Significance
Placebo effects illustrate the power of the human brain; simply expecting an improvement can alter pain processing and produce analgesia. We induced placebo improvement of both negative and positive feelings (painful and pleasant touch) in healthy humans, and compared the brain processing using functional MRI. Pain reduction dampened sensory processing in the brain, whereas increased touch pleasantness increased sensory processing. Neurocircuitry associated with emotion and reward underpinned improvement of both pain and pleasant touch. Our findings suggest that expectation of improvement can recruit common neurocircuitry, which up- or down-regulates sensory processing, depending on whether the starting point is painful or pleasant. These results promote widening the scope of medical research to improvement of positive experiences and pleasure.
Keywords: expectancy, neuroimaging, hedonic feelings
Abstract
Placebo analgesia is often conceptualized as a reward mechanism. However, by targeting only negative experiences, such as pain, placebo research may tell only half the story. We compared placebo improvement of painful touch (analgesia) with placebo improvement of pleasant touch (hyperhedonia) using functional MRI and a crossover design. Somatosensory processing was decreased during placebo analgesia and increased during placebo hyperhedonia. Both placebo responses were associated with similar patterns of activation increase in circuitry involved in emotion appraisal, including the pregenual anterior cingulate, medial orbitofrontal cortex, amygdala, accumbens, and midbrain structures. Importantly, placebo-induced coupling between the ventromedial prefrontal cortex and periaqueductal gray correlated with somatosensory decreases to painful touch and somatosensory increases to pleasant touch. These findings suggest that placebo analgesia and hyperhedonia are mediated by activation of shared emotion appraisal neurocircuitry, which down- or up-regulates early sensory processing, depending on whether the expectation is reduced pain or increased pleasure.
Medical treatments aim to provide relief from pain and aversive states. Consequently, research on placebo effects has focused on relief of negative hedonic feelings, like pain and displeasure (1). In contrast, placebo improvement of positive hedonics has received little attention. However, pain and pleasure processes are tightly linked. Relief from pain can induce a pleasant experience underpinned by activation of reward neurocircuitry (2–4). Moreover, a painful stimulus can even be perceived as pleasant when it represents relief from a more severe outcome (5). In line with this pain–pleasure link, placebo analgesia can be conceptualized as a type of reward mechanism; pain relief is a better outcome than the alternative (6–8) and is typically framed as a gain (improvement of pain) (9).
Like pain, pleasure is greatly affected by context and expectation (10). Manipulation of people’s beliefs about the price of a wine (11), the amount of fruit in a sweet drink (12), the richness of a skin cream (13), and who is caressing them (14) alters the experienced pleasantness of these stimuli.
Placebo-induced improvement of aversive experiences (e.g., pain, anxiety, unpleasant taste) is often underpinned by a decrease in central sensory processing. Placebo analgesia is characterized by decreases in the thalamus, posterior insula (pINS), and primary and secondary somatosensory areas (SI and SII) (15–17). Placebo reduction of affective responses to unpleasant visual stimuli is similarly underpinned by suppression of visual processing (8). It is not, however, known whether placebo-enhanced pleasantness (i.e., hyperhedonia) also alters early stages of sensory processing, or if this change is encoded in higher-level valuation areas.
Functional neuroimaging studies have revealed that the ventromedial prefrontal cortex (vmPFC), amygdala, ventral striatum, and the midbrain are important for mediating placebo analgesia (16, 18–21). Activity in these regions predicts individual placebo analgesia more accurately than regions involved in cognitive control or pain processing (21). This network is dependent on endogenous opioids (16, 19, 22) and interacts with the mesolimbic dopamine system (23–25) to reduce pain by inhibiting nociceptive signaling (15). Because these regions collectively are involved in valuation and reward-related processing more generally (26, 27), and for reasons of clarity and brevity, we will refer to this set of regions as “emotion appraisal circuitry.”
Pleasure and pain show similarities both in terms of neurochemistry and systems neurophysiology (10, 28). If placebo responses build on a generalized mechanism of reward prediction (6–8), a negatively reinforcing effect (e.g., pain relief) should involve processes similar to those encoding positive reinforcement. We hypothesized that placebo improvement of pleasant touch would recruit the same emotion appraisal circuitry that underpins placebo analgesia. Moreover, we investigated whether placebo hyperhedonia, like placebo analgesia, involves modulation of somatosensory processing. Specifically, while expectation of pain relief (placebo analgesia) would reduce sensory processing, we hypothesized that expectation of enhanced pleasantness of an already pleasant touch (placebo hyperhedonia) would increase sensory processing.
To compare brain processing of placebo hyperhedonia and placebo analgesia, we conducted a crossover study using functional MRI (fMRI). Thirty healthy participants received gentle brush strokes, moderately pleasant warmth stimuli, and moderately painful heat stimuli on 2 separate days. These stimuli were applied on the left arm for 10 s in a pseudorandomized order. In the placebo session, participants self-administered an inert nasal spray before the experimental protocol. They were informed that the nasal spray could contain oxytocin, and could thereby: (i) increase the pleasantness of stroking and (ii) warm touch, and (iii) reduce the unpleasantness of painful touch. To strengthen the participants’ belief in the effects of the nasal spray, they were shown a short video documentary summarizing scientific findings of such oxytocin effects. The control session was identical to the placebo session except that there was no nasal spray administration. Session order was counterbalanced, and the experimenter who administered the tactile stimuli was blinded to whether it was the placebo or the control session.
Results
Expectations of Treatment Benefit on Pleasant and Painful Touch.
