Significance
Maximum heart rate (mHR) declines with age, contributing to the reduced aerobic capacity of the elderly. A parallel reduction in "intrinsic heart rate" (which is measured during autonomic blockade) underlies the decline in mHR, and indicates that pacemaker function of the sinoatrial node is compromised during aging. In this study we demonstrate that the slower heart rates in the elderly result from depressed spontaneous activity of individual sinoatrial node myocytes (SAMs). Patch-clamp electrophysiology revealed that aged SAMs have slowed AP firing rates, altered AP waveforms, and changed properties of Ca2+ currents and the cardiac "funny current,"If. Our findings demonstrate that age-dependent changes ion channel activity in SAMs are a major cause of the decline in mHR during aging.
Abstract
An inexorable decline in maximum heart rate (mHR) progressively limits human aerobic capacity with advancing age. This decrease in mHR results from an age-dependent reduction in “intrinsic heart rate” (iHR), which is measured during autonomic blockade. The reduced iHR indicates, by definition, that pacemaker function of the sinoatrial node is compromised during aging. However, little is known about the properties of pacemaker myocytes in the aged sinoatrial node. Here, we show that depressed excitability of individual sinoatrial node myocytes (SAMs) contributes to reductions in heart rate with advancing age. We found that age-dependent declines in mHR and iHR in ECG recordings from mice were paralleled by declines in spontaneous action potential (AP) firing rates (FRs) in patch-clamp recordings from acutely isolated SAMs. The slower FR of aged SAMs resulted from changes in the AP waveform that were limited to hyperpolarization of the maximum diastolic potential and slowing of the early part of the diastolic depolarization. These AP waveform changes were associated with cellular hypertrophy, reduced current densities for L- and T-type Ca2+ currents and the “funny current” (If), and a hyperpolarizing shift in the voltage dependence of If. The age-dependent reduction in sinoatrial node function was not associated with changes in β-adrenergic responsiveness, which was preserved during aging for heart rate, SAM FR, L- and T-type Ca2+ currents, and If. Our results indicate that depressed excitability of individual SAMs due to altered ion channel activity contributes to the decline in mHR, and thus aerobic capacity, during normal aging.
One of the most insidious aspects of growing older is an inevitable decline in maximum heart rate (mHR), which limits maximum aerobic capacity with advancing age (1–3). The decline in mHR proceeds at approximately the same rate for all individuals, without regard for lifestyle or physical fitness (4–8). For many otherwise healthy elderly people, it is the factor that ultimately restricts the ability to live independently (9, 10).
The decrease in mHR with age results primarily from a parallel age-dependent decline in “intrinsic heart rate” (iHR) (11–13), which is measured during autonomic blockade, and thus reflects the spontaneous pacemaker activity of the sinoatrial node of the heart. Although it is known that the intact sinoatrial node from aged animals contracts more slowly (14, 15) and contains fewer pacemaker myocytes (16), little is known about the functional properties of individual myocytes from the sinoatrial node of the aged heart.
Sinoatrial myocytes (SAMs) are highly specialized cells that serve a primarily electrical function as cardiac pacemakers via their production of spontaneous action potentials (APs). Sinoatrial APs are characterized by a spontaneous depolarization during diastole that drives the membrane potential to threshold, thereby triggering the subsequent AP. This “diastolic depolarization” (DD) phase of the sinoatrial AP results from the coordinated activity of numerous membrane conductances, including L- and T-type Ca2+ currents (ICa,L and ICa,T, respectively) and the “funny current” (If), all of which contribute directly to the DD by conducting inward current at diastolic potentials (17–23). ICa,L also contributes indirectly to the DD by stimulating Ca2+ efflux from the sarcoplasmic reticulum of SAMs (24), thereby activating the Na+-Ca2+ exchange current (INCX), which is also known to be critical for normal pacemaker activity (25–29).
In this study, we determined the effects of aging on heart rates (HRs) and on spontaneous APs and membrane currents in acutely isolated SAMs. We observed age-dependent decreases in AP firing rates (FRs) in SAMs that corresponded to the age-dependent reductions in iHRs and mHRs. The slower AP FRs resulted from changes in the AP waveform that were associated with an increase in cell size and with alterations in ICa,L, ICa,T, and If. These findings indicate that changes in expression and/or regulation of ion channels in SAMs comprise part of the molecular program that limits mHR, and thus aerobic capacity, during normal aging.
Results
Similar Reductions in HR and SAM FR in Aged Mice.
