Abstract
Scope
Hyperglycemia-induced vascular inflammation resulting in the adhesion of monocytes to endothelium is a key event in the pathogenesis of atherosclerosis in diabetes. We investigated whether epigallocatechin gallate (EGCG), a major catechin found in green tea, reduces vascular inflammation in diabetes.
Methods and results
Human aortic endothelial cells (HAEC) were pre-treated with green tea catechins before the addition of high glucose (25 mM) for 72 h. EGCG at physiologically achievable concentration (1 µM) significantly inhibited high glucose-induced adhesion of monocytes to HAEC both in static and under flow conditions. EGCG also reduced NFκB-regulated transcriptional activity in ECs. Six-week-old diabetic db/db mice were fed a diet containing 0% or 0.1% EGCG for 8 wk. ECs were isolated from aortic vessels of db/db, db/db-EGCG, and control db/+ mice. EGCG supplementation greatly suppressed diabetes-increased monocytes adhesion to ECs, which is associated with reduced circulating levels of chemokines, and reduced secretions of chemokines and adhesion molecules by aortic ECs from db/db-EGCG mice. EGCG treatment reduced nuclear translocation of NFκB p65 in aortic vessels, decreased blood pressure and serum concentrations of cholesterol and triglycerides in db/db-EGCG mice.
Conclusion
EGCG may have a direct protective effect against vascular inflammation in diabetes.
Keywords: epigallocatechin gallate, vascular inflammation, diabetes, green tea, endothelial cells
1. Introduction
Diabetes is a major risk factor for cardiovascular disease such as atherosclerosis, which accounts for the largest number of all deaths among American diabetic patients [1]. Inflammation-triggered endothelial activation is one of the key early events in the pathogenesis of atherosclerosis which leads to the adhesion of monocytes to the endothelium followed by their transmigration into the subendothelial space [2, 3]. In diabetes, hyperglycemia- induced vascular inflammation and the subsequent endothelial dysfunction play a pivotal role in the development of atherosclerosis [1]. Indeed, enhanced monocyte-endothelial interactions are reported in diabetic animal models [4], diabetic patients [5], and in endothelial cells (ECs) exposed to high glucose in vitro [4]. High glucose can trigger several intracellular signaling events that ultimately up-regulate the expression of a number of pro-inflammatory chemokines such as interleukin-8 (IL-8) and monocyte chemotactic protein-1 (MCP-1), and adhesion molecules such as vascular adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1(ICAM-1) and endothelial-leukocyte adhesion molecule-1 (E-selectin). The chemokines and adhesion molecules then induce monocyte adhesion to ECs, and subsequent transendothelial migration in the vessels [3, 4, 6, 7]. Nuclear factor κB (NFκB) plays a major role in governing the vascular inflammatory process by directly up-regulating these chemokines and adhesion molecules [8]. The most abundant form of NFκB is a p50/p65 heterodimer in which p65 contains the transcriptional activation domain. NFκB activation may arise from the increased nuclear translocation of the p65 subunit and high glucose was shown to increase nuclear levels of p65 [9]. Thus attenuation of hyperglycemia-induced NFκB activation could be a novel molecular target for the treatment or prevention of diabetic vascular inflammation.
Epigallocatechin gallate (EGCG) is a polyphenolic compound abundant in green tea and a number of studies reported the vasculoprotective effects of EGCG [10, 11]. Animal studies showed that EGCG improves endothelial function and reduces blood pressure in hypertensive rats [10]. A human study demonstrated that EGCG can reverse endothelial dysfunction and improve brachial artery flow-mediated dilation in patients with coronary artery disease [12]. The vascular beneficial effects of green tea and EGCG are often explained by their presumably antioxidative and hypolipidemic effects although emerging evidence shows that catechins may exert vascular effects through other mechanisms [13]. Moreover, most of the reported studies used EGCG at doses that are far beyond the physiologically achievable levels (0.6 –1.8 µM) in both humans and animals through dietary ingestion [13, 14]. Therefore, the biological relevance of these findings is largely unclear. Further, studies on the preventive effect of green tea or EGCG in diabetic vascular inflammation are very limited. In the present study, we investigated the role of EGCG at physiologically relevant concentrations in the prevention of high glucose-induced monocyte-EC interaction ex vivo and further examined the effect of dietary intake of EGCG on diabetes-caused vascular inflammation in vivo.
