Abstract
Background
Anemia, a common co-morbidity in older adults with heart failure and a preserved ejection fraction (HFPEF), is associated with worse outcomes. We quantified the effect of anemia treatment on left ventricular (LV) structure and function as measured by cardiac magnetic resonance (CMR) imaging.
Methods
Prospective, randomized single blind clinical trial (NCT NCT00286182) comparing the safety and efficacy of epoetin alfa versus placebo for 24 weeks in which a sub-group (n=22) had cardiac MRI at baseline and after 3 and 6 months to evaluate changes in cardiac structure and function. Pressure volume (PV) indices were derived from MRI measures of ventricular volume coupled with sphygmomanometer-measured pressure and Doppler estimates of filling pressure. The end-systolic and end-diastolic PV relations and the area between them as a function of end-diastolic pressure, the isovolumic PV area (PVAiso), were calculated
Results
Subjects (75±10 years, 64% female) with HFPEF (EF=63±15%) with average hemoglobin of 10.3±1.1 gm/dl were treated with epoetin alfa using a dose adjusted algorithm that increased hemoglobin compared to placebo (p<0.0001). As compared to baseline, there were no significant changes in end diastolic (−7±8 vs. −3±8 ml, p=0.81) or end systolic (−0.4±2 vs. −0.7±5 ml, p= 0.96) volumes at 6 month follow-up between epoetin alfa compared with placebo. LV function as measured based on EF (−1.5±1.6% vs.−2.6±3.3%, p= 0.91) and pressure volume indices (PVa-iso-EDP at 30 mm Hg, −5071±4308 vs. −1662±4140 p=0.58) did not differ between epoetin alfa and placebo.
Conclusion
Administration of epoetin alfa to older adult patients with HFPEF resulted in a significant increase in hemoglobin, without evident change in LV structure, function, or pressure volume relationships as measured quantitatively using CMR.
Keywords: Anemia, heart failure, epoeitin alfa, cardiac MRI
Introduction
Anemia is significant co morbidity among the population with heart failure including those with a preserved ejection fraction (HFPEF) (1–6). It is well established that anemia contributes to the overall morbidity among systolic heart failure patients with prevalence ranging from 4–50% (1,3,7). Numerous studies have shown that patients with systolic heart failure and anemia are at increased risk of morbidity, longer hospitalization, increased diuretic requirement, and greater mortality (8–13). Small scale treatment trials have been conducted in the systolic heart failure population with anemia and have shown that subcutaneous erythropoietin increases peak oxygen consumption, increases ejection fraction, reduces hospitalizations, reduce NYHA class, and reduced diuretic requirements (14–18). Meta-analysis suggests clinical benefits in terms of increase in hemoglobin levels, increase in exercise duration, improvement in New York Heart Association functional class, improvement in 6-minute walk test, decrease in B-type natriuretic peptide, and improvement in peak oxygen consumption (19). A large-scale treatment trial (20) is ongoing. The role of this therapy in subjects with HFPEF is not defined.
A growing body of evidence has emerged indicating that non-cardiac conditions are common in subjects with HFPEF such as anemia, obesity, renal insufficiency, and diabetes (7,21). Adverse outcomes of anemia and heart failure with preserved ejection fraction occur consistently across various populations. The prevalence increases with age, advanced New York Heart Association class, and with certain co-morbidities such as renal insufficiency (7). Evidence reveals the relationship between mortality rates and the level of hemoglobin exhibits a J-shaped curve, noting a higher mortality in patients with hemoglobin levels less than 10 grams per deciliter and greater than 16 grams per deciliter (3,5). Anemia alters cardiac structure by mechanisms of compensatory hypertrophy and dilation of left ventricular (LV) chamber size as noted on non-invasive cardiovascular imaging. This remodeling affects the left atrial volume index, left ventricular mass and filling pressure as measured by 2-D echocardiography (22). Additionally, anemia is associated with an augmentation in ventricular work in HFPEF as evidenced by an enhanced relationship between pressure volume area to end diastolic pressure.(23)
Erythropoietin is a hematopoietic growth factor, which stimulates red blood cell synthesis, that has been used for the treatment of anemia and may have potential cardiovascular effects (24). To date, little is known about the impact of erythropoietin on clinical parameters (i.e. ventricular structure/function, functional capacity, symptoms, renal function) in the subset of heart failure patients with a preserved ejection fraction and anemia. In an open label study, short term (3 month) study (25), erythropoietin administration to elderly anemic patients with HFPEF resulted in significant increases in hemoglobin and red cell volume which was associated with reverse remodeling (e.g. smaller end-diastolic volume [EDV] and rightward shift in the end-diastolic pressure volume relation [EDPVR]), improved sub-maximal and maximal exercise tolerance and quality of life. However, in a larger randomized controlled trial the epoetin alfa was not shown to improve functional capacity, quality of life, nor three dimensional echocardiographic measures of LV volume or mass26.
Cardiac magnetic resonance imaging (CMR) is among the most reproducible techniques to assess LV structure and function. Accordingly, the purpose of the current study is to LV structure and function at baseline and after 3 and 6 months post-randomization using CMR imaging on subgroup of randomized subjects in order to determine if therapy with epoetin alfa was associated with changes in comparison to placebo.
Methods
Study Design
The current analysis is a sub-study prospective, randomized, single blind twenty four week study26. Randomization was to either epoetin alfa or placebo and was stratified based on gender and estimated glomerular filtration rate of > or ≤ 40 ml/min/m2. The endpoints of this sub-study were assessment of cardiac structure and function as quantified by CMR.