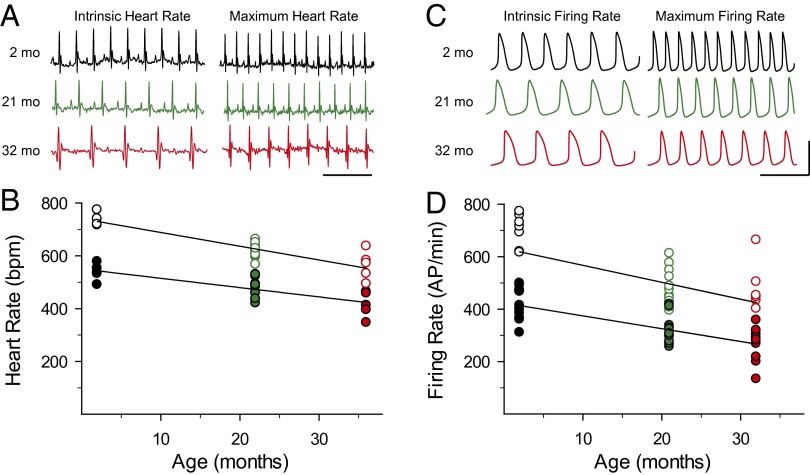
iHR and mHR were determined from ECGs recorded from awake, restrained mice of three age groups: 2–3, 21–24, and 32+ mo (corresponding to ∼17–20, 65–69, and 87+ y in humans) (30, 31) (Fig. 1A). iHR was measured during autonomic blockade with atropine and propranolol, whereas mHR was induced by restraint stress [verified by administration of isoproterenol (ISO); Fig. S1]. Both iHR and mHR were significantly reduced in older mice compared with animals aged 2–3 mo (P < 0.01; Fig. 1B and Table 1). However, the mHR/iHR ratios were similar in each age group (P > 0.05; Table 1), demonstrating that the chronotropic response to β-adrenergic receptor (βAR) stimulation is largely preserved during aging in mice, as it is in humans (11). In agreement with earlier studies (32–35), ECG intervals were also significantly prolonged or altered in the older mice (Table S1).
Fig. 1.
Parallel age-dependent declines in HR and SAM AP FR. (A) Representative ECG recordings of iHRs and mHRs from mice aged 2–3 mo (black), 21–24 mo (green), and 32+ mo (red). (Scale bar: 250 ms.) (B) iHRs (filled circles) and mHRs (open circles) from mice of the three age groups. (C) Representative APs recorded from acutely dissociated SAMs from mice of different ages. (Scale bars: 250 ms, 70 mV). (D) iHR (filled circles) and mHR (open circles) AP FRs from SAMs isolated from mice of different ages. The lines in B and D are linear regressions to the data.
Table 1.
Age-dependent changes in HRs and SAM AP FRs
| Intrinsic |
Maximum |
βAR response (maximum/intrinsic) |
|||||||
| 2–3 mo | 21–24 mo | 32+ mo | 2–3 mo | 21–24 mo | 32+ mo | 2–3 mo | 21–24 mo | 32+ mo | |
| HR, bpm | 537.3 ± 14.3 (5) | 478.7 ± 10.7* (12) | 414.9 ± 21.6* (5) | 737.3 ± 10.3 (5) | 619.1 ± 7.4* (12) | 562.9 ± 24.0* (5) | 1.37 ± 0.04 | 1.30 ± 0.04 | 1.36 ± 0.05 |
| FR rate, AP/min | 414.3 ± 19.3 (10) | 318.5 ± 14.7* (12) | 268.6 ± 21.2* (10) | 626.4 ± 41.7 (10) | 478.4 ± 20.9* (12) | 438.4 ± 33.1* (10) | 1.51 ± 0.06 | 1.50 ± 0.03 | 1.46 ± 0.04 |
Data are averages (±SEM) of 30-s recording windows for HRs from ECG recordings or SAM FRs from perforated-patch recordings. Numbers of mice or cells are shown in parentheses. The βAR response is reported as the average ratio of maximum/intrinsic for both HR and FR.
P < 0.05 vs. animals/cells aged 2–3 mo (one-way ANOVA with a Holm–Sidak posttest).
Because the spontaneous activity of pacemaker myocytes in the sinoatrial node determines HR, we next examined the effects of aging on the intrinsic excitability of individual SAMs. Spontaneous APs were recorded from acutely dissociated SAMs from mice of the three different age groups using the amphotericin perforated-patch technique (Fig. 1C). Stable intrinsic AP firing rate (iFR) was determined in the presence of 1 nM ISO (36), and maximum firing rate (mFR) was recorded in the same cells >2 min after wash-on of a saturating concentration of ISO (1 μM; Fig. S2). Both iFR and mFR were significantly reduced in cells isolated from the older animals (P < 0.01; Fig. 1D and Table 1), with age-dependent declines that were remarkably similar to the declines in iHR and mHR. As in the case of HR, the mFR/iFR ratio did not differ in cells from the three age groups (P > 0.05; Table 1), indicating that βAR responsiveness of individual SAMs did not change appreciably during aging.
Limited Changes in the AP Waveform in Aged SAMs.
