Significance
Regulatory T (Treg) cells expressing the transcription factor FOXP3 play a critical role in suppressing antitumor immune responses. Here we found that, compared with peripheral blood T cells, tumor-infiltrating T cells contained a higher frequency of effector Tregs, which are defined as FOXP3hi and CD45RA−, terminally differentiated, and most suppressive. Effector Treg cells, but not FOXP3lo and CD45RA+ naïve Treg cells, predominantly expressed C-C chemokine receptor 4 (CCR4) in both cancer tissues and peripheral blood. In vivo or in vitro anti-CCR4 mAb treatment selectively depleted effector Treg cells and efficiently induced tumor-antigen-specific CD4+ and CD8+ T cells. Thus, cell-depleting anti-CCR4 mAb therapy is instrumental for evoking and enhancing tumor immunity in humans via selectively removing effector-type FOXP3+ Treg cells.
Keywords: cancer immunotherapy, immunomodulation
Abstract
CD4+ Treg cells expressing the transcription factor FOXP3 (forkhead box P3) are abundant in tumor tissues and appear to hinder the induction of effective antitumor immunity. A substantial number of T cells, including Treg cells, in tumor tissues and peripheral blood express C-C chemokine receptor 4 (CCR4). Here we show that CCR4 was specifically expressed by a subset of terminally differentiated and most suppressive CD45RA−FOXP3hiCD4+ Treg cells [designated effector Treg (eTreg) cells], but not by CD45RA+FOXP3loCD4+ naive Treg cells, in peripheral blood of healthy individuals and cancer patients. In melanoma tissues, CCR4+ eTreg cells were predominant among tumor-infiltrating FOXP3+ T cells and much higher in frequency compared with those in peripheral blood. With peripheral blood lymphocytes from healthy individuals and melanoma patients, ex vivo depletion of CCR4+ T cells and subsequent in vitro stimulation of the depleted cell population with the cancer/testis antigen NY-ESO-1 efficiently induced NY-ESO-1–specific CD4+ T cells. Nondepletion failed in the induction. The magnitude of the responses was comparable with total removal of FOXP3+ Treg cells by CD25+ T-cell depletion. CCR4+ T-cell depletion also augmented in vitro induction of NY-ESO-1–specific CD8+ T cells in melanoma patients. Furthermore, in vivo administration of anti-CCR4 mAb markedly reduced the eTreg-cell fraction and augmented NY-ESO-1–specific CD8+ T-cell responses in an adult T-cell leukemia-lymphoma patient whose leukemic cells expressed NY-ESO-1. Collectively, these findings indicate that anti-CCR4 mAb treatment is instrumental for evoking and augmenting antitumor immunity in cancer patients by selectively depleting eTreg cells.
Naturally occurring CD25+CD4+ regulatory T (Treg) cells expressing the transcription factor forkhead box P3 (FOXP3) are indispensable for the maintenance of immunological self-tolerance and homeostasis (1, 2). Given that most tumor-associated antigens are antigenically normal self-constituents (3–5), it is likely that natural FOXP3+ Treg cells engaged in self-tolerance concurrently hinder immune surveillance against cancer in healthy individuals and also hamper the development of effective antitumor immunity in tumor-bearing patients. Indeed FOXP3+CD25+CD4+ Treg cells are abundant in tumor tissues (6–10), and their depletion augments spontaneous and vaccine-induced antitumor immune responses in animal models (10, 11). In humans, increased numbers of FOXP3+CD25+CD4+ Treg cells and, in particular, decreased ratios of CD8+ T cells to FOXP3+CD25+CD4+ Treg cells among tumor-infiltrating lymphocytes (TIL) are well correlated with poor prognosis in various types of cancers (6, 7, 10). Some clinical studies have shown the potential of depleting CD25-expressing lymphocytes to augment antitumor immune responses (12, 13); yet other similar studies failed to support the effects (10, 14, 15). Because activated effector T cells also express CD25, and their production of IL-2 is required for the expansion of CD8+ cytotoxic lymphocytes, CD25-based cell depletion may reduce activated effector T cells as well, cancelling the effect of Treg-cell depletion to augment antitumor immunity (10). In addition, it has been demonstrated in animal models that depletion of Treg cells as a whole can trigger autoimmunity (1, 16, 17). Therefore, a current key issue is to determine how Treg cells can be controlled to evoke and enhance antitumor immunity without affecting effector T cells or eliciting deleterious autoimmunity.
Human FOXP3+CD4+ T cells are heterogenous in phenotype and function (2). These cells can be dissected into three subpopulations by the expression levels of FOXP3 and the cell-surface molecules CD45RA and CD25: (i) FOXP3hiCD45RA−CD25hi cells, designated effector Treg (eTreg) cells, which are terminally differentiating and highly suppressive; (ii) FOXP3loCD45RA+CD25lo cells, designated naive Treg cells, which differentiate into eTreg cells upon antigenic stimulation; and (iii) FOXP3loCD45RA−CD25lo non-Treg cells, which do not possess suppressive activity but secrete proinflammatory cytokines (18). In principle, these distinct properties of FOXP3+ T-cell subpopulations can be exploited to augment antitumor immunity without inducing autoimmunity, for example, by depleting a particular Treg-cell subpopulation rather than whole Foxp3+-cell population. One of the candidate molecules for such differential control of Treg-cell subpopulations is chemokine receptors, which allow Treg cells to migrate to a specific inflammation site via sensing specific chemokine milieu (19).
It has been shown that tumor-infiltrating macrophages and tumor cells produce the chemokine (C-C motif) ligand 22 (CCL22), which chemoattracts Treg cells as well as effector T cells expressing C-C chemokine receptor type 4 (CCR4) (6, 10, 20). In this report, we have addressed whether CCR4-targeting treatment is able to selectively reduce a particular Treg-cell subpopulation, rather than whole Treg population, and thereby elicit or augment in vitro and in vivo antitumor immune responses in humans.
Results
Depletion of CCR4+ T Cells Predominantly Depletes eTreg Cells.