Study Subjects
The study population including inclusion and exclusion criteria has been described in detail previously26. In brief, subjects were community dwelling, older adult patients with anemia and heart failure with a preserved ejection fraction. Subjects were recruited from outpatient clinics at an urban medical center setting and after acute hospitalization for decompensated heart failure (New York Presbyterian Hospital, New York City.) The diagnosis of heart failure was based on the NHANES CHF Criteria with a score > 327 and were considered to have a preserved ejection fraction if three-dimensional echocardiographically determined ejection fraction was >40%. Anemia was defined as hemoglobin < 12 g/dL.28 Informed consent was obtained in all subjects. Columbia University Medical Center IRB approved the study and the trial was registered at clinical trials.gov (NCT 00286182).
Weekly Monitoring
Subjects were seen each week during which time they underwent an abbreviated physical exam including measurement of vital signs and weight with particular attention to clinical volume status. A vast majority of these examinations were conducted in the participants home, given the frail nature of the study population.29 A venous blood sample was obtained on a weekly basis and evaluated by a point of care system (Hemocue, Angelholm, Sweden) to determine changes in hemoglobin that were used to guide dosing of epoetin alfa.
Study drug administration and dosing
Epogen (Epoetin alpha), (Ortho Biotec, Inc) was administered weekly by subcutaneous injection using a pre-specified dosing algorithm.25 The dosing algorithm was designed to make adjustments based on the rate of rise (ROR) of the hemoglobin over a one week period, as well as the absolute hemoglobin value. Subjects initially received active treatment with 7,500 units of epoetin alfa given weekly by subcutaneous injection. Subjects were carefully monitored (e.g. every week) when beginning therapy to avoid rapid increases in hemoglobin/hematocrit and/or increasing blood pressure control. No dose adjustments were made for the first three doses of erythropoietin (7,500 units/week) unless the hemoglobin rose too rapidly (greater than 0.3 g/dL) in any given weekly interval.
Cardiac MRI
CMR images were acquired using 1.5 Tesla MRI scanners (General Electric, Waukesha, WI) with a dedicated eight-channel phased array surface coil. LV volume and function was assessed using a 2 dimensional breath-held steady-state free precession imaging sequence with short axis images acquired throughout the LV from the level of the mitral valve annulus through the apex. Typical imaging parameters were as follows: repetition time (TR) 3.5 msec, echo time (TE) 1.6 msec, flip angle 60°, temporal resolution 35–40 msec, in-plane spatial resolution 1.9mm × 1.4mm, slice thickness 6.0 mm, inter-slice gap of 4.0 mm.
CMR quantification was performed using manual planimetry. Basal and apical image positions were defined in accordance with established criteria, with the basal LV defined by the basal-most short axis image with at least 50% of circumferential myocardium.30 End-diastole and end-systole were defined based on the respective frames demonstrating the largest and smallest cavity size. Quantification of EDV and end-systolic volume (ESV) was performed using short axis images. Stroke volume (SV) and LV ejection fraction (EF) was calculated based on EDV and ESV (SV= EDV−ESV; EF = [(EDV−ESV)/EDV] × 100%). LV mass was quantified as the volumetric difference between end-diastolic endo- and epicardial chamber volume, multiplied by myocardial specific gravity (1.05 gm/ml). Image acquisition/analysis was performed at a high volume CMR laboratory (Weill Cornell Medical College) by experienced (ACC/AHA level III trained) readers blinded to subject clinical characteristics and treatment assignment.
Estimates of Ventricular Chamber Properties by Non-invasive pressure volume indices
End-systolic pressure volume relation (ESPVR) and EDPVR were estimated in the following manner. The ESPVR, an index of chamber contractility is traditionally measured invasively and defined by a slope, the end-systolic elastance (Ees), and a volume axis intercept, V0. We indexed ventricular contractility by the end-systolic pressure-volume ratio (Res ≡ Pes/ESV)31 where the end systolic pressure Pes ≈ SBP × 0.9.32 Res as a single contractile index, assumes that V0 = 0 ml and simplifies statistical assessment of contractility. Effective arterial elastance (Ea), an index of arterial properties, representable on the pressure-volume plane is defined as Ea≡Pes/SV.32
To characterize the end-diastolic pressure-volume relationship (EDPVR, where EDP=αEDVβ; α is a scaling constant and β is a diastolic stiffness constant), a validated single-beat approach was used.33,34 This approach relies on the empiric observation that volume-normalized EDPVRs share a common shape, thereby allowing estimation of α and β to define the entire EDPVR from a single measured pressure–volume point. Measured EDP (estimated from Doppler echocardiography by previously validated formulas35) and EDV (measured from CMR) were used to derive α and β in each subject. To account for covariance in α and β,36 both of which impact on the shape and position of the EDPVR, the values of these parameters derived from each subject were used to predict the EDV at a common end diastolic pressure (EDP) of 30 mm Hg to yield a pressure-independent index of heart size or ventricular capacitance (EDV30).
The area between the EDPVR and Res measured as a function of EDP was used to index overall pump function (40;41). This specific area is called the isovolumic pressure-volume area (PVAiso), is independent of afterload and can be calculated analytically as a function of LV following curve fitting of the EDPVR and the Res: PVAiso(V)=∫[Pes(V)−Ped(V)]dV = 0.5Res(V−V0)2−Vm(β/α) eα*(V/Vm), where Pes(V) and Ped(V) are the end-systolic and end-diastolic pressures, respectively, as a function of volume.