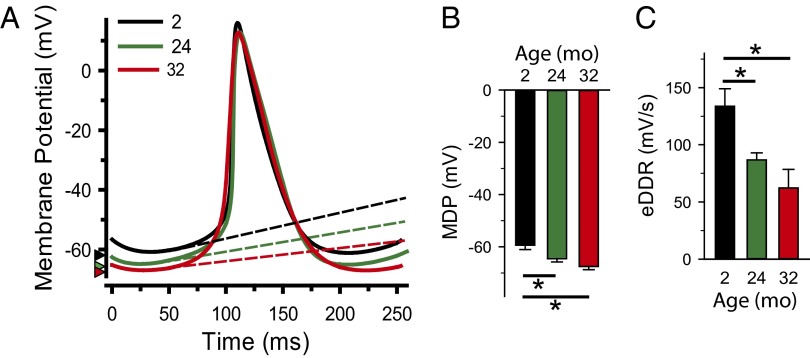
Age-dependent changes in AP waveform parameters were determined from perforated-patch current-clamp recordings using an analysis method adapted from Bucchi et al. (22). In these experiments, we found that the longer cycle length in aged SAMs was associated with substantial changes in only a subset of the AP parameters (Fig. 2 and Table S2). Specifically, aging hyperpolarized the maximum diastolic potential (MDP)and slowed the early part of the DD (P < 0.05; Table S2).In contrast, aging had little or no effect on the late phase of the DD, the AP upstroke velocity, the repolarization rate, or the AP duration (P > 0.05; Table S2). Intermediate effects, apparent only in cells from the oldest animals, were observed for the take-off potential and the AP amplitude (Table S2).
Fig. 2.
Slower AP FRs in aged SAMs result from limited changes in the sinoatrial AP waveform. (A) Representative APs recorded from SAMs in mice aged 2–3 mo (black), 21–24 mo (green), and 32+ mo (red) superimposed at time of peak. Dashed lines indicate the slopes of the DD, and arrowheads mark the MDP. (B) Average (±SEM) MDP and early DD rate (eDDR) in SAMs from mice of different ages. *P < 0.05, one-way ANOVA with a Holm–Sidak posttest.
Reduced Ca2+ Current Densities and Increased Membrane Capacitance in Aged SAMs.
Because ICa,L and ICa,T are important for the generation of spontaneous APs in SAMs (37–39), we next examined their properties in whole-cell voltage-clamp experiments in SAMs isolated from mice aged 2–3 mo, 21–24 mo, and 28 mo (older mice were not available at the time of these experiments). Total ICa was elicited by 200-ms depolarizing voltage steps from a holding potential of −90 mV. ICa,L was then elicited in the same cells from a holding potential of −60 mV [where ICa,T in SAMs is mostly inactivated (37, 39)]. ICa,T was subsequently calculated for each cell as the difference current, ICa − ICa,L. We found that aging reduced the peak current densities for both ICa,L and ICa,T without alteration in the midpoint activation voltage (V1/2) or the rates of inactivation (Fig. 3 and Table S3). These decreases in current density persisted in the presence of ISO and were associated with an age-dependent increase in cell size, as indicated by an increase in cellular capacitance [P < 0.001; Table S3; capacitance = 36.1 ± 1.0, 45.9 ± 1.2, and 48.2 ± 1.0 pF in cells from 2–3 mo, 21–24 mo, and 28+ mo, respectively]. The cellular hypertrophy appeared to account for at least some of the decreased density for ICa,L, ICa,T, and If (see below) because age-dependent differences were reduced when conductance was not normalized to cell size (Table S3). As in the case of the HR and FR, βAR responsiveness for both ICa,L and ICa,T was preserved during aging, as indicated by similar percentage increases in current density in response to ISO in SAMs of different ages (P > 0.05; Table S3).
Fig. 3.
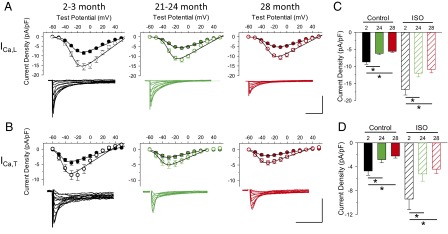
Decreased ICa,L and ICa,T conductance densities in aged SAMs. (A and B) Average (±SEM) current-voltage relationships and representative currents for ICa,L and ICa,T in SAMs in mice aged 2–3 mo (black), 21–24 mo (green), and 28 mo (red) in the absence (filled circles) and presence (open circles) of 1 μM ISO. (Scale bars: 5 pA/pF, 25 ms.) (Insets) Representative current families. (C and D) Average (±SEM) peak ICa,L and ICa,T densities in SAMs in mice aged 2–3 mo (black), 21–24 mo (green), and 28 mo (red) in the absence (filled bars) and presence (hatched bars) of ISO. *P < 0.05, one-way ANOVA with a Holm–Sidak posttest.
Hyperpolarized Voltage Dependence of If in Aged SAMs.
