Thanks to my nominators and the International Stroke Conference program committee for selecting me for this year's Feinberg award, which I would like to accept on behalf of all the investigators and coordinators who participated in the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) trial, the NIH Wingspan registry, and the Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial. This award is particularly meaningful to me because Bill Feinberg was a member of the original WASID Planning Committee when he passed away. He was a generous collaborator and a wonderful person who is still greatly missed by those of us who knew him. It is a particular honor to receive an award that is named after him.
Intracranial atherosclerotic stenosis is an important cause of ischemic stroke particularly in Blacks, Asians, and Hispanics 1-4. Given the racial and ethnic make-up of the world population, intracranial stenosis may be the most common cause of stroke world-wide 4-7. It is also associated with a particularly high risk of recurrent stroke compared with most other stroke subtypes 8. I first became interested in this disease during my residency training at Tufts - New England Medical Center with Lou Caplan and the late Mike Pessin, both master clinicians and teachers, who at the time were describing much of the clinical phenomenology and stroke mechanisms associated with atherosclerosis of the major intracranial arteries 9-12. I owe my fascination with stroke neurology to them. It was during my stroke fellowship at the Cleveland Clinic that Tony Furlan and Cathy Sila, both excellent mentors, stimulated my interest in clinical trials. This was the heyday of some of the most important trials in our field (NASCET, ACAS, SPAF, and NINDS tPA) and it was an exciting time to be a stroke fellow.
At that time when we managed patients with intracranial stenosis at the Cleveland Clinic, we typically prescribed warfarin for stroke prevention, a practice that originated from a small study by Clark Millikan and colleagues at the Mayo Clinic in 1955 13. This study preceded the aspirin era of stroke prevention and by 1990, when I started my first faculty position at the University of Michigan, there were still limited data comparing warfarin versus aspirin for this disease. So our group's first study in this field was a retrospective seven center cohort study that suggested warfarin may lower the risk of major vascular events by almost 50% compared with aspirin in patients with angiographically proven 50-99% intracranial arterial stenosis 14. We also surveyed stroke neurologists in the USA and found that they were roughly equally divided between using warfarin or aspirin for intracranial stenosis 15.
These two studies provided the rationale for a randomized trial to compare warfarin versus aspirin for preventing stroke in patients with symptomatic intracranial stenosis. I decided to submit a grant to the National Institute of Neurological Disorders and Stroke (NINDS) to fund this trial, but there was one big problem - I had no experience designing large randomized trials or writing NIH grants! So I turned to some of the most experienced NIH funded clinical trialists for help: Bob Hart, Henry Barnett, Steve Levine and Phil Gorelick. Despite the fact that I was quite junior, they were extremely supportive and generous with their help. Without that help, WASID would never have happened and I am extremely grateful to them. Ten years later, WASID had been completed and it showed that in patients with 50-99% stenosis of a major intracranial artery who had a TIA or stroke within 90 days prior to enrollment that warfarin and aspirin were equally effective for preventing stroke or vascular death 16, or some may argue equally ineffective given the high event rates in both arms.
Some of the most important findings in WASID were related to risk factor management. We had no pre-specified risk factor management protocols in WASID but asked the study neurologists to work with the patients' primary care physicians to target national guideline levels for risk factors 17. While we had some success in controlling low density lipoprotein (LDL), diabetes and smoking in WASID, we had little success in controlling blood pressure 18. Moreover, control of risk factors, particularly blood pressure and LDL, appeared to be very important in determining outcome of WASID patients, as shown in analyses that Seemant Chaturvedi and Tanya Turan led. These analyses suggested that patients with a mean systolic blood pressure of <140 mmHg and mean cholesterol < 200 mg/dl during follow-up had significantly lower rates of stroke alone as well as major vascular events compared with patients who did not achieve these targets 18,19. These data were the basis for the intensive risk factor management protocols used in the subsequent SAMMPRIS trial.
