Abstract
One feature of neuropathic pain is a reduced spinal GABAergic inhibitory function. However, the mechanisms behind this attenuation remain to be elucidated. This study investigated the involvement of reactive oxygen species in the spinal GABA neuron loss and reduced GABA neuron excitability in spinal nerve ligation (SNL) model of neuropathic pain in mice. The importance of spinal GABAergic inhibition in neuropathic pain was tested by examining the effects of intrathecally administered GABA receptor agonists and antagonists in SNL and naïve mice, respectively. The effects of SNL and antioxidant treatment on GABA neuron loss and functional changes were examined in transgenic GAD67-EGFP mice. GABA receptor agonists transiently reversed mechanical hypersensitivity of the hind paw in SNL mice. On the other hand, GABA receptor antagonists made naïve mice mechanically hypersensitive. Stereological analysis showed that the numbers of enhanced green fluorescent protein positive (EGFP+) GABA neurons were significantly decreased in the lateral superficial laminae (I-II) on the ipsilateral L5 spinal cord after SNL. Repeated antioxidant treatments significantly reduced the pain behaviors and prevented the reduction in EGFP+ GABA neurons. The response rate of the tonic firing GABA neurons recorded from SNL mice increased with antioxidant treatment, whereas no change was seen in those recorded from naïve mice, which suggested that oxidative stress impaired some spinal GABA neuron activity in the neuropathic pain condition. Together the data suggest that neuropathic pain, at least partially, is attributed to oxidative stress which induces both a GABA neuron loss and dysfunction of surviving GABA neurons.
1. Introduction
Gamma-aminobutyric acid (GABA) is one of the main inhibitory neurotransmitters in the mammalian nervous system, including the spinal dorsal horn. The loss of GABA inhibitory tone is an important contributor for the development of pain [10,19,32]. The reduction of inhibitory tone after peripheral nerve injury is evident based on: 1) a reduction in GABA content or GABA synthesizing enzyme immunoreactivity [3-5,11]; 2) presence of apoptotic profiles of spinal GABA neurons [29]; and 3) a reduction in the inhibitory postsynaptic currents in lamina II neurons [1,22]. On the other hand, contradictory findings have shown that nerve injury causes no significant change in GABA content [24,26,31], no loss of GABA neurons [24,25], no significant reduction of GABAergic synapses or GABA receptors in the denervated area [26], or even an increased spinal GABA level [27] and an enhancement of inhibitory tone [12]. Therefore, the fate of GABA neurons in the spinal cord after peripheral nerve injury remains unclear due to the difficulties in labeling GABA neurons and the inconsistent methods used across studies to analyze GABA content.
Several studies have proposed that GABA neurons are particularly susceptible to oxidative stress [21,34]. Reactive oxygen species (ROS) may influence apoptotic gene expression after peripheral nerve injury [31]. Thus, we hypothesize that one of the mechanisms by which ROS contribute to central sensitization is by promoting the loss of GABA neurons and/or hindering GABA functions, consequently, the disruption of GABA inhibitory tone in the spinal dorsal horn.
The present study investigated the role of ROS in neuropathic pain with respect to their interaction with the spinal GABA system. A transgenic mouse line was used that expresses the glutamic acid decarboxylase 67-enhanced green fluorescent protein (GAD67-EGFP) transgene which unequivocally labels a subset of GABA neurons in the spinal cord [8,23]. Behavioral tests for mechanical hyperalgesia examined the effects of intrathecal injections of GABA receptor agonists and antagonists. Stereological analysis of green fluorescent GABA neuron numbers in the spinal dorsal horn was used to determine the effects of repetitive treatments of a ROS scavenger, phenyl N-t-butylnitrone (PBN), in mice with the spinal nerve ligation (SNL) model of neuropathic pain. Whole cell patch recordings were used to determine changes in the physiological properties of spinal GABA neurons after SNL and then after ROS scavenger treatment. Parts of these data have been previously presented in abstract form [35,36].
2. Material and Methods
2.1. Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and are in accordance with NIH guidelines. Two pairs of homozygous transgenic mice expressing Enhanced Green Fluorescent Protein (EGFP) controlled by the mouse Gad1 gene promoter were purchased from the Jackson Laboratory (Bar Harbor, ME, USA; strain name: FVB-TgN(GadGFP)45704Swn) and interbred at an in-house facility. One male and female were housed in a plastic cage with standard bedding and were provided with free access to food and water under a 12/12 hour light-dark cycle (light cycle: 7:00 A.M. – 7:00 P.M.). Weaning took place 21 days after a new litter was born.
2.2. Spinal nerve ligation
Peripheral nerve injury was made by tightly ligating the L5 spinal nerve (SNL) in male mice at 8 weeks of age (at 14-18 day old mice for patch recordings) under isoflurane anesthesia (2% induction and 1.5% maintenance). After a dorsal midline incision, the paraspinal muscles over the L5/6 vertebrae and the L6 transverse process were removed to expose the left L5 spinal nerve. The L5 nerve was tightly ligated with 7-0 silk thread. The surgical site was closed, and the anesthesia was discontinued. Mice were returned to their cages to recover. The same procedures were performed in the sham operated animals, except for ligation of the L5 spinal nerve.
2.3. GABA receptor agonists and antagonists treatment
Four days after SNL, mice were randomly divided into four treatment and one control groups. Two groups of mice were injected intrathecally (i.t.) with the GABAA receptor agonist, muscimol (0.05 or 0.1 μg for each group, Sigma, St. Louis, MO) and 2 other groups with the GABAB receptor agonist, baclofen (0.03 or 0.06 μg for each group, Sigma). Unoperated naïve mice were also divided into four treatment and one control groups. Two groups of naïve mice were injected i.t. with the GABAA receptor antagonist, (-)-bicuculline methiodide (0.5 or 1 μg for each group, Sigma) and the other 2 groups with the GABAB receptor antagonist, CGP46381 (0.25 or 0.5 μg for each group, Tocris, Ellisville, MO). All drugs were dissolved in 5 μl saline. Mice in the control groups were treated with 5 μl saline alone. Drug administration was performed using a modified method of the direct transcutaneous intrathecal injection [16,20].