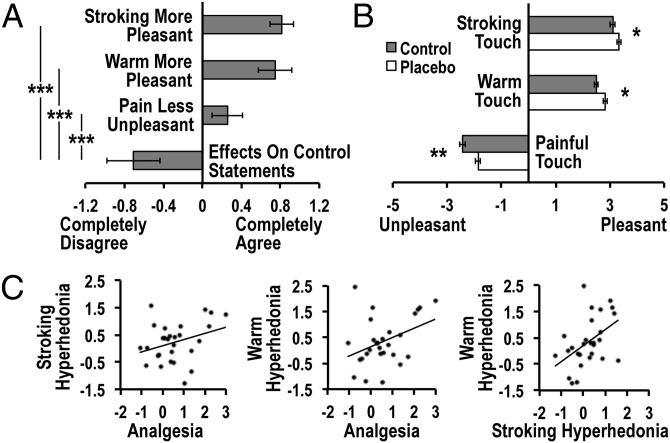
To assess expectations about the effects of the nasal spray administration, participants were asked to indicate on a Likert scale of −3 to 3 how much they agreed with a set of task-relevant and control statements before testing (see SI Materials and Methods). As confirmed by a repeated-measures ANOVA, there was a significant difference in expectation across the different statements [F(3.9, 78.7) = 31.1, P < 0.001]. Planned t tests revealed that ratings of expectations on relevant items [treatment-induced improvement of stroking touch (0.81 ± 0.63, mean rating ± SD, partial eta squared (η2) = 0.62]; warm touch (0.75 ± 0.89, η2 = 0.59); and decreased unpleasantness of painful touch (0.26 ± 0.82, η2 = 0.43) were significantly higher than expectation on irrelevant control items (−0.71 ± 1.42; all P’s < 0.001, one-tailed) (Fig. 1A), confirming the efficacy of the placebo manipulation.
Fig. 1.
Behavioral results. (A) After watching the documentary, participants indicated a positive expectation that intranasal oxytocin treatment would induce stroking touch and warm touch hyperhedonia, as well as analgesia, but no expectation of oxytocin effects on irrelevant control statements. (B) Compared with the control condition, placebo treatment increased pleasantness of stroking and warm touch, and decreased unpleasantness of painful touch. (C) The magnitude of placebo responses [defined as the (placebo > control) difference in VAS scores] correlated across stimulus types. Error bars represent SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Placebo Manipulation Induced Hyperhedonia and Analgesia.
Ratings of pleasantness recorded after each stimulus using a visual analog scale (VAS, unpleasant to pleasant, −5 to 5) confirmed placebo improvement for all three touch stimuli [F(1, 24) = 7.2, P = 0.01, η2 = 0.22] (Fig. 1B). Stroking touch and warm touch were rated as significantly more pleasant after placebo treatment (stroking: 3.3 ± 0.2; warm: 2.8 ± 0.2) compared with the control condition (stroking: 3.1 ± 0.2, P = 0.049; warm: 2.5 ± 0.2, P = 0.03). Correspondingly, painful touch was less unpleasant in the placebo (−1.9 ± 0.2) than in the control condition (−2.4 ± 0.2, P = 0.003). The placebo response magnitude did not significantly differ across stimuli [treatment*stimulus interaction: F(1.9,46.4) = 1.4, P = 0.25, η2 = 0.06].
Magnitude of Placebo Improvement Correlated Across Touch Stimuli.
The placebo response [calculated as the individual (placebo minus control) difference in VAS scores within each stimulus type] correlated across the three stimulus types (all r’s > 0.32, P’s < 0.05) (Fig. 1C).
Opposite Effects on Pleasant and Painful Touch Processing in Sensory Circuitry.
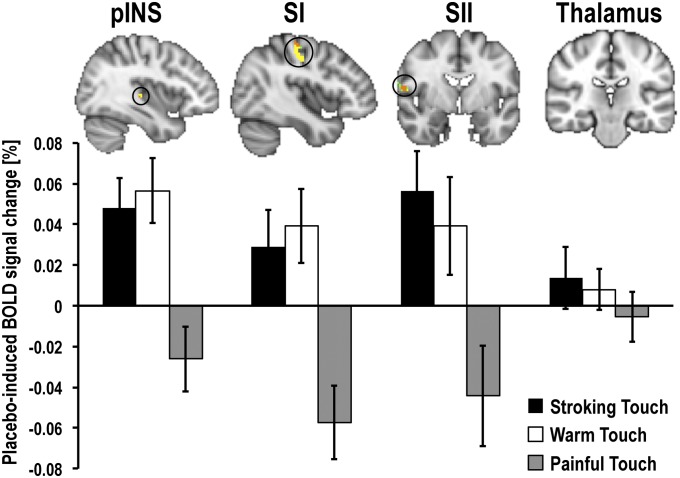
To compare the effects of placebo hyperhedonia and analgesia on somatosensory processing, we first compared placebo-induced (placebo > control) blood-oxygen level-dependent (BOLD) signal changes within each participant for each of the three stimulus types. The group analyses were limited to a priori, independently defined regions of interest (ROI) involved in somatosensory processing (contralateral pINS, SI, SII, and the sensory thalamus). Voxel-wise comparison controlling for multiple comparisons within these regions revealed significant placebo-induced increases in BOLD responses to stroking (pINS: Z = 3.96; SII: Z = 3.25) and warm touch (pINS: Z = 3.26; SII: Z = 2.33), and decreases in responses to painful touch (SI: Z = −4.29; SII: Z = −3.56). Specific contrasts between stimulus types confirmed that placebo-induced BOLD responses to stroking and warm touch differed from those to painful touch in pINS (stroking > pain: Z = 2.27; warm > pain: Z = 2.27), SI (stroking > pain: Z = 3.13; warm > pain: Z = 3.44), and SII (stroking > pain: Z = 3.39; warm > pain: Z = 2.57) (Fig. 2 and Table S1). There were no significant changes in the sensory thalamus ROI.