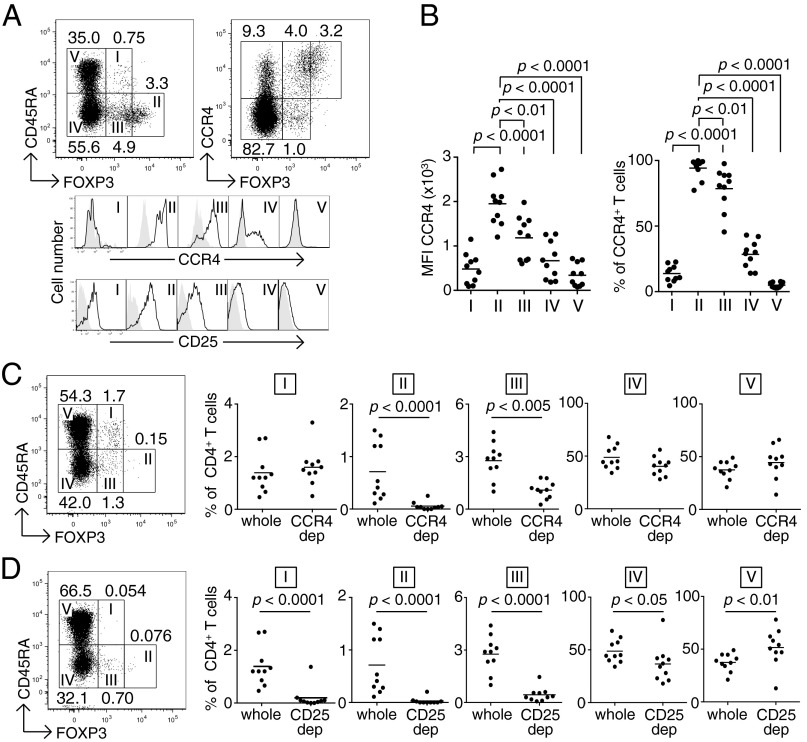
In peripheral blood mononuclear cells (PBMCs) of healthy individuals, CCR4+ T cells were present in both FOXP3+ and FOXP3− T-cell fractions, and FOXP3hi cells in particular were CCR4+ (Fig. 1A). When FOXP3+ T cells were classified into three populations by the levels of FOXP3 and CD45RA expression (18), FOXP3hiCD45RA− eTreg cells (Fr. II) predominantly expressed CCR4 at the protein and mRNA level (Fig. 1A, and Figs. S1 and S2A). In contrast, FOXP3loCD45RA+ naive Treg cells (Fr. I) scarcely expressed the molecule, whereas FOXP3loCD45RA− non-Treg cells (Fr. III) exhibited a moderate expression. Among FOXP3− cells, some CD45RA−CD4+ memory or activated T cells expressed CCR4, whereas CD45RA+CD4+ naive T cells did not. CD25 expression was well correlated with CCR4 expression with the highest CD25 expression by eTreg cells (Fr. II). Analyses of multiple samples of PBMCs from healthy individuals showed similar patterns of CCR4 expression by FOXP3 subsets (Fig. 1B). CD8+ T cells, natural killer (NK) cells, CD14+ monocytes/macrophages, dendritic cells, and B cells hardly expressed CCR4 at the protein and mRNA level (Fig. S2). In vitro depletion of CCR4+ cells from PBMCs by magnet-bead sorting with anti-CCR4 mAb predominantly decreased CD4+FOXP3hiCD45RA− eTreg cells (Fr. II) and, to a lesser extent, CD4+FOXP3loCD45RA− non-Treg cells (Fr. III), but spared CD4+FOXP3loCD45RA+ naive Treg cells (Fr. I) and FOXP3− cells (Fr. IV and V) (Fig. 1C). In contrast with anti-CCR4 mAb treatment, similar in vitro cell depletion with anti-CD25 mAb significantly reduced all of the FOXP3+ subpopulations (Fr I, II, and III) and, to a lesser extent, FOXP3−CD45RA−CD4+ activated or memory T cells (Fr. IV), with a relative increase in FOXP3−CD45RA+CD4+ naive T cells (Fr. V) (Fig. 1D). PBMCs of melanoma patients showed similar patterns of CCR4 expression by FOXP3+ subpopulations and similar changes in the composition of FOXP3+ T-cell subsets after in vitro CCR4+ T-cell depletion (Fig. S3).
Fig. 1.
Reduction of eTreg cells by in vitro depletion of CCR4-expressing T cells. (A) CCR4 and CD25 expression by subpopulations of FOXP3+ Treg cells in PBMCs from healthy donors. CCR4 and CD25 expression levels were evaluated for each fraction. Representative data from 10 healthy donors are shown. (B) Median fluorescence intensity (MFI, Left) and frequency (Right) of CCR4 expression by each fraction of T cells in PBMCs of healthy donors (n =10). (C) Changes in the proportion of T-cell subpopulations after CCR4+ T-cell depletion (CCR4 dep) (n = 10). (D) Changes in the proportion of T-cell subpopulations after CD25+ T-cell depletion (CD25 dep) (n = 10). The numbers in A, C, and D indicate the percentage of gated CD4+ T cells. Representative staining profiles in A, C, and D are from the same donor, and the same PBMC samples were analyzed in B–D.
Taking these data together, we find that CCR4 is predominantly expressed by eTreg cells and depletion of CCR4+ cells results in selective reduction of eTreg cells, while preserving naive Treg cells and the majority of FOXP3−CD4+ T cells.
Tumor-Infiltrating Treg Cells Exhibit the eTreg-Cell Phenotype and Can Be Depleted in Vitro by Anti-CCR4 mAb.