If is thought to be a major determinant of both the MDP and the early DD in SAMs (20–23). To evaluate age-dependent changes in the properties of If in SAMs, we applied 3-s hyperpolarizing voltage steps from a holding potential of −50 mV in whole-cell voltage-clamp recordings. As for ICa,L and ICa,T, we found that the peak If density was substantially reduced in aged SAMs (P < 0.05; Table S3). Interestingly, the voltage dependence of activation of If was also altered by age. Specifically, there was a pronounced hyperpolarizing shift in the V1/2 for If in aged SAMs (P < 0.01; Fig. 4 and Table S3). As for HR, FR, ICa,L, and ICa,T, βAR responsiveness of If was unchanged by age, as evidenced by the similar changes in the V1/2 in the absence or presence of a saturating concentration of ISO (1 μM; Fig. S3 and Table S3).
Fig. 4.
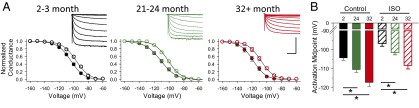
Hyperpolarized activation midpoint for If in aged SAMs. (A) Normalized average (±SEM) conductance-voltage relationships for If in SAMs in mice aged 2–3 mo (black), 21–24 mo (green), and 32+ mo (red) in the absence (filled circles) and presence (open circles) of 1 μM ISO. (Insets) Representative If current families normalized to cellular capacitance. (Scale bars: 15 pA/pF, 750 ms.) (B) Average (±SEM) V1/2 values for If in isolated SAMs from mice of different ages in the absence (filled bars) or presence (hatched bars) of ISO. *P < 0.05, one-way ANOVA with a Holm–Sidak posttest.
Discussion
The major findings of this study are that (i) the age-dependent decline in mHR depends, at least in part, on a corresponding decrease in the spontaneous excitability of SAMs; (ii) the reduced SAM excitability results from changes in a limited set of AP parameters; and (iii) altered membrane currents contribute to the changes in the AP waveform and, as a consequence, to decreased SAM FR and HR.
Preserved βAR Response in the Aged Sinoatrial Node.
Our observation that the chronotropic response to βAR stimulation was preserved in aged mice provides additional support for the long-standing idea that decreased iHR is the primary cause of the age-dependent decrease in mHR (11–13). We also found that aging did not alter βAR responsiveness of AP FR, Ca2+ current density, or the voltage dependence of If in SAMs. These preserved responses to βAR stimulation in aged SAMs differ from the reduced inotropic response to βAR stimulation in aged ventricular myocytes (16, 40), underscoring the high degree of specialization of myocytes from different regions of the heart. In SAMs, βAR-stimulated intracellular signaling pathways appear to be unaltered during aging, which, instead, targeted mechanisms responsible for basal pacemaker activity.
Depressed Excitability in Aged SAMs.
Previous studies in intact sinoatrial node preparations have shown that aging reduces conduction velocity (32–35) and decreases the number of sinoatrial node myocytes (16). These changes are thought to contribute to the age-dependent declines in iHR and mHR by limiting the ability of the relatively small sinoatrial node to overcome the hyperpolarizing load of the much larger surrounding atrial tissue (41, 42). In addition to these mechanisms, a decrease in excitability of individual SAMs has been proposed as a potential factor that could contribute to age-dependent reductions in iHR and mHR (14, 15, 43). Our present data establish that aging does indeed depress the spontaneous activity of SAMs as a result of altered membrane properties. It will be important in future work to determine whether these changes in SAM properties arise from a preferential loss of a subpopulation of smaller cells that fire more rapidly and/or hypertrophy of individual myocytes.
Altered AP Waveform and Membrane Currents in Aged SAMs.
It is noteworthy that aging slowed the FR of SAMs by changing only the MDP and the early DD, with little or no effect on other AP waveform parameters. This result indicates that the slower AP FR of older cells results from changes in the balance of ionic currents that are active during the early DD. As an initial description of candidate currents, we examined the properties of ICa,L, ICa,T, and If, all of which contribute to the DD.
ICa,L in mouse SAMs is produced mainly by the Cav1.3 channel isoform (37), which activates at more negative potentials than Cav1.2 (44), the predominant L-type Ca2+ channel isoform in atrial and ventricular myocytes. The relatively hyperpolarized voltage dependence of Cav1.3 overlaps the voltage range of the DD (e.g., compare Figs. 2 and 3), and SAMs from Cav1.3 KO mice have prolonged cycle lengths (37) and slower DD (38). Thus, the reduced ICa,L density we observed in aged SAMs would be expected to contribute to the age-dependent slowing of the DD, albeit with more substantial effects on the later part of the DD. In contrast, the voltage dependence of ICa,T is considerably more negative than that of ICa,L (e.g., Fig. 3), corresponding more closely to the early part of the DD. KO of the Cav3.1 T-type Ca2+ channel isoform nearly eliminates ICa,T in mouse SAMs and reduces iHR and AP FR via slowing of the DD (39). Consequently, the reduced ICa,T density we observed in aged SAMs would be expected to contribute to depressed pacemaker activity, although the relatively low availability of ICa,T at diastolic potentials due to inactivation may limit its contribution.