When we were designing WASID, we were well aware of the advances in stent technology and interventional techniques that had been made since Sundt's original paper on angioplasty for basilar artery stenosis 20. So the pre-specified secondary aim in WASID was to identify patients at high risk of stroke in the territory of the stenotic artery who would be the target population for a subsequent trial comparing endovascular therapy with medical treatment that became SAMMPRIS. Scott Kasner led this work, which showed that the most important predictor of recurrent stroke was severity of stenosis. Patients with < 70% stenosis had a stroke in the territory rate of 7-8% at 1 year whereas the rate was 18% at 1 year in patients with ≥ 70% stenosis 8. The risk was even higher in patients with 70-99% stenosis if their qualifying event for WASID had occurred within 30 days of enrollment (22.9% at 1 year). However, the risk was modest in patients with 70-99% stenosis if their qualifying event had occurred more than 30 days before enrollment (9% at 1 year). These data led to the decision to restrict enrollment in SAMMPRIS to patients with 70-99% stenosis whose qualifying event occurred within 30 days prior to enrollment.
Just as WASID ended, the Wingspan stent system was approved by the FDA under a humanitarian device exemption for patients with 50-99% stenosis who are “refractory” to medical management 21 based on a single arm trial of 45 patients performed in Europe and Asia 22. In clinical practice, the “refractory” to medical management indication for use of the Wingspan stent was largely interpreted to mean having a TIA or stroke related to intracranial stenosis while taking any antithrombotic therapy. Two multicenter registries performed in the USA following FDA approval of the Wingspan system (the US Multicenter registry 23 and the NIH Wingspan registry 24) and a single center study in Asia 25 showed high technical success rates (96.7% - 98.8%) using the self-expanding Wingspan stent and possibly lower stroke rates at one year compared with WASID patients with 70-99% stenosis. A randomized trial was needed to determine if stenting lowered the risk of stroke compared with medical therapy in high-risk patients, which brings us to the SAMMPRIS trial.
SAMMPRIS is a randomized trial comparing stenting with the Wingspan system plus aggressive medical management versus aggressive medical management alone in patients with 70-99% intracranial stenosis and a qualifying TIA or stroke within 30 days prior to enrollment 26. With the exception of the peri-procedural period, aggressive medical management is the same in both arms of the trial 27. Clopidogrel (75 mg per day) is combined with aspirin (325 mg per day) for 90 days, followed by aspirin alone beyond 90 days. Management of the primary risk factors (systolic blood pressure and LDL) is implemented by the study neurologists and coordinators using pre-specified protocols to target SBP < 140 mmHg and LDL < 70 mg/dl. In addition, a lifestyle modification program 28 and the study medications are provided to each patient. The primary endpoint in SAMMPRIS is stroke or death within 30 days after enrollment or after a revascularization procedure of the qualifying lesion during follow-up, or stroke in the territory of the qualifying artery beyond 30 days.
Enrollment in SAMMPRIS was stopped in April 2011 and the preliminary results of the trial were published in September 2011 29. Follow-up of enrolled patients is ongoing and will end on April 30, 2013, with final results expected by late summer. The data I am presenting today are from April 2011 since I am blinded to events beyond that time. Management of BP and LDL in SAMMPRIS has been much more successful than it was in WASID, with almost 50% of patients meeting target levels by 30 days after enrollment and continued improvement in control of these risk factors by one year and beyond. Fig 1 shows the projected Kaplan-Meier curves for the primary endpoint for the medical and the stenting arms in the trial. The medical curve was based on the outcomes of patients in WASID who met the entrance criteria for SAMMPRIS, with a downward adjustment in rates to account for what we thought would be a modest benefit from aggressive medical management. The stenting curve was based on our primary hypothesis that stenting would reduce this rate by 35%. Fig 2 shows the observed rates in the trial as of April 2011 indicating that the projected Kaplan-Meier curves were very accurate but we just assigned the wrong treatment to each curve! In fact, it is aggressive medical therapy that is significantly more effective than stenting rather than the other way around. Almost 66% of patients enrolled in SAMMPRIS had their qualifying event on antithrombotic therapy. Figure 3 shows that medical therapy is still significantly more effective than stenting in these patients 30, indicating that stenting is not a rescue treatment for patients with 70-99% stenosis who have a TIA or stroke while taking an antithrombotic agent.
Figure 1.
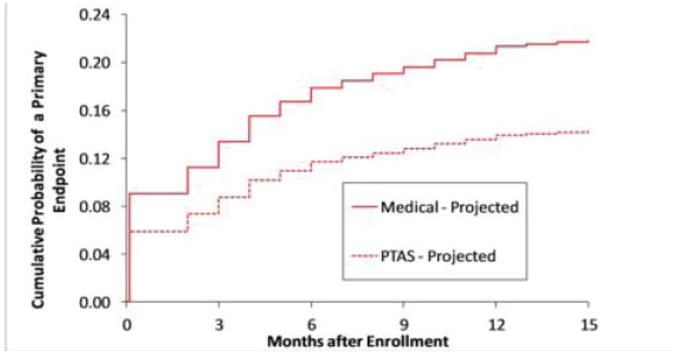
The projected Kaplan-Meier curves for the primary endpoint in the medical and stenting arms in the SAMMPRIS trial.