2.4. Behavioral testing of mechanical hyperalgesia
Testing for mechanical hypersensitivity of the hind paw was performed by examining the response rates of foot withdrawals to 10 stimuli applied to the paw with a von Frey (vF) filament (Stoelting, Wood Dale, IL). All experiments were conducted by a person blinded to the treatment groups. Mice were placed in a plastic box (4 × 4 × 12 cm) on a metal grid floor and acclimated for 8-10 minutes prior to testing. The vF filament #3.0 (equivalent to 0.1 g force) was applied perpendicularly to the skin for 2-3 seconds on the left hind-paw at the base between the 3rd and 4th digits, the most sensitive area after SNL. The vF filament was applied with enough force to bend it slightly. A positive response consisted of an abrupt withdrawal of the foot during or immediately after stimulation. Response rates were calculated as a percentage of the number of positive responses per 10 stimuli.
2.5. Stereological counting of GABA neurons
Stereological analysis of EGFP positive (EGFP+) GABA neurons in the dorsal horn was done in four groups of mice: 1) sham operated (Sham), 2) SNL without any other treatment (SNL), 3) SNL with multiple systemic daily injections of vehicle (saline) (SNL + Veh), and 4) SNL with multiple systemic daily injections of phenyl N-t-butylnitrone (PBN), an ROS scavenger (SNL + PBN). Immediately before SNL surgery and five hours later, mice in groups 3 and 4 received a systemic injection (i.p.) of 0.15-0.18 ml (depending on the body weight) of either vehicle (saline) or PBN solution (20 mg/ml solution, 150 mg/kg). For the next six consecutive days, both groups of mice received four daily injections, at 8 AM, 1 PM, 6 PM, and 11 PM, of either saline or PBN. Behavioral testing for mechanical hyperalgesia was performed daily for all four groups of mice by a person blinded to the treatment, prior to the first daily injection. On day seven, after behavioral testing, all mice were sacrificed, and their L5 spinal cords were processed for stereological analysis.
In stereological analysis for numbers of GABA neurons, mice were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and perfused through the aorta with ice cold saline and then fixative containing 4% paraformaldehyde. The L4-L5 segments of the spinal cord were removed, stored in fixative overnight, and placed into 30% sucrose until equilibration. The L5 cord tissues were serially sectioned with a cryostat into 80 μm thick transverse slices which were then mounted on gelatin coated slides, preserving the correct serial order. Four sections were selected by a systematic random sampling method from each animal and then analyzed for GABA neuron number.
A person who was conducting stereological analyses was blinded for the treatment type of the specimen. The stereological analyses were done by using an Olympus BX51 microscope (Olympus; Tokyo, Japan) with UV fluorescence (using the 10x & 20x UPlanApo objectives), a motorized Z-axis and X and Y stage, and a CX900 color video camera (MicroBrightField Inc., Williston, VT). Using the StereoInvestigator software (version 8, MicroBrightField Inc.), the optical fractionator method was used to estimate the total number of GAD67-EGFP neurons in the L5 spinal dorsal horn in three areas: the medial and lateral halves of superficial laminae (laminae I-II) and the deeper laminae (laminae III-V).
Contours of three sample areas were traced for each ipsilateral and contralateral side of a section under epifluorescence. A 10,000 μm2 counting frame was created in the program and placed randomly within the sampling grids distributed over each sample area. The optical dissector height was set to 25 μm in the Z- direction, leaving guard zones around the dissector height that excluded the regions closest to the slide and the cover slip. Fluorescent green cells were marked positive as their nuclei came into focus within the counting frame when scrolling in the Z- direction. Neurons were not counted if they intersected the “forbidden lines” of the counting frame. Stepwise movements in the X- and Y- directions automatically brought another counting frame into view, and the steps were repeated systematically until all areas were counted.
The total number of EGFP+ neurons (N) for each sample region of the L5 spinal dorsal horn per animal was estimated using the formula N = Q × V where V (volume fraction) equals 1/hsf × 1/asf × 1/ssf and where Q (sum of counts) equals the total count of EGFP+ neurons. The hsf (the tissue height sampling fraction) equals the optical dissector height relative to the average mounted section thickness. The asf (the area sampling fraction) equals the area of the counting frame relative to the grid size area. The ssf (the section sampling fraction) equals ¼ because every fourth section was sampled. The coefficient of error (CE) of the population size estimate according to the Schmitz-Hof equation provided in the software was used, and CE < 0.05 for each estimate.
2.6. Spinal cord preparation and electrophysiological recording
Electrophysiological recordings were made from the spinal cord slices of 21-25 day old mice with or without SNL surgery which was done one week prior to the recordings. Thus SNL surgery was done in 14-18 day old mice. On the day of recording (7 days after SNL), the lumbar spinal cord was quickly removed under pentobarbital anesthesia (50 mg/kg, i.p.) and immediately transferred into ice-cold high-magnesium artificial cerebrospinal fluid (ACSF) (in mM; 117 NaCl, 3.6 KCl, 2.5 CaCl2, 7 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, 11 Glucose) aerated with 95% O2-5% CO2. The spinal cord was then sliced transversely at 350 μm thickness using a Vibratome (Leica, VT1000S). The cord slices were incubated in high-magnesium ACSF at 34°C for 1 hr and then transferred to the recording chamber on the stage of a BX51W1 microscope and perfused with normal ACSF (in mM; 117 NaCl, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, 11 Glucose) at a rate of 2 ml/min at room temperature. GABA neurons in the superficial dorsal horn (laminae I-II) were identified as green fluorescent cells under the fluorescence microscope and then visualized by using infrared differential interference contrast (DIC) optics for whole-cell configuration. Patch-clamp recordings were made in a current-clamp mode using a Multiclamp 700B amplifier (Axon Instruments) and pCLAMP 9 data acquisition software (Molecular Devices). The patch-pipettes (4-6 MΩ) were filled with internal solution (in mM: 120 K-gluconate, 10 KCl, 2 Mg-ATP, 0.5 Na-GTP, 0.5 EGTA, 20 HEPES, 10 phosphocreatine). Only cells with resting membrane potentials greater than -55 mV were included in this study. After the whole-cell patch was established, we waited for 5 min to allow for equilibration.