Strong evidence supports a role for If in determining the FR of SAMs via effects on the DD and MDP (20–23). The exact mechanism(s) by which If contributes to spontaneous activity in SAMs remains enigmatic, given the apparent discrepancy between the voltage dependence of activation as determined by traditional voltage-clamp protocols (e.g., Fig. 4) and the voltage range of the DD. Possible explanations for this mismatch between the importance of the channels and their hyperpolarized voltage dependence include the production of voltage-independent leak current by hyperpolarization-activated, cyclic nucleotide-sensitive (HCN) channels (45, 46), hysteresis in the voltage dependence of activation (47, 48), and slow activation and inactivation that could contribute to persistent opening in response to the relatively rapid voltage changes in mouse SAMs. A precedent for activity of HCN channels at positive membrane potentials is provided by the recent report of a role for HCN3 in repolarization of ventricular myocytes (49).
Numerous molecular mechanisms likely conspire to produce all the age-dependent changes we saw in ICa,L, ICa,T, and If. However, the similar reductions in ICa,L, ICa,T, and If current densities and increase in membrane capacitance raise the intriguing possibility that these changes may be related. For instance, down-regulation of Ca2+ channels could contribute to the hypertrophy of older SAMs, because decreased Ca2+ flux via L-type channels is a known regulator of cardiac gene expression (50). Alternatively, there may be a common mechanism that controls channel expression in aged SAMs such that the number of Ca2+ and HCN channels per unit membrane does not increase sufficiently to compensate for the increased cell size. In this regard, the unchanged voltage dependence of activation and rate of inactivation of ICa,L in aged SAMs strongly suggest that Cav1.2 and Cav1.3 are regulated in parallel during aging (because they activate at different potentials and have markedly different kinetics of inactivation). However, it is difficult to compare the reduced current densities in our study with previous studies of transcript expression in the aging rat sinoatrial node in which Cav1.3 and Cav3.1 transcripts were unchanged (51), Cav1.2 transcripts were increased (51), and HCN4 transcripts were either unchanged (51) or decreased (52) with age. These apparent discrepancies could result from species-dependent differences or from differential transcript expression in SAMs (as assayed in our patch-clamp experiments) vs. other cell types present in the entire sinoatrial node (as included in expression studies). Altered channel regulation could also contribute to the age-dependent reductions in current densities and shift in If voltage dependence in our studies. However, any such mechanism(s) could not depend on soluble cytoplasmic factors, because they persisted in whole-cell voltage-clamp studies in which soluble factors such as cAMP would be highly diluted. Finally, although age-dependent changes in HCN isoform expression are also possible, such changes are unlikely to account for the negative shift in the V1/2 of If in aged SAMs, given that the activation of HCN2 is similar to that of HCN4 and that HCN1 and HCN3 activate at substantially more positive potentials (53).
Predicted Changes in Other Membrane Currents in Aged SAMs.
Our observation that the AP upstroke, duration, and repolarization were largely preserved in aged SAMs indicates the there must be age-related changes in outward currents that are active during these phases of the sinoatrial AP. Without a decrease in net outward current, the age-dependent reduction in ICa,L density would tend to decrease the action potential duration (APD), as it does in SAMs from Cav1.3 KO mice (38). Candidate outward currents that could offset the reduced ICa,L density include the rapid delayed rectifier K+ current (IKr) and the inward rectifier Cl− current mediated by ClC-2, both of which influence MDP, APD, and repolarization in SAMs (36, 54). The decrease in If density in aged SAMs could also contribute to a reduction in repolarizing K+ current, as it does in ventricular myocytes (49).
We also consider it likely that aging alters Ca2+ release from the sarcoplasmic reticulum (SR) in SAMs, a process that is known to be critical for pacemaker activity (27–29, 55). At very least, the age-dependent decrease in ICa,L density would be expected to reduce the probability of SR Ca2+ release during diastole (24). Although Ca2+ release from the SR via ryanodine receptors and the ensuing inward INCX are thought to be associated with the later part of the DD (55), which was not altered in aged SAMs, the relationship between SR Ca2+ release and membrane voltage is complex and includes Ca2+-dependent inactivation of L-type channels, regulation of cAMP production [which links Ca2+ levels to If activation in guinea pig SAMs (56)], and activation of large conductance Ca2+-activated K+ (BK) channels [which also contribute to pacemaking (57)].
Summary.
Given that the spontaneous activity of SAMs determines HR, our central observation that pacemaker activity of individual SAMs is depressed during aging indicates that the slower AP FR of aged SAMs contributes to the age-dependent reductions in iHR and mHR (11–13). Furthermore, because the decrease in mHR with age is thought to be a major determinant of the age-dependent decrease in the maximum rate of oxygen consumption (VO2-max) (1–3), our data support a model in which depressed excitability of SAMs underlies at least part of the reduction in aerobic capacity with age. This decline in VO2-max is a fundamental aspect of aging that has an enormous impact on individuals and on society. For athletes, the decrease in VO2-max is the main reason for the decline in exercise performance with age (1, 58). For many elderly individuals, a low VO2-max is the factor that limits functional independence by restricting the ability to perform daily activities (9, 10, 59). Elucidation of the molecular mechanisms responsible for the age-dependent reduction in SAM pacemaker activity may identify novel drug targets that could forestall the reduction in aerobic capacity during aging.