Figure 2.
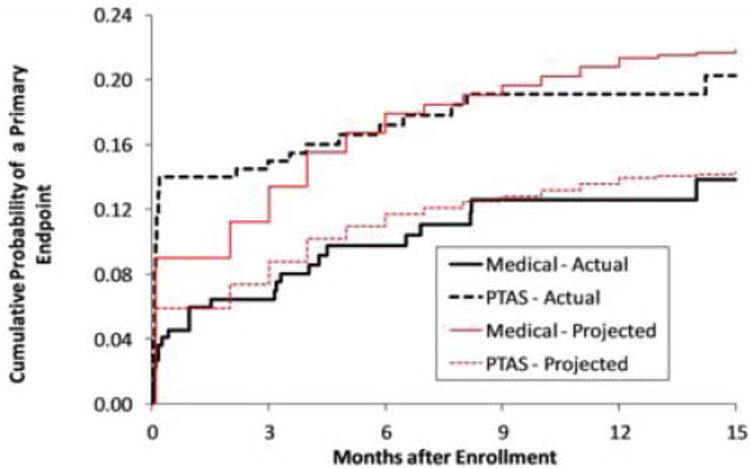
The observed Kaplan-Meier curves for the primary endpoint in the SAMMPRIS trial for the medical and stenting arms superimposed on the projected Kaplan-Meier curves.
Figure 3.
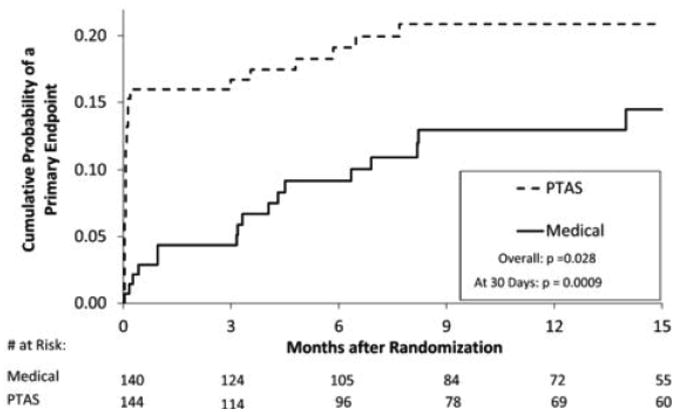
The observed Kaplan-Meier curves for the primary endpoint in the SAMMPRIS trial in patients whose qualifying event for the trial occurred while on antithrombotic therapy
Why was the stroke rate so much higher than projected in the stenting arm in SAMMPRIS? It was driven by the very high peri-procedural (30-day) rate of stroke of 14.7%, which was much higher than the anticipated rate of 6%. This was largely because of the unexpectedly high rates of perforator strokes in proximity to the stent, parenchymal brain hemorrhage, and subarachnoid hemorrhage. Stenting of the basilar artery was particularly high risk for perforator strokes 31. More details on the peri-procedural strokes in SAMMPRIS are provided in analyses led by Dave Fiorella 31 and Colin Derdyen 32, the interventional principal investigators in the trial.
Which components of the medical treatment resulted in the much lower rate of stroke than projected in the medical arm? We can't answer that question definitively yet but we can speculate. Figure 4 shows the projected and observed Kaplan-Meier curves for the medical arm in SAMMPRIS. As indicated previously, the projected curve was based on the outcome of WASID patients who met the SAMMPRIS entrance criteria and were treated with either aspirin or warfarin and usual risk factor management. Recall that clopidogrel was only used for 90 days after enrollment and these curves continue to diverge beyond 3 months, suggesting that intensive risk factor management is playing an important role in lowering the risk of a primary endpoint in SAMMPRIS patients beyond 3 months. Within the first 3 months, the curves diverge the most in the first month when risk factor control in SAMMPRIS had not been optimized yet and one might not expect that risk factor control would have such a rapid impact on lowering the risk of stroke. This suggests that the combination of aspirin and clopidogrel may be playing a key role during this period. This hypothesis is supported by the results of the CLAIR trial led by Lawrence Wong from Hong Kong, which showed that patients with recently symptomatic intracranial stenosis who were treated with aspirin and clopidogrel had fewer microembli detected by transcranial Doppler (TCD) ultrasound at 2 and 7 days after enrollment than patients treated with aspirin alone 33.