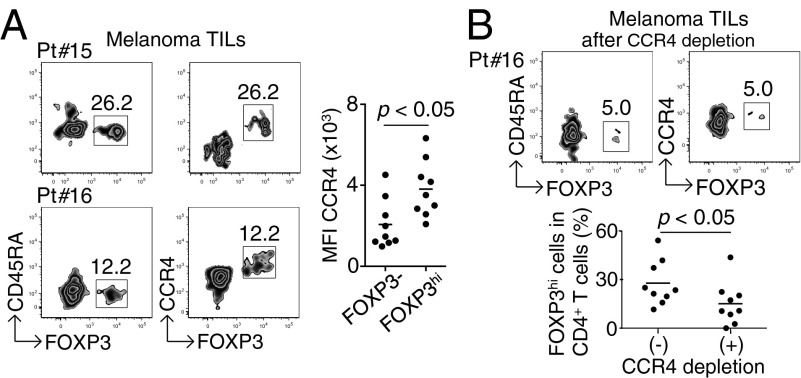
Although there is accumulating data that FOXP3+ T cells predominantly infiltrate into tumor tissues (6, 7, 10, 21), their detailed phenotypes remain to be determined. Our analysis of TILs in nine melanoma samples revealed infiltration of a high percentage of CCR4+ T cells, the majority of which were CD4+FOXP3hiCD45RA− eTreg cells (Fr. II), with only a small number of CD4+FOXP3loCD45RA+ naive Treg cells (Fr. I) (Fig. 2A). In vitro depletion of CCR4+ T cells indeed dramatically reduced these tumor-infiltrating eTreg cells (Fig. 2B), indicating that anti-CCR4 mAb treatment is able to selectively deplete eTreg cells abundantly infiltrating into tumors.
Fig. 2.
Predominant infiltration of CCR4+ eTreg cells into melanoma tissues. (A) CCR4 expression by melanoma-infiltrating T cells. CD4+ T cells from melanoma sites were fractionated into subpopulations based on the expression of CCR4, CD45RA, and FOXP3; CCR4 expression by each fraction was analyzed. Data from two representative patients are shown. (Right) Summary of MFI of CCR4 expression by FOXP3− or FOXP3+ cells (n = 9). (B) CCR4+CD4+ T cells from melanoma tissues (Pt #16) were depleted of CCR4+ T cells and then analyzed for the proportion of FOXP3hi eTreg cells. (Lower) Percentages of FOXP3hi cells among CD4+ T cells after CCR4+ cell depletion or nondepletion (n = 9). The numbers in A and B indicate the percentage of gated CD4+ T cells.
In Vitro Induction of NY-ESO-1–Specific CD4+ T Cells After CCR4+ T-Cell Depletion from PBMCs of Healthy Donors and Melanoma Patients.
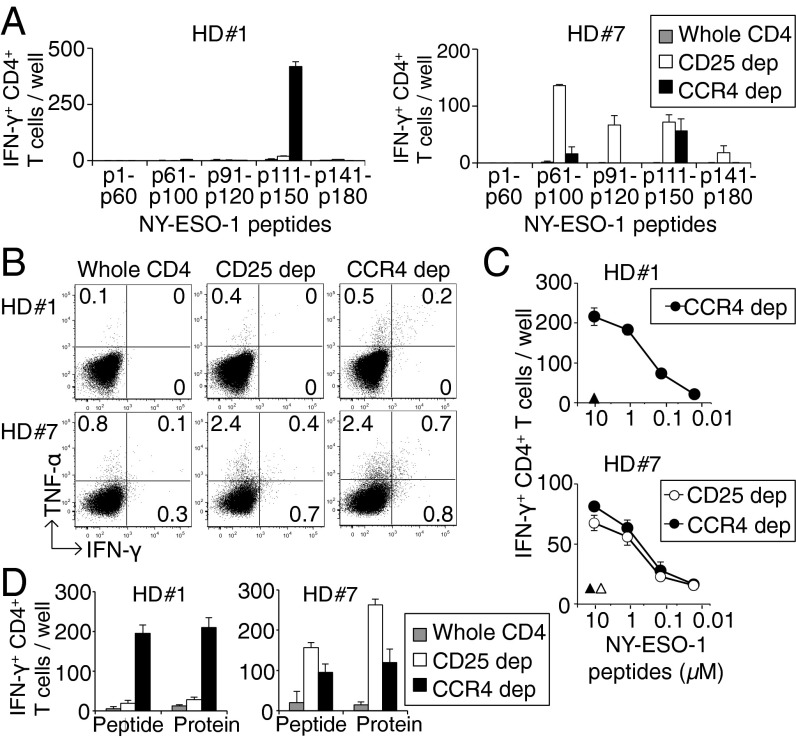
With the efficient depletion of the eTreg-cell population by in vitro anti-CCR4 mAb treatment, we next examined whether CCR4+ T-cell depletion from PBMCs of healthy donors was able to induce tumor antigen-specific CD4+ T cells. We assessed specific T-cell responses to NY-ESO-1, a cancer/testis antigen, which is normally expressed by human germ-line cells and also by various types of cancer cells (4, 22). CCR4−CD4+ T cells or CD25−CD4+ T cells were cultured with CD4−CD8− PBMCs as antigen-presenting cells (APCs), which were pulsed overnight with series of overlapping peptides covering the entire sequence of the NY-ESO-1 protein and X-irradiated (35 Gy) before use, as previously described (23, 24). Fifteen to 20 d later, NY-ESO-1–specific CD4+ T cells secreting IFN-γ were enumerated by enzyme-linked immunospot (ELISpot) assay. Significant numbers of IFN-γ–secreting NY-ESO-1–specific CD4+ T cells were induced in 7 of 16 healthy donors (43.8%), but only in the cultures with CCR4+ or CD25+ T-cell–depleted T cells (Fig. 3A, and summarized in Table S1). Furthermore, the frequencies of IFN-γ–secreting NY-ESO-1–specific CD4+ T cells were higher after CCR4+ T-cell depletion compared with CD25+ T-cell depletion in five of seven healthy donors (71.4%) (Table S1). This result could be attributed in part to possible depletion of NY-ESO-1–specific CD25+ activated T cells by anti-CD25 mAb treatment. The NY-ESO-1–specific CD4+ T cells produced IFN-γ and TNF-α (Fig. 3B). Those cells induced in vitro after CCR4+ T-cell depletion recognized NY-ESO-1 peptides at the concentration as low as 0.1 μM (Fig. 3C), and also NY-ESO-1 peptides produced by natural processing of the NY-ESO-1 protein by APCs, as previously shown with CD25+ T-cell depletion (22, 24) (Fig. 3D).