2. Materials and methods
2.1. Materials
Primary human aortic endothelial cells (HAEC), bovine aortic endothelial cells (BAEC), endothelial growth supplements EGM2, and DMEM medium were purchased from Lonza; THP-1 monocytes were purchased from ATCC and WEHI78/24 mouse monocytes were provided by Dr. Judith A Berliner (UCLA). Fetal bovine serum (FBS), other cell culture supplements, transfection reagent lipofectamine, calcein-AM, and M199 and Opti-Mem media were from Invitrogen; protein assay kits were from Bio-Rad; NFκB promoter linked to firefly luciferase reporter gene was from Clontech; dual luciferase reporter assay system was from Promega; EGCG (for animal study) was from Taiyo International. ELISA kits for mouse MCP-1/JE, KC, VCAM-1, and ICAM-1 were from R&D Systems; fluorescent probe -3,3’-dioctadecyloxacarbocyanine perchlorate labeled acetylated low density lipoprotein (DiO-Ac-LDL) from Biomedical Technologies Inc; dispase was from BD Biosciences; nuclear extraction kit was from Epigentek; NFκB p65 and β-actin antibodies were from Santa Cruz; chemiluminescence reagent was from Thermo Scientific; protease and phosphatase inhibitor cocktails, epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and other general chemicals were from Sigma. Stock solution of 20 mM EGCG in dimethylsulfoxide (DMSO) was stored at −80°C before use.
2.2. Cell culture
HAEC were cultured in M199 medium containing 2% FBS and endothelial growth supplements EGM2 at 37°C in a 5% CO2/95% air environment and passages 4–6 were used in all experiments. THP-1 cells were cultured in RPMI-1640 medium containing 10% FBS. WEHI78/24 cells, a mouse monocyte cell line, were cultured in DMEM medium containing 10% FBS.
2.3. Monocyte Adhesion assay
The determination of monocyte adhesion to ECs was conducted using THP-1 cells as described previously [15] with few modifications. In brief, HAEC were grown to confluence in 96-well plates and treated with EGCG or other structurally related catechins such as EC, ECG, EGC for 1 h before addition of high glucose (25 mM) for 72 h. Calcein-AM labeled THP-1 cells were then added to HAEC and incubated for 1 h and the monolayer was gently washed with PBS to remove the unbound monocytes. The adhered monocytes were determined by measuring the fluorescence using a FLX800 multi-detection microplate reader (Bio-Tek Instruments, Inc) at excitation and emission wavelengths of 496 and 520 nm, respectively. Parallel experiments were conducted with 20 mM mannitol as osmotic control.
2.4. Monocyte adhesion assay under flow conditions
An automated microfluidic system, Bioflux 200 (Fluxion Biosciences, CA). was used to monitor live EC-monocyte adhesion under flow condition, which simulates physiological conditions by precisely controlling shear flow. HAEC monolayers were grown on fibronectin-coated microfluidic channels that were integrated into a 24-well plate. The cells were incubated with normal (5.5 mM) or high glucose (25 mM) for 72 h. To determine the EGCG effect, cells were pre-perfused with 1 µmol/L EGCG for 1 h at 1 dyn/cm2 before addition of high glucose (25 mM). 72 h later, THP-1 cells were then perfused into channels for 5 min at 3 dyn/cm2, followed by 5 min at 1.5 dyn/cm2. Images (20 fields per channel) were captured using a Nikon microscope under flow and the adhered immune cells were enumerated and the adhesion was calculated as number of cells/ field.
2.5. NFκB transcriptional activity assay
BAEC were used for the transfection study, because it is difficult to yield adequate and consistent transfection efficiency for this construct in HAEC. BAEC plated in 24-well plate were co-transfected with 2 µg of NFκB promoter-luciferase vector and pRL reporter control plasmid using Lipofectamine transfection reagent. 24 h after transfection, BAEC were treated with EGCG for 1 h before addition of high glucose (25 mM) for 72 h. Treated cells were then washed with PBS and lysed in reporter lysis reagent. Luciferase activity, normalized to pRL activity in the cell extracts, was determined by using the dual luciferase assay system.