Figure 4.
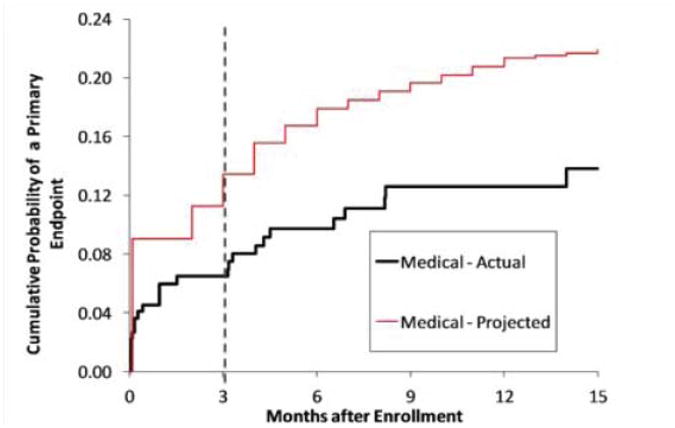
The projected and observed Kaplan-Meier curves for the primary endpoint in the medical arm of SAMMPRIS.
What treatment recommendations emerge from WASID and SAMMPRIS? Patients with < 70% stenosis or patients with a TIA or stroke more than 30 days ago (regardless of the severity of stenosis), who represented about 75% of the patients in WASID, should be treated with aspirin and intensive risk factor management. Their stroke rate on usual risk factor management was in the 3-9% range in WASID and will likely be even lower with intensive risk factor management. For patients with 70-99% stenosis and events within the past 30 days, intensive risk factor management plus a combination of clopidogrel with aspirin for 90 days followed by aspirin alone seems warranted. With this treatment, patients in SAMMPRIS had a primary endpoint rate of 12.2% at 1 year (as of April 2011) 29, suggesting that some patients have a risk of < 12.2% at one year while others have a risk that exceeds 12.2% at 1 yr. We need to determine who the latter patients are, why they are failing aggressive medical therapy, and develop better therapies to improve their outcome.
Studies to identify these patients should evaluate clinical characteristics, biomarkers, and imaging features that predict an increased risk of stroke despite aggressive medical management. SAMMPRIS and other ongoing studies will provide important data in these three areas. Michael Frankel at Emory is leading an NINDS study called BIOSIS 34 that is affiliated with SAMMPRIS to determine whether inflammatory and endothelial cell biomarkers are predictors of stroke in the territory of the stenotic artery in SAMMPRIS patients. He has been assisted by Juan Arenillas from Spain who has done important preliminary work in this area 35. The results of BIOSIS will also likely be available later this year.
Regarding image predictors of stroke, David Liebeskind has done excellent work correlating impaired collaterals on angiography with poor outcome in the WASID patients 36. However, we need non-invasive tests to screen for high-risk patients. In this regard, Sepi Amin-Hanjani is leading an NIH funded study called VERiTAS to correlate impaired flow detected by quantitative magnetic resonance angiography (QMRA) with outcome in patients with vertebro-basilar stenosis 37. Also, Jose Romano and Shyam Prabhakaran have submitted a grant application to NINDS to study QMRA, MR perfusion, and vasomotor reactivity and emboli detection using TCD in patients with intracranial stenosis 38. Another promising technique that David Liebeskind and Ed Feldmann have been working on is using time-of-flight MRA to calculate fractional flow across a stenotic lesion. They will be presenting encouraging results using this technique to predict stroke risk in patients in the WASID and SONIA trials at this meeting 39,40. Several groups in North America, Europe and Asia have been using HR MRI to identify features of intracranial atherosclerotic plaque such as intraplaque hemorrhage, fibrous cap rupture, and lipid core that may predict an increased risk of stroke in patients with intracranial stenosis from a variety of mechanisms, including artery-to-artery embolism, branch artery occlusive disease, or perforator involvement at the site of the stenosis 41-45.
