Significance
Traumatic brain injury (TBI) occurs when a strong jolt to the head causes damage to brain cells, resulting in immediate and long-term consequences including physical, behavioral, and cognitive problems. Despite the importance of TBI as a major health issue, our understanding of the underlying cellular and molecular mechanisms is limited. To unravel these mechanisms, we have developed a model of TBI in the fruit fly, Drosophila melanogaster, where we can apply many powerful experimental tools. The main features of human TBI also occur in flies, suggesting that the underlying mechanisms are conserved. Our studies demonstrate the value of a fly model for understanding the consequences of TBI and may ultimately enable development of therapies for their prevention and treatment.
Keywords: concussion, insect, chronic traumatic encephalopathy
Abstract
Traumatic brain injury (TBI) is a substantial health issue worldwide, yet the mechanisms responsible for its complex spectrum of pathologies remains largely unknown. To investigate the mechanisms underlying TBI pathologies, we developed a model of TBI in Drosophila melanogaster. The model allows us to take advantage of the wealth of experimental tools available in flies. Closed head TBI was inflicted with a mechanical device that subjects flies to rapid acceleration and deceleration. Similar to humans with TBI, flies with TBI exhibited temporary incapacitation, ataxia, activation of the innate immune response, neurodegeneration, and death. Our data indicate that TBI results in death shortly after a primary injury only if the injury exceeds a certain threshold and that age and genetic background, but not sex, substantially affect this threshold. Furthermore, this threshold also appears to be dependent on the same cellular and molecular mechanisms that control normal longevity. This study demonstrates the potential of flies for providing key insights into human TBI that may ultimately provide unique opportunities for therapeutic intervention.
Traumatic brain injury (TBI) is a leading cause of neurological deficits and mortality worldwide (1, 2). TBI outcomes result from primary and secondary injuries that cause cell damage and death in the brain. Primary injuries occur during the initial impact and are triggered by external mechanical forces that deform the brain, whereas secondary injuries are triggered by cellular and molecular responses that occur over time in reaction to the primary injuries. TBI outcomes are heterogeneous in the human population owing to variation in the location and strength of primary injuries as well as genetic and environmental factors that affect the severity of primary and secondary injuries. This heterogeneity is one of the most significant barriers to the development of therapeutic interventions (3–5). Therefore, research aimed at determining the genetic and environmental factors that affect the severity of primary and secondary injuries is essential for developing treatments for TBI.
To investigate the underlying cellular and molecular basis of TBI, we developed a Drosophila melanogaster model. Key advantages of flies are that (i) large numbers of animals can be rapidly and inexpensively analyzed to establish causality between injuries and outcomes, (ii) many molecular and genetic tools are available to investigate the molecules and pathways that underlie injuries, and (iii) outcomes can readily be evaluated over the entire animal lifespan. Thus, flies provide unique opportunities to understand TBI.
It is reasonable to expect that TBI can be modeled in flies because Drosophila has already proved to be an extremely useful model for studying other human neurodegenerative disorders (6). In fact, research in flies has already provided novel insights into neurodegeneration, memory, and sleep, all of which are affected in human TBI (6–8). Additionally, fly and human brains have common architectural, cellular, and molecular features. The fly brain is bilaterally symmetrical and is joined to the ventral ganglion that innervates the body, similar to the way that the spinal cord innervates the human body (9). The fly brain is organized into three regions, the protocerebrum, deutocerebrum, and tritocerebrum, which are homologous to the forebrain, midbrain, and hindbrain, respectively, of humans (10). The fly brain is composed of functionally diverse neurons with all of the structural features, neurotransmitters, and ion channels found in human neurons (11). Glial cell types and functions in flies are also similar to those in humans (12). For instance, surface glial cells cover brain neurons to form the blood–brain barrier, and glial cells are part of the innate immune system (13, 14). The fly brain is encapsulated by an exoskeleton, also known as the cuticle (15). Like the human cranium, the cuticle is relatively inelastic. It provides protection from the environment, and it defines the structure of the head. The brain is further separated from the cuticle by fluid hemolymph, a blood-like carrier system for macrophages and nutrients (16). The topology of the fly brain relative to the cuticle is such that an impact to the head or body most likely causes the brain to ricochet and deform against the cuticle resulting in TBI.
Here, we describe the development and initial characterization of a fly model of TBI. Using the model, we found that fundamental characteristics of human TBI also occur in flies. We also found that primary injuries exacerbate the normal age-related decline in flies. This may explain why human TBI is associated with cognitive and neurodegenerative disorders that are typical of older individuals and why TBI outcomes are worse in older individuals.
Results
The HIT Device Reproducibly Inflicts Closed Head TBI in Flies.
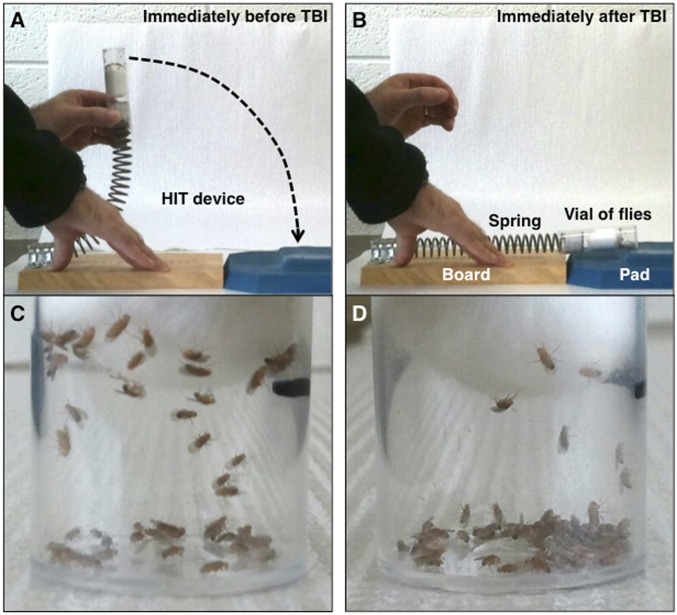
To inflict TBI in flies, we built the “high-impact trauma” (HIT) device, which consists of a metal spring clamped at one end to a wooden board with the free end positioned over a polyurethane pad (Fig. 1A and Fig. S1). A standard plastic vial containing unanesthetized flies that are confined to the bottom quarter of the vial by a stationary cotton ball is connected to the free end of the spring. When the spring is deflected and released, the vial rapidly contacts the polyurethane pad, and a mechanical force is delivered to the flies as they contact the vial wall and rebound (Fig. 1B). Individual flies presumably contact the wall with different regions of their head and/or body and with different forces, so primary injuries will vary among flies in the same vial. The lack of penetrating injuries and the randomness of impact location and strength are features of closed head TBI in the human population. Since closed head TBI is the most common form of TBI occurring in falls, sports collisions, and automobile crashes, a fly model is of significant potential value (17). As shown in Fig. 2, the phenotypic effects on flies subjected to HIT device-induced injury are highly reproducible.