Fig. 3.
Induction of cancer/testes antigen-specific CD4+ T cells by depletion of CCR4- or CD25-expressing T cells in healthy donors. (A) CD4+ T-cell responses to NY-ESO-1 peptides after depletion of CCR4+ or CD25+ T cells. CD4+ T cells prepared from PBMCs of healthy donors were presensitized with APCs pulsed with NY-ESO-1 peptide covering the entire sequence of NY-ESO-1. Results of 2 (HD#1 and HD#7) among 16 healthy donors are shown. The numbers of IFN-γ–secreting CD4+ T cells were assessed by ELISpot assay. (B) Intracellular cytokine secretion of CD4+ T cells shown in A. The numbers in figures indicate the percentage of gated CD4+ T cells. (C) Peptide dose-dependent recognition of NY-ESO-1–specific IFN-γ–secreting CD4+ T cells. NY-ESO-1–specific CD4+ T cells derived from CCR4+ or CD25+ T-cell–depleted cells (CCR4 dep and CD25 dep, respectively) were cultured with autologous activated T-cell APCs pulsed with graded amounts of NY-ESO-1 peptides and assessed for the number of IFN-γ–secreting cells as in A. Triangles indicate responses to control peptide at 10 μM. (D) Recognition of naturally processed NY-ESO-1 protein antigen by NY-ESO-1–specific CD4+ T cells derived from whole CD4+, CCR4+ cell-depleted, or CD25+ cell-depleted cells. NY-ESO-1–specific CD4+ T cells from two healthy donors were cultured with autologous dendritic cells pulsed with NY-ESO-1 or control protein, or with NY-ESO-1 or control peptide. The experiments were independently performed twice with similar results.
We also attempted to determine whether Treg-cell depletion would evoke anti–NY-ESO-1 responses in apparently nonresponsive melanoma patients. With PBMCs from patients bearing NY-ESO-1–expressing melanomas, but without detectable NY-ESO-1–specific Ab in the sera, in vitro depletion of CCR4+ or CD25+ T cells and subsequent in vitro peptide stimulation induced IFN-γ– and TNF-α–secreting NY-ESO-1–specific CD4+ T cells in three of eight patients (37.5%) (Fig. S4 A and B and Table S2). These NY-ESO-1–specific CD4+ T cells appeared to express high-avidity T-cell receptors that recognized NY-ESO-1 peptides at a concentration as low as 0.1 μM, as seen with healthy donor T cells (Fig. S4C).
Thus, in healthy individuals as well as melanoma patients who had not raised spontaneous NY-ESO-1 immune responses, removal of eTreg cells by CCR4+ T-cell depletion is able to efficiently induce high-avidity NY-ESO-1–specific CD4+ T cells secreting effector cytokines.
CCR4+ T-Cell Depletion Augments in Vitro Induction of NY-ESO-1–Specific CD8+ T Cells from PBMCs of Melanoma Patients.
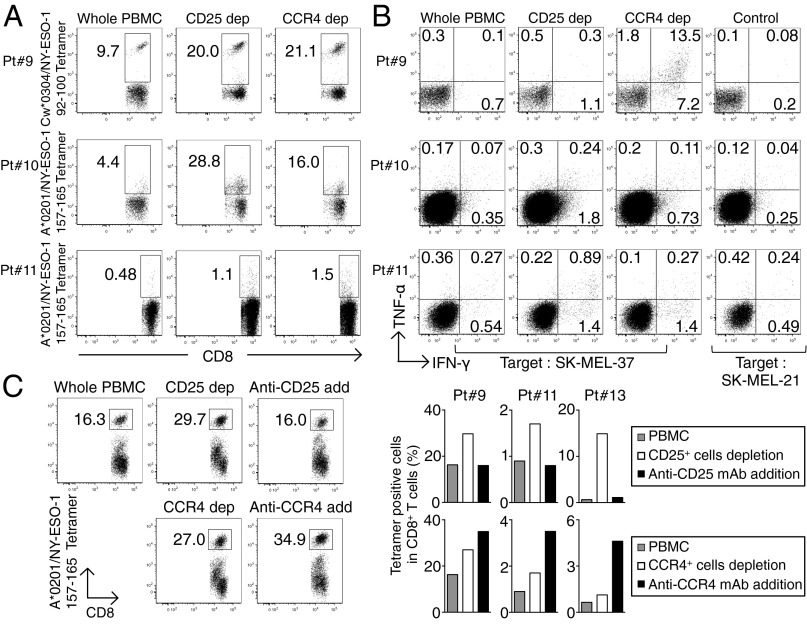
PBMCs from melanoma patients were subjected to in vitro depletion with anti-CCR4 mAb or anti-CD25 mAb, and cultured with NY-ESO-1 peptide capable of binding to HLA class I of each patient. Seven to 10 d later, NY-ESO-1–specific CD8+ T cells were detected by NY-ESO-1/HLA tetramers and analyzed for intracellular cytokine production. NY-ESO-1–specific CD8+ T cells were induced in four of six patients (66.7%), and the responses were markedly augmented after depletion of CCR4+ or CD25+ cells (Fig. 4A). In addition, these NY-ESO-1–specific CD8+ T cells recognized an HLA-matched malignant melanoma cell line and secreted IFN-γ and TNF-α (Fig. 4B). For example, Pt. #9 (HLA-A*02/29, B*44/27, C*03/04) harbored not only HLA-C*03–restricted NY-ESO-1–specific CD8+ T-cells detected by HLA Cw*0304/NY-ESO-1 tetramers, but also those NY-ESO-1–specific CD8+ T cells that recognized the SK-MEL 37 melanoma line (A*0201+, NY-ESO-1+) in an HLA-A2–restricted manner.