Statistical analyses
Results are expressed as mean ± standard error unless otherwise noted. Changes in principle measures were compared from baseline to both 3 and 6 month values by a student’s t-test for paired comparisons. The primary endpoint of this sub-study was changes in left ventricular end diastolic volume (LVEDV) after six months of study. Preliminary data indicates that the standard deviation of the LVEDV by MRI was ~55 and 45 ml and the correlation coefficient of repeated measures was high (r=0.95), such that with a calculated standard deviation of the difference of 19 ml, with 8 subjects in each group accounting for dropouts, we would have an 80% power at an alpha of 0.05 to detect a 22 ml difference in LV volumes. Given that the mean LVEDV is ~120 ml in this population, then we had sufficient power to detect a ~20% change over the course of the study. SAS for Windows (Version 8.0, SAS Institute Inc., Cary, North Carolina) was used for all analyses.
Results
The study population was older adult (~77 years of age), predominately female of Hispanic ethnicity, with concomitant hypertension and several other co-morbid conditions, similar to large demographic series of patients with HFPEF. Almost all subjects were taking diuretics and on average two other cardio-active medications including angiotensin converting enzyme inhibitors, angiotensin receptor antagonists, beta blockers and calcium channel blockers. Laboratory testing revealed anemia (as required by the protocol), chronic kidney disease and elevated b-type natriuretic peptides (Table 1). None of the demographic and clinical characteristics differed significantly in subjects randomized to active drug (epoetin alfa) compared to placebo except for use of beta blockers. The subjects in this sub-study did not meaningfully differ from subjects in the full trial26.
Table 1.
Demographic and Clinical Characteristics of Study Subjects
| Parameter | Overall (n=22) |
Epoetin alfa (n=11) |
Placebo (n=11) |
P Value |
|---|---|---|---|---|
| Age (years) | 75±2 | 77±3 | 72±4 | 0.26 |
| Gender (% females) | 14(64%) | 8 (72%) | 6 (55%) | 0.65 |
| Race (W/B/O) | 13/9/0 | 7/4/0 | 6/5/0 | 0.81 |
| Ethnicity (% Hispanic) | 14 (64%) | 7 (64%) | 7 (64%) | 0.67 |
| BSA (m2) | 1.89±0.04 | 1.84±0.07 | 1.94±0.06 | 0.29 |
| BMI | 32±6 | 32±7 | 33±6 | 0.93 |
| Medications (%) | ||||
| Diuretics | 20 (91%) | 10 (91%) | 10 (91%) | 0.99 |
| Beta Blockers | 16 (73%) | 5 (45%) | 11 (100%) | 0.006 |
| ACE/ARB | 15 (68%) | 9 (82%) | 6 (55%) | 0.19 |
| Ca Channel blockers | 11 (50%) | 6 (55%) | 5 (45%) | 0.69 |
| Aldosterone antagonists | 5 (23%) | 3 (27%) | 2 (18%) | 0.63 |
| Systolic Blood Pressure | 147±4 | 150±6 | 144±6 | 0.49 |
| Diastolic Blood Pressure | 66±2 | 64±3 | 68±4 | 0.36 |
| Co-morbid conditions (%) | ||||
| Hypertension | 22 (100%) | 11 (100%) | 11 (100%) | - |
| Diabetes | 16 (72%) | 9 (82%) | 7 (64%) | 0.63 |
| Coronary Artery Disease | 12 (55%) | 6 (55%) | 6 (55%) | 0.99 |
| Obesity | 15 (68%) | 7 (64%) | 8 (73%) | 0.99 |
| COPD | 3 (14%) | 2 (18%) | 1 (9%) | 0.99 |
| Lab Results | ||||
| BNP (pg/ml) | 402±71 | 406±78 | 398±122 | 0.96 |
| Hemoglobin (gm/dl) | 10.3±0.2 | 10.4±0.4 | 10.3±0.2 | 0.81 |
| Creatinine (mg/dl) | 1.7±0.2 | 1.8±0.2 | 1.7±0.3 | 0.83 |
| eGFR (ml/min) | 44±4 | 41±6 | 47±5 | 0.49 |
Values are mean ± S.E.
P values were calculated using chi-squared, Fisher’s exact, or students t-test as appropriate.