To test the ROS contribution to GABA neuron dysfunction, the effect of PBN on the excitability of GABA neurons was examined in the SNL mice (n=5) and compared with that of the unoperated naïve mice (n = 3). GABA neuron excitability was quantified by examining the number of action potentials (AP; spikes/second) generated in response to a current injection (60 pA, 500 msec) from the base membrane potential of -70 mV. This depolarizing current was applied three times with 1 min intervals, and the resulting number of action potentials was averaged. The action potential recordings were done for 3 min before and then again 3 min after the application of a ROS scavenger, PBN (1 mM in ACSF solution), for 5 min. The number of action potentials per second was compared before and after PBN treatment in the naïve and SNL groups. Usually, two to five recordings were made from the spinal cord slices prepared from each animal.
2.7. Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). The SigmaStat program (Version 3.1, Systat Software, San Jose, CA) was used to analyze all data. A p value less than 0.05 was considered to be statistically significant. For the behavioral tests for mechanical hyperalgesia, changes from the vehicle controls were compared using the two-way analysis of variance (ANOVA) with one repeated factor followed by Holm-Sidak post hoc tests. For the GABA cell stereological counts, the differences in the number of GAD67 EGFP+ neurons between groups were compared using one-way ANOVA followed by the Holm-Sidak post-hoc test for group comparisons. Electrophysiology data were analyzed using the paired Student’s t test.
3. Results
3.1. Effects of GABA receptor antagonists on pain behavior
Before investigating the ROS modulation on the spinal GABAergic system in neuropathic pain, the reduced spinal GABA function in neuropathic pain and their inhibitory role in pain processing were re-evaluated. The effects of intrathecally administered GABA antagonists were tested to determine whether suppressing GABA transmission in the spinal cord would induce pain behaviors in unoperated naïve mice. A single intrathecal injection of the GABAA antagonist, bicuculline (0.5 or 1 μg) dose-dependently increased paw withdrawal response rates and the increases lasted over 1.5 h (Fig. 1A). For example, 1 μg bicuculline changed response rates from the pre-injection value of 3 ± 2% (mean ± SEM) to 64 ± 7% at 0.5 h after injection, which was also significantly different from that after the vehicle injection (20 ± 6%). Likewise, a single intrathecal injection of the GABAB antagonist, CGP46381 (0.25 or 0.5 μg), dose-dependently increased paw withdrawal response rates, which lasted over 1.5 h (Fig. 1B). For example, 0.5 μg CGP46381 changed response rates from the pre-injection value of 8 ± 3% to 80 ± 7% at 0.5 h after injection, which was also significantly different from that after vehicle injection (19 ± 4%). Therefore, the data show that antagonism of the GABAA or GABAB receptors in the spinal cord resulted in mechanical hyperalgesia in unoperated naïve mice, indicating that decreasing GABAergic inhibitory tone in the spinal cord is important for pain behaviors.
Figure 1. The effects of GABA receptor agonists and antagonists on paw withdrawal responses in mice.
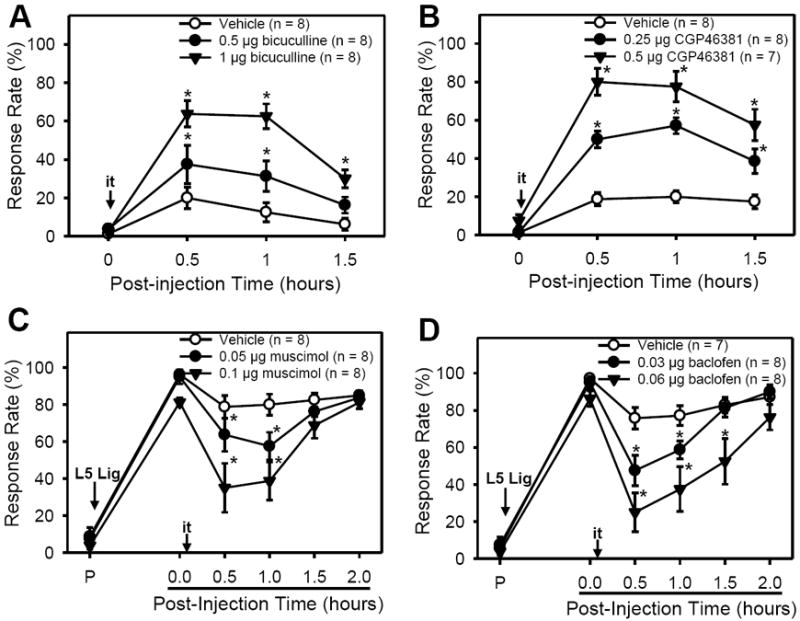
A & B: In normal (naïve) mice, a single i.t. injection of bicuculline (GABAA antagonist, 0.5 or 1 μg [A]) or CGP43681 (GABAB antagonist, 0.25 or 0.5 μg [B]) dose-dependently increased paw withdrawals to von Frey (vF #3.0) stimuli, which lasted up to 1.5 h. Vehicle injection did not affect response rates. C & D: SNL resulted in significantly increased response rates to vF # 3.0 stimuli from pre-surgical levels (P). Four days after surgery, i.t. injection of either muscimol (GABAA agonist, 0.05 or 0.1 μg [C]) or baclofen (GABAB agonist, 0.03 or 0.06 μg [D]) dose-dependently decreased paw withdrawal responses up to 1.5 h. Vehicle treatment did not affect response rates. Data are presented as means ± SEM. P, pre-surgical time; L5 Lig, time of L5 spinal nerve ligation; i.t., intrathecal injection; *, the value significantly (p < 0.05) different from that of the vehicle control by two-way ANOVA with one repeated factor (time) followed by the Holm-Sidak post-hoc test.