Materials and Methods
Animal procedures were approved by the University of Colorado Institutional Animal Care and Use Committee. ECGs were recorded from awake, restrained mice using the ECG Tunnel system (EMKA Technologies) and a Powerlab amplifier (AD Instruments), and they were analyzed offline using Labchart 7 Pro software (AD Instruments). SAMs were isolated from WT C57BL/6 male mice as previously described (60) and were patch-clamped at 35 ± 1 °C in current-clamp mode (for APs) and voltage-clamp mode (for membrane currents). Detailed methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Peter DeWitt, MS, and the Colorado Biostatistics Consortium for consultation regarding statistical analysis [supported by National Institutes of Health (NIH) Grant UL1 TR000154 to the Colorado Clinical Translational Sciences Institute]. This work was supported by NIH Grants HL088427 (to C.P.) and AG038778 (to R.A.B.). E.D.L. was partially supported by National Institute of Neurological Disorders and Stroke Grant T32NS007083.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308477110/-/DCSupplemental.
References
- 1.Hagberg JM, et al. A hemodynamic comparison of young and older endurance athletes during exercise. J Appl Physiol. 1985;58(6):2041–2046. doi: 10.1152/jappl.1985.58.6.2041. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: Implications for exercise training. Sports Med. 2003;33(12):877–888. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- 3.Heath GW, Hagberg JM, Ehsani AA, Holloszy JO. A physiological comparison of young and older endurance athletes. J Appl Physiol. 1981;51(3):634–640. doi: 10.1152/jappl.1981.51.3.634. [DOI] [PubMed] [Google Scholar]
- 4.Higginbotham MB, Morris KG, Williams RS, Coleman RE, Cobb FR. Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol. 1986;57(15):1374–1379. doi: 10.1016/0002-9149(86)90221-3. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa T, et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86(2):494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 6.Robinson S. Experimental studies of physical fitness in relation to age. Eur J Appl Physiol. 1938;10(3):251–323. [Google Scholar]
- 7.Robinson S, Dill DB, Tzankoff SP, Wagner JA, Robinson RD. Longitudinal studies of aging in 37 men. J Appl Physiol. 1975;38(2):263–267. doi: 10.1152/jappl.1975.38.2.263. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald MD, Tanaka H, Tran ZV, Seals DR. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: A meta-analysis. J Appl Physiol. 1997;83(1):160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 9.Paterson DH, Govindasamy D, Vidmar M, Cunningham DA, Koval JJ. Longitudinal study of determinants of dependence in an elderly population. J Am Geriatr Soc. 2004;52(10):1632–1638. doi: 10.1111/j.1532-5415.2004.52454.x. [DOI] [PubMed] [Google Scholar]
- 10.Shephard RJ. Maximal oxygen intake and independence in old age. Br J Sports Med. 2009;43(5):342–346. doi: 10.1136/bjsm.2007.044800. [DOI] [PubMed] [Google Scholar]
- 11.Christou DD, Seals DR. Decreased maximal heart rate with aging is related to reduced β-adrenergic responsiveness but is largely explained by a reduction in intrinsic heart rate. J Appl Physiol. 2008;105(1):24–29. doi: 10.1152/japplphysiol.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jose AD, Collison D. The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res. 1970;4(2):160–167. doi: 10.1093/cvr/4.2.160. [DOI] [PubMed] [Google Scholar]
- 13.Jose AD, Stitt F, Collison D. The effects of exercise and changes in body temperature on the intrinsic heart rate in man. Am Heart J. 1970;79(4):488–498. doi: 10.1016/0002-8703(70)90254-1. [DOI] [PubMed] [Google Scholar]
- 14.Cavoto FV, Kelliher GJ, Roberts J. Electrophysiological changes in the rat atrium with age. Am J Physiol. 1974;226(6):1293–1297. doi: 10.1152/ajplegacy.1974.226.6.1293. [DOI] [PubMed] [Google Scholar]
- 15.Di Gennaro M, Bernabei R, Sgadari A, Carosella L, Carbonin PU. Age-related differences in isolated rat sinus node function. Basic Res Cardiol. 1987;82(6):530–536. doi: 10.1007/BF01907222. [DOI] [PubMed] [Google Scholar]
- 16.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73(2):413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa Y, Ogiwara Y, Akahane K, Chiba S. Different antagonism of the positive chronotropic and inotropic responses of the isolated, blood-perfused dog atrium to Bay k 8644 by nicardipine and verapamil. Eur J Pharmacol. 1988;156(2):231–237. doi: 10.1016/0014-2999(88)90326-3. [DOI] [PubMed] [Google Scholar]
- 18.Satoh K, Wada Y, Taira N. Differential effects of Bay k 8644, a presumed calcium channel activator, on sinoatrial nodal and ventricular automaticity of the dog heart. Naunyn Schmiedebergs Arch Pharmacol. 1984;326(2):190–192. doi: 10.1007/BF00517319. [DOI] [PubMed] [Google Scholar]