Fig. 4.
Augmentation of NY-ESO-1–specific CD8+ T-cell induction in melanoma patients by in vitro CCR4+ T-cell depletion. (A) Induction of NY-ESO-1–specific CD8+ T cells. Unfractionated PBMCs, or PBMCs depleted of CD25+ or CCR4+ cells, were prepared from melanoma patients (n = 6), and presensitized in peptides capable of binding to patients’ HLA. NY-ESO-1–specific CD8+ T cells were analyzed with NY-ESO-1/HLA tetramers (Pt. #9: A*02/29, B*44/27, C*03/04, Pt. #10: A*02/11, B*35/44, C*04/05, and Pt. #11: A*02/-, B*13/18, C*06/07). (B) Cytokine secretion of NY-ESO-1–specific CD8+ T cells upon recognition of the HLA-A*0201+ melanoma cell line SK-MEL 37 (NY-ESO-1+), or SK-MEL-21 (NY-ESO-1−) analyzed by intracellular cytokine staining. Data from three representative patients are shown. (C) Induction of antigen-specific CD8+ T cells by addition (add) of anti-CD25 or anti-CCR4 mAb (KM2160) to cell cultures, or by CCR4+ or CD25+ cell depletion or nondepletion, as shown in A (Pt. #13 A02/03, B07/41, C07/17). A representative result (Left) and summary of three melanoma patients (Right) are shown. The numbers in the panels indicate the percentage of gated CD8+ T cells. These experiments were performed independently at least twice with similar results.
We also examined whether NY-ESO-1–specific CD8+ T cells could be induced by directly adding mAb into cell cultures. Addition of anti-CD25 mAb or anti-CCR4 mAb reduced the frequency of CD4+FOXP3hiCD45RA− eTreg cells (Fr. II) (Fig. S5). Interestingly, although NY-ESO-1–specific CD8+ T-cell induction was augmented in the cell culture containing anti-CCR4 mAb, the addition of anti-CD25 mAb reduced the frequency of NY-ESO-1–specific CD8+ T cells (Fig. 4C), indicating that it might have killed some CD25+CD8+ activated effector T cells in addition to CD25+CD4+ Treg cells.
These results indicate that depletion of CCR4+ T cells before in vitro induction or even simple incubation with anti-CCR4 mAb during the induction effectively augments NY-ESO-1–specific CD8+ T-cell responses by selectively reducing eTreg cells.
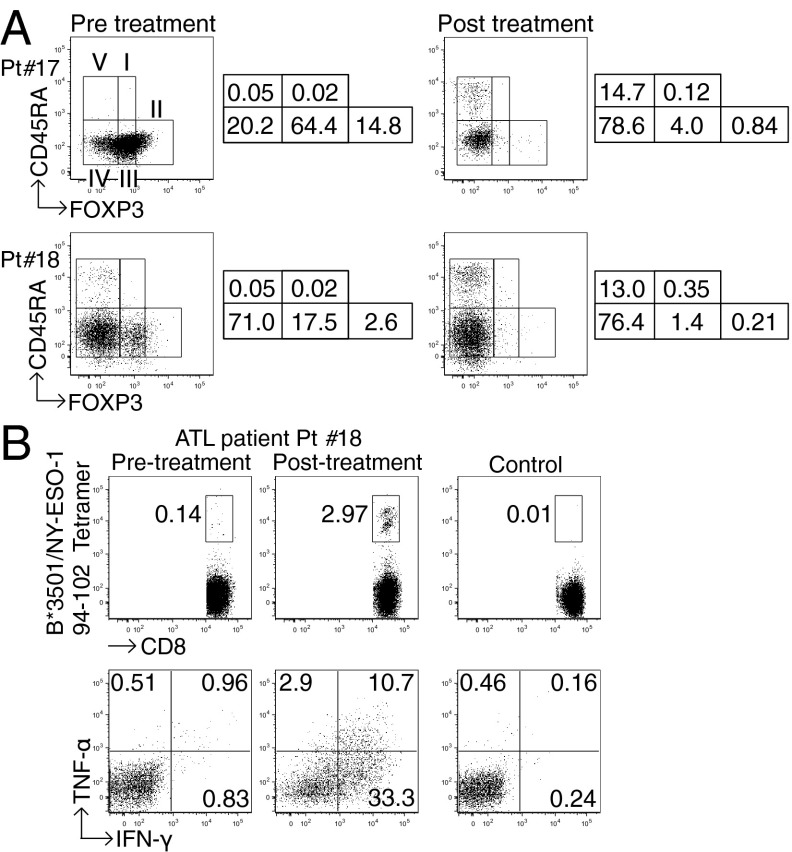
Anti-CCR4 mAb Administration into Adult T-Cell Leukemia-Lymphoma Patients Reduces CD4+FOXP3hiCD45RA− eTreg Cells and Augments NY-ESO-1–Specific CD8+ T-Cell Responses.
In adult T-cell leukemia-lymphoma (ATL), which is caused by human T-lymphotropic virus 1 infection, ATL cells are CD4+ and the majority—if not all—of them express FOXP3, CD25, CTLA-4, and CCR4, thus resembling naturally occurring FOXP3+ Treg cells (25–28). Although it is currently difficult to discriminate whether anti-CCR4 mAb reduces ATL cells or normal FOXP3+ Treg cells (29), we examined whether in vivo administration of anti-CCR4 mAb (Mogamulizumab), which has a cell-depleting effect by antibody-dependent cellular cytotoxicity, was able to reduce FOXP3+ cells or a subpopulation thereof. Analysis of PBMCs from ATL patients collected before and after anti-CCR4 mAb therapy revealed that CD4+FOXP3hiCD45RA− cells including both ATL cells and eTreg cells were markedly reduced after the therapy (Fig. 5A). In addition, in a patient whose ATL cells expressed NY-ESO-1, NY-ESO-1–specific CD8+ T cells producing IFN-γ and TNF-α were induced after several rounds of anti-CCR4 mAb administration (Fig. 5B). NY-ESO-1–specific CD8+ T cells producing these cytokines were much higher in frequency than NY-ESO-1–specific CD8+ T cells detected by NY-ESO-1/HLA-B*3501 tetramers, suggesting that this patient additionally possessed CD8+ T cells recognizing other epitopes of NY-ESO-1. These results collectively indicate that anti-CCR4 mAb therapy for ATL is able to selectively deplete eTreg cells as well as ATL cells in vivo, and induce/augment tumor antigen-specific T-cell responses, although it is possible that anti-CCR4 mAb-induced reduction of FOXP3+ ATL cells, which reportedly exhibit a Treg-cell–like in vitro suppressive activity (27, 28), might also contribute to the augmentation of immune responses.