3.2. Effects of GABA receptor agonists on pain behavior
Conversely, the GABA action on existing pain conditions was tested by examining the effects of GABA receptor agonists on SNL mice, as shown in Figs. 1C & 1D. Four days after SNL, a single intrathecal injection of a GABAA agonist, muscimol (0.05 or 0.1 μg), reduced response rates in a dose dependent manner. After 0.1 μg muscimol injection, the foot withdrawal responses were reduced from 81 ± 2% to 35 ± 13% at 0.5 h (Fig. 1C). Similarly, a single intrathecal injection of the GABAB agonist, baclofen (0.03 or 0.06 μg), significantly decreased the response rates up to 1.5 - 2 h when compared to vehicle injection. For instance, 0.06 μg baclofen reduced pain behavior from 86 ± 4% to 25 ± 10% at 0.5 h while vehicle reduced response rates from 97 ± 2% to 76 ± 6% at 0.5 h (Fig. 1D). Therefore, supplementation with GABA receptor agonists in the spinal cord transiently alleviates pain behaviors in SNL mice, confirming that a reduced spinal GABA inhibitory function contributes to pain processing in neuropathic animals.
3.3. Effects of PBN treatment on mechanical hyperalgesia in SNL animals
To determine whether a reduction in the spinal GABAergic tone is a consequence of ROS-induced GABA neuron loss in SNL animals, stereological counting of EGFP+ GABA neurons was performed in the L5 spinal cord of four groups of mice seven days following SNL or sham surgery. Those groups include: 1) Sham (n=6), 2) SNL (n=6), 3) SNL + Veh (n=6) and 4) SNL + PBN (n=7). Behavioral testing for mechanical hyperalgesia was performed daily, prior to the first injection of PBN or vehicle. All SNL mice, regardless of treatments, developed significant mechanical hyperalgesia (Fig. 2D), as opposed to the sham-operated mice (n = 6). However, PBN treated mice (SNL+PBN, n = 7) showed partially but significantly reduced nociceptive responses starting from the third day up to one week after surgery, when compared to the vehicle treated mice (SNL+Veh, n = 6) (Fig. 2D). For example, on day 7, the vehicle treated mice exhibited a response rate of 92 ± 2% while response rates of the PBN treated mice were 67 ± 5%. There was no difference in the magnitude and duration of hyperalgesia between SNL + Veh and SNL groups (data not shown).
Figure 2. The effects of SNL and PBN treatment on paw withdrawal responses and EGFP+ GABA neuron numbers.
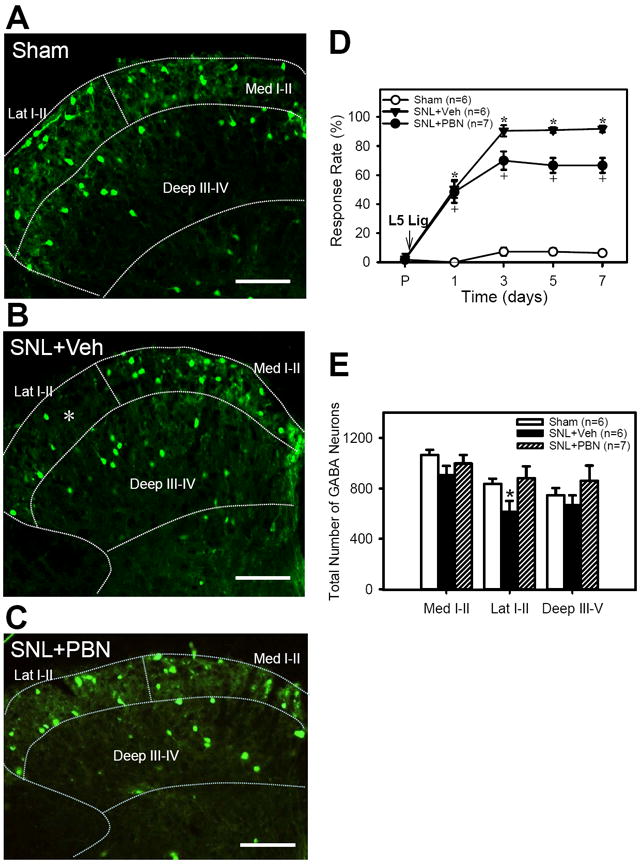
A - C: examples of micrographs of EGFP+ GABA neurons in the ipsilateral L5 spinal dorsal horn one week after sham (A) and SNL surgery with daily vehicle injections of vehicle(B) or PBN (C). The boundaries of medial and lateral laminae I-II (Med I-II and Lat I-II) and deep laminae (Deep III-IV) are outlined with dotted lines. Note that the appearance of EGFP+ GABA neurons were decreased in the lateral half of the superficial dorsal horn after SNL+Veh (indicated by white *) but recovered after SNL+PBN. Scale bar = 100 μm. D: Foot withdrawal response rates to vF stimuli immediately before (P) and on 1, 3, 5 and 7 days after surgery in Sham, SNL+Vehicle, and SNL+PBN mice (tests done just before the first injection of drug on a day). SNL significantly increased the response rates, thus indicating the development of mechanical hyperalgesia. Repetitive PBN treatment (SNL+PBN) partially reduced response rates when compared to vehicle treatment (SNL+Veh). E: The number of EGFP+ GABA neurons for the medial (Med I-II) and lateral halves (Lat I-II) of superficial laminae (I-II) and the deep laminae (Deep III-V) of ipsilateral L5 cord. The EFGP+ GABA neuron numbers were significantly decreased only in the Lat I-II on the ipsilateral side as compared to that of sham. Repetitive PBN treatment (SNL+PBN) reversed the SNL-induced EGFP+ GABA neuron decrease in the Lat I-II. Data are presented as means ± SEM. *, the value is significantly (p < 0.05) different from the sham by ANOVA followed by the Holm-Sidak post-hoc test.