2.6. Mice
Five-wk-old male diabetic (db/db; B6.Cg-m+/+Leprdb) mice were obtained from Jackson Laboratory. This is a widely used type 2 diabetic animal model which spontaneously develops metabolic features, including vascular complications similar to those observed in humans with diabetes. Age matched db/+ mice were used as the controls. Mice were housed in micro-isolator cages in a pathogen-free facility. After one- wk acclimation, the diabetic mice were divided into two groups db/db and db/db-EGCG with 20 mice per group and fed AIN-93G rodent diet (Dyet, Inc.) or this basal diet containing 1g EGCG/kg diet. Based on allometric scaling, this EGCG dose is equivalent to consumption of 2–3 cups of green tea (containing 2 g tea leaves/cup) for an average person requiring 8374 kJ (2000 kcal)/day [16]. After 8 wk of treatment, the mice were killed after feed-deprived for 12 h. Blood samples were collected into chilled tubes and centrifuged and serum was frozen at −80°C for the analysis. All experimental protocols (08-053-HNFE), were approved by the Institutional Animal Care and Use Committee at Virginia Tech.
2.7. Measurements of blood pressure, body composition, and serum lipids
At the end of treatment period, blood pressure was determined using the Kent CODA 2 blood pressure system [17]. Body composition was determined by using LF90 Minispec Time Domain Nuclear Magnetic Resonance Spectrometer (Bruker Optics Inc., MA). Serum total cholesterol, HDL-cholesterol and triglycerides levels were measured using PTS CardioChek blood analysis meters (Maria Stein Inc., OH). Serum levels of JE/MCP-1, KC, ICAM-1, VCAM-1 were measured by using ELISA kits according to the manufacturer’s instructions.
2.8. Isolation of mouse aortic endothelial cells
Mouse aortic endothelial cells (MAEC) from control db/+ and db/db mice were harvested under sterile conditions as previously described [4]. Briefly, the aorta was excised and cleansed of periadventitial fat. The aorta was cut into rings and the aortic ring pieces were placed onto Matrigel. The pieces were then incubated in DMEM containing 1% penicillin-streptomycin, 15% FBS, 180 µg/ml heparin, and 20 µg/ml endothelial cell growth supplement [4, 18]. The aortic explants were removed once cell outgrowth was observed and ECs were allowed to grow until they reaches confluence. The cells were then passaged using dispase and cultured for 2 days in DMEM medium containing D-valine to eliminate fibroblast contamination. ECs were then cultured in DMEM medium without D-valine until confluence and these cells exhibit a cobblestone-like morphology with contact-inhibited growth at confluence [19]. The purity of ECs was verified by DiO-Ac-LDL uptake. The passages 2–3 were used for experiments.
2.9. Mouse monocyte adhesion assay
For adhesion assay, MAEC were cultured to confluence in 96-well plates. Calcein-AM labeled WEHI 78/24 cells were then added to MAEC and cell adhesion was determined as describe above. As a positive control, MAEC were incubated with 10 µg/L tumor necrosis factor α (TNFα) for 6 h before adhesion assay.
2.10. Measurements of chemokines and adhesion molecules
JE/MCP-1, KC, and soluble forms of ICAM-1 (sICAM-1) and VCAM-1 (sVCAM-1) in the serum and cell culture supernatants were measured by ELISA kits according the manufacturer’s instructions.
2.11. Analysis of NFκB activation in mouse aortic vessels
Total and nuclear NFκB p65 in mouse aortic vessels was analyzed by using Western blot. The nuclei were extracted from the aortas using Nuclear Extraction Kit and nuclear proteins were subjected to immunoblot and membranes were probed with antibody against NFκB p65. Immunoreactive proteins were detected using chemiluminescence reagent and the band densities were determined using Genetools software (Syngene). Proteins extracted from aortic vessels were used to measure total p65. NFκB p65 expression levels were normalized to β-actin contents in the same sample.