Fig. 2.
Placebo-induced BOLD responses in somatosensory circuitry. Placebo improvement of painful and pleasant touch experiences was underpinned by opposite BOLD effects in contralateral somatosensory areas (pINS, SI, and SII). After placebo treatment, BOLD responses to pleasant touch were increased, but BOLD responses to painful touch were decreased. Averaged activation maps [Z > 2, uncorrected for illustration purposes, superimposed on the Montreal Neurological Institute (MNI) standard template brain] show voxels where placebo-induced BOLD changes during stroking touch (green) and warm touch (yellow) were significantly more positive than during painful touch (orange represents overlap between stroking and warm touch). Averaged percent signal change values (placebo > control) from the ROIs (bottom) are plotted for illustration purposes. Error bars represent SEM.
Placebo Hyperhedonia and Analgesia Recruited Similar Emotion Appraisal Circuitry.
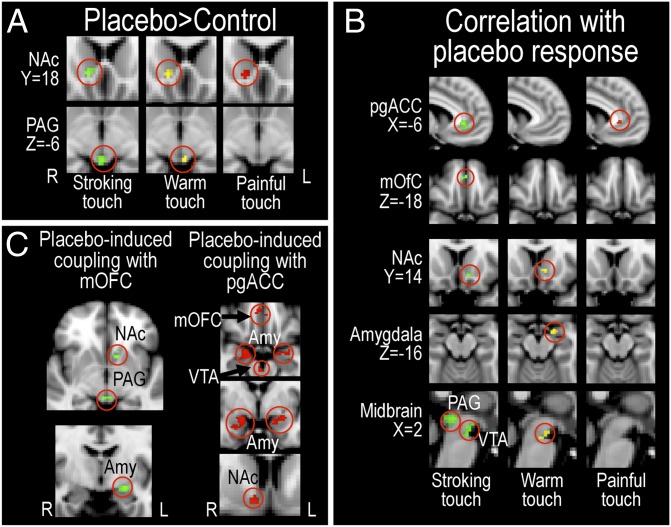
To investigate placebo-induced processing changes in emotion appraisal circuitry, we performed voxel-wise comparisons within each stimulus type, controlling for multiple comparisons within a priori-defined ROIs encompassing emotion appraisal circuitry. The results revealed a significant placebo-induced increase in activation for the whole group (placebo > control) in the nucleus accumbens (NAc) during stroking (Z = 2.92), warm (Z = 4.69), and painful touch (Z = 3.51) (Fig. 3A), shown by a conjunction analysis to involve overlapping parts of the NAc (Z = 2.9) (see SI Materials and Methods for details). A significant increase was also found in the periaqueductal gray (PAG) during stroking (Z = 3.16) and warm touch (Z = 2.59) (Fig. 3A and Table S2). Further placebo-induced BOLD increases were found in the amygdala for warm touch (Z = 2.06), and in the ventral tegmental area (VTA) for warm (Z = 2.31) and painful touch (Z = 2.29). Placebo-induced recruitment of emotion appraisal circuitry did not significantly differ between the three touch stimuli, as assessed by voxel-wise comparisons between placebo > control parameter estimates of the three stimuli [e.g., painful (placebo > control) > stroking (placebo > control)].
Fig. 3.
Placebo-induced BOLD responses in a priori-defined emotion appraisal neurocircuitry. (A) The group contrast (placebo > control) revealed overlapping placebo-induced BOLD increases in the NAc during stroking, warm, and painful touch (as revealed by conjunction analysis), and in the PAG during stroking and warm touch. (B) Regions where individual placebo response (placebo > control) correlated with placebo-induced (placebo > control) BOLD increase. High placebo responses correlated with high placebo-induced increases in these regions. (C) Magnitude of stroking touch hyperhedonia correlated with increased functional coupling between the mOFC, left NAc, left amygdala, and the PAG. Magnitude of placebo analgesia correlated with increased functional coupling between pgACC and mOFC, and bilateral amygdalae as well as mesolimbic reward regions (right NAc and VTA). Green represents stroking touch; yellow represents warm touch; red represents painful touch. Averaged activation maps (Z > 2, uncorrected for illustration purposes) were superimposed on the MNI standard template brain.
Placebo Responses Correlated with BOLD Signal Increases in Emotion Appraisal Circuitry.
A well-known feature of placebo treatment is individual variability in the magnitude of the placebo response. These behavioral differences are known to reflect differences in central placebo processing (15, 23). We identified covariance with the behavioral placebo response within emotion appraisal circuitry by adding a regressor with each subject’s average placebo improvement (placebo > control) for each stimulus type to the fMRI group analysis setup (placebo > control) for each stimulus. This correlation analysis confirmed that the larger the reported benefit of placebo treatment, the higher placebo-induced BOLD increases in the medial orbitofrontal cortex (mOFC, stroking: Z = 2.78), pregenual anterior cingulate cortex (pgACC, stroking: Z = 3.69; pain: Z = 3.18), NAc (stroking: Z = 3.24; warm: Z = 2.98), amygdala (warm: Z = 2.9), PAG (stroking: Z = 4.13), and VTA (stroking: Z = 3.75; warm: Z = 2.75) (Fig. 3B and Table S2).
