, Veuillet E, Isnard J, Guenot M, Ryvlin P, Fischer C, Collet L. Evidence of peripheral auditory activity modulation by the auditory cortex in humans. Neuroscience. 2001;104:347–358. doi: 10.1016/s0306-4522(01)00072-0. [DOI] [PubMed] [Google Scholar]
- 30.Liegeois-Chauvel C, Musolino A, Chauvel P. Localization of the primary auditory area in man. Brain. 1991;114:139–151. [PubMed] [Google Scholar]
- 31.Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage. 2001;13:684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- 32.Mullen S, Penfield W. Illusions of comparative interpretation and emotion. Archives Neurology and Psychiatry. 1959;81:269–284. [PubMed] [Google Scholar]
- 33.Muller-Preuss P, Ploog D. Inhibition of auditory cortical neurons during phonation. Brain Research. 1981;215:61–76. doi: 10.1016/0006-8993(81)90491-1. [DOI] [PubMed] [Google Scholar]
- 34.Ojemann GA, Engel J., Jr . Acute and chronic intracranial recording and stimulation. In: Engel J Jr, editor. Surgical Treatment of Epilepsies. Raven Press; New York: 1986. pp. 263–288. [Google Scholar]
- 35.Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–26. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- 36.Ojemann GA. Cortical organization of language. J Neurosci. 1991;11:2281–2287. doi: 10.1523/JNEUROSCI.11-08-02281.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandya DN, Rosene DL, Doolittle AM. Corticothalamic connections of auditory-related areas of the temporal lobe in the rhesus monkey. J Comp Neurol. 1994;345:447–71. doi: 10.1002/cne.903450311. [DOI] [PubMed] [Google Scholar]
- 38.Penfield W, Rasmussen T. The Cerebral Cortex of Man - A Clinical Study of Localization of Function. MacMillan; New York: 1950. [Google Scholar]
- 39.Penfield W, Jasper H. Epilepsy and the functional anatomy of the human brain. Little; Brown, Boston: 1954. [Google Scholar]
- 40.Penfield W. The excitable cortex in conscious man. Liverpool Press; Liverpool: 1958. [Google Scholar]
- 41.Penfield W, Perot P. The brain’s record of auditory and visual experience - a final summary and discussion. Brain. 1963;86:595–696. doi: 10.1093/brain/86.4.595. [DOI] [PubMed] [Google Scholar]
- 42.Plewnia C, Bartels M, Gerloff C. Transient suppression of tinnitus by transcranial magnetic stimulation. Ann Neurol. 2003;53:263–266. doi: 10.1002/ana.10468. [DOI] [PubMed] [Google Scholar]
- 43.Rademacher J, Caviness VS, Jr, Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cerebral Cortex. 1993;3:313–29. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- 44.Sams M, Aulanko R, Hamalainen M, Hari R, Lounasmaa OV, Lu ST, Simola J. Seeing speech: visual information from lip movements modifies activity in the human auditory cortex. Neurosci, Lett. 1991;127:141–145. doi: 10.1016/0304-3940(91)90914-f. [DOI] [PubMed] [Google Scholar]
- 45.Schaffler L, Luders HO, Beck GJ. Quantitative comparison of language deficits produced by extraoperative electrical stimulation of Broca’s, Wernicke’s, and basal temporal language areas. Epilepsia. 1996;37:463–75. doi: 10.1111/j.1528-1157.1996.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 46.Sinha SR, Crone NE, Fotta R, Lenz F, Boatman DF. Transient unilateral hearing loss induced by electrocortical stimulation. Neurology. 2005;64:383–385. doi: 10.1212/01.WNL.0000149524.11371.B1. [DOI] [PubMed] [Google Scholar]
- 47.Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- 48.Tramo MJ, Shah GD, Braida LD. Functional role of auditory cortex in frequency processing and pitch perception. J Neurophysiology. 2002;87:122–39. doi: 10.1152/jn.00104.1999. [DOI] [PubMed] [Google Scholar]
- 49.Wallace MN, Johnston PW, Palmer AR. Histochemical identification of cortical areas in the auditory region of the human brain. Exp Br Res. 2002;143:499–508. doi: 10.1007/s00221-002-1014-z. [DOI] [PubMed] [Google Scholar]
- 50.Weedman DL, Ryugo DK. Projections from auditory cortex to the cochlear nucleus in rats: synapses on granule cell dendrites. J Comparative Neurology. 1996a;371:311–24. doi: 10.1002/(SICI)1096-9861(19960722)371:2<311::AID-CNE10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 51.Weedman DL, Ryugo DK. Pyramidal cells in primary auditory cortex project to cochlear nucleus in rat. Brain Res. 1996b;706:97–102. doi: 10.1016/0006-8993(95)01201-x. [DOI] [PubMed] [Google Scholar]
- 52.Wessinger CM, VanMeter J, Tian B, Van Lare J, Pekar J, Rauschecker JP. Hierarchical organization of the human auditory cortex revealed by functional magnetic resonance imaging. J Cognitive Neuroscience. 2001;13:1–7. doi: 10.1162/089892901564108. [DOI] [PubMed] [Google Scholar]
- 53.Whitfield IC, Diamond IT, Chiveralls K, Williamson TG. Some further observations on the effects of unilateral cortical ablation on sound localization in the cat. Exp Brain Res. 1978;31:221–34. doi: 10.1007/BF00237601. [DOI] [PubMed] [Google Scholar]
- 54.Wright TM, Pelphrey KA, Allison T, McKeown MJ, McCarthy G. Polysensory interactions along lateral temporal regions evoked by audiovisual speech. Cereb Cortex. 2003;13:1034–1043. doi: 10.1093/cercor/13.10.1034. [DOI] [PubMed] [Google Scholar]


