Abstract
The last decades of neuroscience research have produced immense progress in the methods available to understand brain structure and function. Social, cognitive, clinical, affective, economic, communication, and developmental neurosciences have begun to map the relationships between neuro-psychological processes and behavioral outcomes, yielding a new understanding of human behavior and promising interventions. However, a limitation of this fast moving research is that most findings are based on small samples of convenience. Furthermore, our understanding of individual differences may be distorted by unrepresentative samples, undermining findings regarding brain–behavior mechanisms. These limitations are issues that social demographers, epidemiologists, and other population scientists have tackled, with solutions that can be applied to neuroscience. By contrast, nearly all social science disciplines, including social demography, sociology, political science, economics, communication science, and psychology, make assumptions about processes that involve the brain, but have incorporated neural measures to differing, and often limited, degrees; many still treat the brain as a black box. In this article, we describe and promote a perspective—population neuroscience—that leverages interdisciplinary expertise to (i) emphasize the importance of sampling to more clearly define the relevant populations and sampling strategies needed when using neuroscience methods to address such questions; and (ii) deepen understanding of mechanisms within population science by providing insight regarding underlying neural mechanisms. Doing so will increase our confidence in the generalizability of the findings. We provide examples to illustrate the population neuroscience approach for specific types of research questions and discuss the potential for theoretical and applied advances from this approach across areas.
Keywords: neuroimaging, life course, statistics, survey methodology, physics
Why Population Neuroscience?
How do biology, social situations, and the broader environmental context interact to guide behavior, health, and development? This question is fundamental to most, if not all, social and behavioral sciences. We argue that to effectively address the many topics that stem from this larger question across disciplines, it is necessary to (i) bring a “population perspective” to neuroscience and (ii) leverage neuroscience tools within population sciences, which are subdisciplines of many fields, areas, and departments focused on documenting and understanding the dynamics of human populations, including outcomes such as health, well-being, behavior, etc.
Although recent advances in neuroscience research, and neuroimaging in particular, speak to how social, cognitive, and emotional processes unfold (1–5), the extent to which existing knowledge in human neuroscience applies to broader, theoretically relevant populations, and the ways that macrolevel structures (e.g., social structure, neighborhood safety, school quality, media exposure) influence neural processes is often unknown (6). Thus, in parallel with a broader social science focus on the limitations of nonrepresentative samples (7, 8), we are now at a critical juncture for social and biological science. What would a “representative group of brains” tell us about the generalizability of current samples and current findings regarding brain-behavior mechanisms? How do individual differences in brain structure and function affect cognitive, affective, and behavioral outcomes and how do social situations and broader environmental contexts interact with these processes? Current methods in much of neuroscience research and the absence of neural measures in most population-based research limit our ability to answer these questions (9).
At the same time, most social scientists are interested in thoughts and behaviors (e.g., decision making, empathy, attitudes), which must have some relationship to the brain. As such, neural measures, especially neuroimaging, have become widely used in several specific social science disciplines (e.g., psychology, decision science) (1–5, 10–12). However, this trend toward brain science has not been as true for social sciences that deal in large and representative samples (e.g., social demography) or long-term development (e.g., the life course), leading to a view of the brain as a black box in those disciplines. A more recent focus within population sciences on how the broader environment “gets under the skin” suggests that this may be a key moment to look to the brain. Health psychologists have demonstrated that the broader environment becomes biologically embedded in the brain over the course of development (5, 13–17), but how does this yield observed variations within populations? Therefore, we argue that the critical juncture described for neuroscience research also poses an opportunity for population science research more broadly. Taken together, how can neuroscience research usefully inform broader understanding in the population sciences and how can these sciences be brought to bear on neuroscience research?
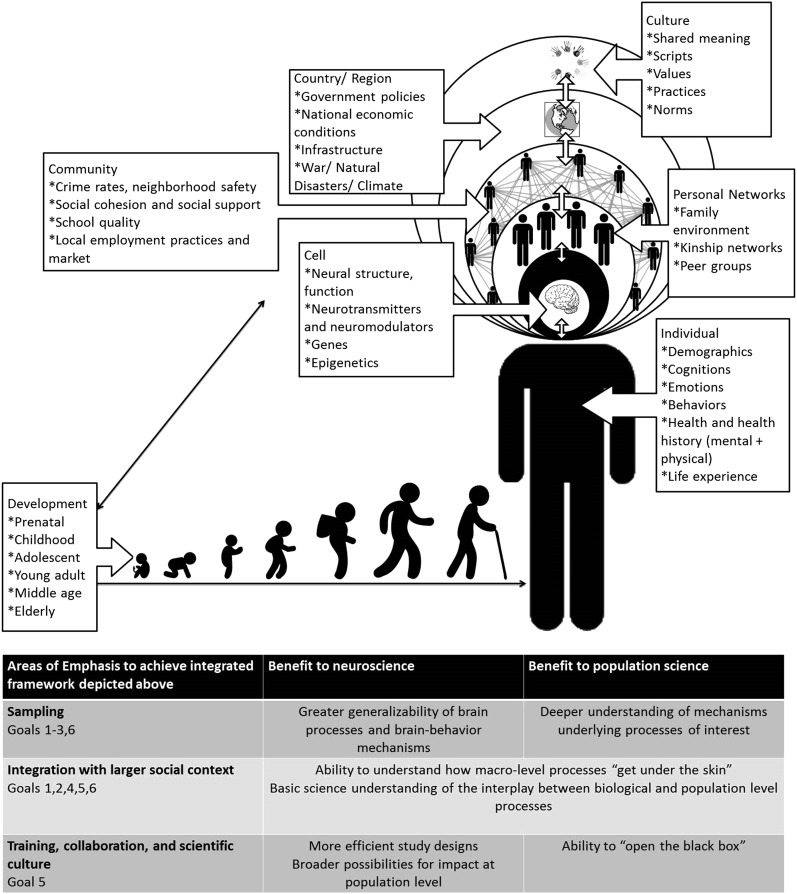
In the present article, we point to building momentum of a new subfield—population neuroscience (6, 18)—and the opportunities it affords. A population neuroscience perspective emphasizes an understanding of human behavior across multiple levels of influence (e.g., from culture to social structure, to experience, to behavior, to genes, to neural connectivity and function guided by a multilevel ecological model (12, 19–21) (Fig. 1). We encourage readers to read Paus's initial treatment of population neuroscience cited above. Although our thesis focuses on the interchange between population sciences and neuroscience, we believe that this thesis fits within the larger and emerging field Paus has described as population neuroscience and thus we use his term to characterize our goals for this emerging discipline.
Fig. 1.
This framework highlights the interplay between multiple levels of analysis from biology to macro level environments across development. Adapted from Harrison et al. (125) and Antonucci et al. (126).