Placebo Responses Correlated with Increases in Functional Connectivity Within Emotion Appraisal Circuitry.
Previous studies showed that placebo analgesia increases functional connectivity of the pgACC and mOFC with PAG and amygdala (16, 20, 22). We used a psychophysiological interaction (PPI) analysis (29, 30) to assess whether placebo-induced functional coupling between these prefrontal regions and subcortical emotion appraisal circuitry increased in proportion to the behavioral placebo effect. We extracted the mean time series from pgACC and mOFC from each individual run, and added these as regressors in separate first-level generalized linear model (GLM) analyses for each subject. Statistical maps based on interactions between the time series and each stimulus regressor were included in group-level analyses assessing the correlation between the placebo-induced (placebo – control) change in PPI parameter estimates and the individual behavioral placebo response. As above, this analysis controlled for multiple comparisons within the a priori-defined ROIs. We confirmed significant placebo-related increases in functional connectivity between prefrontal and subcortical emotion appraisal regions. Specifically, the stronger the placebo-induced increases in functional connectivity between the mOFC and the amygdala (Z = 2.94), PAG (Z = 2.98), and NAc (Z = 2.35), the larger the reported benefit of placebo treatment on stroking touch pleasantness (Fig. 3C and Table S2). Similarly, placebo-induced increases in functional connectivity between the pgACC and the mOFC (Z = 3.18), amygdala (left: Z = 2.84; right: Z = 3.24), NAc (Z = 3.46), and VTA (Z = 3.04) correlated with the magnitude of the placebo analgesic response (Fig. 3C).
Placebo-Induced Functional Coupling Strength Correlated with Opposite Modulation of Sensory Processing During Placebo Hyperhedonia and Analgesia.
To investigate how placebo-induced functional coupling within this circuitry related to sensory processing, we first extracted each individual’s mean parameter estimate within the PAG from the PPI-analysis (placebo > control) seeded in the mOFC for each stimulus type. This value, reflecting individual placebo-induced functional coupling between the mOFC and PAG, was then added as a regressor in the group level GLM (placebo > control) for each stimulus. This correlation analysis revealed that placebo-induced (placebo > control) functional coupling between the mOFC and PAG correlated with placebo-induced (placebo > control) modulation of sensory regions in opposite directions during hyperhedonia and analgesia. Specifically, participants with high placebo-induced increases in mOFC–PAG coupling strength had larger increases in SII responses to stroking touch (Z = 3.01), but larger decreases in SII responses to painful touch (Z = −2.85) (Fig. 4). To formally test whether these relationships differed between placebo hyperhedonia and placebo analgesia, we calculated the corresponding correlation coefficients (based on mean placebo-induced OFC–PAG functional coupling vs. mean percent BOLD signal change within sensory regions). Direct comparison between these correlation coefficients confirmed that the correlation during stroking touch was significantly more positive than the correlation during painful touch for SII (rstroking = 0.31, rpain = −0.25; Z = 2.04; P = 0.02) and for pINS (stroking: Z = 2.37; pain: Z = −2.51; stroking > pain: rstroking = 0.43, rpain = −0.27; Z = 2.6, P < 0.001).
Fig. 4.
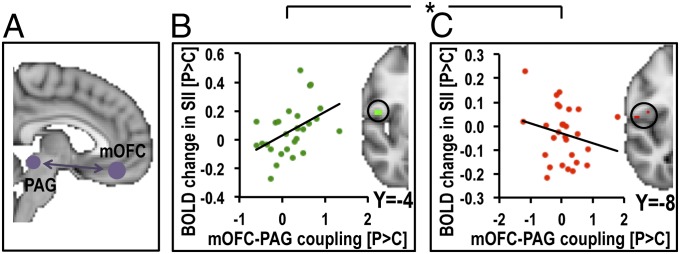
mOFC–PAG coupling strength was associated with opposite modulation of SII in placebo analgesia and hyperhedonia. Strong placebo-induced functional coupling between mOFC and PAG (A) correlated with increased SII responses to stroking touch (B) but decreased SII responses to painful touch (C), a pattern that was replicated also for the pINS. Averaged activation maps were thresholded at Z > 2, uncorrected, for illustrational purposes. The scatterplots illustrate the correlations, which are significantly different from each other. *P > 0.05.
A similar pattern was revealed for the functional coupling between pgACC and PAG. High placebo-induced pgACC–PAG coupling correlated significantly with increases in SII responses to stroking (Z = 2.79) and warm (Z = 2.38) touch, and decreases in SI responses to painful touch (Z = −4.77). These findings are consistent with a general pattern of modulation across sensory circuitry.
Discussion
This study investigated the central mechanisms by which positive expectations to the same inert nasal spray enhance the pleasantness of stroking and warm touch, and reduce the unpleasantness of painful touch. These beneficial placebo effects were reflected in opposite modulation of sensory processing. Specifically, placebo-induced improvement of pleasant experiences involved an up-regulation of activity in the pINS, SI, and SII, the earliest cortical targets of somatosensory processing, and placebo-induced analgesia involved a down-regulation of activity in these areas. Our results indicate that increased sensory processing of a stimulus of positive valence (e.g., pleasant touch) underpins hyperhedonia, in a similar manner as reduced processing of an aversive stimulus (e.g., painful touch) underpins analgesia.