2.12. Statistics
The data were derived from at least three independent experiments for ex vivo experiments and six mice in each group for animal studies. All data were analyzed with one-way ANOVA using SPSS/10 software. Values are expressed as the mean ± SEM. Treatment differences were subjected to Tukey’s multiple comparison tests, where P < 0.05 was considered significantly different.
3. Results
3.1. EGCG inhibits high glucose-induced adhesion of monocytes to ECs and this effect of EGCG is structure-specific
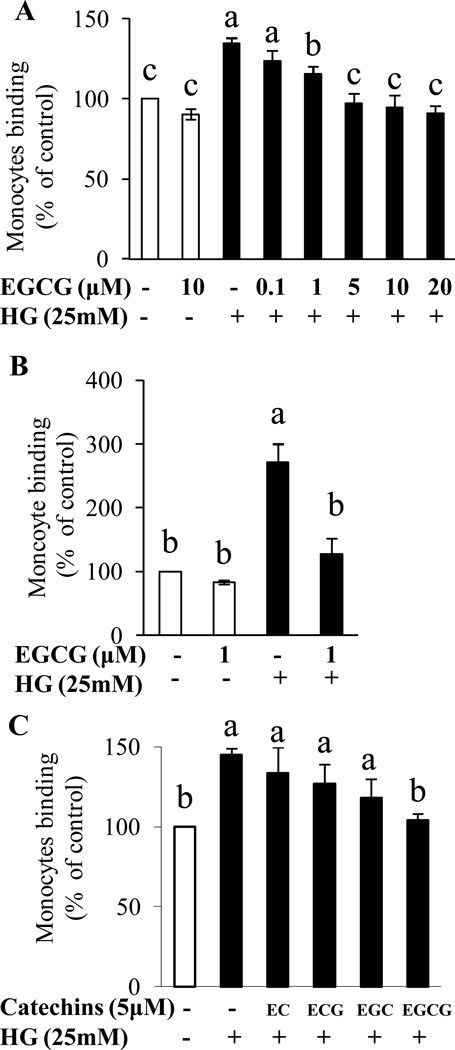
Exposure of HAEC to high glucose significantly stimulated adhesion of monocytes to ECs compared with control, both in static condition and under flow. Pretreatment with EGCG as low as 1 µM concentration suppressed high glucose-induced adhesion of monocytes to HAEC in static condition (Fig. 1A). As ECs under flow may have different functional characteristics from cells cultured in static condition, we further determined whether high glucose induces monocyte adhesion to ECs under flow condition using a shear flow imaging system, which creates an environment simulating physiological flow conditions in the vessels. High glucose induced 3-fold increase in EC-monocyte interaction as compared to control. Consistent with the results of static assay, 1 µM EGCG significantly inhibited high glucose-induced EC-monocyte interaction under flow (Fig. 1B and Supporting Information Figure S1). While EGCG suppressed EC inflammation induced by high glucose, other structurally related catechins including EC, EGC and ECG failed to exert such an effect (Fig. 1C), suggesting the unique effect of EGCG on vascular inflammation that is not shared by other structurally related catechin compounds. D-Mannitol, which was used as osmotic control, didn’t alter monocyte adhesion to HAEC (data not shown).
Figure 1.
HAEC were pre-treated with catechins for 1 h before the addition of high glucose (25 mM) for 72 h and cell adhesion was measured using fluorescent labeled THP-1 monocytes. (A) EGCG inhibited high glucose-induced monocyte adhesion to ECs in static condition. Values are mean ± SEM, n=4 (means of quadruplicates). (B) EGCG inhibited high glucose stimulated adhesion of monocytes to ECs under shear flow control. Values are mean ± SEM, n=3 (means of 20 fields). Please also see supplemental online material for the images. (C) The effect of EGCG on high glucose-induced monocyte adhesion to ECs is structure-specific. Values are mean ± SEM, n=3 (means of quadruplicates). EC, epicatechin; ECG, epicatechin gallate; EGC, epigallocatechin; EGCG, epigallocatechin gallate; and HG, high glucose. Means without a common letter differ, P < 0.05.