Fig. 5.
Reduction of CD4+FOXP3hiCD45RA− T cells and augmentation of NY-ESO-1–specific CD8+ T-cell responses in ATL patients after anti-CCR4 mAb (Mogamulizumab) therapy. (A) FOXP3+ Treg-cell subpopulations in PBMCs from two ATL patients (Pt. #17: acute type, HLA-A*2402/-, B*3901/5401, C*0102/0702 and Pt. #18: lymphoma type, HLA-A*0201/3101, B*3501/4002, C*0303/0401) before and after anti-CCR4 mAb therapy. These experiments were performed at least twice with similar results. The numbers indicate the percentage of gated CD4+ T cells. (B) Analysis of NY-ESO-1–specific CD8+ T-cell induction before and after anti-CCR4 mAb therapy. PBMCs from Pt. #18 were presensitized in the presence of APCs pulsed with NY-ESO-191–110 peptide corresponding to the patient’s HLA. NY-ESO-1–specific CD8+ T cells were detected with NY-ESO-1/HLA tetramers, and cytokine secretion of these NY-ESO-1–specific CD8+ T cells upon recognition of autologous activated T-cell APCs pulsed with NY-ESO-191–110 or control peptide was analyzed by intracellular cytokine staining. The numbers in figures indicate the percentage of gated CD8+ T cells. The result was derived from a single assay because of limited availability of the patient’s samples.
Discussion
Accumulating evidence indicates that effective cancer immunotherapy needs to control FOXP3+ Treg cells naturally present in the immune system and abundantly infiltrating into tumor tissues (10, 11, 30). Here, we have shown that CD4+FOXP3hiCD45RA− eTreg cells, which are terminally differentiated and most suppressive, highly express CCR4, that they are predominant among FOXP3+ T cells infiltrating into tumor tissues (e.g., melanoma), and that specific depletion of eTreg cells in vivo or in vitro by anti-CCR4 mAb evoked tumor antigen-specific immune responses mediated by CD4+ and CD8+ T cells in healthy individuals and cancer patients.
Besides high expression of CCR4 in eTreg cells, CCR4 is expressed, although to a lesser extent, in non-Treg CD4+ T-cell fractions [i.e., the FOXP3loCD45RA− cells (Fr. III) and FOXP3−CD45RA− cells (Fr. IV)]. The former are capable of secreting cytokines, such as IL-4 and IL-17, as previously reported with PBMCs of healthy individuals (18). It has also been shown that Th2 cells and a fraction of central memory CD8+ T cells express CCR4 (31–33). It is thus likely that tumor-infiltrating activated macrophages, and presumably some tumor cells produce CCL22, which predominantly chemoattracts and recruits from peripheral blood both CCR4+ eTreg and CCR4+ effector T cells that recognize tumor-associated antigens (such as cancer/testis antigen) and presumably self-antigens released from tumor cells (6, 10, 21, 34). However, the frequency of IL-4– or IL-17–secreting CD4+ T cells were much lower than eTreg cells among CCR4+CD4+ T cells in PBMCs and TILs in melanoma tissues of nontreated patients; and CCR4 expression by CD8+ TILs were limited. Moreover, addition of anti-CCR4 mAb into in vitro peptide stimulation more effectively induced antigen-specific CD8+ T cells than CCR4+ T-cell depletion, indicating that anti-CCR4 mAb had reduced eTreg cells but spared CD8+ effector T cells. The result contrasted with the addition of anti-CD25 mAb, which appeared to deplete CD25+CD8+ T cells and cancel the enhancing effect of Treg-cell depletion. These results taken together indicate that anti-CCR4 mAb treatment to augment antitumor immunity mainly target CCR4+ eTreg cells in tumor tissues and the regional lymph nodes, as well as peripheral blood, which would otherwise be a reservoir of fresh tumor-infiltrating Treg cells. Further study is warranted to determine whether depletion of CCR4+CD4+ and CD8+ effector T cells in vivo affects antitumor immunity to a clinically significant extent.
Both NY-ESO-1–specific CD4+ and CD8+ T cells induced by in vitro anti-CCR4 mAb treatment possessed high-avidity T-cell receptors, and responded to dendritic cells processing tumor antigens and histocompatible tumor cell lines, respectively. This finding raises the issue of whether Treg depletion by anti-CCR4 mAb activates and expands already present antigen-primed effector T cells or newly induces effector T cells from a naive T-cell pool. We previously showed that in vitro NY-ESO-1-peptide stimulation following CD25+CD4+ T-cell depletion could activate NY-ESO-1–specific naive CD4+ T-cell precursors in healthy individuals and in melanoma patients who possessed NY-ESO-1–expressing tumors but failed to develop anti-NY-ESO-1 Ab (23). In contrast, most NY-ESO-1–specific CD4+ T cells in melanoma patients who had spontaneously developed anti–NY-ESO-1 Ab were derived from a memory population and could be activated even in the presence of CD25+CD4+ Treg cells (23). In addition, following vaccination of ovarian cancer patients with a HLA-DP–restricted NY-ESO-1 peptide, development of NY-ESO-1–specific high-avidity effector T cells from naive T cells was hampered by the presence of CD25+CD4+ Treg cells, although the vaccination could expand low-avidity NY-ESO-1–specific CD4+ T cells that were apparently present in an effector/memory fraction before the vaccination (24). These results collectively indicate that elimination of eTreg cells by CCR4+ T-cell depletion abrogates Treg cell-mediated suppression on NY-ESO-1–specific high-avidity naive T-cell precursors, allowing their activation and differentiation into high-avidity effector T cells capable of mediating strong antitumor immune responses. This successful induction of tumor antigen-specific CD4+ and CD8+ T cells indicates that the combination of anti-CCR4 mAb administration and vaccination with tumor antigens, such as NY-ESO-1, could be an ideal strategy for immunotherapy of a variety of cancers including ATL, which express NY-ESO-1 (35).