3.4. Effects of PBN treatment on GABA neuron numbers in SNL mice
In sham operated mice, EGFP+ GABA neurons are detected throughout the spinal dorsal horn, with higher concentrations in the lamina II (Fig. 2A). Gross examination of the cord sections revealed a small area of markedly reduced EGFP+ GABA neurons consistently in the lateral part of the lamina II on the side ipsilateral to the SNL surgery (indicated by * in Fig. 2B). However, the EGFP+ neurons reappeared in this vacant spot in the SNL + PBN mice, thus the EGFP+ neuron distribution in the SNL + PBN was similar to the sham tissue (Fig. 2C). The estimated total number of EGFP+ GABA neurons in the L5 spinal dorsal horn was calculated for the ipsilateral and contralateral sides in the three areas of the dorsal horn in the SNL mice, SNL mice treated with either vehicle (SNL + Veh) or PBN (SNL + PBN) and compared to that of the sham operated mice (Table 1, Fig. 2E). When EGFP+ GABA neurons were estimated for the three regions of each L5 spinal dorsal horn, there were significantly less GABA neurons in the lateral laminae I-II on the ipsilateral side of SNL and SNL + Veh groups (641 ± 42 for SNL and 614 ± 36 for SNL + Veh,) as compared to that of sham animals (Sham, 836 ± 42) (Fig. 2E) or to that of the contralateral side (948 ± 42 for SNL and 865 ± 16 for SNL + Veh). Although the numbers of EGFP+ neurons are slightly less in the ipsilateral Deep III-IV of SNL and SNL + Veh groups compared to that of the contralateral side, they are not significantly different. In the sham operated mice, there was no significant difference in the number of EGFP+ neurons between ipsi- and contra- sides in all examined regions. For the Med I-II region, there was no significant difference in the numbers of EGFP+ neurons among all the experimental groups regardless of the ipsi- or contra- lateral sides. In PBN treated SNL mice (SNL + PBN), the number of EGFP+ GABA neurons in the lateral laminae I-II (Lat I-II, 882 ± 35) was not significantly different from that of the sham operated mice (836 ± 42) or the contralateral side (791 ± 52). Thus, repetitive treatment with PBN effectively prevented the loss of EGFP+ GABA neurons. However, this GABA neuron saving provides an explanation for only a small reduction in mechanical hyperalgesia in SNL mice (Fig. 2D).
Table 1. The estimated number of EGFP-GABA neurons in the dorsal horn of the L5 spinal cord.
The estimated number of EGFP+ GABA neurons in the dorsal horn of the L5 spinal cord in four groups of mice one week after the SNL or sham surgery.
| Group | Side | Med I-II | Lat I-II | Deep III-V |
|---|---|---|---|---|
| Sham (n=6) | Ipsi | 1065 ± 41 | 836 ± 42 | 746 ± 57 |
| Contra | 1019 ± 30 | 920 ± 45 | 704 ± 46 | |
| SNL (n=6) | Ipsi | 989 ± 47 | *641 ± 42 | 708 ±61 |
| Contra | 1054 ± 46 | 948 ± 42 | 845 ± 74 | |
| SNL+Veh (n=6) | Ipsi | 904 ± 30 | *614 ± 36 | 668 ± 31 |
| Contra | 953 ± 29 | 865 ± 16 | 720 ± 31 | |
| SNL+PBN (n=7) | Ipsi | 998 ± 25 | 882 ± 35 | 860 ± 46 |
| Contra | 984 ± 26 | 791 ± 52 | 838 ± 52 |
Sham: sham operated, SNL: L5 spinal nerve ligation, SNL+Veh: L5 spinal nerve ligation with repetitive systemic injection of vehicle (saline), SNL+PBN: L5 spinal nerve ligation with repetitive systemic injection of PBN (150 mg/kg, i.p.), a ROS scavenger. Ipsi: ipsilateral side, contra: contralateral side, Med I-II: medial half of laminae I-II, Lat I-II: lateral half of laminae I-II, Deep III-IV: deep laminae III-IV.
3.5. Effects of PBN treatment on GABA neuron excitability in SNL mice
Despite the complete recovery of GABA neuron numbers by repetitive PBN treatments, there was only a partial recovery in pain behaviors (~20%) in the SNL mice. The data suggested that GABA cell loss might have contributed to a small portion of pain behaviors following the SNL. On the other hand, a reduction of GABA release was reported in the superficial laminae of the spinal dorsal horn after either SNL or application of a ROS donor [37]. Thus, it was speculated that the SNL not only produced GABA neuronal cell loss but also the function of surviving GABA neurons may be suppressed due to oxidative stress. This possibility was tested by examining the excitability of naïve and neuropathic (after SNL) GABA neurons with or without a treatment with PBN. Whole cell patch clamp recordings of action potentials were performed on the EGFP+ GABA neurons in spinal cord slices.
Based on action potential discharge patterns induced by a current injection into the cell, GABA neurons are classified as tonic firing, delayed firing, initial bursting, and single spiking types as previously observed in the spinal dorsal horn [2,6,7,9]. In addition, our preliminary study [17] showed that the numbers of action potentials generated by current injection in GABA neurons were decreased by an application of a ROS donor, t-BOOH and then recovered by PBN. These changes were most prominent in GABA neurons with tonic firing patterns. Thus, the GABA neurons with tonic firing patterns were used to test the effect of PBN on GABA excitability following SNL.