Fig. 1.
The HIT device was used to inflict TBI in 0- to 4-d-old w1118 flies. Images show the HIT device and flies before and after a strike. Shown are (A) the HIT device deflected to 90° before a strike, (B) the HIT device immediately after a strike, (C) 60 flies in a vial before a strike, and (D) after a strike.
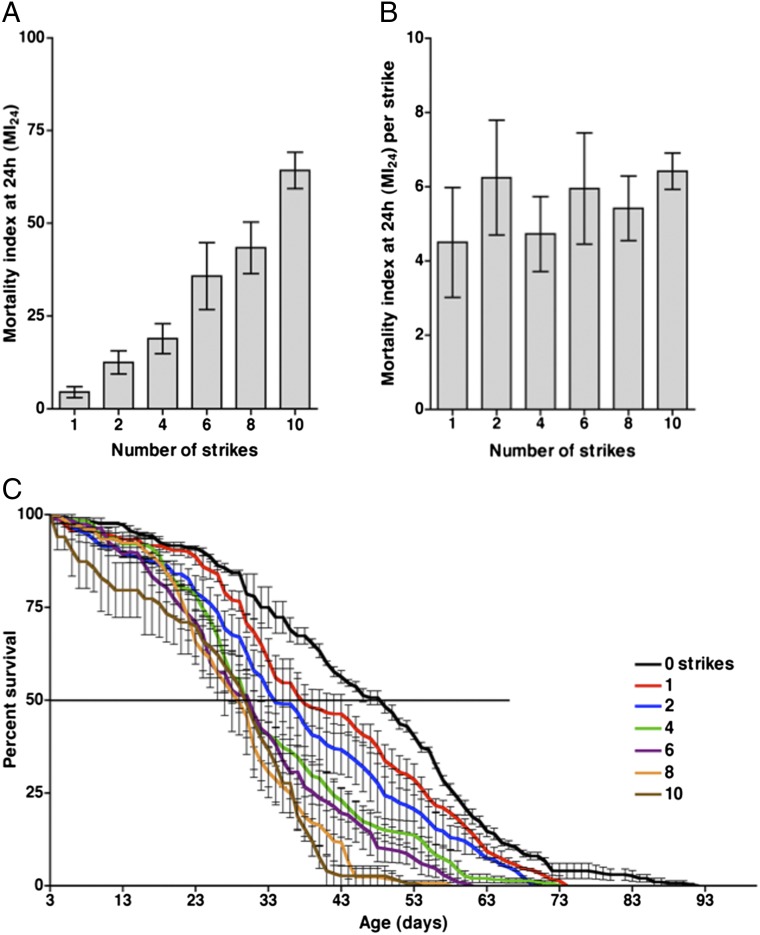
Fig. 2.
The effect of the number of strikes on the MI24 and the lifespan of w1118 flies. (A) The MI24 is graphed vs. the number of strikes. MI24 values were normalized to those of untreated flies. (B) Data from A are graphed as the MI24 per strike vs. the number of strikes. The MI24 per strike was not significantly affected by the number of strikes (P = 0.82, one-way ANOVA). (C) The percent survival is graphed vs. age for flies that received the indicated number of strikes. Only flies that survived 24 h after treatment were included in the analysis. The line at 50% indicates the median lifespan. Error bars indicate the SD for at least three independent trials of 60 flies each. Exact values and SD for median and maximum lifespans are provided in Table S2.
The strength of the primary injuries inflicted by the HIT device can be adjusted, either by varying the extent of spring deflection or by varying the number of strikes. Deflection of the spring to 90° resulted in an impact velocity of ∼3.0 m/s (6.7 miles/h) and an average force of 2.5 N. Some flies subjected to a single strike with the spring deflected to 90° became temporarily incapacitated and fell to the bottom of the vial; however, they did not incur obvious external damage to the head, body, or appendages (Fig. 1 C and D). During the first minute after a strike, 8.8 ± 3.8% of flies were incapacitated, lying on their back or side on the bottom of the vial. Most of the incapacitated flies recovered locomotor activity within 5 min (Fig. S2A). Subsequently, their mobility, as measured by climbing ability, was reduced but gradually returned to normal over a 2-d period (Fig. S2B and Table S1). The immediate loss of motor ability, followed by ataxia and gradual recovery of mobility are reminiscent of concussion in humans and are consistent with the idea that the HIT device inflicts brain injury in flies.
Primary Injuries That Exceed a Threshold Cause Death Within 24 h.
To determine the effect of subjecting flies to multiple TBI incidents, we varied the number of strikes that flies received and measured the percentage of flies that died within 24 h. We define the percentage of flies that died within 24 h after injury as the mortality index at 24 h (MI24). To minimize any variation in outcome associated with differences in age, genotype, or sex, we used 0- to 3-d-old white (w1118) flies in every experiment, with an approximately equal number of males and females. Flies received 0–10 strikes with the spring deflected to 90° and with 5 min recovery periods between strikes. After a single strike, the MI24 was 4.5 ± 1.2 (Fig. 2A). Additional strikes resulted in an increase in the MI24; however, additional strikes did not significantly increase the MI24 per strike (Fig. 2B). The fact that not all flies died after a single strike indicates that primary injuries cause death within 24 h only if the injuries exceed a specific threshold, where threshold is presumably a composite measure of impact location and strength. Furthermore, the fact that the number of strikes did not affect the MI24 per strike indicates that flies subjected to primary injuries below the threshold do not have increased susceptibility for mortality from subsequent primary injuries.