Individual differences in behavioral placebo hyperhedonia and analgesia responses correlated with placebo-induced activity increases and functional coupling strength within circuitry involved in reward, valuation, and emotion appraisal. Moreover, placebo-induced functional coupling between the vmPFC and PAG correlated with increased sensory processing to stroking touch but decreased processing to painful touch. We suggest that similar modulatory circuits can up- and down-regulate early sensory processing, depending on whether the expectation is improvement of positive or negative hedonic feelings.
Opposite Modulation of Sensory Processing.
The placebo-related decrease in sensory regions (pINS, SI, and SII) during painful touch confirmed previous findings (15–17). A unique finding is the increase in sensory processing when placebo treatment increased the pleasantness of pleasant touch. Thus, a cognitively induced increase in pleasantness was underpinned by modulation of the earliest cortical relay stations of somatosensory processing, and not only in higher-level valuation areas. This modulation affected cortical targets of both myelinated A-fibers (SI and SII) and unmyelinated C-fibers (pINS). This result is consistent with recent findings that modulation of touch affect is reflected in both pINS and SI (14, 31).
The somatosensory cortices are also prone to attentional modulation (32). However, the opposite effects during pleasant and painful stimuli indicate that placebo treatment did not induce a general effect of attention. Moreover, recent evidence suggests that placebo treatment and distraction work additively in reducing pain when combined in the same challenge, supporting the view that placebo responses and attention provide analgesia through independent mechanisms (33).
Common Emotion Appraisal Circuitry Mediated Behavioral and BOLD Placebo Responses of Hyperhedonia and Analgesia.
Placebo treatment increased BOLD responses in the NAc for stroking, warm, and painful touch, and in the VTA for painful and warm touch, potentially constituting a common component of placebo hyperhedonia and analgesia. The NAc is a key structure of the mesolimbic reward network and receives heavy dopaminergic projections from the VTA. Dopaminergic and opioidergic NAc activity underpins placebo analgesia responses (22–25). The ventral striatum (including the NAc) is involved in a variety of expectation effects: motor improvement in patients with Parkinson disease (34), anxiety reduction (8), and enhanced pleasantness of a sweet drink (35). Moreover, dopamine release in the ventral striatum is related to motivational and learning aspects of rewards (1). Placebo responses across conditions are mediated by expectations and desire for a benefit from the treatment. The dopamine and opioid systems have thus been proposed to be motors of placebo modulation across different conditions (6, 36).
We observed that individuals with the largest placebo hyperhedonia responses also had the largest placebo-related increases in the pgACC, mOFC, NAc, amygdala, VTA, and PAG processing: regions where placebo analgesia is underpinned by opioid release (18, 19, 22, 24). Stroking touch hyperhedonia and analgesia responses both correlated with increases in the pgACC. In addition to its role in pain modulation and placebo responses, this region is paramount for emotion appraisal and valuation processing (27), and is activated by pleasant tactile stimuli, such as warmth (37), massage (38), and soft gentle strokes to the skin (39).
Placebo analgesia is characterized by increases in functional connectivity between the the pgACC, mOFC, and the PAG and amygdala (16, 20, 22). We found that high placebo analgesia responders had the greatest increases in functional coupling between the pgACC and mOFC, amygdala, NAc, and VTA. These findings extend upon previous research because this modulatory network was also functionally connected with the NAc and VTA, parts of the mesolimbic reward system. Intriguingly, stroking touch hyperhedonia responses correlated positively with functional connectivity strength in a similar network, comprising the mOFC, amygdala, PAG, and NAc.
Proposed Mechanisms of Placebo Hyperhedonia and Analgesia.
Positive expectations, conditioning, or desire for pain relief activates an opioid network involving the vmPFC and amygdala, which in turn engages the antinociceptive brainstem-spinal cord/dorsal horn circuit (40), resulting in placebo analgesia. We found that placebo-induced functional coupling between the vmPFC and PAG correlated with modulation of sensory processing of pleasant and painful touch in an opposite manner. Although a large increase in mOFC–PAG coupling strength was associated with reduced SII responses to painful touch, it was associated with greater SII responses to stroking touch. A similar pattern was found for the posterior insula, as well as for the modulation of sensory responses by placebo-induced coupling of the pgACC to the PAG.
This influence of vmPFC–PAG coupling on sensory processing may potentially reflect a descending modulatory mechanism acting at the spinal cord level, facilitating “positive” touch signals and suppressing nociceptive signals (7, 41). However, the PAG has bidirectional connections to a wide range of cortical and subcortical structures (42), and the modulation of sensory circuitry may be entirely central in origin. For example, bidirectional modulation of one central region by another region has been reported in studies of reappraisal of negative affect (43). Further research is needed to pinpoint the exact mechanism whereby placebo-induced engagement of cortical and subcortical circuitry modulates sensory systems, but it is likely to emerge from a synergy of both descending action at the spinal cord level (40), and interaction of dopaminergic (23–25) and opioidergic (16, 19, 22, 36) cortico-limbic networks.
The opposite influence of vmPFC–PAG connectivity on sensory processing of pleasant and painful touch points to a potential shared mechanism of placebo improvement of positive and negative hedonic feelings. Mu opioid signaling in this circuitry induces powerful analgesia, but also has reinforcing effects, promoting reward seeking (28). In the framework of the motivation–decision model of pain, opioid inhibition of pain reduces the motivation to escape pain, allowing the individual to endure the pain to survive a threat or to seek a reward (41). In a pain context, successful opioid and dopamine activation in the vmPFC, amygdala, NAc, PAG, and VTA is associated with a large placebo analgesic response (22, 24). We show here that activation of this circuitry also correlates with an increase in pleasantness of appetitive stimuli, likely through corresponding influences on sensory processing. It will be interesting to see, in future studies, whether placebo hyperhedonia, similarly to analgesia, relies on opioid or dopaminergic transmission.