Below, we highlight work on predictors, outcomes, moderators, and mediators of the brain–behavior relationship in studies that inform our understanding of broader populations (22). We place specific emphasis on integrating perspectives on sampling methodology from demography and survey research (23, 24) into a population neuroscience approach (6). We provide more concrete examples to illustrate the necessity of a population neuroscience approach for certain types of research questions and the benefits it can afford to both neuroscientists and population scientists. We then outline specific goals to make population neuroscience a reality and discuss the need for theoretical and applied advances from this approach.
Current Practices in Neuroimaging Research: How Universal Is What We Know?
Researchers in both social and biological sciences have pointed to the negative consequences of extrapolating from small, nonrepresentative samples based on the systematic biases these samples can introduce (9). For example, for years, research suggested that IQ was highly heritable, but more recent research using more representative samples found that genetic heritability was decidedly lower for the whole population (25). Previous researchers had used samples primarily consisting of high socioeconomic status (SES) participants. SES, however, was shown to moderate the genetic heritability such that for high SES genetic heritability was above 70%, but for low SES participants genetic heritability was closer to 10% (7, 25). Similarly there are many examples across other domains of research where early nonrepresentative convenience samples and/or small sample sizes led to incorrect or inconsistent estimates of outcomes, including errors such as misconceptions of age patterns on morbidity and cognition (26, 27), the assumption that basic tenants in social psychology (e.g., the fundamental attribution error) generalize to all people (7, 28, 29), and relationships between socioeconomic position, neuropathology, and dementia (30). Beyond these consequences, social scientists have long noted the constraints and problems imposed by reliance on student subject pools (8, 29) and Western, Educated, Industrialized, Rich, and Democratic (WEIRD) populations more broadly (7). These samples differ in many concrete ways from broader populations of interest, which has led to a greater emphasis by the National Institutes of Health on including women and minorities in studies (31, 32). Additionally, even within medical clinical trials there has been a call for greater use of practical clinical trials to improve the external validity of the results (33, 34).
Learning from these examples, the need for population neuroscience for certain types of research questions is clear. Population neuroscience leverages well-known sampling techniques that are routinely used in other fields such as demography, epidemiology, and survey research (11, 12) to strengthen the link between sample and target population and enhance the generalizability of results. The extent to which neuroscience findings can inform and be informed by disciplines that focus on macro level structures (e.g., demography, sociology) rests on our ability to maximize generalizability to relevant populations. It should be noted that what constitutes a relevant population is often subjective and project specific. Researchers may target different populations (e.g., by disease status/risk, age, geographic region, or SES level) based on their research goals and substantive theoretical questions, but a key aspect of moving research forward is identifying and describing the relevant population the research is intended to characterize or address. Not explicating the population could lead to problems of comparability, replication, and inference.
This is not to say that all studies must use samples that represent entire nations or that treat variability across major cultural groups. In fact, collecting larger representative data without clear hypotheses or a target population to generalize to may not yield helpful conclusions either. Rather, the goal is for neuroscience studies to consider sampling as one critical component of the design, just as researchers would consider functional MRI (fMRI) pulse sequences, task, stimuli, and/or analysis strategy. In fact, many clinical studies and high-risk population studies are excellent examples where neuroimaging is used on a carefully selected and characterized sample that generalizes to a specific, relevant population (but see ref. 27). Ideally, in the study design phase, researchers should consider to whom their research question should ideally apply and then take steps to recruit participants who represent the target group and report information relevant to this process. Through a focus on theory-relevant sampling and interdisciplinary discussion between neuroscientists and population scientists, population neuroscience can improve the external validity, replicability, and generalizability of neuroscience findings to relevant populations and clarify to which population(s) the results can be generalized. In parallel, this creates opportunities to make the results more applicable to a wider range of social scientific fields and to test key theoretical questions of interest to population scientists. To achieve these goals, however, requires a cross-discipline emphasis on both the internal validity (a greater focus in neuroscience) and the external validity of studies (a greater focus within population studies). It also requires multidisciplinary communication and collaboration (e.g., development of methodology and common language shared across areas), so that critical methods and findings across the disciplines that study these phenomena can inform each other.
Getting Inside the Black Box: Adding Neuroimaging and Other Neuroscience Methods to Broader Population Science
Over the last several years, there has been a rapid expansion of social and population researchers using biomarkers (e.g., cortisol response, cholesterol levels, epigenetics) to examine how the social environment gets under the skin (17, 35). Examining these biological mechanisms of social environment and health has produced important justifications for funding of continued work in the social and population sciences (5, 36). However, very little current population-focused work has examined the brain, which may be an optimal biomarker to examine the wide variety of variables typically found in population-based studies (e.g., health, decision-making, educational achievement, acceptance of new ideas). Thus, the current practice of many population researchers using large, omnibus studies is well suited to allow even a small group of population neuroscience experts to make immediate impacts on a wide variety of research areas. With the help of neuroscientists, population scientists can begin to open the black box of the brain that has long been assumed, but rarely examined, in most population-based models.
For example, one set of topics of interest to population scientists includes the effects of neighborhood and family poverty and social inequality on later outcomes such as family instability, educational attainment, health, employment, crime, and psychiatric disorders. Although population research has established robust effects of poverty and inequality on these outcomes (37–42), neural mechanisms of these effects have not been a primary focus in population approaches. However, within neuroscience and health psychology, research has begun to show that early life experiences such as parenting and SES have effects on brain areas such as the amygdala and prefrontal cortex (43–47), areas that have also been linked to a variety of relevant outcomes such as crime and violence (48, 49), depression (50), social cognition (51, 52), drug use (53, 54), and cognitive control (55). For example, a recent study demonstrated that early life stress predicted stress responses in the hypothalamic-pituitary-adrenal axis, which in turn predicted connectivity between the amygdala and prefrontal cortex and later risk for depression (56). Therefore, emerging research showing that early experience can affect the function, structure, and connections within and between key brain areas may help explain why experiences such as poverty lead to deleterious health and behavioral outcomes and also why some individuals are more susceptible to these experiences.
Although this research is beginning to elucidate biological embedding of experience at the neural level, it has not yet addressed a second related key process linking experience and behavior: the effect of culture in developing cognitive maps that allow us to understand and navigate the world (57). For example, our brains help us to acquire and then use complex information specific to our culture(s), such as knowing the difference between breakfast and snack foods, how to respond to authority, whether smoking is bad, etc., but the content of these cognitive maps is not currently accessible through neuroimaging, suggesting the importance of interdisciplinary partnerships (58–60). Furthermore, additional work linking such processes with macrolevel variables (e.g., social network structure) and biological variables (e.g., neural responses to cognitive tasks) also stands to advance both population and neuroscientific theory.
Overall, by partnering with neuroscientists, population scientists can specify new biological processes such as brain structure and function, which would shape cognition and perception of experiences thereby influencing behavior. This process leads to an interaction through which experience and biology shape each other over time (61, 62). Although neuroscience cannot capture experience at all levels, it can help to specify how some experience is biologically embedded and how experience and biology interact over time to explain questions of interest to population scientists.