We also examined whether varying the recovery time between repeated strikes had any effect on the MI24. The expectation was that if the time between primary injuries was increased, then secondary injuries or injury repair mechanisms would have more time to occur, thereby altering the threshold for death caused by subsequent primary injuries. Thus, the MI24 would change as the time between strikes changed. To test this hypothesis, flies received four strikes with time intervals from 1 to 60 min between successive strikes. The temporal spacing of strikes did not significantly affect the MI24 (Fig. S3A). Therefore, over the times tested, the time between successive strikes neither exacerbates nor ameliorates the consequences of multiple primary injuries. The fact that the time between strikes did not affect the MI24 indicates that secondary injuries and injury repair mechanisms either neutralize one another or do not contribute to the primary injury threshold for death within 24 h.
Another parameter that could affect the MI24 is the number of flies per vial. To investigate this possibility, we placed 10–60 flies per vial in increments of 10 and subjected the vials to four strikes with 5-min recovery periods between strikes. The number of flies in a vial did not significantly affect the MI24 (Fig. S3B). Thus, in vials containing 10–60 flies, contact among flies does not appear to affect the primary injury threshold for death within 24 h.
TBI Reduces Lifespan.
To investigate whether multiple TBI incidents have long-term effects on survival, we determined the lifespan of flies that survived beyond 24 h after receiving 0–10 strikes with 5 min recovery periods between strikes. We assumed that death from primary injuries was complete by 24 h because the percent survival at 24 h was not substantially different from the percent survival at 48 h. Relative to untreated flies, flies that received one strike had a substantially reduced median lifespan (48.3 ± 1.2 d vs. 38.3 ± 3.5 d) and significantly reduced maximum lifespan (85.7 ± 3.6 d vs. 73.0 ± 0.6 d) (Fig. 2C and Table S2). Additional strikes caused further reductions in the median and maximum lifespan, but an upper limit for median lifespan was reached at four strikes; additional strikes did not further reduce the median lifespan. These results demonstrate that primary injuries that are below the threshold for death within 24 h nonetheless cause a reduction in lifespan that likely results from the long-term consequences of secondary injuries.
Age-Associated Processes Lower the Primary Injury Threshold.
For subsequent experiments, we developed a standard TBI protocol consisting of four strikes separated by 5 min intervals. This protocol resulted in an MI24 of 19.0 ± 4.0 for 0- to 3-d-old w1118 flies (Fig. 2A). This moderate MI24 is convenient for identifying genetic and environmental factors that enhance or suppress the effect of primary injuries.
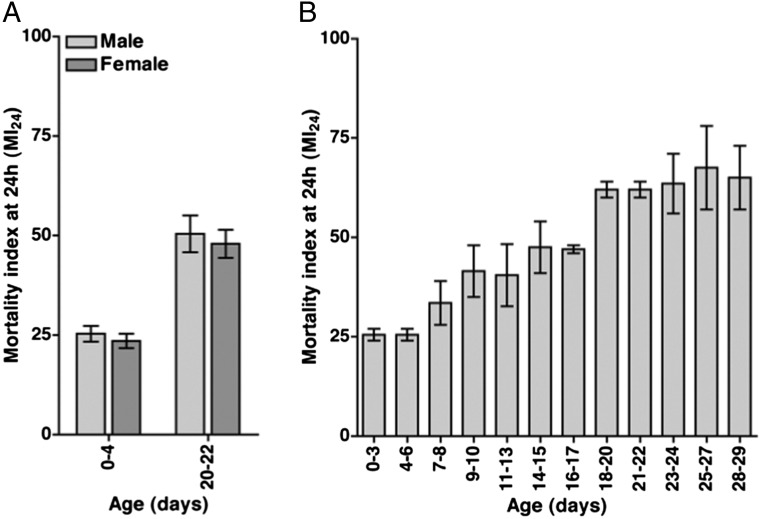
We used the standard TBI protocol to determine the effect of sex and age on the MI24. w1118 male and female flies at 0–4 and 20–22 d old were assayed separately. Sex did not have a significant effect on the MI24 at either age (Fig. 3A). In contrast, age did have a significant effect; the MI24 was approximately twofold higher for older flies compared with younger flies.
Fig. 3.
The MI24 of w1118 flies subjected to the standard TBI protocol is not affected by sex but is affected by age at the time of injury. (A) For flies treated with the standard TBI protocol, the MI24 is graphed vs. sex. The experiment was performed with 0- to 4-d-old and 20- to 22-d-old flies. MI24 values were normalized to those of untreated flies. Sex did not have a significant effect on the MI24 for either 0- to 4-d-old flies (P = 0.27, one-tailed t test) or 20- to 22-d-old flies (P = 0.34, one-tailed t test). Age at the time of injury did have a significant effect on the MI24 for both males (P = 0.004, one-tailed t test) and females (P = 0.002, one-tailed t test). (B) The MI24 is graphed vs. age at the time of treatment with the standard TBI protocol. MI24 values were normalized to those of untreated flies. Error bars indicate the SD for at least three independent trials of 60 flies each.
To more systematically examine the effect of age on the MI24, we examined flies in 12 age groups ranging from 0–3 to 28–29 d old that were treated with the standard TBI protocol. The data revealed an increase in the MI24 as age increased (Fig. 3B). These results suggest that cellular and molecular changes occur during aging that lower the primary injury threshold for death within 24 h.
Age-Associated Processes Enhance Neurodegeneration Due to Brain Injuries.