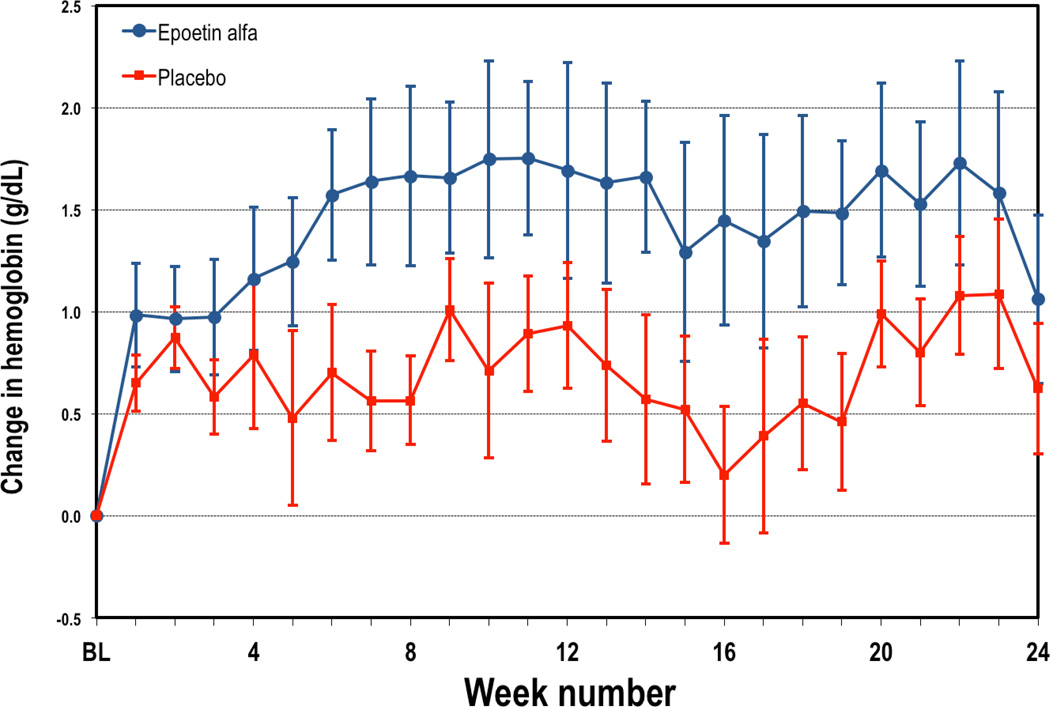
Overall the course of the trial, hemoglobin increased in the group assigned to active therapy in comparison to placebo (See Figure 1). Hemoglobin increases were seen within the first weeks of starting therapy and reached a plateau by the 7–8 week after starting therapy. Overall the course of the trial, subjects on active therapy had an ~1.5 gm/dL increase in hemoglobin while those on placebo has a ~0.7 gm/dL increase in hemoglobin, resulting in an average difference between groups of ~0.8 gm/dL (p<0.0001). The average dose of epoetin alfa in the active therapy group was 4655 units per week.
Figure 1.
Weekly changes in hemoglobin from baseline during study period in subjects randomized to ESA (red) or placebo (blue). Data represents mean +/− standard error.
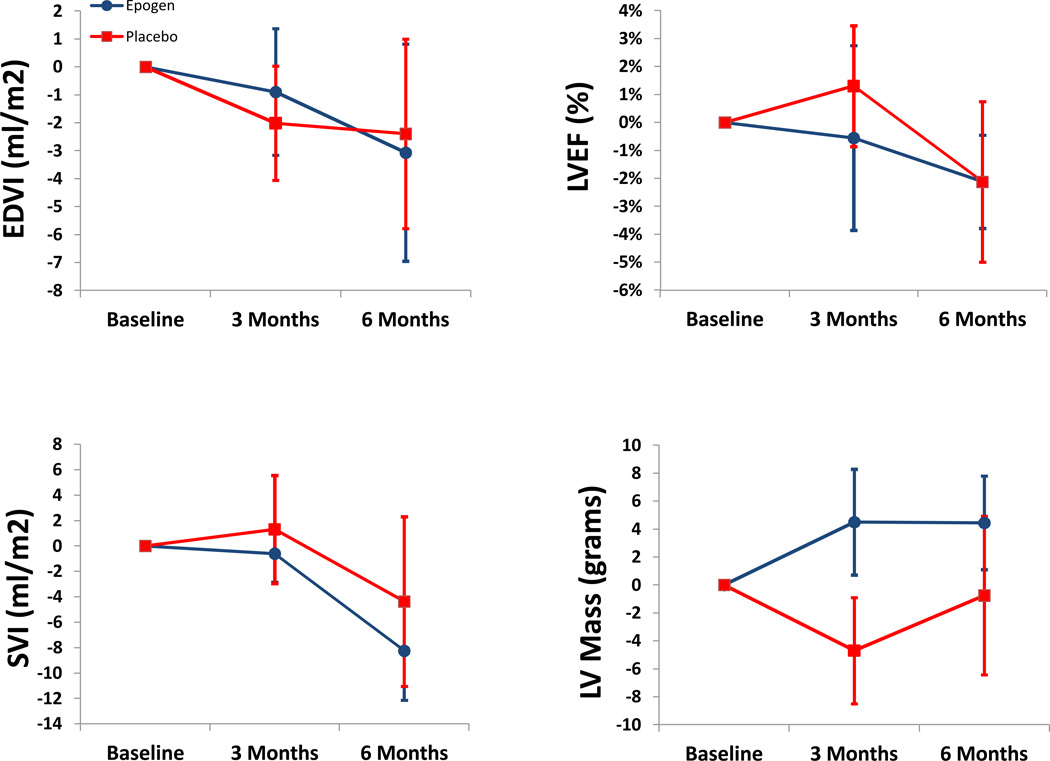
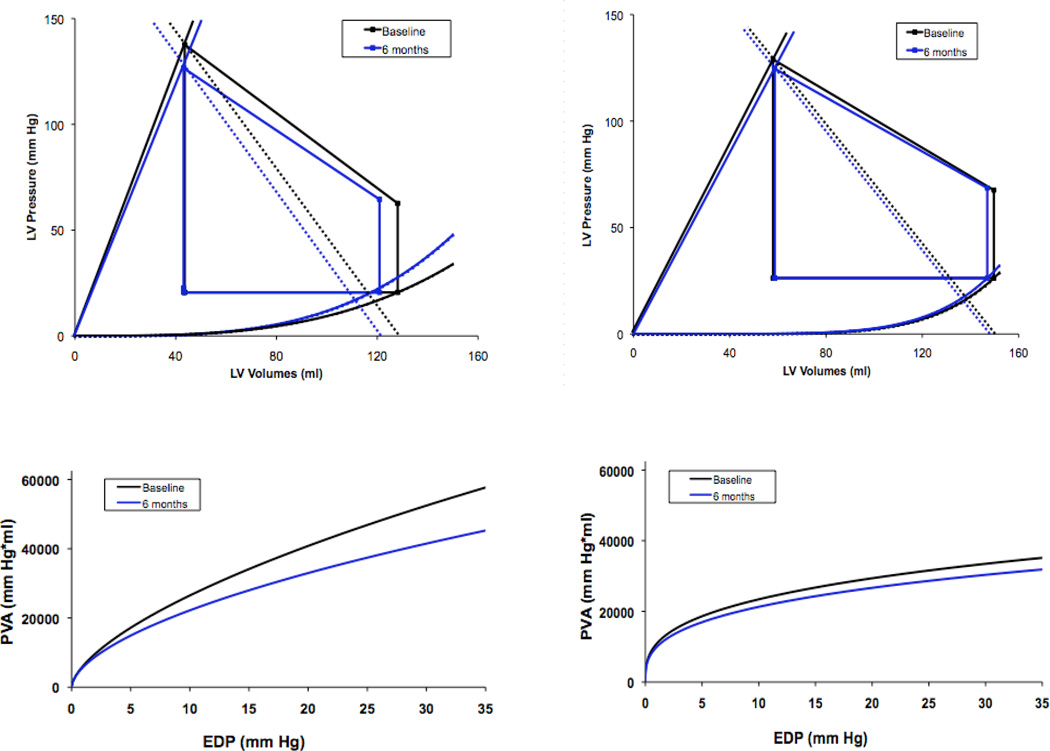
As shown in table 2, there were minimal changes in LV volumes and mass as well as ejection fraction over the course of this 6 month trial. Changes in LV volumes, mass and EF did not differ between subjects randomized to active therapy as compared to placebo (Figure 2). Additionally, PV analysis (Figure 3) did demonstrate a reduction in chamber capacitance after six months in subjects receiving epoetin alfa with concomitant declines in SV but these changes were not statistically significant in comparison to baseline values nor did they differ from controls. There was no difference in the change in pressure volume area to EDP relationship in the placebo group compared to the epoetin alfa group (p=0.58, non-paired t-test). Additionally, the 6 month pressure volume area at an EDP of 30 mmHg was not different from baseline in the placebo group (p=0.7, paired t test) or in the epoetin alfa group (p=0.3, paired t-test).
Table 2.
LV volumes, mass and pressure volume indices with treatment of Epoetin alfa versus placebo.
| Parameter | Baseline | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|
| Placebo (n=11) |
Epoetin Alfa (n=11) |
Placebo (n=10) |
Epoetin Alfa (n=9) |
Placebo (n=8) |
Epoetin Alfa (n=7) |
|
| Cardiac MRI | ||||||
| LV EDV (ml/m2) | 79±6 | 73±8 | 77±6 | 67±5 | 77±6 | 66±4 |
| LV ESV (ml/m2) | 30±4 | 27±6 | 27±3 | 23±4 | 29±3 | 23±4 |
| LV Stroke Volume (ml/m2) | 49±3 | 46±4 | 50±4 | 45±4 | 45±4 | 43±2 |
| LV Mass (grams/m2) | 77±6 | 66±5 | 71±6 | 65±4 | 73±6 | 67±5 |
| LV Ejection Fraction (%) | 64±3 | 66±4 | 65±3 | 70±3 | 61±4 | 71±3 |