All these studies will help clarify who is at highest risk of recurrent stroke but we need better therapies for these patients. So what's on the horizon? For endovascular therapy to have a role, the rate of peri-procedural stroke will need to be reduced substantially. It is unlikely that this will be accomplished with stenting with currently available devices since enrollment in another intracranial stenosis trial, which is comparing the balloon expandable Pharos intracranial stent with medical management in patients with 70-99% stenosis 46, was also stopped early but the data have not been presented yet. Angioplasty alone has been proposed as an alternative endovascular approach for intracranial stenosis 47-49, which may be safer than stenting for the following reasons:it is a one step procedure (vs. 2 steps with stenting), which could decrease the risk of SAH from distal wire perforation; there is no residual metal left in the artery after angioplasty, which could lower the risk of thrombo-embolism; and angioplasty balloons are lower profile than stent bearing catheters, which might result in less “snow plowing” of the atherosclerotic plaque into perforators with balloon dilation. Although there are promising retrospective data on angioplasty alone for intracranial stenosis 47-49, there are no prospective, multicenter studies of angioplasty alone in a well defined high-risk population of patients with symptomatic intracranial stenosis. Our group is currently planning a multicenter pilot study to evaluate the safety of angioplasty alone.
Is there a neurosurgical option for intracranial stenosis? Extracranial – intracranial (EC-IC) bypass was not effective for intracranial stenosis in the EC-IC bypass study 50 but Nestor Gonzalez and his group at UCLA are evaluating another surgical procedure to deliver flow distal to an intracranial stenosis called encephaloduroarteriosynangiosis or EDAS 51. With EDAS, a network of collaterals forms between the donor artery and the adjacent brain vessels without a surgical anastomosis. We look forward to the results of Nestor's study evaluating this surgical technique.
Regarding non-surgical treatments, Meng et al. recently reported a small randomized trial in which brief repetitive cycles of occluding both brachial arteries with a blood pressure cuff twice daily for 300 days was compared with usual care in patients with symptomatic intracranial stenosis. They found a substantially lower rate of stroke at 300 days in the upper limb ischemic preconditioning group (7.9% vs. 26.7% (p<0.01) 52. The mechanisms of action of remote ischemic preconditioning on the brain are uncertain but could involve improved cerebral perfusion as well as systemic effects such as reduction in oxidative stress 52, 53. The remarkable results in this small study warrant a larger multicenter randomized trial to validate these findings.
Coming full circle back to WASID, the proponents of anticoagulation have not forgotten the data from the original WASID publication suggesting that if the international normalized ratio (INR) could be kept between 2-3, the ischemic stroke and myocardial infarct rates in the warfarin arm were low and the major hemorrhages were limited 16. This raises the possibility that the direct thrombin and Xa inhibitors, now approved for atrial fibrillation 54, might be effective and safe for the treatment of intracranial stenosis and worthy of further study. Lastly, several other therapies are being developed for atherosclerosis, including novel anti-inflammatory therapies 55, dyslipidemic drugs 56, and endothelial homeostasis therapies such as endothelial progenitor cell based treatment 57. It is possible that one or more of these treatments may be worth evaluating in future trials of patients with intracranial stenosis.
In summary, substantial progress has been made in lowering the risk of stroke in patients with atherosclerotic intracranial stenosis over the last 10 years. Aggressive medical management is the treatment of choice and is effective for most patients with intracranial stenosis. A subgroup of patients with intracranial stenosis fails aggressive medical therapy - this subgroup still accounts for a large number of patients in the USA and worldwide. Future research should focus on detecting clinical and imaging features as well as biomarkers that identify high-risk patients who would be the target group to enroll in new clinical trials testing novel therapies that will hopefully lower their risk of stroke.
In closing, I would like to thank NINDS for supporting my research over the years. This support goes well beyond providing funding - Scott Janis has been a valued member of the SAMMPRIS Executive Committee, and Walter Koroshetz, Claudia Moy, Peter Gilbert, and Petra Kaufmann have all assisted in other important ways. A special thanks to my main research partner for the past 18 years, Mike Lynn at Emory who has done a magnificent job helping with study design and overseeing all aspects of data management and statistical analyses for WASID, the NIH Registry and SAMMPRIS. Additionally, I'd like to thank the leadership of SAMMPRIS and WASID who are too numerous to name individually (see list in on-line appendix). They deserve much of the credit for this research, as do the hardworking investigators and coordinators at the participating sites. And finally, a very special thanks to my wife and colleague Tanya Turan, my daughters Justine and Hannah, and my parents for their love, support and the sacrifices they have made for my career. Thank you for your attention.