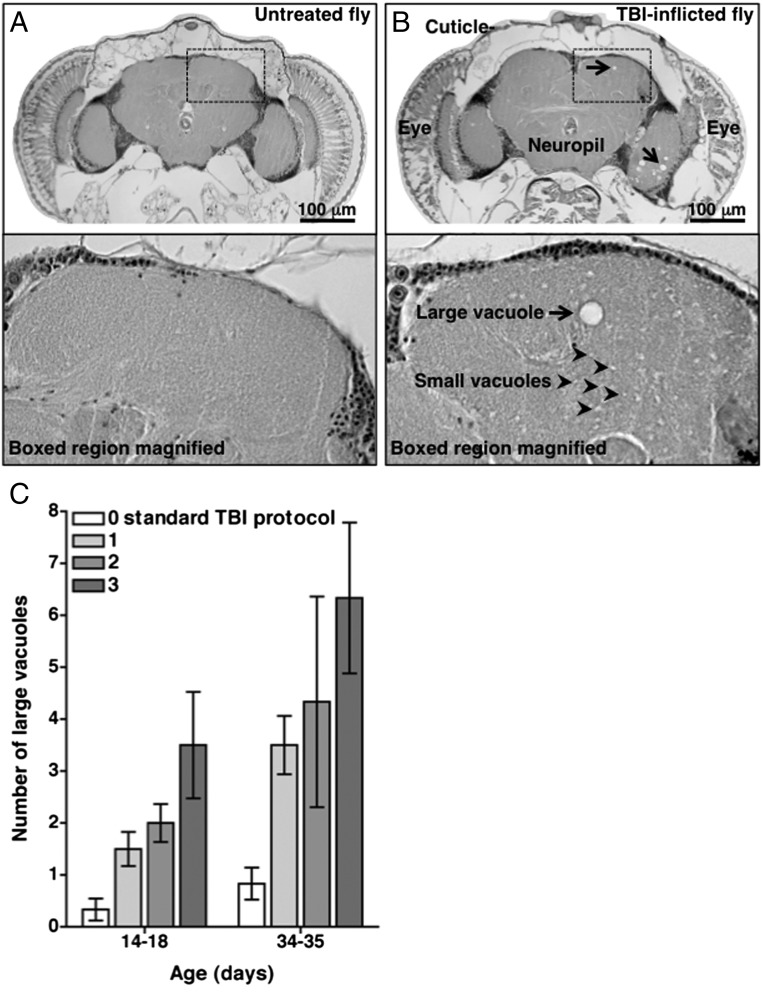
TBI in rodents and humans increases the occurrence of chronic traumatic encephalopathy (CTE), a form of neurodegeneration that generally appears long after (years to decades in the case of humans) the primary injury (18). To determine if neurodegeneration is also a long-term outcome of TBI in flies and whether it is affected by age at the time of primary injury, we performed histological analysis on brains of younger (0- to 4-d-old) and older (20- to 21-d-old) flies 14 d after they were subjected to the standard TBI protocol. Neurodegeneration in flies is commonly manifested by the appearance of vacuolar lesions in the brain neuropil, a region enriched for axons, synaptic terminals, and glial cells (6). In contrast with untreated flies whose brains had a smooth and uniform appearance throughout the neuropil (Fig. 4A), treated flies exhibited neuropathology manifested by the appearance of numerous small vacuolar lesions (∼1.0 μm in diameter) (Fig. 4B). We also observed large vacuolar lesions (5.0–10.0 μm in diameter) in injured flies. The incidence of large vacuolar lesions in the central region of the brain increased with the number of times flies were subjected to the standard TBI protocol (Fig. 4C). In addition, the incidence of large vacuolar lesions was higher in older flies subjected to TBI compared with similarly treated younger flies. These data indicate that brain injuries in flies elicit neurodegeneration as a long-term consequence and that the extent of neurodegeneration is determined by the severity of primary injuries and the age at the time of primary injuries.
Fig. 4.
Treatment of w1118 flies with the standard TBI protocol causes neurodegeneration. Images of sections of fly brains from 14- to 18-d-old (A, Upper and Lower) untreated and (B, Upper and Lower) treated flies are shown. The treated fly received the standard TBI protocol at 0–4 d old. The large vacuole indicated by the arrow in the magnified image (B, Lower) is 7.75 μm in diameter. (Scale bars in A, Upper and B, Upper: 100 μm. Images in Lower panels are magnified an additional 5×.) (C) The number of large, i.e., 5.0- to 10.0-μm-diameter vacuoles in the central region of the brain is graphed vs. the number of times flies were subjected to the standard TBI protocol. Brain sections chosen for analysis were at equivalent depths in the brain, as exemplified by A and B. Multiple treatments with the standard TBI protocol occurred over successive days. All flies were analyzed 14 d after the time of the first treatment, i.e., flies labeled 14–18 d were treated beginning at 0–4 d old, and flies labeled 34–35 d were treated beginning at 20–21 d old. Error bars indicate the SD for at least three heads. When treated with one standard TBI protocol, young flies (14–18 d old) developed significantly fewer large vacuoles that older flies (34–35 d old) (P = 0.003, one-tailed t test), but the difference between young and older flies was not significant for treatment with two or three standard TBI protocols (P = 0.07 and P = 0.07, one-tailed t test, respectively). Both young and older flies developed significantly more large vacuoles when treated with three vs. one standard TBI protocol (P = 0.029 for young flies and P = 0.03 for older flies, one-tailed t test).
Primary Injuries Activate the Innate Immune Response.
One important cause of secondary injuries in TBI is activation of the innate immune response (19). The innate immune response is triggered by pathogen-derived molecules and by endogenous molecules generated by stressed and injured cells (20). A component of this response is the production of proinflammatory cytokines, such as tumor necrosis factor (TNF), by microglial cells and astrocytes. In some studies, elevated levels of TNF in the cerebrospinal fluid of TBI patients are correlated with unfavorable clinical outcomes, providing evidence of the injurious role of cytokines (21). Likewise, inhibition of TNF shortly after primary injuries in rodents reduces the severity of some deleterious outcomes, such as tissue loss (22). On the other hand, long-term recovery of neurological damage in TBI is impaired in TNF-deficient mice, providing evidence of a beneficial role of cytokines (23). Therefore, the innate immune response plays a critical but not yet fully understood role in TBI. Flies provide an opportunity to advance our understanding of this role because innate immune response pathways are highly conserved between flies and humans (24). The Toll pathway in flies is analogous with mammalian Toll-like receptor (TLR) pathways, and the Immune deficiency (Imd) pathway is analogous with the mammalian TNF pathway. Among the key functions of both the Toll and Imd pathways is the transcriptional activation of antimicrobial peptide (AMP) genes (24).