Integrative Framework
How can these goals become reality? How can we link multiple levels of analysis (i.e., move from synapse to cell to brain to individual to groups to regions to nations)? Below, we outline six concrete steps toward the big picture goal of promoting generalizability in neuroscience investigations and harnessing neuroscience tools to understand processes of interest to population scientists.
Goal 1: Integrate Brain Imaging into Existing Representative (Sub)samples.
By using techniques such as sample stratification, cluster sampling, subsampling, and “planned missingness” (63), neuroscience methods can be integrated with ongoing and large-scale population-level studies without needing to collect neural data on every sample participant. This strategy can afford greater generalizability of brain processes and insights about underlying mechanisms that contribute to macro level processes (10). Larger-scale studies of the type typically conducted by survey researchers and demographers often contain rich longitudinal measures of behavior and experience. Presently, it is rare for these types of data to be examined in dialogue with neuroimaging data [although a growing number of studies have scanned subsamples of participants within specific existing longitudinal or archival studies (64–66)]. One large-scale example that might be considered a model for integration of brain imaging into existing representative samples is the National Institutes of Health Pediatric MRI Database (NIH-PD) (6, 67), which used population-based sampling at six sites, based on the 2000 census data. This study includes information about social and environmental variables (e.g., socioeconomic position, prenatal exposure to risk factors such as alcohol), cognitive tests, and behavioral measures, including laboratory-based tests of executive function and academic skills, and a range of structural brain images. The key to this point is that using these sampling techniques, along with “piggybacking” on another study, means that neuroimaging studies need not necessarily be of large magnitude to yield large findings if sampled thoughtfully within the context of a larger study. Moreover, when piggybacked on another study, the neuroimaging data are enhanced through more precise (and often longitudinal) behavioral phenotypes at both the individual and macro level of behavioral analysis (6, 64). Substantially more work is needed to broaden this understanding and to more fully integrate what is known about large-scale social phenomena with the individual level processes that yield these larger-scale phenomena.
Goal 2: Development of Methods to Scale Up Neuroimaging Studies to Larger and More Representative Samples with Methods Allowing for Cross-Study, Cross-Age, and Cross-Culture Comparisons.
Recently, several large-scale neuroimaging studies have emerged by expertly piecing together smaller convenience samples (68, 69), scanning larger and larger samples of individuals (6, 70, 71), using data sharing and open access data (68, 72–74), consortium models (75–77), and neuroimaging meta-analysis (78, 79). These models emphasize that neuroimaging approaches can be done on a larger scale and across research teams. However, these studies also highlight many of the current methodological challenges to going big with neuroimaging studies (68, 75, 78). For example, neuroscience methods have lagged in terms of addressing the use of multiple scanners (69, 80, 81), standardizing tasks used for functional (and resting-state: ref. 82) MRI studies, understanding the effect of different pulse sequences on findings (83, 84), standardization of single data processing streams (85, 86), and statistical approaches for issues such as multiple comparisons (68). Understanding the extent to which different laboratories, scanners, and methods can reliably collect data (80) is also vital to population-based studies because most large studies rely on cluster sampling, which is conducive to using multiple laboratories for imaging. Cross-team data sharing also highlights the need for better methods for secure data sharing and computation across sites. Moreover, research is needed to examine factors that affect MRI results (e.g., head motion, ability to attend to a task) that covary with health factors and behaviors (e.g., chronic hypertension, smoking), which may be linked to environmental factors being studied (e.g., SES, geography). We also need designs and analytic tools that allow us to combine variables and processes at different time scales and levels of analysis. Finally, research that examines translation from neuroimaging to neural methods or proxies including self-report measures that are cost-effective and can be implemented at a population level are also critical in the translation of this research from smaller samples to larger samples and to wide-scale clinical relevance.
Ultimately, methodological advances (including sampling approaches) will be inherently intertwined with theory and the hypotheses being tested in these studies. Although sampling for large-scale representativeness may not be appropriate for all research questions, goal 2 emphasizes the need for methods in cases where the scientific question calls for this type of generalizability and/or where theoretical questions cannot be adequately addressed using data from one level of analysis alone.
Goal 3: Use Strategic Sampling When Recruiting for Stand-Alone fMRI Studies.
Statisticians, survey methodologists, and demographers can partner with neuroscientists to improve the inferential and statistical quality of smaller studies by helping neuroscientists clarify to whom it is important for their results to generalize and then to sample accordingly. Population scientists have extensive experience using a variety of methods to reduce bias, increase power, and improve causal inference in smaller sample studies (87). These methods include knowledge of matching sampling frames and target populations to reduce coverage error, reducing sampling costs by using cluster samples and subsampling within clusters, and improving representativeness through stratified sampling (23, 88–91). For example, developmental scientists interested in the role of psychological resources on limbic system reactivity during adolescence might benefit from input from population scientists in selecting specific subgroups of adolescents who represent the demographic profile of high- and low-risk adolescents within the United States. The process of more precisely specifying the target population can help clarify theoretical predictions and advance understanding of boundary conditions for effects observed. In considering the demographic profile of teens, developmental neuroscientists and population scientists might both benefit from selecting adolescents who vary along some key dimension (e.g., SES, risk-taking status) and examining the interplay between the target macro level processes and the brain.
Further, there may be great interest in examining the predictors of participation and nonresponse in brain imagining studies with the goal of improving and adjusting for nonresponse bias in our statistical models. Combined with goal 1 of scanning existing participants of current population-based studies, a particularly useful concept may be using a large population-based study as the control for several smaller case-control neuroimaging studies. This way the controls can be used to provide generalizability, whereas the case studies provide the necessary power for disease or behavior conditions that may be too rare to power a study from a standard population-based study (e.g., see the Welcome Trust Case Control Consortium in genetic research as an exemplar; ref. 92).
Goal 4: Explore Moderators of Brain–Behavior Links and Neural Predictors of Relevant Outcomes.
A growing body of research has demonstrated that environmental factors influence brain development. For example, childhood SES predicts brain structure (46) and function (45). Likewise, the size of our social networks relates to brain structure (93, 94). Given that the social environment is known to affect a wide array of biological responses (5, 17, 19, 95), a next important goal for neuroscience will be to further understand how experience at multiple levels (e.g., culture, family, social networks, SES) affects neural structure and function (46, 96–101). In parallel, it is certain that social and environmental variables moderate the link between brain and behavior (12), but further research is needed to examine such interactions. For example a recent study has demonstrated that level of perceived social support moderates the previously much replicated relationship between amygdala reactivity and trait anxiety, indicating that many brain–behavior relationships may vary by environment and experience (102). However, if research does not explore these moderators, then these relationships may be assumed to be invariant across people and environments.