| Pressure Volume Indices | ||||||
| V120 (mm Hg/ml) | 50±10 | 35±13 | 52±11 | 37±13 | 52±7 | 22±10 |
| V30 (ml) | 158±12 | 145±20 | 153±13 | 124±15 | 146±10 | 114±12 |
Values are mean ± standard error
Figure 2.
Left ventricular end diastolic volume index (EDVI), ejection fraction (LVEF), stroke volume index (SVI) and cardiac output by cardiac resonance imaging in subjects randomized to ESA (red) or placebo (blue). Data represents mean +/− standard error.
Figure 3.
Discussion
The primary results of the CMR substudy of this prospective, randomized trial with blinded endpoint assessment is that correction of anemia in subjects with HFPEF with epoetin alfa did not result in significant changes in left ventricular structure (volume and mass) nor function; as assessed by ejection fraction and indices of pressure volume analysis including sophisticated measures of chamber function. Minimal changes in stroke volume, left ventricular end diastolic volume and ejection fraction were observed during the course of this six month trial, which were of smaller magnitude than observed in previous studies of epoetin therapy. These differences are likely attributable to several factors including differences in the: 1) population under investigation, 2) degree of anemia and associated chronic renal disease, and 3) erythropoietin dosing regimens used, targets achieved and treatment duration.
Our cohort consists solely of symptomatic HFPEF subjects the majority of whom were females and older than 75 years of age. Previous cross sectional data of the European Survey of Anemia Management indicate that women achieve a ~ 0.25 gm/dL lower hemoglobin than men with erythropoietin stimulating agents (ESAs) and are more likely to be classified as poor responders to epoetin.37 Additionally, studies of ESAs in chronic kidney disease populations have identified older age, higher body mass index, angiotensin converting enzyme inhibitor or angiotensin receptor antagonist use and diabetes as being associated with increased epoetin requirements when normalizing hemoglobin.38,39 Thus, the population characteristics of subjects with HFPEF include many factors that make them particularly susceptible to hypo-responsiveness with ESAs. However, despite these characteristics, we were able to achieve a significant difference in the hemoglobin levels between subjects randomized to epoetin alfa compared to placebo using relatively low doses of epoetin alfa (4655 units per week).