On the other hand, it was noted that not all healthy individuals or melanoma patients developed NY-ESO-1–specific T cells in vitro after Treg depletion for several possible reasons. For example, individuals who do not have a proper HLA haplotype may fail to select NY-ESO-1–reactive T cells thymically (22), hence possessing few NY-ESO-1–specific T-cell precursors. Other types of suppressor cells (such as myeloid-derived suppressor cells, immunosuppressive macrophages, and Foxp3− Treg cells) might contribute to inhibiting the induction of the responses (30). Alternatively, T cells specific for NY-ESO-1, a cancer/testis antigen, may also be subjected to other mechanisms of immunological self-tolerance—for example, anergy—hence being hyporesponsive to the antigen (36). These possibilities are under investigation to make anti-CCR4 mAb therapy more effective.
Would in vivo anti-CCR4 mAb treatment to deplete Treg cells elicit harmful autoimmunity? It has been shown in animal models that a longer period and a more profound degree of Treg-cell depletion is required to elicit clinically and histologically evident autoimmunity than evoking effective antitumor immunity (37, 38). In humans, naive Treg cells are generally well preserved in peripheral blood in cancer patients, even if they are low in frequency in tumor tissues. Furthermore, CCR4+ T-cell depletion selectively eliminates eTreg cells but spares naive Treg cells. Assuming that effective tumor immunity can be evoked without significant autoimmunity via controlling the degree and duration of Treg-cell depletion, it is likely that, although anti-CCR4 mAb administrations reduce eTreg cells in the immune system during the treatment, the residual CCR4− eTreg cells (as shown in Fig. 2), including those which have newly differentiated from naive Treg cells, are sufficient to prevent deleterious autoimmunity. Supporting this notion, only a minor population of ATL patients treated with anti–CCR4 mAb experienced severe immune-related adverse events, except skin rashes (29). Anti-CCR4 mAb therapy can therefore be a unique cancer immunotherapy aiming at depleting eTreg cells without clinically serious adverse effects that would be incurred by total Treg-cell depletion or functional blockade (39).
The critical roles of CCR4 in Treg-cell recruitment to tumors have been reported with various types of human cancers, such as malignant lymphomas, gastric, ovarian, and breast cancers (10). CCR4+ eTreg cells abundantly and predominantly infiltrated into gastric and esophageal cancers as observed with melanoma. Although it remains to be determined whether every cancer tissue has predominant infiltration of CCR4+ eTreg cells, it is envisaged that possible combination of anti-CCR4 mAb treatment, tumor antigen immunization, and antibody-mediated immune checkpoint blockade will further increase clinical efficacy of cancer immunotherapy.
Materials and Methods
Donor Samples.
PBMCs were obtained from healthy donors, malignant melanoma patients with NY-ESO-1 expression, and ATL patients. To collect tumor-infiltrating T cells, melanoma tissues were minced and treated with gentleMACS Dissociator (Miltenyi Biotec). All healthy donors were subjects with no history of autoimmune disease. All donors provided written informed consent before sampling according to the Declaration of Helsinki. The present study was approved by the institutional ethics committees of Osaka University, Osaka, Japan and Landesarztekammer Hessen, Frankfurt, Germany.
Antibodies and Peptides.
The information of antibodies and synthetic peptides is provided in SI Materials and Methods.
Preparation of CD25− or CCR4− Cells.
PBMCs or CD4+ T cells were treated with biotin-anti-CD25 mAb (BC96) or biotin-anti-CCR4 (1G1) mAb (0.01 mg/mL), otherwise specified, for 15 min at 4 °C. Subsequently, anti-Biotin MicroBeads (Miltenyi Biotec) were added as described in the manufacturer’s protocol, then washed using PBS containing 2% (vol/vol) FCS. CD25− or CCR4− cells were separated on autoMACS Pro Separator (Miltenyi Biotec).
In Vitro Sensitization of NY-ESO-1–Specific CD4+ T Cells.
NY-ESO-1–specific CD4+ T cells were presensitized as previously described (23, 24) and in SI Materials and Methods.
In Vitro Sensitization of NY-ESO-1–Specific CD8+ T Cells.
For in vitro sensitization of NY-ESO-1–specific CD8+ T cells, 1.5–2 × 106 cells were cultured with NY-ESO-1 peptides (NY-ESO-1157–165 for HLA-A*0201 restricted, NY-ESO-192–100 for HLA-Cw*0304 restricted, NY-ESO-191–110 for HLA-B*3501 restricted, 10 μM) (22, 23) in a 48-well dish or round-bottom 96-well plate. After 8 h, one-half of the medium was replaced by fresh medium containing IL-2 (20 U/mL) and IL-7 (40 ng/mL) and repeated twice per week. In some assays, purified anti-CD25 (M-A251) mAb or anti-CCR4 (KM2160) mAb (1 μg/mL) was included in some wells during the entire period of culture.
ELISpot Assay.
The number of IFN-γ–secreting NY-ESO-1–specific CD4+ T cells was assessed by ELISpot assay as previously described (23, 24) and in SI Materials and Methods.
Intracellular Cytokine Secretion Assay.