To fully leverage the fruits of the methodological goals outlined above, neuroscientists and population scientists might also reconsider the ways that neural variables are conceptualized (103). Traditional neuroimaging research has focused on the brain as a dependent measure (e.g., Where do certain processes take place in the brain? What structures support those processes?). Decades of neuroimaging literature have now characterized several processes that may be able to predict outcomes of interest to population scientists [e.g., neural activity in response to health communications predicts large-scale effects of media campaigns (104)]. With this in mind, neural variables (e.g., structure, function, connectivity) can be hypothesized in advance and treated as predictor variables of relevant population level outcomes (103). This use of neuroimaging methods may contribute explanatory power that is not readily available from other sources and may be a source of convergent validity for identifying the best measures of behavior (103–105). Moreover, increasingly, there has been a shift from the assumption that the brain is only a dependent or independent variable but also can be a moderator and mediator of paths from experience to behavior or between genes, brain, and behavior (56, 61, 102, 103, 106). Moreover, evidence is mounting that that brain–behavior links may be powerfully moderated by experience, context, and culture (102, 107).
Goal 5: Changing of the Cultures in Neuroscience and Population Research.
As population research has recently begun to acknowledge the usefulness and importance of including genetic and other biomarker data (108–113), scholars note a lack of brain research in the examination of the influence of macrolevel processes on health and behavior (111, 114). Moreover, we argue that even though most population researchers may not use MRI data, a better general understanding of the behaviorally relevant elements of basic brain research will be important for the progress of the field, especially as cross-discipline studies are becoming the norm rather than exception. Likewise, for many neuroscience questions, smaller targeted samples make sense, and many findings within the social, affective, and cognitive neurosciences have replicated well across laboratories, across geographical locations, and across time. However, neuroscience has compelling insight to add to population level investigations and will benefit from increased focus on who the relevant sample is. Both goals will be advanced with minimal burden by researchers providing more basic data on the sample and how the sample relates to larger populations that might be of interest in both publications and grant applications. Building on the excellent checklist developed by Poldrack and colleagues (115), we propose the addition of some basic sampling strategy information, as well as the facts that would typically be included in a Consolidated Standards of Reporting Trials (CONSORT) flow diagram (www.consort-statement.org/consort-statement/) to the checklist (Table 1).
Table 1.
Guidelines for presentation of neuroimaging studies with a focus on population neuroscience areas of emphasis
| Specific subject and recruitment details to report | Advantages of reporting this information regularly |
| Target population: Author note about who the sample may generalize to in the larger population | Draws attention to the author specified relevant population and draws attention to when samples may be limited in generalizing to other populations |
| Sample design: Sampling strategy used to select potential participants from a larger population pool | Allows readers to understand the strengths of the strategy used, as well as possible sampling error and bias within the study, and what methods will be needed to appropriately account for the strategy |
| Recruitment strategy and response rate: Techniques used to find, contact and encourage study participation | Allows for assessment of selectivity of sample by learning the extent to which nonresponse bias may influence the findings |
| Analysis exclusion criteria: Measures used to distinguish analysis sample | Delimits the sample and population of who and who is not included |
| Attrition bias: (longitudinal studies) Rates of continued participation | Allows for the assessment of selectivity of a longitudinal sample due various types of attrition |
| Demographics: age, race, and ethnicity, SES at each step from recruitment to scanning to those with usable data | Draws attention to the diversity of characteristics of the sample and potential bias in who is retained at any step along the way |
| Efforts to standardize across multiple scanners if the study includes such data: If multiple scanners or scan sites were used in data collection, what specific steps were taken to standardize pulse sequences, protocols, and other factors that might affect the imaging data? What steps were taken to adjust for scanner variability without removing variability that is due to differing demographics or participant characteristics across sites? | Draws attention to portions of the protocol that are standard across sites, and elements that may introduce variability |
A complement to the guidelines suggested by Poldrack et al. (115), which include suggestions for reporting design specification, task specification, planned comparisons, details of the subject sample (e.g., inclusions/exclusion criteria), ethics approval, behavioral performance, image properties, preprocessing, first level modeling, group level modeling, inferences related to statistical images, ROI analysis, and figures/tables.
Funding for interdisciplinary training and interaction, support for interdisciplinary working groups, and consultation across disciplines will promote true cross-pollination. For example, conferences that bring together scientists across these disciplines can lead to more explicit collaboration and a better understanding of methods and the benefits of each discipline’s area of expertise. Funding that focuses explicitly on these specific aims may yield studies that have big impacts, not only on the study’s specific question but more broadly on how we interpret and understand neuroimaging and population science. Although these studies may seem risky to funding agencies, these are the types of studies that can have big rewards.
Goal 6: Emphasis on Development and Ecological and Interactional Models.
One other major theme population science brings to a population neuroscience approach is an appreciation of development and multilevel ecological models (18, 19). Many fundamental neuroscience questions require longitudinal data and a developmental perspective (17, 61, 79). Social ecological models of development and life course will be fundamental to the organization of population neuroscience through their emphasis on the multiple layers of influence on behavior and cognition. Life course and development frameworks imply that the relationships between social context and the brain may not be constant across an individual’s life, may vary as a function of the context in which individuals operate, and may not be constant over cohorts or historical periods (116). Developmental theory has as its primary focus the interaction of person, process, and context, as studied with regard to age and age-graded transitions in processes and relationships (20, 62, 117). Life course theories focus on context as well (cohort, period, and historical contexts) and will be relevant here. Thus, these theories highlight the (individual, societal, and historical) timing of transitions and adaptation to various transitions, which are likely to influence and qualify brain–behavior relationships in powerful ways that are yet to be examined. Beyond thinking of these contexts as predictors and moderators of brain processes, ecological models emphasize that brain processes are nested and embedded within larger social contexts at multiple levels and are likely to be influenced by and influence these contexts (Fig. 1). Thus, partnerships across these disciplines may bring a more complex and interactional framework to our understanding of neuroscience (20, 61, 62).
Benefits to Neuroscience, Population Science, and Broader Social Sciences
Implementation of the six steps outlined above would promote advances in the integration of knowledge across levels of analysis (Table 2). In turn, neuroscience, population sciences, and related social and biological sciences all stand to benefit: Population neuroscience complements other movements toward larger team-based science (118–121), which have accomplished goals that could not be achieved with a single principal investigator (e.g., recent work in high energy physics on the Higgs Boson; the human genome project). Recent advances in handling “Big Data” (e.g., new methods for secure data sharing) should also inform this work. Most centrally, neuroscience and population science will benefit from knowing how brain structure and function varies across groups and what can be generalized, and population-based social sciences like demography and sociology will benefit by looking into the black box that has previously been a stand-in for the brain.
Table 2.