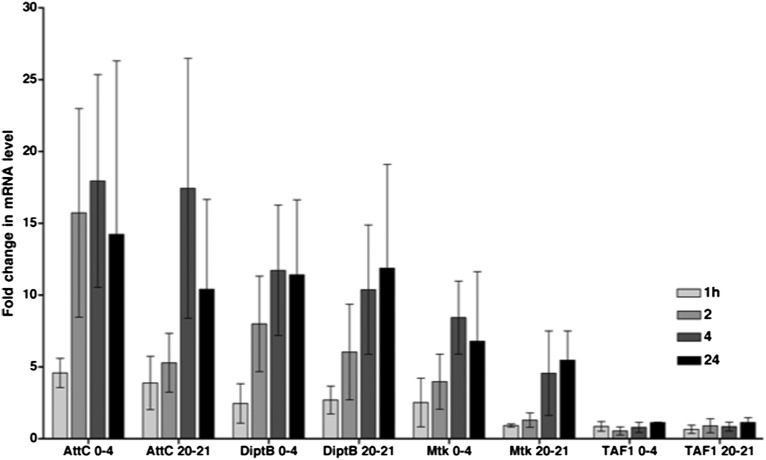
To assess activation of the innate immune response in flies following primary injuries, we used quantitative real-time reverse transcription–PCR (qRT-PCR) to determine AMP gene mRNA levels in heads of w1118 flies subjected to the standard TBI protocol. To control for the usual increase in expression of innate immunity genes that occurs as a function of age, treated flies were normalized to age-matched untreated flies (25). Flies that survived the standard TBI protocol exhibited an increase in AMP gene expression within 24 h after the primary injuries (Fig. 5). Similar results were observed in 0- to 4-d-old and 20- to 21-d-old flies. Some AMP genes were activated within 1 h after the primary injury and all AMP genes were activated within 24 h. Activation of innate immunity genes following primary injuries is at least somewhat specific because the expression of other genes such as TAF1 was not similarly increased. Thus, TBI in flies, as in mammals, elicits activation of an innate immune response pathway.
Fig. 5.
Treatment of w1118 flies with the standard TBI protocol activates the innate immune response. The histogram shows the fold-increase in mRNA levels in treated vs. untreated flies at the indicated timepoints after treatment of 0- to 4- or 20- to 21-d-old flies. The AMP genes examined were Attacin-C (AttC), Diptericin B (DiptB), and Metchnikowin (Mtk), and the control gene examined was TBP-associated factor 1 (TAF1). Error bars indicate the SEM for at least three independent trials.
Genetic Background Affects the Primary Injury Threshold.
An increasing body of evidence implicates genetic factors in the variable clinical outcomes of TBI in humans (26). To investigate the effect of genetic background on the primary injury threshold in flies, we determined the MI24 for 42 fly lines (aged 0–7 d) that were subjected to the standard TBI protocol. Five of these lines are commonly used as wild-type controls in various Drosophila experiments; the remaining 37 lines contain mutations in genes that encode components of the Imd or Toll pathways (24).
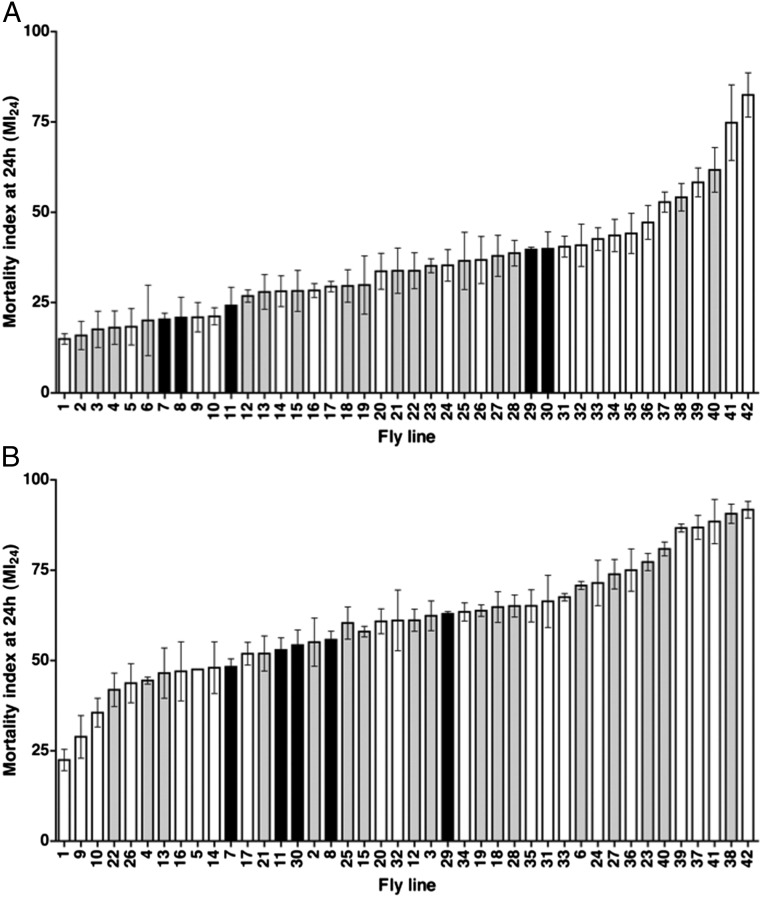
The MI24 showed wide variation among the lines, ranging from 14.9 ± 1.2 to 82.5 ± 5.0 (Fig. 6A). Variability was even observed among wild-type controls, for which the MI24 ranged from 20.3 ± 1.4 to 39.9 ± 3.8. Among the mutant lines there were striking differences for different alleles of the same gene. For example, the MI24 for ImdSDK and Imd10191 was 21.0 ± 3.3 and 40.9 ± 4.8, respectively. Additionally, the MI24 did not correlate with either the Imd or the Toll pathway. For example, mutations in Relish (Rel), which encodes the NF-κB transcription factor in the Imd pathway, had a significantly different effect on the MI24 than mutations in kenny (key), which encodes an activator of Rel (RelE20, 47.2 ± 2.3; RelE38, 41.7 ± 3.3; keyf05097, 8.5 ± 1.2; and keyc02831, 7.9 ± 1.9) (24). The wide range of effects for wild-type lines and the lack of coherent effects for Imd and Toll pathway lines suggests that in young flies many genes in the genetic background affect the primary injury threshold for death within 24 h. We suspect that, in some cases, the genetic background masks the effect of mutations in Imd or Toll pathway genes making it impossible to determine from these data whether the innate immune response specifically influences the MI24.