Seven out of the eight GABA neurons recorded from normal mice showed tonic firing and one showed delayed firing. On the other hand, the 26 GABA neurons recorded from SNL mice showed tonic (n=14), delayed (n=11), and transient (n=1) firing patterns. Thus those seven units from naïve mice and fourteen units from SNL mice that showed tonic firing patterns were tested for PBN effects. Examples of tonic firing units recorded from naïve and SNL neurons are shown in the top panel of Fig. 3A and 3B (Before), respectively. After PBN treatment, the firing frequency was not changed in the unit recorded from the naïve mouse (Fig. 3A, Naïve, PBN). In contrast, the action potential firing frequency was increased in the unit recorded from a SNL mouse (Fig. 3B, SNL, PBN). When the number of spikes after PBN treatment was compared to that before treatment, none of the seven units recorded from the naïve mice showed >10% increase, but 6/14 units recorded from the SNL mice showed >10% increases (Fig. 3C & 3D). When the average increases were calculated from all recorded units in each group, only the SNL group showed a significant increase in the number of action potential spikes after PBN (Fig. 3E). The results suggest that the excitability of some spinal GABA neurons is suppressed by oxidative stress in SNL mice, and PBN treatment restored the excitability.
Figure 3. Effect of an ROS scavenger, PBN, on the excitability of GABA neurons in the superficial dorsal horn of normal and SNL mice.
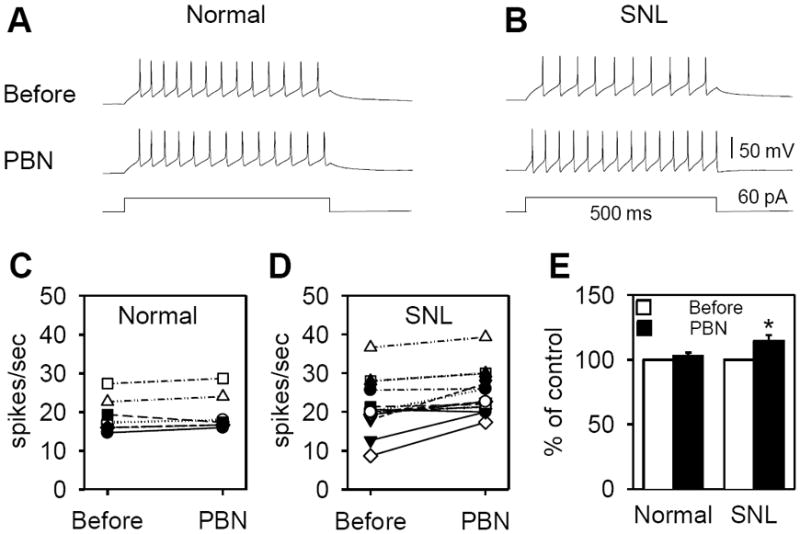
A & B: Representative recordings of tonic firings from 2 GABA neurons, one normal (A) and one SNL (B), during depolarization before and after PBN (1 mM) treatment. Membrane depolarization was induced by a current injection of 60 pA for 500 msec. C, D & E: Changes of action potential numbers (spikes/sec) before and after PBN treatment were plotted individually in normal (C; n=7 neurons) and SNL (D; n=14 neurons) mice. None of 7 units recorded in normal changed their firing rate >10% after PBN but 6/14 units recorded from SNL showed >10% increase. E: When changes of all units were averaged, a significant increase in the number of action potentials after PBN treatment was seen only in the SNL group, but not in the normal group. Data were expressed as percent of control (Before, value before the PBN treatment).
4. Discussion
This study demonstrates that L5 SNL resulted in mechanical hyperalgesia of the hind paw and a significant loss of GABA neurons in the lateral half of laminae I-II in the ipsilateral L5 spinal dorsal horn. In SNL mice, repeated treatments with the antioxidant moderately attenuated pain behaviors and completely prevented the loss of GABA neurons. In addition, many GABA neurons in SNL neuropathic mice increased their depolarization-induced firing rates with an application of PBN as compared to that in naïve mice, suggesting that the excitability of GABA neurons is compromised by oxidative stress in the neuropathic condition.
The results of previous studies, that investigated a possible GABA neuron death contributing to neuropathic pain, have been inconsistent [4,5,11,24,25]. For instance, while some studies found a dramatic loss of GABA-immunoreactivity in the chronic constriction injury model (CCI) [5,11], other studies using either CCI [24] or spared nerve injury model [25] found no significant reduction in the GABA neuron population nor GABA content in GABAergic terminals [26]. While some studies speculate the appearance of apoptotic cells following a nerve injury as an indicator of neuronal death [22], another study concludes that apoptosis is limited to glia rather than neurons [25]. Therefore, controversy remains about a possible contribution of GABA neuron loss for the reduction of GABA inhibitory influence after nerve injury.
The present study avoids previously encountered technical challenges inherent to immunohistochemistry, such as the extent of antibody penetration, reproducibility of the staining, and reduced GABA expression rather than cell death. The transgenic mouse contains fluorescently labeled GABA neurons in the spinal dorsal horn and their physiological properties are well characterized [8,23]. Using these transgenic mice has several advantages. First, EGFP positively identifies GABA neurons without further staining procedures that impose many technical problems for proper identification. Second, EGFP+ neurons can be considered GABA neurons even if they are not actively expressing GABA at the time of examination. Thus, the controversy rooted on the technical problems is much reduced. However, using these transgenic mice impose some other problems. First, not all GABA neurons are EGFP+ in these mice. In fact, only 73% of the EGFP+ neurons in lamina I and 86% of the EGFP+ neurons in lamina II are immunopositive for GABA, thus indicating a small portion of EGFP+ neurons are not expressing enough GABA to be detected by GABA immunostaining [8,23]. Furthermore, only about 1/3 of all spinal dorsal horn GABA immunopositive neurons are found to be EGFP positive [8]. Furthermore, it is possible that the disappearance of EGFP+ neurons is due to a down-regulation of GAD67 production. A similar reduction of GABA neuron numbers is found when GABA neurons are identified by in situ hybridization for GAD67 mRNA after spared nerve injury (SNI) [29].