Acknowledgments
Source of Funding: This research has been funded through the following grants to the author from NIH / NINDS for support of the WASID Trial (R01 NS36643), the NIH Wingspan Registry (R01 NS051688), the SAMMPRIS Trial (U01 NS058728), and a K24 grant (K24 NS050307).
Industry Support: Corporate support for this research has consisted of Bayer providing aspirin and placebo aspirin for the WASID trial, Bristol-Myers Squibb supplying warfarin and placebo warfarin for the WASID trial, Stryker Neurovascular (formerly Boston Scientific Neurovascular) providing study devices and supplemental funding for third party device distribution, site monitoring and study auditing in the SAMMPRIS trial, and the Investigator-Sponsored Study Program of AstraZeneca that donated rosuvastatin (Crestor) to study patients in SAMMPRIS.
Footnotes
SAMMPRIS Executive Committee: Colin Derdeyn, David Fiorella, Mike Lynn, Tanya Turan, Scott Janis, Bethany Lane, Jean Montgomery, Janice Malloy, Mary Evelyn Armstrong
SAMMPRIS Steering Committee: Helmi Lutsep, Stan Barnwell, Michael Walters, Brian Hoh, Maurice Hourihane, Elad Levy, Andrei Alexandrov, Mark Harrigan, David Chiu, Richard Klucznik, Joni Clark, Cameron McDougall, Mark Johnson, Lee Pride, Michel Torbey, Osama Zaidat, Harry Cloft
WASID Steering Committee: Barney Stern, Vicki Hertzberg, Harriet Howlett-Smith, Michael Frankel, Steven Levine, Seemant Chaturvedi, Scott Kasner, Curt Benesch, Cathy Sila, Tudor Jovin, Jose Romano
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke. 2011;42(1 Suppl):S20–3. doi: 10.1161/STROKEAHA.110.597278. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26(1):14–20. doi: 10.1161/01.str.26.1.14. [DOI] [PubMed] [Google Scholar]
- 3.Wityk R, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974–1980. doi: 10.1161/01.str.27.11.1974. [DOI] [PubMed] [Google Scholar]
- 4.Wong LK. Global burden of intracranial atherosclerosis. International Journal of Stroke. 2006;1:158–9. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 5.Kumar G, Kalita J, Kumar B, Bansal V, Jain SK, Misra U. Magnetic resonance angiography findings in patients with ischemic stroke from. North India J Stroke Cerebrovasc Dis. 2010;19:146–52. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Moustafa RR, Moneim AA, Salem HH, Shalash AS, Azmy HA. Intracranial steno-occlusive arterial disease and its associations in Egyptian ischemic stroke patients. Stroke. 2013;44:538–41. doi: 10.1161/STROKEAHA.112.679050. [DOI] [PubMed] [Google Scholar]
- 7.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–9. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 8.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 9.Caplan LR. “Top of the basilar” syndrome. Neurology. 1980;30:72–9. doi: 10.1212/wnl.30.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989;39:1246–50. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- 11.Pessin MS, Gorelick PB, Kwan ES, Caplan LR. Basilar artery stenosis: middle and distal segments. Neurology. 1987;37:1742–6. doi: 10.1212/wnl.37.11.1742. [DOI] [PubMed] [Google Scholar]
- 12.Pessin MS, Kwan ES, DeWitt LD, Hedges TR, 3rd, Gale D, Caplan LR. Posterior cerebral artery stenosis. Ann Neurol. 1987;21:85–9. doi: 10.1002/ana.410210115. [DOI] [PubMed] [Google Scholar]
- 13.Millikan CH, Siekert RG, Shick RM. Studies in cerebrovascular disease. III. The use of anticoagulant drugs in the treatment of insufficiency or thrombosis within the basilar arterial system. Proceedings of the staff meetings Mayo Clinic; 1955; pp. 116–26. [PubMed] [Google Scholar]
- 14.Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488–93. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- 15.Chimowitz MI, Williams J, Stern BJ, Cotsonis G, Swanson S, Lynn M, et al. Physician preferences for diagnosis and treatment of symptomatic intracranial arterial stenosis. Neurology. 2004;(Suppl 5):A266–A267. Abstract. [Google Scholar]
- 16.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. NEJM. 2005;352:1305–16. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 17.The Warfarin Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Design, progress and challenges of a double blind trial of warfarin versus aspirin for symptomatic intracranial arterial stenosis. Neuroepidemiology. 2003;22:106–117. doi: 10.1159/000068744. [DOI] [PubMed] [Google Scholar]