- 19.Verheijck EE, van Ginneken AC, Wilders R, Bouman LN. Contribution of L-type Ca2+ current to electrical activity in sinoatrial nodal myocytes of rabbits. Am J Physiol. 1999;276(3 Pt 2):H1064–H1077. doi: 10.1152/ajpheart.1999.276.3.H1064. [DOI] [PubMed] [Google Scholar]
- 20.Alig J, et al. Control of heart rate by cAMP sensitivity of HCN channels. Proc Natl Acad Sci USA. 2009;106(29):12189–12194. doi: 10.1073/pnas.0810332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrmann S, Stieber J, Stöckl G, Hofmann F, Ludwig A. HCN4 provides a ‘depolarization reserve’ and is not required for heart rate acceleration in mice. EMBO J. 2007;26(21):4423–4432. doi: 10.1038/sj.emboj.7601868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bucchi A, Baruscotti M, Robinson RB, DiFrancesco D. Modulation of rate by autonomic agonists in SAN cells involves changes in diastolic depolarization and the pacemaker current. J Mol Cell Cardiol. 2007;43(1):39–48. doi: 10.1016/j.yjmcc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Stieber J, Wieland K, Stöckl G, Ludwig A, Hofmann F. Bradycardic and proarrhythmic properties of sinus node inhibitors. Mol Pharmacol. 2006;69(4):1328–1337. doi: 10.1124/mol.105.020701. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, Wu Y, Mohler PJ, Anderson ME, Song LS. Local control of Ca2+-induced Ca2+ release in mouse sinoatrial node cells. J Mol Cell Cardiol. 2009;47(5):706–715. doi: 10.1016/j.yjmcc.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogdanov KY, Vinogradova TM, Lakatta EG. Sinoatrial nodal cell ryanodine receptor and Na(+)-Ca(2+) exchanger: Molecular partners in pacemaker regulation. Circ Res. 2001;88(12):1254–1258. doi: 10.1161/hh1201.092095. [DOI] [PubMed] [Google Scholar]
- 26.Gao Z, et al. Genetic inhibition of Na+-Ca2+ exchanger current disables fight or flight sinoatrial node activity without affecting resting heart rate. Circ Res. 2013;112(2):309–317. doi: 10.1161/CIRCRESAHA.111.300193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ju YK, Allen DG. Intracellular calcium and Na+-Ca2+ exchange current in isolated toad pacemaker cells. J Physiol. 1998;508(Pt 1):153–166. doi: 10.1111/j.1469-7793.1998.153br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rigg L, Terrar DA. Possible role of calcium release from the sarcoplasmic reticulum in pacemaking in guinea-pig sino-atrial node. Exp Physiol. 1996;81(5):877–880. doi: 10.1113/expphysiol.1996.sp003983. [DOI] [PubMed] [Google Scholar]
- 29.Rubenstein DS, Lipsius SL. Mechanisms of automaticity in subsidiary pacemakers from cat right atrium. Circ Res. 1989;64(4):648–657. doi: 10.1161/01.res.64.4.648. [DOI] [PubMed] [Google Scholar]
- 30.Fox JG. The Mouse in Biomedical Research. Boston: Elsevier; 2007. [Google Scholar]
- 31.Nadon NL, et al. Design of aging intervention studies: The NIA interventions testing program. Age (Dordr) 2008;30(4):187–199. doi: 10.1007/s11357-008-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones SA, Lancaster MK, Boyett MR. Ageing-related changes of connexins and conduction within the sinoatrial node. J Physiol. 2004;560(Pt 2):429–437. doi: 10.1113/jphysiol.2004.072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox TT, Weaver JC, Francis RL. Further studies on electrocardiographic changes in old age. Geriatrics. 1948;3(1):35–41. [PubMed] [Google Scholar]
- 34.Khane RS, Surdi AD, Bhatkar RS. Changes in ECG pattern with advancing age. J Basic Clin Physiol Pharmacol. 2011;22(4):97–101. doi: 10.1515/JBCPP.2011.017. [DOI] [PubMed] [Google Scholar]
- 35.Xing S, et al. Genetic influence on electrocardiogram time intervals and heart rate in aging mice. Am J Physiol Heart Circ Physiol. 2009;296(6):H1907–H1913. doi: 10.1152/ajpheart.00681.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark RB, et al. A rapidly activating delayed rectifier K+ current regulates pacemaker activity in adult mouse sinoatrial node cells. Am J Physiol Heart Circ Physiol. 2004;286(5):H1757–H1766. doi: 10.1152/ajpheart.00753.2003. [DOI] [PubMed] [Google Scholar]
- 37.Mangoni ME, et al. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci USA. 2003;100(9):5543–5548. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, et al. Functional Roles of Ca(v)1.3 (alpha(1D)) calcium channel in sinoatrial nodes: Insight gained using gene-targeted null mutant mice. Circ Res. 2002;90(9):981–987. doi: 10.1161/01.res.0000018003.14304.e2. [DOI] [PubMed] [Google Scholar]