Note that activity patterns in emotion appraisal circuitry were similar, but not identical for placebo analgesia and hyperhedonia, consistent with nonidentical top-down mechanisms for these two placebo modulations. For example, a significant conjunction was found only in the NAc, and basic contrasts (placebo-control) showed significant activations in the PAG during stroking and warm touch, but not pain. Nevertheless, there were no significant differences between analgesia and hyperhedonia within emotion appraisal circuitry. Moreover, the observation that placebo-induced vmPFC–PAG coupling correlated with reduction in sensory processing during pain supports a role of the PAG in the current study consistent with previous investigations of placebo analgesia (15, 16).
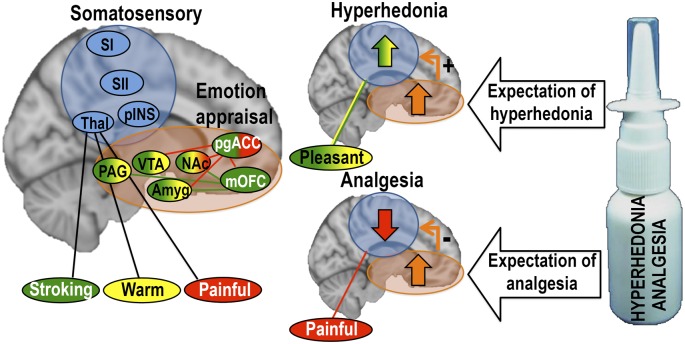
We suggest that expectation of treatment benefit, whether increased pleasantness or reduced unpleasantness, engages shared modulatory neurocircuitry, consistent with investigations of placebo suppression of pain (15) and negative emotions (8). The consequence is top-down modulation of processing in sensory areas in an opposite manner, with expectation of hyperhedonia leading to up-regulation of sensory processing, and expectation of analgesia leading to down-regulation of sensory processing (Fig. 5).
Fig. 5.
Proposed mechanism of placebo analgesia and hyperhedonia. During expectation of hyperhedonia and analgesia, a shared modulatory network up-regulates pleasant touch processing and down-regulates painful touch processing in somatosensory areas, possibly through similar dopaminergic/opioidergic connections. Color-coding of the regions represent areas where placebo treatment induced activation for stroking touch (green), warm touch (yellow), and painful touch (red). Connecting lines represent placebo-related increases in functional connectivity for stroking touch (green) and painful touch (red). Somatosensory regions are shown in blue.
Placebo Hyperhedonia and Analgesia in the Clinic.
In clinical settings, placebo responses can confound assumptions about the physical or “true” effects of the treatment, but can also be used to optimize a treatment (44–46). Although the amount of expectancy, desirability (47), personality traits (48), and biomarkers, like gray matter density of the NAc (25), sometimes correlate with placebo responses, it has been notoriously difficult to predict placebo responses across different contexts (49, 50). Most placebo research has addressed the effect of a treatment on one clinical outcome in isolation (e.g., reduction of pain). However, in randomized controlled trials, it has been proposed that the severity of side effects may give rise to larger placebo effects, because this increases the participants’ confidence that they are receiving a potent treatment (51). Here, individuals with large placebo analgesia responses also had large placebo hyperhedonia responses. This finding could reflect a placebo-induced hyperhedonic and analgesic state affecting both positive and negative hedonic feelings. If so, one would predict hyperhedonic responses after treatment that is presented as purely analgesic (i.e., without conscious expectation of hyperhedonia) and vice versa.
Furthermore, if hyperhedonia and analgesia share common mechanisms, it is possible that one contributed to the other, and that inducing hyperhedonia bolstered the analgesic effect. Such an account would highlight the importance of focusing on positive effects of a treatment (e.g., regained ability to enjoy pleasures, or increased life quality), analogous to the importance of avoiding focus on negative side effects to reduce nocebo effects (46). Future studies should address these questions.
In conclusion, this study demonstrated that placebo improvement of pleasant and painful touch involved opposite modulation of somatosensory processing. Placebo increases in touch pleasantness increased sensory activation, whereas placebo reductions in pain unpleasantness decreased sensory activation. Furthermore, similar emotion appraisal neurocircuitry was recruited during both analgesia and hyperhedonia. Increases in functional coupling between the vmPFC and PAG specifically correlated with increased sensory processing of stroking touch, but reduced sensory processing of painful touch, potentially constituting a shared mechanism of placebo hyperhedonia and analgesia. Overall, our results suggest that emotion appraisal circuitry is recruited by expectations of a benefit, whether it is pain relief or enhanced pleasantness of a positive stimulus, and modulates sensory processing accordingly to meet these predictions.
Materials and Methods
Subjects.
Thirty healthy volunteers (mean age 25.5 ± 4.5 y; range 20–41 y; 10 females) participated. Two datasets were incomplete, leaving a final sample size of 28 participants. All participants gave informed written consent and were paid 400 NOK (∼70 USD). The study was approved by the Regional Committee for Medical and Health Research Ethics (2009/208/REK sør-øst C) and followed the guidelines of the Declaration of Helsinki (1996).
Study Design.