Areas of emphasis within a population neuroscience framework
| Areas of emphasis | Why? | How? |
| Increase the representativeness of samples using neuroimaging approaches | • Neuroimaging studies based on convenience samples may not optimally address target research questions or may come to erroneous conclusions | • Increased emphasis on sampling approaches (goal 5) |
| • All brains are not the same | • Use of sophisticated sampling and analytic techniques to decrease N needed in samples (goal 3) | |
| Increased collection of larger, well-characterized neuroimaging samples at multiple points across the life span | • Understand developmental trajectories of brain development | • Merging existing data sets and meta-analysis (goal 2) |
| • Increase replicability and generalizability of results | • Large-scale collaborative studies | |
| • Piggybacking neuroimaging on existing behavioral studies | ||
| • Increased work on cross-site imaging and standardization of protocols to allow for combining samples (goal 1) | ||
| • Longitudinal imaging (goal 6) | ||
| Increase the emphasis on larger social context and experience as a predictor and moderator of brain-behavior links | • Evidence in social sciences emphasizes the importance of broader context and culture on behavior | • Examination of moderators and collection of data from diverse groups (both cross- and within-culture) (goal 4) |
| • Ignoring these variables assumes uniform brain-behavior relationships which is unlikely | • Examination of ecological and interactional models (goal 6) | |
| Increased training and collaboration between neural and social scientists | • Neural science can gain from increased focus on samples and on contextual effects | • Funding focused on this “high-risk, high-reward,” large-scale collaboration |
| • Population science can gain from increased understanding of the brain as a mediator of context-behavior links | • Conferences and national meetings for collaboration and learning | |
| • Emphasis on making each discipline’s methods accessible (goal 5) |
In addition, the mechanisms uncovered by neuroscience research can be incorporated within larger-scale models of human behavior. For example, longitudinal neuroimaging of representative samples, with repeat scans starting early and continuing across development, will advance developmental science, as well as our broad understanding of brain plasticity and relationship to experience (12, 18, 61, 122). Sociologists and social psychologists will also have a major stake in this research and will be particularly important in helping to formulate the possible chronic influences on brain structure and function, such as discrimination, poverty, SES, and social support (5, 17, 43, 44). As well, these influences of brain function and structure may act as moderators of brain–behavior relationships, thus leading to a more dynamic model of social context, brain function, and behavior. Communication scholars are also increasingly interested in how neuroscience methods may inform our understanding of media effects (e.g., effects of violent media), as well as ways to predict individual differences in response to health communication, political communication, and inform media campaigns (123). However, a full understanding of how larger-scale mediated variables interact with individual level processes requires a more sophisticated set of methods for linking micro and macro level processes. Similarly, neuroimaging tools can be more efficiently applied within psychiatry, medicine, and clinical psychology with advances that allow tighter linkage between the samples under study and the broader populations that require treatment.
It should be noted that several relevant fields already have models for success: research in demography has transitioned from relatively coarse measures from the census to an emphasis on mechanisms and processes, statistical methods, and recent integration of biomarkers to the field (90, 110, 111). Methods from demography might also be harnessed as best practices for cross-cultural/cross-national analysis and work across broader sets of multilevel problems (i.e., international, national, regional). Likewise, in human genetics, genetic information is used as a predictor of behavior and moderator of the social environment. As illustrated by imaging genetics approaches, one meeting place of genetics and society is in the brain (61, 124). Therefore, neuroscience (e.g., MRI, psychophysiology) data could easily be seen as an outcome of great interest to population sciences, a moderator of environmental influences, a mediator of gene × environment interactions (61), or at least, an important confounder to account for in their models.
Conclusion
We outlined a framework to better understand influences and mechanisms of behavior from culture to experience to brain structure and function, which would also improve confidence in the generalizability of neuroimaging findings. To take action on this framework, collaboration is needed between neuroscientists, survey methodologists, biophysicists, biostatisticians, and representatives from across social sciences, and population-based sciences in particular. These stakeholders include members of multiple social and behavioral sciences (psychology, sociology, economics, epidemiology, medicine, education, communication). Research across each of these disciplines will benefit from the resulting knowledge. However, our point is not simply that researchers should collaborate more across disciplines, rather it is more pressing: social and neural sciences are building huge literatures that could be more efficient and informative; however, at the present, “we don’t know what we don’t know”: human neuroimaging studies are limited in the extent to which results might generalize based on relatively less sophisticated sampling methods, whereas social science disciplines that ignore brain science may be missing a critical piece to understanding behavior phenomena even at macro levels. Thus, this is a critical moment for these disciplines. Collaboration can and should happen through funding opportunities, summer institutes, cross-disciplinary training of future scientists, graduate and postdoctoral training opportunities across areas, and with each area making their methods accessible to others. This framework is meant to be dynamic and will be refined as members of each of these groups agree on principles for the collection and analysis of representative brain imaging data. Although this goal is ambitious, the groundwork is in place, and several large-scale neuroimaging studies and existing nationally representative surveys with interest in adding neuroscience data provide jumping off points (6). Accomplishment of this overarching goal will provide deeper insights about how biology, social situations, and broader environmental context interact to guide behavior and development. In turn, this will advance basic science and provide concrete insight for the design of better interventions and policies.
Acknowledgments
This paper was made possible by the collective efforts of the Social Environment and Neural Development (SEND) working group within the Survey Research Center (SRC) at the University of Michigan. We gratefully acknowledge the SRC for support of this group, as well as funding supporting group members: National Institutes of Health (NIH)-1 Grants DP2 DA035156-01 (to E.B.F.), U01AG009740 (to. J.F.), R01 DA027261 (to M.M.H.), R01 AA12217 (to M.M.H.), and U01 AG09740 (to. K.L.), and the Robert Wood Johnson Foundation Health and Society Scholars program (J.M.).