Considering all the above conditions, our stereological analysis of the number of EGFP+ GABA neurons in the spinal dorsal horn revealed a loss of ~26% of EGFP+ neurons in the lateral laminae I-II after SNL. This represents an approximately 16% reduction of EGFP+ GABA neurons in the entire laminae 1-II of the ipsilateral L5 spinal cord. Since only about 1/3 of the total GABA neuron population is EGFP+, we speculate the total loss of inhibitory GABA neurons would be significant if the loss of other EGFP negative GABA neurons occurred in a similar ratio, while it would be small (~3%), if neuronal loss occurred preferentially to the EGFP+ neurons. Together with the findings of the total recovery of EGFP+ neurons and a partial recovery of pain behaviors after repetitive PBN treatments, our results support that a loss of GABA neurons partially contributes to pain behaviors following a peripheral nerve injury. In addition, the results suggest that this neuron loss is due to oxidative stress.
We still do not know why some studies did not observe any change in GABA neuron population in the spinal dorsal horn following peripheral nerve injury. The possible causes include: the difference in the region where the cell population is analyzed, such as medial vs. lateral part of laminae I-II; and the difference of the technique for identification of GABA neurons, such as genetic tagging of EGFP vs. immunostaining of neurons; and the location of nerve injury, such as at proximally located segmental spinal nerve vs. distally at the tibial and peroneal nerves. The reason that EGFP+ GABA neuron loss is found only in the lateral laminae I-II is not clear although it is expected that most of medio-lateral regions would receive the afferent inputs from the L5 spinal nerve. However, one might speculate that the patterns of peripheral inputs to neurons in the medial and lateral superficial laminae are different in terms of neuronal dendritic organization, afferent input density, and/or differential nociceptive afferent inputs. To get a definitive answer, it seems necessary to explore the detailed somatotopic representation of nociceptive afferent fiber projections to the spinal dorsal horn from the L5 spinal nerve in relation to the dendritic fields of subpopulations of dorsal horn neurons.
Besides the issue of GABA neuron survival, an important question regarding the role of GABA system in pain after nerve injury includes a functional viability of GABA neurons. One study reported that there was no significant change in electrophysiological properties, such as membrane excitability, firing patterns, or synaptic input of the lamina II EGFP positive GABA neurons in CCI mice [28]. However, other studies showed a reduction in the inhibitory postsynaptic currents in lamina II neurons [1,22] and the loss of GABA inhibitory tone for the development of pain [10,19,32]. The frequency of GABA mediated mIPSC is significantly reduced in SNL dorsal horn, suggesting an impairment of GABA release in neuropathic condition and this decrease can be reversed by a ROS scavenger [37]. This reversal of GABA inhibitory function seems to be related to the fact that scavenging ROS effectively alleviates persistent pain behaviors [30] as well as decreases central sensitization [15,18]. Thus, the results suggest that reduced GABA inhibitory function in neuropathic pain is not entirely due to a loss of GABA neurons but partially due to GABA dysfunction. In agreement with this notion, the present study shows that excitability is enhanced after scavenging ROS with PBN in many GABA neurons recorded in SNL mice, suggesting the GABA neuron excitability is reduced in neuropathic condition. Thus, this study suggests the presence of dysfunctional GABA neurons due to oxidative stress in mice with the SNL model. It is, however, possible that different neuropathic pain models may produce pain with different spinal mechanisms.
The results of the present study suggest that repeated treatment with PBN prevents GABA neuronal loss in the spinal cord after a nerve injury. The effect is likely due to the PBN’s antioxidant effect because PBN is a powerful ROS scavenger [13]. However, PBN is known to have actions other than directly scavenging ROS, such as an inhibition of gene induction of inducible nitric oxide synthase which produces nitric oxide (another ROS), and a suppression of the nuclear factor kappa B, which is responsible for mediating inflammatory processes and apoptosis-associated genes [14]. PBN was found to induce over-expression of the anti-apoptotic gene, bcl-2, which was thought to be partly responsible for reducing the number of apoptotic profiles seen in the CCI model [31]. Therefore, it is possible that the effect of the PBN treatment could have been, at least in part, caused by actions other than directly scavenging ROS.
In conclusion, this study demonstrates that ROS may be involved in the loss of the inhibitory tone in the neuropathic pain state since reducing ROS levels can recover to a certain extent the loss of EGFP+ GABA neurons and reduced GABA function seen after SNL. Further studies are needed to clarify whether the disappearance of EGFP labeled neurons is a result of neuronal death or a down-regulation of the synthesis of GAD67 and why EGFP+ neuron loss is limited to the lateral lamina II. One interesting point is that treatment with antioxidant produced only partial reduction in pain while GABA cell reduction was completely prevented. Thus the data indicate that the GABA neuron reduction contributes to only a small portion of the hyperalgesia after nerve injury. Despite the small antihyperalgesic effect of PBN, its prevention of GABA cell loss is a much desirable property, making the antioxidant treatment an attractive therapeutic remedy for neuropathic pain.
Acknowledgments
This work was supported by NIH Grants R01 NS031680 and P01 NS011255.