The presensitized CD4+ and CD8+ T cells were restimulated with peptide-pulsed autologous activated T-cell APCs, SK-MEL-21 cells (NY-ESO-1−, HLA-A*0201+), or SK-MEL-37 cells (NY-ESO-1+, HLA-A*0201+) for 1 h, after which GolgiStop reagent (BD Biosciences) was added. Subsequently, cells were cultured for another 6–8 h at 37 °C. Cells were stained for cell surface markers and then for intracellular cytokines using BD Cytofix/Cytoperm Buffer and BD Perm/Wash Buffer (BD Biosciences). Results were analyzed by flow cytometry (BD LSRFortessa; BD Biosciences) and FlowJo v9.6.2 software (TreeStar).
Tetramer Assay.
Tetramer staining was performed as previously described (35, 40) and in SI Materials and Methods.
Preparation of Dendritic Cells.
Dendritic cells were prepared as previously described (24) and in SI Materials and Methods.
Statistical Analysis.
The significance of the difference in each data between two groups was assessed by a Mann–Whitney test using Prism version 6 software (GraphPad). P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Drs. J. B. Wing and D. O. Adeegbe for helpful discussion and critical reading of this manuscript, and Ms. Y. Tada, K. Teshima and Y. Funabiki for technical assistance. SK-MEL21 and SK-MEL37 were kindly provided by Dr. Lloyd J. Old; anti-CCR4 mAb (KM2160) was a generous gift from Kyowa Hakko Kirin Co., Ltd. This study was supported by Grants-in-Aid for Specially Promoted Research 20002007 (to S.S.) and for Scientific Research (B) 23300354 (to H.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Core Research for Evolutional Science and Technology from the Japan Science and Technology Agency (S.S.); Health and Labor Sciences Research Grants, Research on Applying Health Technology H24-Clinical Cancer Research-general-006 and H23-Third Term Comprehensive Control Research for Cancer-general-011 (to H.N.) from the Ministry of Health, Labor, and Welfare, Japan; a Cancer Research Institute Designated grant and CLIP grant (to H.N.); and a research grant from Kyowa Hakko Kirin Co., Ltd. (to H.N.).
Footnotes
Conflict of interest statement: H.N. received a research grant from Kyowa Hakko Kirin Co., Ltd.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1316796110/-/DCSupplemental.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 3.Kawakami Y, Rosenberg SA. Human tumor antigens recognized by T-cells. Immunol Res. 1997;16(4):313–339. doi: 10.1007/BF02786397. [DOI] [PubMed] [Google Scholar]
- 4.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: An expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 5.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 6.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 7.Sato E, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi T, et al. Control of immune responses by antigen-specific regulatory T cells expressing the folate receptor. Immunity. 2007;27(1):145–159. doi: 10.1016/j.immuni.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Mitsui J, et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin Cancer Res. 2010;16(10):2781–2791. doi: 10.1158/1078-0432.CCR-09-3243. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 11.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 12.Dannull J, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115(12):3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rech AJ, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med. 2012;4(134):134ra162. doi: 10.1126/scitranslmed.3003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28(6):582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litzinger MT, et al. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110(9):3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 17.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 18.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97(11):1139–1146. doi: 10.1111/j.1349-7006.2006.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonertz A, et al. Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J Clin Invest. 2009;119(11):3311–3321. doi: 10.1172/JCI39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnjatic S, et al. NY-ESO-1: Review of an immunogenic tumor antigen. Adv Cancer Res. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 23.Nishikawa H, Jäger E, Ritter G, Old LJ, Gnjatic S. CD4+ CD25+ regulatory T cells control the induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood. 2005;106(3):1008–1011. doi: 10.1182/blood-2005-02-0607. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa H, et al. Influence of CD4+CD25+ regulatory T cells on low/high-avidity CD4+ T cells following peptide vaccination. J Immunol. 2006;176(10):6340–6346. doi: 10.4049/jimmunol.176.10.6340. [DOI] [PubMed] [Google Scholar]
- 25.Yoshie O, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99(5):1505–1511. doi: 10.1182/blood.v99.5.1505. [DOI] [PubMed] [Google Scholar]
- 26.Ishida T, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: Its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9(10 Pt 1):3625–3634. [PubMed] [Google Scholar]
- 27.Matsubara Y, Hori T, Morita R, Sakaguchi S, Uchiyama T. Phenotypic and functional relationship between adult T-cell leukemia cells and regulatory T cells. Leukemia. 2005;19(3):482–483. doi: 10.1038/sj.leu.2403628. [DOI] [PubMed] [Google Scholar]
- 28.Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7(4):270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 29.Ishida T, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J Clin Oncol. 2012;30(8):837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- 30.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 31.Imai T, et al. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11(1):81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- 32.Lim HW, Lee J, Hillsamer P, Kim CH. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J Immunol. 2008;180(1):122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- 33.Kondo T, Takiguchi M. Human memory CCR4+CD8+ T cell subset has the ability to produce multiple cytokines. Int Immunol. 2009;21(5):523–532. doi: 10.1093/intimm/dxp019. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa H, et al. Definition of target antigens for naturally occurring CD4+CD25+ regulatory T cells. J Exp Med. 2005;201(5):681–686. doi: 10.1084/jem.20041959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa H, et al. Cancer/testis antigens are novel targets of immunotherapy for adult T-cell leukemia/lymphoma. Blood. 2012;119(13):3097–3104. doi: 10.1182/blood-2011-09-379982. [DOI] [PubMed] [Google Scholar]
- 36.Chappert P, Schwartz RH. Induction of T cell anergy: Integration of environmental cues and infectious tolerance. Curr Opin Immunol. 2010;22(5):552–559. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: A common basis between tumor immunity and autoimmunity. J Immunol. 1999;163(10):5211–5218. [PubMed] [Google Scholar]
- 38.Ko K, et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005;202(7):885–891. doi: 10.1084/jem.20050940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: Recent successes and next steps. Nat Rev Cancer. 2011;11(11):805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishikawa H, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116(7):1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.