- 39.Mangoni ME, et al. Voltage-dependent calcium channels and cardiac pacemaker activity: From ionic currents to genes. Prog Biophys Mol Biol. 2006;90(1-3):38–63. doi: 10.1016/j.pbiomolbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Lakatta EG, Gerstenblith G, Angell CS, Shock NW, Weisfeldt ML. Diminished inotropic response of aged myocardium to catecholamines. Circ Res. 1975;36(2):262–269. doi: 10.1161/01.res.36.2.262. [DOI] [PubMed] [Google Scholar]
- 41.Verheijck EE, et al. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc Res. 2001;52(1):40–50. doi: 10.1016/s0008-6363(01)00364-9. [DOI] [PubMed] [Google Scholar]
- 42.Verheijck EE, Wilders R, Bouman LN. Atrio-sinus interaction demonstrated by blockade of the rapid delayed rectifier current. Circulation. 2002;105(7):880–885. doi: 10.1161/hc0702.104128. [DOI] [PubMed] [Google Scholar]
- 43.Rose RA. Keeping the clocks ticking as we age: Changes in sinoatrial node gene expression and function in the ageing heart. Exp Physiol. 2011;96(11):1114–1115. doi: 10.1113/expphysiol.2011.060418. [DOI] [PubMed] [Google Scholar]
- 44.Xu W, Lipscombe D. Neuronal Ca(V)1.3α(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21(16):5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Proenza C, Angoli D, Agranovich E, Macri V, Accili EA. Pacemaker channels produce an instantaneous current. J Biol Chem. 2002;277(7):5101–5109. doi: 10.1074/jbc.M106974200. [DOI] [PubMed] [Google Scholar]
- 46.Proenza C, Yellen G. Distinct populations of HCN pacemaker channels produce voltage-dependent and voltage-independent currents. J Gen Physiol. 2006;127(2):183–190. doi: 10.1085/jgp.200509389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azene EM, Xue T, Marbán E, Tomaselli GF, Li RA. Non-equilibrium behavior of HCN channels: Insights into the role of HCN channels in native and engineered pacemakers. Cardiovasc Res. 2005;67(2):263–273. doi: 10.1016/j.cardiores.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Männikkö R, Pandey S, Larsson HP, Elinder F. Hysteresis in the voltage dependence of HCN channels: Conversion between two modes affects pacemaker properties. J Gen Physiol. 2005;125(3):305–326. doi: 10.1085/jgp.200409130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fenske S, et al. HCN3 contributes to the ventricular action potential waveform in the murine heart. Circ Res. 2011;109(9):1015–1023. doi: 10.1161/CIRCRESAHA.111.246173. [DOI] [PubMed] [Google Scholar]
- 50.Goonasejera SA, et al. Decreased cardiac L-type CA2+ channel activity induces hypertrophy and heart failure in mice. J Clin Invest. 2012;122(1):280–290. doi: 10.1172/JCI58227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tellez JO, et al. Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlying sino-atrial node pacemaking. Exp Physiol. 2011;96(11):1163–1178. doi: 10.1113/expphysiol.2011.057752. [DOI] [PubMed] [Google Scholar]
- 52.Huang P, et al. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA. 2001;98(24):14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stieber J, Stöckl G, Herrmann S, Hassfurth B, Hofmann F. Functional expression of the human HCN3 channel. J Biol Chem. 2005;280(41):34635–34643. doi: 10.1074/jbc.M502508200. [DOI] [PubMed] [Google Scholar]
- 54.Huang ZM, et al. Functional role of CLC-2 chloride inward rectifier channels in cardiac sinoatrial nodal pacemaker cells. J Mol Cell Cardiol. 2009;47(1):121–132. doi: 10.1016/j.yjmcc.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakatta EG, Maltsev VA, Vinogradova TM. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ Res. 2010;106(4):659–673. doi: 10.1161/CIRCRESAHA.109.206078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattick P, et al. Ca2+-stimulated adenylyl cyclase isoform AC1 is preferentially expressed in guinea-pig sino-atrial node cells and modulates the I(f) pacemaker current. J Physiol. 2007;582(Pt 3):1195–1203. doi: 10.1113/jphysiol.2007.133439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imlach WL, Finch SC, Miller JH, Meredith AL, Dalziel JE. A role for BK channels in heart rate regulation in rodents. PLoS ONE. 2010;5(1):e8698. doi: 10.1371/journal.pone.0008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka H, Seals DR. Endurance exercise performance in Masters athletes: Age-associated changes and underlying physiological mechanisms. J Physiol. 2008;586(1):55–63. doi: 10.1113/jphysiol.2007.141879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleg JL, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 60.Liao Z, Lockhead D, Larson ED, Proenza C. Phosphorylation and modulation of hyperpolarization-activated HCN4 channels by protein kinase A in the mouse sinoatrial node. J Gen Physiol. 2010;136(3):247–258. doi: 10.1085/jgp.201010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.