Previous studies of ESA have shown statistical reductions in left ventricular mass and volumes and improvements in ejection fraction both in patient with chronic kidney disease not on dialysis40,41,42–44 and in those on dialysis45,46. Similar results have been shown in patients with systolic heart failure.14,15,47 However, not all trials have demonstrated a clinical effect48. Collectively these trials suggest that the more severe the anemia, greater the LV mass, lower the EF and worse the renal function, the greater the benefit from correction of anemia with ESA therapy. Most of these studies enrolled subjects with more severe anemia than the population studied in this trial with more significant decrements in renal function and higher LV mass. The decrements in LV mass in these previous studies ranged from 6 to 31 grams per m2, typically within 6 months of therapy. Additionally, all of the previous trials used echocardiography rather than CMR imaging, which is considered a non-invasive reference standard for assessment of LV structure and function.49,50 Most previous investigations did not have imaging performed by investigators blinded to the patients’ treatment assignment. These differences may explain the discrepant results in this trial compared to previous investigations.
Regarding regression of LV hypertrophy, a previous study has shown that the normalization of hematocrit was more effective than a partial correction of anemia during therapy with ESAs. These investigators observed more than a doubling of the LV mass reduction observed over a 12 month time period in the normalized hematocrit versus partial correction cohort.44 Additionally, other trials that demonstrated benefit in terms of remodeling used higher doses of ESA41 and demonstrated no difference between epoetin alfa and darbepoetin alfa in their efficacy in this regard.40 However, in patients with CKD in whom anemia was prevented by use of epoetin alfa, maintenance of the hemoglobin above 12.0 g/d, compared with subjects allowed to develop anemia in the range of 9.0 to 10.0 g/dL, did not differ in terms of the effects on LV mass index and did not affect the development or progression of LV hypertrophy.48 Similarly, in our study subjects had relatively mild degrees of anemia (average hemoglobin of 10.3 gm/dL) and achieved final hemoglobins of ~12.0 gm/d, using a dosing algorithm that was conservative and designed to address concerns about the risks of ESAs to achieve higher targets.51,52 While more aggressive dosing protocols could have resulted in greater differences between cohorts studied, this may be associated with adverse outcomes as noted in previous investigations.51,52
The duration of treatment in this trial was similar to a majority of previous studies that demonstrated an effect. However, some previous studies only demonstrated effects after 12 months of therapy and the duration of anemia correction has been shown to be a factor influencing the rate of LV regression; hence improving LV performance and structure. Such data raise the possibility that structural remodeling may be noted after a longer period of time. Indeed, the duration of this trial was intermediate in length, compared to trials performed in this arena (e.g. 6 months) and hemoglobin targets were not achieved until 6–8 weeks after starting therapy, limiting the time in which the effect of reversing anemia could be observed to affect measured cardiac structural and functional parameters. Whether the lack of an effect of treatment on measured outcomes could be a result of the 24 week duration of this study is unknown.
The reported data is a relatively small cohort of a larger clinical trial. However, despite the modest sample size, we had adequate power to detect a 20 ml change in EDV. Whether a smaller change, which could be observed with a larger cohort of subject receiving CMR, would be detected and have clinical significance is unknown. While we did observe statistically significant changes in hemoglobin in subjects randomized to active therapy versus placebo, the observed increase in hemoglobin in the placebo group negated the absolute differences between cohorts.
In conclusion, the administration of subcutaneous erythropoietin in older adult patients with HFPEF and anemia did not demonstrate significant effect on LV structure or function as measured by CMR.
Citations
- 1.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003 Jan 21;107(2):223–225. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 2.Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001 Oct;38(4):955–962. doi: 10.1016/s0735-1097(01)01470-x. [DOI] [PubMed] [Google Scholar]
- 3.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Anemia and heart failure: a community study. Am J Med. 2008 Aug;121(8):726–732. doi: 10.1016/j.amjmed.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Felker GM, Shaw LK, Stough WG, O'Connor CM. Anemia in patients with heart failure and preserved systolic function. Am Heart J. 2006 Feb;151(2):457–462. doi: 10.1016/j.ahj.2005.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Yang J, Ackerson LM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006 Jun 13;113(23):2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 6.He SW, Wang LX. The impact of anemia on the prognosis of chronic heart failure: a meta-analysis and systemic review. Congest Heart Fail. 2009 May-Jun;15(3):123–130. doi: 10.1111/j.1751-7133.2008.00030.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindenfeld J. Prevalence of anemia and effects on mortality in patients with heart failure. Am Heart J. 2005 Mar;149(3):391–401. doi: 10.1016/j.ahj.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 8.Maggioni AP, Opasich C, Anand I, et al. Anemia in patients with heart failure: prevalence and prognostic role in a controlled trial and in clinical practice. J Card Fail. 2005 Mar;11(2):91–98. doi: 10.1016/j.cardfail.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 9.O'Meara E, Murphy C, McMurray JJ. Anemia and heart failure. Curr Heart Fail Rep. 2004 Dec;1(4):176–182. doi: 10.1007/s11897-004-0006-7. [DOI] [PubMed] [Google Scholar]