- 18.Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, et al. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063–8. doi: 10.1212/01.wnl.0000279338.18776.26. [DOI] [PubMed] [Google Scholar]
- 19.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007;115:2969–75. doi: 10.1161/CIRCULATIONAHA.106.622464. [DOI] [PubMed] [Google Scholar]
- 20.Sundt TM, Jr, Smith HC, Campbell JK, Vlietstra RE, Cucchiara RF, Stanson AW. Transluminal angioplasty for basilar artery stenosis. Mayo Clin Proc. 1980;55:673–80. [PubMed] [Google Scholar]
- 21.http://www.fda.gov/ohrms/dockets/dockets/05m0308/05m-0308-aav0001-Summary-of-Safety-vol1.pdf
- 22.Bose A, Hartmann M, Henkes H, Liu HM, Teng MM, Szikora I, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38:1531–7. doi: 10.1161/STROKEAHA.106.477711. [DOI] [PubMed] [Google Scholar]
- 23.Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38:881–7. doi: 10.1161/01.STR.0000257963.65728.e8. [DOI] [PubMed] [Google Scholar]
- 24.Zaidat OO, Klucznik R, Alexander MJ, Chaloupka J, Lutsep H, Barnwell S, et al. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. 2008;70:1518–24. doi: 10.1212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang WJ, Yu W, Du B, Gao F, Cui LY. Outcome of patients with ≥70% symptomatic intracranial stenosis after Wingspan stenting. Stroke. 2011;42:1971–5. doi: 10.1161/STROKEAHA.110.595926. [DOI] [PubMed] [Google Scholar]
- 26.Chimowitz MI, Lynn MJ, Turan TN, Fiorella D, Lane BF, Janis S, et al. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. J Stroke Cerebrovasc Dis. 2011;20:357–68. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turan TN, Lynn MJ, Nizam A, Lane B, Egan BM, Le NA, et al. Rationale, design, and implementation of aggressive risk factor management in the Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. Circ Cardiovasc Qual Outcomes. 2012;5:e51–60. doi: 10.1161/CIRCOUTCOMES.112.966911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon NF, Salmon RD, Franklin BA, et al. Effectiveness of therapeutic lifestyle changes in patients with hypertension, hyperlipidemia, and/or hyperglycemia. Am J Cardiol. 2004;94:1558–61. doi: 10.1016/j.amjcard.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. NEJM. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lutsep HL, Barnwell SL, Larsen DT, Lynn MJ, Turan TN, Lane BF, et al. Outcome of patients in the SAMMPRIS Trial who had failed antithrombotic therapy at study enrollment. Stroke. 2012;43:LB5. Abstract. [Google Scholar]
- 31.Fiorella D, Derdeyn CP, Lynn MJ, Barnwell SL, Hoh BL, Levy EI, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Stroke. 2012;43:2682–8. doi: 10.1161/STROKEAHA.112.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derdeyn CP, Fiorella D, Lynn MJ, Barnwell SL, Zaidat OO, Meyers P, et al. Impact of operator and site experience on outcomes after angioplasty and stenting in the SAMMPRIS Trial. Journal of Neurointerventional Surgery. 2012 doi: 10.1136/neurintsurg-2012-010504. Epub Sept 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The CLAIR Study Investigators. The effectiveness of dual antiplatelet treatment in acute ischemic stroke patients with intracranial arterial stenosis: a subgroup analysis of CLAIR study. Int J Stroke. 2012 Aug 7; doi: 10.1111/j.1747-4949.2012.00828.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.BIOSIS Trial. http://www.researchgrantdatabase.com/g/5R01NS064162-02/BIOMARKERS-OF-ISCHEMIC-OUTCOMES-IN-SYMPTOMATIC-INTRACRANIAL-STENOSIS--BIOSIS-/
- 35.Arenillas JF, Alvarez-Sabín J, Molina CA, et al. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. 2008;39:1456–63. doi: 10.1161/STROKEAHA.107.498600. [DOI] [PubMed] [Google Scholar]