Each volunteer participated in two sessions on separate days, with and without intranasal placebo treatment (counterbalanced order), but identical in every other manner. To induce expectation of intranasal oxytocin’s beneficial effects on painful and pleasant touch experience, participants viewed a 6-min locally developed video documentary about oxytocin’s putative prosocial effects, such as involvement in bonding, love, grooming, affective touch, and healing (see SI Materials and Methods for details). Following this presentation, the subjects either self-administered 10 puffs (five in each nostril) of a saline nasal spray that they were told could contain oxytocin (placebo session), or directly moved on to the next part of the procedure (control session). Next, fMRI data were collected throughout the 15-min experiment, in which the participants received a total of 27 10-s tactile stimuli: stroking touch, warm touch, and painful touch presented in a pseudorandomized order (no more than two of the same stimulus in a row; at least 1 min between each painful touch stimulus to avoid skin sensitization). Eight seconds after each stimulus, the subjects rated their subjective experience on a VAS.
Stroking Touch.
Gentle strokes were delivered to the dorsum of the left forearm (20 cm distance) at a velocity of ∼5 cm/s using a 7-cm-wide soft artist’s goat hair brush (31). The brush strokes were administered for 10 s (two strokes) in proximal-to-distal direction. This type of tactile stimulation is consistently perceived as pleasant and effectively activates C-tactile afferents, which signal affective aspects of touch (52).
Warm Touch.
A soft, gel-filled “heat pad” (ColdHot Pack, 3M Health Care) was heated for 60 s in a microwave oven (∼42.5 °C surface temperature) immediately before the experiment. The ColdHot Pack, wrapped in thin nylon cloth, was placed gently on the dorsum of the left forearm for 10 s and then removed, resembling the touch of a warm human hand.
Painful Touch.
Heat stimuli were delivered using an MRI compatible peltier thermode (Pathway model ATS, 30 × 30 mm, Medoc). A moderately painful temperature, which was selected for each participant before the first fMRI session (5 on a numeric rating scale, NRS, with anchors 0 = no pain; 1 = pain threshold; 10 = intense pain), was used in both fMRI sessions (mean temperature = 47.1 ± 0.73 °C). An experimenter placed the thermode on the dorsum of the left hand for 10 s, and then removed it. Participants were not informed that the same temperature was used for all stimuli in the fMRI sessions, and were instructed to focus on their experience of each individual stimulus. To avoid skin sensitization that could affect the positive touch experience, painful touch was applied on a location adjacent to the pleasant touch stimuli.
Hedonic Ratings.
A VAS (−5 to +5) with anchors “unpleasant” and “pleasant” was presented on a screen 8 s after each stimulus, and remained on the screen for 6 s. Participants used a button-box to indicate their rating. Average per-session ratings for each stimulus were calculated and analyzed using repeated-measures ANOVA (Greenhouse–Geisser correction) with the within-subjects factors treatment (placebo, control) and stimulus type (stroking, warm, pain), and between-subjects factors treatment order (placebo first, control first) and sex (male, female). Planned paired t tests (one-tailed) between placebo and control were calculated within each stimulus type. See SI Results and Figs. S1–S3 for details about temporal characteristics of ratings, effects of order and sex, and the relationship between expectation and hedonic ratings.
fMRI Preprocessing and Analysis.
Imaging was performed using a Philips Achieva 3 Tesla whole-body MR unit (Philips Medical Systems). See SI Materials and Methods for image-acquisition details. fMRI data analysis was performed in a multistage process using FEAT (fMRI Expert Analysis Tool) v5.98, part of FSL [Functional MRI of the Brain (FMRIB)’s Software Library]. Prestatistics processing was applied within each individual run (see SI Materials and Methods, Fig. S4, and Table S3 for details). A unique input stimulus function was defined for each stimulus type (stroking, warm, and pain), and for the VAS rating intervals. Input stimulus functions were convolved with the γHRF to yield regressors for the GLM. Time-series statistical analysis was carried out using FILM with local autocorrelation correction (53). Registration to high-resolution structural and standard space images was carried out using FLIRT (54, 55). Higher-level (group) analyses were performed using FLAME 1+2 (FMRIB’s Local Analysis of Mixed Effects) (see SI Materials and Methods, Fig. S5, and Table S4). All a priori ROIs were defined from independent sources (SI Materials and Methods and Fig. S6).
Supplementary Material
Acknowledgments
We thank Tomas Karlsson and Simon Bergstrand for technical assistance; Karin Saar for help with testing; the people at FMRIB (Functional MRI of the Brain), University of Oxford, especially Jesper Andersson; Guido Biele, University of Oslo, for valuable guidance on MRI design and analysis; and Tom Johnstone for helpful comments on earlier drafts of the manuscript. We thank the Department of Psychology/Intervention Centre, University of Oslo for access to the MR scanner. This study was supported by the Swedish Research Council and the Sahlgrenska University Hospital (J.W. and H.O.); the Marianne and Marcus Wallenberg Fundation (H.O.); and the Research Council of Norway (S.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305050110/-/DCSupplemental.