- 10.Anand I, McMurray JJ, Whitmore J, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004 Jul 13;110(2):149–154. doi: 10.1161/01.CIR.0000134279.79571.73. [DOI] [PubMed] [Google Scholar]
- 11.O'Meara E, Clayton T, McEntegart MB, et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006 Feb 21;113(7):986–994. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 12.Felker GM, Gattis WA, Leimberger JD, et al. Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure. Am J Cardiol. 2003 Sep 1;92(5):625–628. doi: 10.1016/s0002-9149(03)00740-9. [DOI] [PubMed] [Google Scholar]
- 13.Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008 Sep 2;52(10):818–827. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 14.Palazzuoli A, Silverberg D, Iovine F, et al. Erythropoietin improves anemia exercise tolerance and renal function and reduces B-type natriuretic peptide and hospitalization in patients with heart failure and anemia. Am Heart J. 2006 Dec;152(6):1096. doi: 10.1016/j.ahj.2006.08.005. e1099-1015. [DOI] [PubMed] [Google Scholar]
- 15.Palazzuoli A, Silverberg DS, Iovine F, et al. Effects of beta-erythropoietin treatment on left ventricular remodeling, systolic function, and B-type natriuretic peptide levels in patients with the cardiorenal anemia syndrome. Am Heart J. 2007 Oct;154(4):645. doi: 10.1016/j.ahj.2007.07.022. e649-615. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg DS, Wexler D, Blum M, Iaina A. The cardio renal anemia syndrome: correcting anemia in patients with resistant congestive heart failure can improve both cardiac and renal function and reduce hospitalizations. Clin Nephrol. 2003 Jul;60(Suppl 1):S93–S102. [PubMed] [Google Scholar]
- 17.Silverberg DS, Wexler D, Blum M, et al. Effect of correction of anemia with erythropoietin and intravenous iron in resistant heart failure in octogenarians. Isr Med Assoc J. 2003 May;5(5):337–339. [PubMed] [Google Scholar]
- 18.Silverberg DS, Wexler D, Sheps D, et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001 Jun 1;37(7):1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 19.Tehrani F, Dhesi P, Daneshvar D, et al. Erythropoiesis stimulating agents in heart failure patients with anemia: a meta-analysis. Cardiovasc Drugs Ther. 2009 Dec;23(6):511–518. doi: 10.1007/s10557-009-6203-6. [DOI] [PubMed] [Google Scholar]
- 20.McMurray JJ, Anand IS, Diaz R, et al. Design of the Reduction of Events with Darbepoetin alfa in Heart Failure (RED-HF): a Phase III, anaemia correction, morbidity-mortality trial. Eur J Heart Fail. 2009 Aug;11(8):795–801. doi: 10.1093/eurjhf/hfp098. [DOI] [PubMed] [Google Scholar]
- 21.Lang CC, Mancini DM. Non-cardiac comorbidities in chronic heart failure. Heart. 2007 Jun;93(6):665–671. doi: 10.1136/hrt.2005.068296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YR, Pyun WB, Shin GJ. Relation of anemia to echocardiographically estimated left ventricular filling pressure in hypertensive patients over 50 year-old. J Cardiovasc Ultrasound. 2010 Sep;18(3):86–90. doi: 10.4250/jcu.2010.18.3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abramov DHK, Wang J, Burkhoff D, Maurer MS. The Impact of Extra Cardiac Comorbidities on Pressure Volume Relations in Heart Failure and Preserved Ejection Fraction. J Card Fail. 2011 doi: 10.1016/j.cardfail.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Meer P, Voors AA, Lipsic E, van Gilst WH, van Veldhuisen DJ. Erythropoietin in cardiovascular diseases. Eur Heart J. 2004 Feb;25(4):285–291. doi: 10.1016/j.ehj.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 25.Cohen RS, Karlin P, Yushak M, Mancini D, Maurer MS. The effect of erythropoietin on exercise capacity, left ventricular remodeling, pressure-volume relationships, and quality of life in older patients with anemia and heart failure with preserved ejection fraction. Congest Heart Fail. 2010 May;16(3):96–103. doi: 10.1111/j.1751-7133.2009.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurer MS, Teruya S, Chakraborty B, Helmke S, Mancini D. Treating Anemia in Older Adults with Heart Failure with a Preserved Ejection Fraction (HFPEF) with Epoetin Alfa: Single Blind Randomized Clinical Trial of Safety and Efficacy. Circ Heart Fail. 2012 Dec 20; doi: 10.1161/CIRCHEARTFAILURE.112.969717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992 Aug;20(2):301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 28.Parker KP, Mitch WE, Stivelman JC, Macon EJ, Bailey JL, Sands JM. Safety and efficacy of low-dose subcutaneous erythropoietin in hemodialysis patients. J Am Soc Nephrol. 1997 Feb;8(2):288–293. doi: 10.1681/ASN.V82288. [DOI] [PubMed] [Google Scholar]
- 29.Teruya SL, Gil HR, Teresi JA, et al. Facilitating clinical trials of anemia in older adults: a point-of-care system to measure hemoglobin in the home and its agreement with a hospital core laboratory. J Am Geriatr Soc. 2009 Dec;57(12):2362–2364. doi: 10.1111/j.1532-5415.2009.02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papavassiliu T, Kuhl HP, Schroder M, et al. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology. 2005 Jul;236(1):57–64. doi: 10.1148/radiol.2353040601. [DOI] [PubMed] [Google Scholar]