Fig. 6.
The MI24 is strongly affected by genetic background. Histograms show the MI24 for 42 different fly lines treated with the standard TBI protocol and tested at (A) 0–7 d old and (B) 20–27 d old. The MI24 vs. fly genotype is graphed. MI24 values were normalized to those of untreated flies. Genotypes are listed in Table S3. White bars indicate fly lines containing mutations in genes implicated in the Imd pathway. Gray bars indicate fly lines containing mutations in genes implicated in the Toll pathway. Black bars indicate fly lines commonly used as wild-type controls in Drosophila experiments. For a reference, fly line number 7 is w1118. Error bars indicate the SD for at least three independent trials of 60 flies each.
Genetic Background Affects the Age-Dependent Reduction of the Primary Injury Threshold.
To investigate the effect of genetic background on the primary injury threshold in older flies, we reexamined the 42 fly lines at 20–27 d old. The results largely paralleled those observed in young flies in that wide variability was observed in the MI24, and the variability did not correlate with the Imd or the Toll pathway (Fig. 6B). In addition, for every line, the MI24 for 20- to 27-d-old flies was higher than the MI24 for 0- to 7-d-old flies (Fig. S4). MIs24 of younger and older flies had a correlation coefficient (r) of 0.77. Thus, in all genetic backgrounds, age appears to be a critical determinant of the primary injury threshold for death within 24 h. However, the MI24 in younger flies was not entirely predictive of the extent of increase in the MI24 in older flies. For example, 0- to 7-d-old keyf05097 and spatzle (spz3) flies had similar MIs24 (14.9 ± 1.2 and 15.9 ± 3.2, respectively) but 20- to 27-d-old keyf05097 and spz3 flies had substantially different MIs24 (22.5 ± 4.2 and 55.1 ± 9.4, respectively) (Fig. S4). Conversely, 0- to 7-d-old necrotic (nec2) and TAK1-associated binding protein 2 (Tab2EY00380) flies had substantially different MIs24 (17.6 ± 4.1 and 44.2 ± 4.6, respectively) but 20- to 27-d-old nec2 and Tab2EY00380 flies had similar MIs24 (62.4 ± 5.9 and 65.1 ± 6.3, respectively). These data indicate that the genes that affect the primary injury threshold for death within 24 h in young flies are different from those that affect the age-dependent reduction in the primary injury threshold.
Variation in Primary Injury Threshold Correlates with Variation in Longevity.
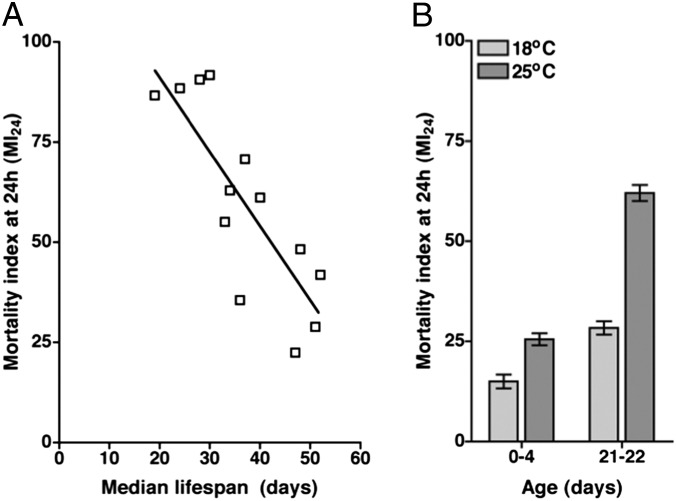
Because the primary injury threshold varies with age, we examined the correlation between MI24 and longevity in the absence of TBI. We analyzed 14 fly lines, including lines with low, average, or high MI24 after treatment with the standard TBI protocol. We found a negative linear relationship between the MI24 and the median lifespan for both 0- to 7-d-old and 20- to 27-d-old flies (Fig. 7A). The correlation coefficient r between the MI24 and the median lifespan was −0.67 for 0- to 7d-old flies and −0.84 for 20- to 27-d-old flies. Thus, the longevity of a particular fly line and its primary injury threshold for death within 24 h are largely determined by the same genetic factors.
Fig. 7.
The susceptibility of different fly lines to TBI-induced mortality is inversely correlated with their respective longevity. (A) The MI24 of 20- to 27-d-old flies is graphed vs. the median lifespan for 14 of the fly lines that were analyzed in Fig. 6. The lines that were analyzed are listed in Table S3. RelE20 and RelE38 flies had the same MI24 and median lifespan, so they appear as a single open box on the graph. (B) The MI24 is graphed for w1118 flies of the indicated age that were raised at either 18 °C or 25 °C. MI24 values were normalized to those of untreated flies. Temperature had a significant effect on the MI24 for both 0- to 4-d-old flies (P = 0.012, one-tailed t test) and 21- to 22-d-old flies (P = 0.001, one-tailed t test). Error bars indicate the SD for at least three independent trials of 60 flies each.
To test this proposal, we extended the lifespan of flies and examined the effect on the MI24. To extend the lifespan, we raised flies at 18 °C rather than at 25 °C, the temperature at which flies were raised for all of the prior experiments. Untreated w1118 flies raised at 18 °C had a significantly longer median lifespan relative to flies raised at 25 °C (68.5 ± 0.8 d vs. 48.3 ± 1.2 d, P < 0.0001, one-tailed t test). After treatment with the standard TBI protocol, w1118 flies raised at 18 °C had a significantly lower MI24 than equivalent-age flies raised at 25 °C (Fig. 7B). The negative correlation between natural longevity and MI24 was observed for both 0- to 3-d-old and 21- to 22-d-old flies. Thus, flies of the same genotype and chronological age but different median lifespan differed in their primary injury threshold for death within 24 h. This result indicates that environmental factors such as temperature determine both the longevity of a particular line in the absence of injury and its primary injury threshold for death within 24 h.