Footnotes
The authors report no conflicts of interest through financial or other relationships.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–30. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 2.Balasubramanyan S, Stemkowski PL, Stebbing MJ, Smith PA. Sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons. J Neurophysiol. 2006;96:579–90. doi: 10.1152/jn.00087.2006. [DOI] [PubMed] [Google Scholar]
- 3.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993;620:287–91. doi: 10.1016/0006-8993(93)90167-l. [DOI] [PubMed] [Google Scholar]
- 5.Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- 6.Graham BA, Brichta AM, Schofield PR, Callister RJ. Altered potassium channel function in the superficial dorsal horn of the spastic mouse. J Physiol. 2007;584:121–36. doi: 10.1113/jphysiol.2007.138198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinke B, Ruscheweyh R, Forsthuber L, Wunderbaldinger G, Sandkuhler J. Physiological, neurochemical and morphological properties of a subgroup of GABAergic spinal lamina II neurones identified by expression of green fluorescent protein in mice. J Physiol. 2004;560:249–66. doi: 10.1113/jphysiol.2004.070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochman S, Garraway SM, Pockett S. Membrane properties of deep dorsal horn neurons from neonatal rat spinal cord in vitro. Brain Res. 1997;767:214–9. doi: 10.1016/s0006-8993(97)00578-7. [DOI] [PubMed] [Google Scholar]
- 10.Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- 11.Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–58. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- 12.Kontinen VK, Stanfa LC, Basu A, Dickenson AH. Electrophysiologic evidence for increased endogenous gabaergic but not glycinergic inhibitory tone in the rat spinal nerve ligation model of neuropathy. Anesthesiology. 2001;94:333–9. doi: 10.1097/00000542-200102000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Kotake Y. Pharmacologic properties of phenyl N-tert-butylnitrone. Antioxid Redox Signal. 1999;1:481–99. doi: 10.1089/ars.1999.1.4-481. [DOI] [PubMed] [Google Scholar]
- 14.Kotake Y, Sang H, Wallis GL, Stewart CA. Phenyl N-tert-butylnitrone provides protection from endotoxin shock through amplified production of the anti-inflammatory cytokine interleukin-10. Arch Biochem Biophys. 1999;371:129–31. doi: 10.1006/abbi.1999.1417. [DOI] [PubMed] [Google Scholar]
- 15.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee I, Park ES, Kim HK, Wang JG, Chung K, Chung JM. A modified direct lumbar puncture method in rats. Neurosci Abstr. 2006:832.820. [Google Scholar]
- 17.Lee KY, Chung K, Chung JM. ROS are involved in pain mechanisms by a reduction of GABA function in the spinal cord. Neurosci Abstr. 2008:772.2. [Google Scholar]
- 18.Lee KY, Chung K, Chung JM. Involvement of reactive oxygen species in long-term potentiation in the spinal cord dorsal horn. J Neurophysiol. 2010;103:382–91. doi: 10.1152/jn.90906.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malan TP, Mata HP, Porreca F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology. 2002;96:1161–7. doi: 10.1097/00000542-200205000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 21.Minami T, Uda R, Horiguchi S, Ito S, Hyodo M, Hayaishi O. Allodynia evoked by intrathecal administration of prostaglandin E2 to conscious mice. Pain. 1994;57:217–23. doi: 10.1016/0304-3959(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 22.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–31. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliva AA, Jr, Jiang M, Lam T, Smith KL, Swann JW. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J Neurosci. 2000;20:3354–68. doi: 10.1523/JNEUROSCI.20-09-03354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polgar E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–39. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 25.Polgar E, Hughes DI, Arham AZ, Todd AJ. Loss of neurons from laminas I-III of the spinal dorsal horn is not required for development of tactile allodynia in the spared nerve injury model of neuropathic pain. J Neurosci. 2005;25:6658–6666. doi: 10.1523/JNEUROSCI.1490-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polgar E, Todd AJ. Tactile allodynia can occur in the spared nerve injury model in the rat without selective loss of GABA or GABAA receptors from synapses in laminae I-II of the ipsilateral spinal dorsal horn. Neuroscience. 2008;156:193–202. doi: 10.1016/j.neuroscience.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoh O, Omote K. Roles of monoaminergic, glycinergic and GABAergic inhibitory systems in the spinal cord in rats with peripheral mononeuropathy. Brain Res. 1996;728:27–36. [PubMed] [Google Scholar]
- 28.Schoffnegger D, Heinke B, Sommer C, Sandkuhler J. Physiological properties of spinal lamina II GABAergic neurons in mice following peripheral nerve injury. J Physiol. 2006;577:869–78. doi: 10.1113/jphysiol.2006.118034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–23. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138:514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, Rossi F, Roth KA, Maione S, de Novellis V. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2007;55:158–66. doi: 10.1016/j.phrs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–79. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 33.Somers DL, Clemente FR. Dorsal horn synaptosomal content of aspartate, glutamate, glycine and GABA are differentially altered following chronic constriction injury to the rat sciatic nerve. Neurosci Lett. 2002;323:171–4. doi: 10.1016/s0304-3940(02)00157-x. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi A, Mikami M, Yang J. Hydrogen peroxide increases GABAergic mIPSC through presynaptic release of calcium from IP3 receptor-sensitive stores in spinal cord substantia gelatinosa neurons. Eur J Neurosci. 2007;25:705–16. doi: 10.1111/j.1460-9568.2007.05323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Yowtak J, Chung K, Chung JM. Spinal GABA expression in a murine model of spinal nerve ligation-induced neuropathic pain. Neurosci Abstr. 2007:186.1. [Google Scholar]
- 36.Wang J, Yowtak J, Chung K, Chung JM. A ROS scavenger, PBN, prevents spinal GABA neuron death in spinal nerve ligated neuropathic mice. Neurosci Abstr. 2008:467.3. [Google Scholar]
- 37.Yowtak J, Lee KY, Kim HY, Wang J, Kim HK, Chung K, Chung JM. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain. 2011;152:844–52. doi: 10.1016/j.pain.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