As we advocate cross-disciplinary collaboration, we describe how (i) our group has come together representing many disciplines and (ii) how this paper was written as an example of the potential of this type of group. (i) In 2010, the University of Michigan challenged social science researchers to cross the traditional bounds of their disciplines to think of emerging cross-disciplinary work that would inform the science in the future. P.D.-K. and F.J.M. received a grant from this initiative centered on documenting important changes in the brain related to socioeconomic differences of children and families. This research, however, was based on small sample sizes and a fairly basic understanding of indicators of socioeconomic differences. Thus, a conference was assembled to bring together researchers across the social sciences and neuroscience to discuss the state of this research and ways to improve and validate findings. One of outcome of this conference was that investigators across the University began to meet and identify important synergies across broad areas of social and neural sciences. With support from the Institute for Social Research, the senior authors began hosting monthly meetings for these discussions. The group continues to grow and represent multiple disciplines and career stages, often with junior members contributing “cutting edge” new approaches. (ii) This manuscript was the result of discussions that the group has had from 2012–2013 and emerged as a way to organize our collective vision. Key to the production of the paper was that the three first authors were junior investigators with three very different backgrounds (e.g., demography, social neuroscience, and developmental neurogenetics) interested in collaborating and synthesizing interests from across our fields. As the three first authors were somewhat representative of the larger group, we were able to structure a paper and receive feedback from the larger group, especially in parts of the manuscript core to each member’s expertise. We also received excellent feedback from two reviewers: their thoughtful input significantly strengthened the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 2.Cacioppo JT, Berntson GG, Sheridan JF, McClintock MK. Multilevel integrative analyses of human behavior: Social neuroscience and the complementing nature of social and biological approaches. Psychol Bull. 2000;126(6):829–843. doi: 10.1037/0033-2909.126.6.829. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman MD. Social cognitive neuroscience. In: Fiske S, Gilbert D, Lindzey G, editors. Handbook of Social Psychology. 5th Ed. New York: McGraw-Hill; 2010. pp. 143–193. [Google Scholar]
- 4.Sanfey AG, Loewenstein G, McClure SM, Cohen JD. Neuroeconomics: Cross-currents in research on decision-making. Trends Cogn Sci. 2006;10(3):108–116. doi: 10.1016/j.tics.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberger NI, Cole SW. Social neuroscience and health: Neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15(5):669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- 6.Paus T. Population neuroscience: Why and how. Hum Brain Mapp. 2010;31(6):891–903. doi: 10.1002/hbm.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henrich J, Heine SJ, Norenzayan A. The weirdest people in the world? Behav Brain Sci. 2010;33(2-3):61–83, discussion 83–135. doi: 10.1017/S0140525X0999152X. [DOI] [PubMed] [Google Scholar]
- 8.Sears DO. College sophomores in the laboratory: Influences of a narrow data base on social psychology's view of human nature. J Pers Soc Psychol. 1986;15(3):515–530. [Google Scholar]
- 9.Button KS, et al. Power failure: Why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 10.Stiles J. On genes, brains, and behavior: Why should developmental psychologists care about brain development? Child Dev Perspect. 2009;3(3):196–202. [Google Scholar]
- 11.Zelazo PD, Paus T. Developmental social neuroscience: An introduction. Soc Neurosci. 2010;5(5-6):417–421. doi: 10.1080/17470919.2010.510002. [DOI] [PubMed] [Google Scholar]
- 12.Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- 13.Keating DP. Nature and Nurture in Early Child Development. New York: Cambridge Univ Press; 2010. [Google Scholar]
- 14.Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- 15.Boyce WT, Sokolowski MB, Robinson GE. Toward a new biology of social adversity. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17143–17148. doi: 10.1073/pnas.1121264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Dev. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 17.Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci USA. 2010;107(19):8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paus T. Some thoughts on the relationship of developmental science and population neuroscience. Int J Develop Sci. 2012;6(1):9–11. [Google Scholar]
- 19.Bronfenbrenner U. The Ecology of Human Development: Experiments by Nature and Design. Cambridge, MA: Harvard Univ Press; 1979. [Google Scholar]
- 20.Cicchetti D, Toth SL. Transactional ecological systems in developmental psychopathology. In: Luthar SS, Burack JA, Cicchetti D, Weisz JR, editors. Developmental Psychopathology: Perspectives on Adjustment, Risk, and Disorder. Cambridge, UK: Cambridge Univ Press; 1997. p. 317. [Google Scholar]
- 21.Li S-C. Brain in macro experiential context: Biocultural co-construction of lifespan neurocognitive development. Prog Brain Res. 2009;178:17–29. doi: 10.1016/S0079-6123(09)17802-0. [DOI] [PubMed] [Google Scholar]
- 22.Chiao JY, Cheon BK. The weirdest brains in the world. Behav Brain Sci. 2010;33(2-3):88–90. doi: 10.1017/S0140525X10000282. [DOI] [PubMed] [Google Scholar]
- 23.Groves RM, et al. Survey Methodology. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 24.Kish L. Survey Sampling. Hoboken, NJ: Wiley; 1995. [Google Scholar]
- 25.Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychol Sci. 2003;14(6):623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- 26. Camp CJ, West RL, Poon LW (1989) Recruitment practices of psychology research in gerontology. Special Research Methods for Gerontology, eds Lawton MP, Herzog AR (Baywood, Amityville, NY). pp 163–189.
- 27.Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299(17):926–930. doi: 10.1056/NEJM197810262991705. [DOI] [PubMed] [Google Scholar]
- 28.Miller JG. Culture and development of everyday social explanation. J Pers Soc Psychol. 1984;46(5):961–978. doi: 10.1037//0022-3514.46.5.961. [DOI] [PubMed] [Google Scholar]
- 29.Peterson RA. On the use of college students in social science research: Insights from a second-order meta-analysis. J Consum Res. 2001;28(3):450–461. [Google Scholar]
- 30.Brayne C, et al. EClipSE Collaborative Members Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133(Pt 8):2210–2216. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- 31.Blehar MC. Public health context of women’s mental health research. Psychiatr Clin North Am. 2003;26(3):781–799. doi: 10.1016/s0193-953x(03)00039-x. [DOI] [PubMed] [Google Scholar]
- 32.Brawley OW, Freeman HP. Race and outcomes: Is this the end of the beginning for minority health research? J Natl Cancer Inst. 1999;91(22):1908–1909. doi: 10.1093/jnci/91.22.1908. [DOI] [PubMed] [Google Scholar]
- 33.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: Increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290(12):1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- 34.Glasgow RE, et al. External validity: We need to do more. Ann Behav Med. 2006;31(2):105–108. doi: 10.1207/s15324796abm3102_1. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe B, Evans W, Seeman TE. The Biological Consequences of Socioeconomic Inequalities. New York: Russell Sage Foundation Publications; 2012. [Google Scholar]