Discussion
Flies Can Model Human TBI.
Here, we have described the development and initial characterization of a fly model of TBI. We found that inflicting mechanical injury on flies by rapid acceleration and deceleration produces outcomes that are similar to outcomes characteristic of closed head TBI in humans (1, 2). These outcomes include temporary incapacitation (Fig. S2A), ataxia (Fig. S2B and Table S1), activation of the innate immune response (Fig. 5), neurodegeneration (Fig. 4), and death (Fig. 2). In addition, we found that risk factors for mortality in flies are shared with humans. Our data indicate that the MI24 following injury does not vary with sex (Fig. 3A). Similarly, a study of 914 women and 916 men by Coimbra et al. concluded that sex is not a risk factor for mortality in TBI patients (27). The MI24, however, is dependent on the age of flies at the time of injury (Figs. 3B and 6). Likewise, in a study of 5,612 TBI patients, Hukkelhoven et al. found that the percent mortality within 6 mo of primary injuries increased with age, 21% at <35 y and 52% at >55 y (28). Also, in a study of 244 TBI patients, Dhandapani et al. found that the percent mortality within 1 mo of primary injuries was significantly associated with increasing age, 15% at <18 y, 44% at 18–59 y, and 52% at >59 y (29). These similarities between flies in our TBI model and human TBI patients strongly indicate that flies and humans incur TBI through similar cellular and molecular mechanisms.
The Fly Model of TBI Can Provide Unique Insights into Human TBI.
Because the fly TBI model enables us to analyze many animals throughout their lifespan, we have found several properties of TBI that may be of clinical relevance: age at the time of primary injury as well as the number of primary TBI incidents are risk factors for neurodegeneration (Fig. 4C); the number of primary TBI incidents affects longevity (Fig. 2C and Table S2); the MI24 following TBI varies with genetic background (Fig. 6); and finally, the genes that affect the susceptibility to mortality are different at different ages (Fig. 6 and Fig. S4). Although it is difficult to obtain exactly comparable information from human TBI patients, available data are consistent with our conclusions. For example, as in flies, human studies suggest that age and the severity of primary injury are important factors in CTE development (30, 31). In addition, studies in different rodent strains as well as genetic association studies in TBI patients indicate that, as in flies, TBI outcomes depend on genetic background (26, 32).
Finally, we found that the genetic and environmental factors that affect longevity in the absence of injury also affect the susceptibility to mortality following TBI (Fig. 7). Two lines of evidence suggest that fractional age rather than chronological age per se is a risk factor for TBI-induced mortality in humans as it is in flies, where fractional age is defined as chronological age relative to the maximum longevity of flies of that genotype. First, drugs such as rapamycin and resveratrol that alleviate TBI outcomes in mammals also extend normal longevity (33–36). Second, the gene apolipoprotein E (apoE), implicated in controlling longevity, is also found to be a risk factor for TBI outcomes in mammals (37, 38). Interestingly, rapamycin, resveratrol, and apoE also appear to affect the severity of neurodegeneration in Alzheimer’s disease, which is phenotypically similar to CTE (18, 39). Collectively, these findings suggest that secondary injuries in TBI accelerate some normal aging processes in the brain. This idea may explain why the average age of onset (AAO) for CTE is substantially earlier than for Alzheimer’s disease (CTE: AAO = 42.8 ± 12.7 y, range = 25–76 y; Alzheimer’s disease: AAO = 72.8 ± 6.8 y, range = 49–97 y) (40, 41).
In summary, our studies demonstrate that a fly TBI model offers considerable potential for understanding the cellular and molecular mechanisms that underlie the pathological consequences of human TBI and may ultimately facilitate development of unique therapeutic strategies.
Materials and Methods
Fly Lines and Culturing.
Flies were maintained on standard molasses medium at 25 °C unless otherwise stated. All fly lines were obtained from the Bloomington Stock Center except for ImdSDK and Imd10191, which were obtained from Mimi Shirasu-Hiza (Columbia University, New York). Genotypes of flies used in Figs. 6 and 7A and Fig. S4 are described in Table S3.
Assays for TBI Outcomes.
Construction and use of the HIT device is described in Fig. S1. The impact velocity of the HIT device was determined by analyzing images from a high-speed camera. MIs24 were determined by analyzing at least 180 flies (three experiments of 60 flies). The percentage of flies that were incapacitated was determined by analyzing movie recordings of flies after a single strike. Flies that did not show obvious locomotor activity were considered incapacitated. Before each timepoint, hitting the benchtop upon which the fly vial sat was used to stimulate locomotion. Climbing, lifespan, histology, and qRT-PCR assays were performed as described in Petersen et al. (14, 42).
Supplementary Material
Acknowledgments
We thank Nicole Bertram, Grace Boekhoff-Falk, members of the D.A.W. and B.G. laboratories, and Ron Kalil for their insights that greatly improved this research; Russell Little for help in determining the impact velocity; and Satoshi Kinoshita for performing the histology. This work was supported by National Institutes of Health Grants R01 NS059001 (to D.A.W.) and R01 AG033620 (to B.G.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316895110/-/DCSupplemental.