- 31.Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res. 1974 Jul;35(1):117–126. doi: 10.1161/01.res.35.1.117. [DOI] [PubMed] [Google Scholar]
- 32.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992 Aug;86(2):513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 33.Klotz S, Dickstein ML, Burkhoff D. A computational method of prediction of the end-diastolic pressure-volume relationship by single beat. Nat Protoc. 2007;2(9):2152–2158. doi: 10.1038/nprot.2007.270. [DOI] [PubMed] [Google Scholar]
- 34.Klotz S, Hay I, Dickstein ML, et al. Single-beat estimation of end-diastolic pressure-volume relationship: a novel method with potential for noninvasive application. Am J Physiol Heart Circ Physiol. 2006 Jul;291(1):H403–H412. doi: 10.1152/ajpheart.01240.2005. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura RA, Appleton CP, Redfield MM, Ilstrup DM, Holmes DR, Jr, Tajik AJ. Noninvasive doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol. 1996 Nov 1;28(5):1226–1233. doi: 10.1016/S0735-1097(96)00315-4. [DOI] [PubMed] [Google Scholar]
- 36.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol. 2005 Aug;289(2):H501–H512. doi: 10.1152/ajpheart.00138.2005. [DOI] [PubMed] [Google Scholar]
- 37.Richardson D. Clinical factors influencing sensitivity and response to epoetin. Nephrol Dial Transplant. 2002;17(Suppl 1):53–59. doi: 10.1093/ndt/17.suppl_1.53. [DOI] [PubMed] [Google Scholar]
- 38.Rossert J, Gassmann-Mayer C, Frei D, McClellan W. Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrol Dial Transplant. 2007 Mar;22(3):794–800. doi: 10.1093/ndt/gfl716. [DOI] [PubMed] [Google Scholar]
- 39.Biesenbach G, Schmekal B, Eichbauer-Sturm G, Janko O. Erythropoietin requirement in patients with type 2 diabetes mellitus on maintenance hemodialysis therapy. Wien Klin Wochenschr. 2004 Dec 30;116(24):844–848. doi: 10.1007/s00508-004-0286-7. [DOI] [PubMed] [Google Scholar]
- 40.Chen HH, Tarng DC, Lee KF, Wu CY, Chen YC. Epoetin alfa and darbepoetin alfa: effects on ventricular hypertrophy in patients with chronic kidney disease. J Nephrol. 2008 Jul-Aug;21(4):543–549. [PubMed] [Google Scholar]
- 41.Pappas KD, Gouva CD, Katopodis KP, et al. Correction of anemia with erythropoietin in chronic kidney disease (stage 3 or 4): effects on cardiac performance. Cardiovasc Drugs Ther. 2008 Feb;22(1):37–44. doi: 10.1007/s10557-007-6075-6. [DOI] [PubMed] [Google Scholar]
- 42.Portoles J, Torralbo A, Martin P, Rodrigo J, Herrero JA, Barrientos A. Cardiovascular effects of recombinant human erythropoietin in predialysis patients. Am J Kidney Dis. 1997 Apr;29(4):541–548. doi: 10.1016/s0272-6386(97)90335-8. [DOI] [PubMed] [Google Scholar]
- 43.Ayus JC, Go AS, Valderrabano F, et al. Effects of erythropoietin on left ventricular hypertrophy in adults with severe chronic renal failure and hemoglobin <10 g/dL. Kidney Int. 2005 Aug;68(2):788–795. doi: 10.1111/j.1523-1755.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 44.Hayashi T, Suzuki A, Shoji T, et al. Cardiovascular effect of normalizing the hematocrit level during erythropoietin therapy in predialysis patients with chronic renal failure. Am J Kidney Dis. 2000 Feb;35(2):250–256. doi: 10.1016/s0272-6386(00)70334-9. [DOI] [PubMed] [Google Scholar]
- 45.Hampl H, Hennig L, Rosenberger C, et al. Effects of optimized heart failure therapy and anemia correction with epoetin beta on left ventricular mass in hemodialysis patients. Am J Nephrol. 2005 May-Jun;25(3):211–220. doi: 10.1159/000085881. [DOI] [PubMed] [Google Scholar]
- 46.Low I, Grutzmacher P, Bergmann M, Schoeppe W. Echocardiographic findings in patients on maintenance hemodialysis substituted with recombinant human erythropoietin. Clin Nephrol. 1989 Jan;31(1):26–30. [PubMed] [Google Scholar]
- 47.Parissis JT, Kourea K, Panou F, et al. Effects of darbepoetin alpha on right and left ventricular systolic and diastolic function in anemic patients with chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am Heart J. 2008 Apr;155(4):751. doi: 10.1016/j.ahj.2008.01.016. e751-757. [DOI] [PubMed] [Google Scholar]
- 48.Roger SD, McMahon LP, Clarkson A, et al. Effects of early and late intervention with epoetin alpha on left ventricular mass among patients with chronic kidney disease (stage 3 or 4): results of a randomized clinical trial. J Am Soc Nephrol. 2004 Jan;15(1):148–156. doi: 10.1097/01.asn.0000102471.89084.8b. [DOI] [PubMed] [Google Scholar]
- 49.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002 Jul 1;90(1):29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 50.Dewey M, Muller M, Eddicks S, et al. Evaluation of global and regional left ventricular function with 16-slice computed tomography, biplane cineventriculography, and two-dimensional transthoracic echocardiography: comparison with magnetic resonance imaging. J Am Coll Cardiol. 2006 Nov 21;48(10):2034–2044. doi: 10.1016/j.jacc.2006.04.104. [DOI] [PubMed] [Google Scholar]
- 51.Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis-stimulating agents--time for a reevaluation. N Engl J Med. 2010 Jan 21;362(3):189–192. doi: 10.1056/NEJMp0912328. [DOI] [PubMed] [Google Scholar]
- 52.Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010 Sep 16;363(12):1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]