- 36.Muscatell KA, Eisenberger NI. A social neuroscience perspective on stress and health. Social and Personality Psychological Compass. 2012;6(12):890–904. doi: 10.1111/j.1751-9004.2012.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brooks-Gunn J, Duncan GJ. The effects of poverty on children. Future Child. 1997;7(2):55–71. [PubMed] [Google Scholar]
- 38.Xue Y, Leventhal T, Brooks-Gunn J, Earls FJ. Neighborhood residence and mental health problems of 5- to 11-year-olds. Arch Gen Psychiatry. 2005;62(5):554–563. doi: 10.1001/archpsyc.62.5.554. [DOI] [PubMed] [Google Scholar]
- 39.Sirin SR. Socioeconomic status and academic achievement: A meta-analytic review of research. Rev Educ Res. 2005;75(3):417–453. [Google Scholar]
- 40.Appleyard K, Egeland B, van Dulmen MH, Sroufe LA. When more is not better: The role of cumulative risk in child behavior outcomes. J Child Psychol Psychiatry. 2005;46(3):235–245. doi: 10.1111/j.1469-7610.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson RG, Pickett KE. Income inequality and population health: A review and explanation of the evidence. Soc Sci Med. 2006;62(7):1768–1784. doi: 10.1016/j.socscimed.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 42.McLanahan S. Fragile families and the reproduction of poverty. Ann Am Acad Pol Soc Sci. 2009;621(1):111–131. doi: 10.1177/0002716208324862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gianaros PJ, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2(3):161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gianaros PJ, et al. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35(2):795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gianaros PJ, et al. Parental education predicts corticostriatal functionality in adulthood. Cereb Cortex. 2011;21(4):896–910. doi: 10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanson JL, et al. Brain development and poverty: A first look. In: Wolfe B, Evans W, Seeman TE, editors. The Biological Consequences of Socioeconomic Inequalities. New York: Russell Sage Foundation; 2013. [Google Scholar]
- 47.Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat Rev Neurosci. 2010;11(9):651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hyde LW, Shaw DS, Hariri AR. Neuroscience, developmental psychopathology and youth antisocial behavior: Review, integration, and directions for research. Dev Rev. 2013;33:168–223. doi: 10.1016/j.dr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crowe SL, Blair RJR. The development of antisocial behavior: What can we learn from functional neuroimaging studies? Dev Psychopathol. 2008;20(4):1145–1159. doi: 10.1017/S0954579408000540. [DOI] [PubMed] [Google Scholar]
- 50.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- 52.Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 53.Kelley AE, Berridge KC. The neuroscience of natural rewards: Relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Everitt BJ, Cardinal RN, Hall J, Parkinson J, Robbins T. In: Differential Involvement of Amygdala Subsystems in Appetitive Conditioning and Drug Addiction. The Amygdala: A Functional Analysis. Aggleton J, editor. Oxford Univ Press, Oxford; 2000. pp. 353–390. [Google Scholar]
- 55.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 56.Burghy CA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaisey S. Motivation and justification: A dual-process model of culture in action. AJS. 2009;114(6):1675–1715. doi: 10.1086/597179. [DOI] [PubMed] [Google Scholar]
- 58.Damasio A. Self Comes to Mind: Constructing the Conscious Brain. New York: Random House; 2012. [Google Scholar]
- 59.Strauss C, Quinn N. A Cognitive Theory of Cultural Meaning. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 60.Sewell WH. Logics of History: Social Theory and Social Transformation. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 61.Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene-environment interactions. Trends Cogn Sci. 2011;15(9):417–427. doi: 10.1016/j.tics.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sameroff A. A unified theory of development: A dialectic integration of nature and nurture. Child Dev. 2010;81(1):6–22. doi: 10.1111/j.1467-8624.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- 63.Graham JW, Hofer SM, MacKinnon DP. Maximizing the usefulness of data obtained with planned missing value patterns: An application of maximum likelihood procedures. Multivariate Behav Res. 1996;31(2):197–218. doi: 10.1207/s15327906mbr3102_3. [DOI] [PubMed] [Google Scholar]
- 64.Fakra E, et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66(1):33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Persson J, et al. Longitudinal structure-function correlates in elderly reveal MTL dysfunction with cognitive decline. Cereb Cortex. 2012;22(10):2297–2304. doi: 10.1093/cercor/bhr306. [DOI] [PubMed] [Google Scholar]
- 66.Weiland BJ, et al. Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend. 2013;128(1-2):130–139. doi: 10.1016/j.drugalcdep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans AC, Group TBDC. Brain Development Cooperative Group The NIH MRI study of normal brain development. Neuroimage. 2006;30(1):184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 68. Yan C-G, Craddock RC, Zuo X-N, Zang Y-F, Milham MP (2013) Standardizing the intrinsic brain: Towards robust measurement of interindividual variation in 1000 functional connectomes. NeuroImage 80:246–262. [DOI] [PMC free article] [PubMed]
- 69.Fennema-Notestine C, et al. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5(4):235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- 70. Nikolova YS, Singhi EK, Drabant EM, Hariri AR (2013) Reward-related ventral striatum reactivity mediates gender-specific effects of a galanin remote enhancer haplotype on problem drinking. Genes Brain Behav 12(5):516–524. [DOI] [PubMed]
- 71.Satterthwaite TD, et al. Being right is its own reward: Load and performance related ventral striatum activation to correct responses during a working memory task in youth. Neuroimage. 2012;61(3):723–729. doi: 10.1016/j.neuroimage.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marcus DS, et al. Open access series of imaging studies (OASIS): Cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19(9):1498–1507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- 73.Jack CR, Jr, et al. The Alzheimer's disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Potkin SG, Ford JM. Widespread cortical dysfunction in schizophrenia: The FBIRN imaging consortium. Schizophr Bull. 2009;35(1):15–18. doi: 10.1093/schbul/sbn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thyreau B, et al. IMAGEN Consortium Very large fMRI study using the IMAGEN database: Sensitivity-specificity and population effect modeling in relation to the underlying anatomy. Neuroimage. 2012;61(1):295–303. doi: 10.1016/j.neuroimage.2012.02.083. [DOI] [PubMed] [Google Scholar]
- 76.Toga AW, Clark KA, Thompson PM, Shattuck DW, Van Horn JD. Mapping the human connectome. Neurosurgery. 2012;71(1):1–5. doi: 10.1227/NEU.0b013e318258e9ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fjell AM, et al. Pediatric Imaging, Neurocognition, and Genetics Study Multimodal imaging of the self-regulating developing brain. Proc Natl Acad Sci USA. 2012;109(48):19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jahanshad N, et al. (2013) Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA–DTI Working Group. NeuroImage 81:455–469. [DOI] [PMC free article] [PubMed]