References
- 1.Masel BE, DeWitt DS. Traumatic brain injury: A disease process, not an event. J Neurotrauma. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 2.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76(5):886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Maas AI, et al. Re-orientation of clinical research in traumatic brain injury: Report of an international workshop on comparative effectiveness research. J Neurotrauma. 2012;29(1):32–46. doi: 10.1089/neu.2010.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor WT, Smyth A, Gilchrist MD. Animal models of traumatic brain injury: A critical evaluation. Pharmacol Ther. 2011;130(2):106–113. doi: 10.1016/j.pharmthera.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Lessing D, Bonini NM. Maintaining the brain: Insight into human neurodegeneration from Drosophila melanogaster mutants. Nat Rev Genet. 2009;10(6):359–370. doi: 10.1038/nrg2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahsai L, Zars T. Learning and memory in Drosophila: Behavior, genetics, and neural systems. Int Rev Neurobiol. 2011;99:139–167. doi: 10.1016/B978-0-12-387003-2.00006-9. [DOI] [PubMed] [Google Scholar]
- 8.Bushey D, Cirelli C. From genetics to structure to function: Exploring sleep in Drosophila. Int Rev Neurobiol. 2011;99:213–244. doi: 10.1016/B978-0-12-387003-2.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyan GS, Reichert H. Mechanisms for complexity in the brain: Generating the insect central complex. Trends Neurosci. 2011;34(5):247–257. doi: 10.1016/j.tins.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Reichert H. A tripartite organization of the urbilaterian brain: Developmental genetic evidence from Drosophila. Brain Res Bull. 2005;66(4-6):491–494. doi: 10.1016/j.brainresbull.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 11.Littleton JT, Ganetzky B. Ion channels and synaptic organization: Analysis of the Drosophila genome. Neuron. 2000;26(1):35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 12.Stork T, Bernardos R, Freeman MR. Analysis of glial cell development and function in Drosophila. Cold Spring Harb Protoc. 2012;2012(1):1–17. doi: 10.1101/pdb.top067587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeSalvo MK, Mayer N, Mayer F, Bainton RJ. Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila. Glia. 2011;59(9):1322–1340. doi: 10.1002/glia.21147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen AJ, Rimkus SA, Wassarman DA. ATM kinase inhibition in glial cells activates the innate immune response and causes neurodegeneration in Drosophila. Proc Natl Acad Sci USA. 2012;109(11):E656–E664. doi: 10.1073/pnas.1110470109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fristrom D, Fristrom JW. The metamorphic development of the adult epidermis. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 843–897. [Google Scholar]
- 16.Piyankarage SC, Featherstone DE, Shippy SA. Nanoliter hemolymph sampling and analysis of individual adult Drosophila melanogaster. Anal Chem. 2012;84(10):4460–4466. doi: 10.1021/ac3002319. [DOI] [PubMed] [Google Scholar]
- 17.Faul M, Xu L, Wald MM, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 18.Baugh CM, et al. Chronic traumatic encephalopathy: Neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 2012;6(2):244–254. doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- 19.Hellewell SC, Morganti-Kossmann MC. Guilty molecules, guilty minds? The conflicting roles of the innate immune response to traumatic brain injury. Mediators Inflamm. 2012;2012:356494. doi: 10.1155/2012/356494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ransohoff RM, Brown MA. Innate immunity in the central nervous system. J Clin Invest. 2012;122(4):1164–1171. doi: 10.1172/JCI58644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csuka E, et al. IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: Relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J Neuroimmunol. 1999;101(2):211–221. doi: 10.1016/s0165-5728(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 22.Shohami E, Bass R, Wallach D, Yamin A, Gallily R. Inhibition of tumor necrosis factor alpha (TNFalpha) activity in rat brain is associated with cerebroprotection after closed head injury. J Cereb Blood Flow Metab. 1996;16(3):378–384. doi: 10.1097/00004647-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan PG, et al. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999;19(15):6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 25.Ramsden S, Cheung YY, Seroude L. Functional analysis of the Drosophila immune response during aging. Aging Cell. 2008;7(2):225–236. doi: 10.1111/j.1474-9726.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 26.Dardiotis E, et al. Genetic association studies in patients with traumatic brain injury. Neurosurg Focus. 2010;28(1):E9. doi: 10.3171/2009.10.FOCUS09215. [DOI] [PubMed] [Google Scholar]
- 27.Coimbra R, Hoyt DB, Potenza BM, Fortlage D, Hollingsworth-Fridlund P. Does sexual dimorphism influence outcome of traumatic brain injury patients? The answer is no! J Trauma. 2003;54(4):689–700. doi: 10.1097/01.TA.0000058314.31655.5F. [DOI] [PubMed] [Google Scholar]
- 28.Hukkelhoven CW, et al. Patient age and outcome following severe traumatic brain injury: An analysis of 5600 patients. J Neurosurg. 2003;99(4):666–673. doi: 10.3171/jns.2003.99.4.0666. [DOI] [PubMed] [Google Scholar]
- 29.Dhandapani S, Manju D, Sharma B, Mahapatra A. Prognostic significance of age in traumatic brain injury. J Neurosci Rural Pract. 2012;3(2):131–135. doi: 10.4103/0976-3147.98208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: A potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 2011;30(1):179–188, xi. doi: 10.1016/j.csm.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavett BE, Stern RA, Cantu RC, Nowinski CJ, McKee AC. Mild traumatic brain injury: A risk factor for neurodegeneration. Alzheimers Res Ther. 2010;2(3):18. doi: 10.1186/alzrt42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al Nimer F, et al. Strain influences on inflammatory pathway activation, cell infiltration and complement cascade after traumatic brain injury in the rat. Brain Behav Immun. 2013;27(1):109–122. doi: 10.1016/j.bbi.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Singleton RH, Yan HQ, Fellows-Mayle W, Dixon CE. Resveratrol attenuates behavioral impairments and reduces cortical and hippocampal loss in a rat controlled cortical impact model of traumatic brain injury. J Neurotrauma. 2010;27(6):1091–1099. doi: 10.1089/neu.2010.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Timmers S, Auwerx J, Schrauwen P. The journey of resveratrol from yeast to human. Aging (Albany, NY Online) 2012;4(3):146–158. doi: 10.18632/aging.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo D, Zeng L, Brody DL, Wong M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS ONE. 2013;8(5):e64078. doi: 10.1371/journal.pone.0064078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493(7432):338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahley RW, Huang Y. Apolipoprotein e sets the stage: Response to injury triggers neuropathology. Neuron. 2012;76(5):871–885. doi: 10.1016/j.neuron.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ang LS, Cruz RP, Hendel A, Granville DJ. Apolipoprotein E, an important player in longevity and age-related diseases. Exp Gerontol. 2008;43(7):615–622. doi: 10.1016/j.exger.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(10):a006296. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKee AC, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68(7):709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li YJ, et al. Age at onset in two common neurodegenerative diseases is genetically controlled. Am J Hum Genet. 2002;70(4):985–993. doi: 10.1086/339815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen AJ, Katzenberger RJ, Wassarman DA. The innate immune response transcription factor relish is necessary for neurodegeneration in a Drosophila model of ataxia-telangiectasia. Genetics. 2013;194(1):133–142. doi: 10.1534/genetics.113.150854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.