References
- 1.Berridge KC, Kringelbach ML. Neuroscience of affect: Brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol. 2013;23(3):294–303. doi: 10.1016/j.conb.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: Hedonic and neural responses to safety from pain. PLoS ONE. 2011;6(4):e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leknes S, Brooks JC, Wiech K, Tracey I. Pain relief as an opponent process: A psychophysical investigation. Eur J Neurosci. 2008;28(4):794–801. doi: 10.1111/j.1460-9568.2008.06380.x. [DOI] [PubMed] [Google Scholar]
- 4.Seymour B, et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci. 2005;8(9):1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- 5.Leknes S, et al. The importance of context: When relative relief renders pain pleasant. Pain. 2013;154(3):402–410. doi: 10.1016/j.pain.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Fuente-Fernández R. The placebo-reward hypothesis: Dopamine and the placebo effect. Parkinsonism Relat Disord. 2009;15(Suppl 3):S72–S74. doi: 10.1016/S1353-8020(09)70785-0. [DOI] [PubMed] [Google Scholar]
- 7.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5(7):565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 8.Petrovic P, et al. Placebo in emotional processing—Induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46(6):957–969. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. 1981;211(4481):453–458. doi: 10.1126/science.7455683. [DOI] [PubMed] [Google Scholar]
- 10.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 11.Plassmann H, O’Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci USA. 2008;105(3):1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods AT, et al. Expected taste intensity affects response to sweet drinks in primary taste cortex. Neuroreport. 2011;22(8):365–369. doi: 10.1097/WNR.0b013e3283469581. [DOI] [PubMed] [Google Scholar]
- 13.McCabe C, Rolls ET, Bilderbeck A, McGlone F. Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Soc Cogn Affect Neurosci. 2008;3(2):97–108. doi: 10.1093/scan/nsn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gazzola V, et al. Primary somatosensory cortex discriminates affective significance in social touch. Proc Natl Acad Sci USA. 2012;109(25):E1657–E1666. doi: 10.1073/pnas.1113211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp. 2013;34(3):738–752. doi: 10.1002/hbm.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eippert F, et al. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Lu HC, et al. Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: A 3T-fMRI study. Pain. 2010;148(1):75–83. doi: 10.1016/j.pain.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—Imaging a shared neuronal network. Science. 2002;295(5560):1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 19.Zubieta JK, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25(34):7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1-2):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia: Contributions of brain activity during anticipation and pain experience. J Neurosci. 2011;31(2):439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci USA. 2007;104(26):11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott DJ, et al. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55(2):325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Scott DJ, et al. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65(2):220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 25.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: A link between personality and the placebo analgesic response. J Neurosci. 2009;29(15):4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. The brain basis of emotion: A meta-analytic review. Behav Brain Sci. 2012;35(3):121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fields HL. Mu opioid receptor mediated analgesia and reward. In: Pasternak GW, editor. The Opiate Receptors, The Receptors. Vol 23. Humana, New York; 2011. pp. 239–264. [Google Scholar]
- 29.O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: Psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7(5):604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 31.Morrison I, Björnsdotter M, Olausson H. Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. J Neurosci. 2011;31(26):9554–9562. doi: 10.1523/JNEUROSCI.0397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mima T, Nagamine T, Nakamura K, Shibasaki H. Attention modulates both primary and second somatosensory cortical activities in humans: A magnetoencephalographic study. J Neurophysiol. 1998;80(4):2215–2221. doi: 10.1152/jn.1998.80.4.2215. [DOI] [PubMed] [Google Scholar]
- 33.Buhle JT, Stevens BL, Friedman JJ, Wager TD. Distraction and placebo: Two separate routes to pain control. Psychol Sci. 2012;23(3):246–253. doi: 10.1177/0956797611427919. [DOI] [PubMed] [Google Scholar]
- 34.de la Fuente-Fernández R, et al. Expectation and dopamine release: Mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 35.Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: Top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18(7):1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- 36.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2(8091):654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 37.Rolls ET, Grabenhorst F, Parris BA. Warm pleasant feelings in the brain. Neuroimage. 2008;41(4):1504–1513. doi: 10.1016/j.neuroimage.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Lindgren L, et al. Pleasant human touch is represented in pregenual anterior cingulate cortex. Neuroimage. 2012;59(4):3427–3432. doi: 10.1016/j.neuroimage.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Gordon I, et al. Brain mechanisms for processing affective touch. Hum Brain Mapp. 2013;34(4):914–922. doi: 10.1002/hbm.21480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eippert F, Finsterbusch J, Bingel U, Büchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326(5951):404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 41.Fields HL. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007;32(3):242–246. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D. Neuroimaging of the periaqueductal gray: State of the field. Neuroimage. 2012;60(1):505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bingel U, et al. The effect of treatment expectation on drug efficacy: Imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3(70):70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- 45.Jensen KB, et al. Sharing pain and relief: neural correlates of physicians during treatment of patients. Mol Psychiatry. 2013 doi: 10.1038/mp.2012.195. 10.1038/mp.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells RE, Kaptchuk TJ. To tell the truth, the whole truth, may do patients harm: The problem of the nocebo effect for informed consent. Am J Bioeth. 2012;12(3):22–29. doi: 10.1080/15265161.2011.652798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105(1–2):17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 48.Peciña M, et al. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology. 2013;38(4):639–646. doi: 10.1038/npp.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liberman R. An experimental study of the placebo response under three different situations of pain. J Psychiatr Res. 1964;33:233–246. doi: 10.1016/0022-3956(64)90010-x. [DOI] [PubMed] [Google Scholar]
- 50.Whalley B, Hyland ME, Kirsch I. Consistency of the placebo effect. J Psychosom Res. 2008;64(5):537–541. doi: 10.1016/j.jpsychores.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Max MB, Schafer SC, Culnane M, Dubner R, Gracely RH. Association of pain relief with drug side effects in postherpetic neuralgia: A single-dose study of clonidine, codeine, ibuprofen, and placebo. Clin Pharmacol Ther. 1988;43(4):363–371. doi: 10.1038/clpt.1988.44. [DOI] [PubMed] [Google Scholar]
- 52.Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12(5):547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- 53.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 54.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 55.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.