- 79.Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: A review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33(8):1987–2002. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Glover GH, et al. Function biomedical informatics research network recommendations for prospective multicenter functional MRI studies. J Magn Reson Imaging. 2012;36(1):39–54. doi: 10.1002/jmri.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sutton BP, et al. Investigation and validation of intersite fMRI studies using the same imaging hardware. J Magn Reson Imaging. 2008;28(1):21–28. doi: 10.1002/jmri.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. Characterizing variation in the functional connectome: Promise and pitfalls. Trends Cogn Sci. 2012;16(3):181–188. doi: 10.1016/j.tics.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zuo X-N, et al. Toward reliable characterization of functional homogeneity in the human brain: Preprocessing, scan duration, imaging resolution and computational space. Neuroimage. 2013;65(15):374–386. doi: 10.1016/j.neuroimage.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yendiki A, et al. Multi-site characterization of an fMRI working memory paradigm: Reliability of activation indices. Neuroimage. 2010;53(1):119–131. doi: 10.1016/j.neuroimage.2010.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Evans AC, Janke AL, Collins DL, Baillet S. Brain templates and atlases. Neuroimage. 2012;62(2):911–922. doi: 10.1016/j.neuroimage.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 86.Sutton BP, Ouyang C, Karampinos DC, Miller GA. Current trends and challenges in MRI acquisitions to investigate brain function. Int J Psychophysiol. 2009;73(1):33–42. doi: 10.1016/j.ijpsycho.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. 2nd Ed. Boston: Houghton-Mifflin; 2002. [Google Scholar]
- 88.LaVange LM, Koch GG, Schwartz TA. Applying sample survey methods to clinical trials data. Stat Med. 2001;20(17-18):2609–2623. doi: 10.1002/sim.732. [DOI] [PubMed] [Google Scholar]
- 89.Korn EL, Graubard BI. Analysis of large health surveys: Accounting for the sampling design. J R Stat Soc Ser A Stat Soc. 1995;158:263–295. [Google Scholar]
- 90.Xie Y. Demography: Past, present, and future. J Am Stat Assoc. 2000;95(450):670–673. [Google Scholar]
- 91.Kalsbeek W, Heiss G. Building bridges between populations and samples in epidemiological studies. Annu Rev Public Health. 2000;21(1):147–169. doi: 10.1146/annurev.publhealth.21.1.147. [DOI] [PubMed] [Google Scholar]
- 92.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kanai R, Bahrami B, Roylance R, Rees G (2012) Online social network size is reflected in human brain structure. Proc R Soc Biol Sci 279(1732):1327–1334. [DOI] [PMC free article] [PubMed]
- 94. Lehmann J & Dunbar RI (2009) Network cohesion, group size and neocortex size in female-bonded Old World primatesProc R Soc Biol Sci 276(1677):4417–4422. [DOI] [PMC free article] [PubMed]
- 95.Taylor SE, Repetti RL, Seeman T. Health psychology: what is an unhealthy environment and how does it get under the skin? Annu Rev Psychol. 1997;48(1):411–447. doi: 10.1146/annurev.psych.48.1.411. [DOI] [PubMed] [Google Scholar]
- 96. Issa FA, Drummond J, Cattaert D, Edwards DH (2012) Neural circuit reconfiguration by social status. J Neurosci 32(16):5638–5645. [DOI] [PMC free article] [PubMed]
- 97.Morrison KE, Curry DW, Cooper MA. Social status alters defeat-induced neural activation in Syrian hamsters. Neuroscience. 2012;210:168–178. doi: 10.1016/j.neuroscience.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muscatell KA, et al. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60(3):1771–1777. doi: 10.1016/j.neuroimage.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chiao JY, et al. Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia. 2009;47(2):354–363. doi: 10.1016/j.neuropsychologia.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 100.Wasserman CR, Shaw GM, Selvin S, Gould JB, Syme SL. Socioeconomic status, neighborhood social conditions, and neural tube defects. Am J Public Health. 1998;88(11):1674–1680. doi: 10.2105/ajph.88.11.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hyde LW, Gorka A, Manuck SB, Hariri AR. Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia. 2011;49(4):651–656. doi: 10.1016/j.neuropsychologia.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berkman ET, Falk EB. Beyond brain mapping: Using the brain to predict real-world outcomes. Curr Dir Psychol Sci. 2013;22(1):45–55. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Falk EB, Berkman ET, Lieberman MD. From neural responses to population behavior: Neural focus group predicts population-level media effects. Psychol Sci. 2012;23(5):439–445. doi: 10.1177/0956797611434964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Falk EB, Berkman ET, Mann T, Harrison B, Lieberman MD. Predicting persuasion-induced behavior change from the brain. J Neurosci. 2010;30(25):8421–8424. doi: 10.1523/JNEUROSCI.0063-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychol Bull. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- 107.Chiao JY, et al. Theory and methods in cultural neuroscience. Soc Cogn Affect Neurosci. 2010;5(2-3):356–361. doi: 10.1093/scan/nsq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sastry N, McGonagle K, Schoeni RF (2009) Introduction to the special issue on the scientific assessment of biomeasures in the panel study of income dynamics. Biodemography and Social Biology 55(2):113–117. [DOI] [PMC free article] [PubMed]
- 109.Freese J, Shostak S. Genetics and social inquiry. Annu Rev Sociol. 2009;35(1):107–128. [Google Scholar]
- 110.McDade TW, Williams S, Snodgrass JJ. What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44(4):899–925. doi: 10.1353/dem.2007.0038. [DOI] [PubMed] [Google Scholar]
- 111.Freese J, Li J-CA, Wade LD. The potential relevances of biology to social inquiry. Annu Rev Sociol. 2003;29(1):233–256. [Google Scholar]
- 112.Boardman JD, et al. Population composition, public policy, and the genetics of smoking. Demography. 2011;48(4):1517–1533. doi: 10.1007/s13524-011-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mitchell C, et al. (2013) Differential sensitivity to social environments: Implications for research. Am J Public Health 103(S1):S102–S110. [DOI] [PMC free article] [PubMed]
- 114.Massey DS. A brief history of human society: The origin and role of emotion in social life. Am Sociol Rev. 2002;67(1):1–29. [Google Scholar]
- 115.Poldrack RA, et al. Guidelines for reporting an fMRI study. Neuroimage. 2008;40(2):409–414. doi: 10.1016/j.neuroimage.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–293. [PubMed] [Google Scholar]
- 117.Lerner RM. Developmental Science, Developmental Systems, and Contemporary theories of human development. 2006. eds Darmon W, Lerner RM, (Wiley, Hoboken, NJ), 6th Ed, Vol 1. [Google Scholar]
- 118.Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Buckner RL, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kalcher K, et al. (2012) Fully exploratory network independent component analysis of the 1000 functional connectomes database. Front Hum Neurosci 6:1–11. [DOI] [PMC free article] [PubMed]
- 121.Yarkoni T, Poldrack RA, Van Essen DC, Wager TD. Cognitive neuroscience 2.0: building a cumulative science of human brain function. Trends Cogn Sci. 2010;14(11):489–496. doi: 10.1016/j.tics.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Giedd JN, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4-18. Cereb Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 123.Falk EB. Can neuroscience advance our understanding of core questions in Communication Studies? An overview of Communication Neuroscience. In: Jones S, editor. Communication @ the Center. New York: Hampton Press; 2013. pp. 77–94. [Google Scholar]
- 124.Hariri AR. The neurobiology of individual differences in complex behavioral traits. Annu Rev Neurosci. 2009;32:225–247. doi: 10.1146/annurev.neuro.051508.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harrison K, et al. Toward a developmental conceptualization of contributors to overweight and obesity in childhood: the six-Cs model. Child Development Perspectives. 2011;5(1):50–58. [Google Scholar]
- 126.Antonucci TC, et al. The right to move: A multidisciplinary lifespan conceptual framework. Current Gerontology and Geriatrics Research. 2012:873937. doi: 10.1155/2012/873937. [DOI] [PMC free article] [PubMed] [Google Scholar]