- 36.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Annals of Neurology. 2011;69:963–74. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amin-Hanjani S, Rose-Finnell L, Richardson D, Ruland S, Pandey D, Thulborn KR, et al. Vertebrobasilar Flow Evaluation and Risk of Transient Ischaemic Attack and Stroke study (VERiTAS): rationale and design. Int J Stroke. 2010;5:499–505. doi: 10.1111/j.1747-4949.2010.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prabhakaran S, Romano JG. Current diagnosis and management of symptomatic intracranial atherosclerotic disease. Curr Opin Neurol. 2012;25:18–26. doi: 10.1097/WCO.0b013e32834ec16b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liebeskind DS, Kosinski AS, Lynn MJ, et al. Noninvasive fractional flow on MRA predicts stroke risk of intracranial stenosis in SONIA/WASID. Stroke. 2013;44:ATP166. doi: 10.1111/jon.12101. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68:2099–106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 41.Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. 2013;44:287–92. doi: 10.1161/STROKEAHA.112.664680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–34. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 43.Klein IF, Lavallée PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke. 2010;41:1405–9. doi: 10.1161/STROKEAHA.110.583534. [DOI] [PubMed] [Google Scholar]
- 44.Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. 2010;212:507–11. doi: 10.1016/j.atherosclerosis.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 45.Park JK, Kim SH, Kim BS, Choi G, Jeong SY, Choi JC. Imaging of intracranial plaques with black-blood double inversion recovery MR imaging and CT. Journal of Neuroimaging. 2011;21:e64–8. doi: 10.1111/j.1552-6569.2010.00499.x. [DOI] [PubMed] [Google Scholar]
- 46.Zaidat OO, Castonguay AC, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, et al. Design of the Vitesse Intracranial Stent Study for Ischemic Therapy (VISSIT) Trial in Symptomatic Intracranial Stenosis. Journal of Stroke and Cerebrovascular Diseases. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.10.021. Epub 2012/12/25. [DOI] [PubMed] [Google Scholar]
- 47.Connors JJ, 3rd, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. Journal of Neurosurgery. 1999;91:415–23. doi: 10.3171/jns.1999.91.3.0415. [DOI] [PubMed] [Google Scholar]
- 48.Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006;37:1016–20. doi: 10.1161/01.STR.0000206142.03677.c2. [DOI] [PubMed] [Google Scholar]
- 49.Dumont TM, Kan P, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI. Revisiting angioplasty without stenting for symptomatic intracranial atherosclerotic stenosis after the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) study. Neurosurgery. 2012;71:1103–10. doi: 10.1227/NEU.0b013e318271bcb8. [DOI] [PubMed] [Google Scholar]
- 50.The EC/IC Bypass Study Group. Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. NEJM. 1985;313:1191–200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 51.Dusick JR, Liebeskind DS, Saver JL, Martin NA, Gonzalez NR. Indirect revascularization for nonmoyamoya intracranial arterial stenoses: clinical and angiographic outcomes. J Neurosurg. 2012;117:94–102. doi: 10.3171/2012.4.JNS111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–61. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 53.Hu S, Dong H, Zhang H, Wang S, Hou L, Chen S, et al. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 2012;1459:81–90. doi: 10.1016/j.brainres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 54.Contractor T, Levin V, Martinez MW, Marchlinski FE. Novel oral anticoagulants for stroke prevention in patients with atrial fibrillation: dawn of a new era. Postgrad Med. 2013;125:34–44. doi: 10.3810/pgm.2013.01.2622. [DOI] [PubMed] [Google Scholar]
- 55.van der Valk FM, van Wijk DF, Stroes ES. Novel anti-inflammatory strategies in atherosclerosis. Curr Opin Lipidol. 2012;23:532–9. doi: 10.1097/MOL.0b013e3283587543. [DOI] [PubMed] [Google Scholar]
- 56.Wierzbicki AS, Hardman TC, Viljoen A. New lipid-lowering drugs: an update. Int J Clin Pract. 2012;66:270–80. doi: 10.1111/j.1742-1241.2011.02867.x. [DOI] [PubMed] [Google Scholar]
- 57.Du F, Zhou J, Gong R, Huang X, Pansuria M, Virtue A, et al. Endothelial progenitor cells in atherosclerosis. Front Biosci. 2012;17:2327–49. doi: 10.2741/4055. [DOI] [PMC free article] [PubMed] [Google Scholar]