3.2. EGCG suppresses high glucose-induced NFκB activity in ECs
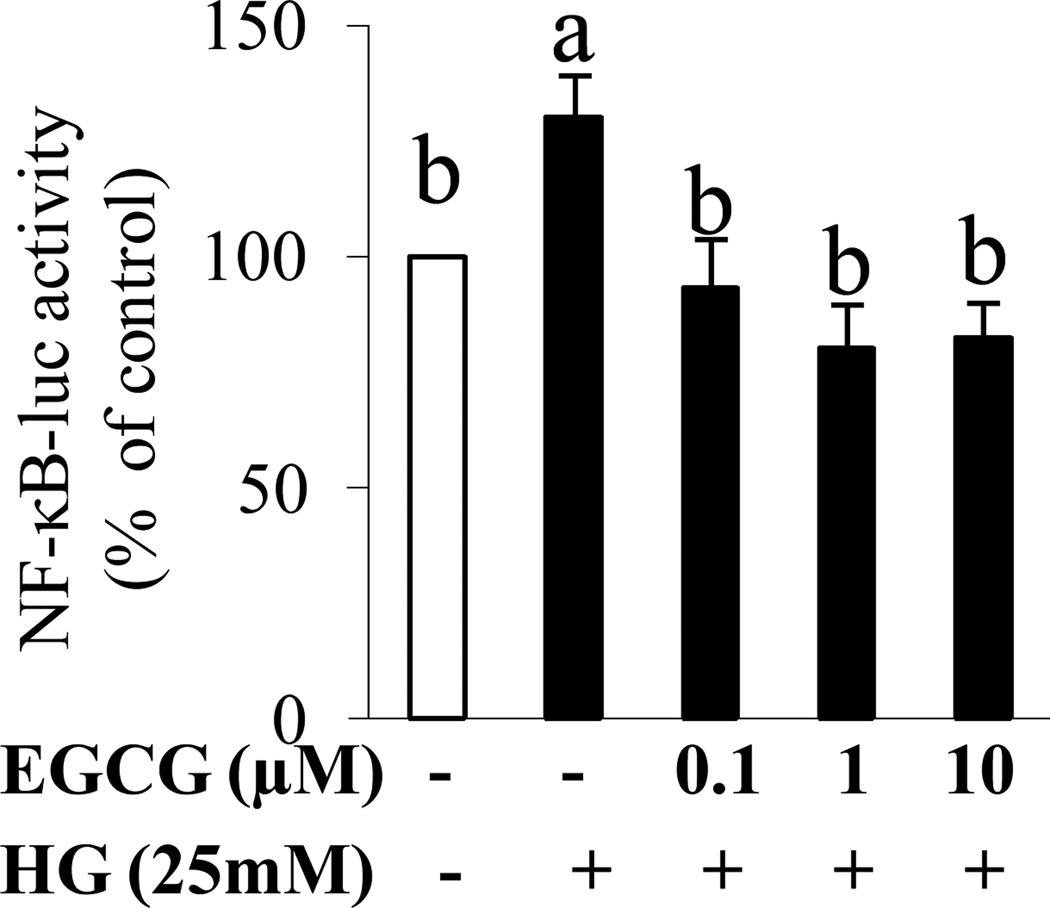
Monocyte adhesion to endothelium is mediated through chemokines and adhesion molecules that are critically up-regulated by NFκB and hence we determined NFκB transcriptional activity. Exposure of BAEC to high glucose potently increased luciferase activity, indicating the induction of NFκB-regulated gene expression (Fig. 2). However, co-incubation with EGCG normalized high-glucose-induced NFκB activation, suggesting that EGCG may inhibit inflammation through suppressing NFκB activity (Fig. 2).
Figure 2.
BAEC were co-transfected with NFκB promoter-luciferase vector and pRL reporter control plasmid. 24 h after transfection, BAEC were treated with EGCG for 1 h before addition of high glucose (25 mM) for 72 h. Luciferase activity, normalized to pRL activity in the cell extracts, was determined. EGCG inhibited high glucose induced NFκB-regulated gene transcription in BAEC. Values are mean ± SEM, n=3 (means of duplicates). EGCG, epigallocatechin gallate; HG, high glucose. Means without a common letter differ, P < 0.05.
3.3. Effects of dietary intake of EGCG on metabolic variables
EGCG treatment to db/db mice has no effect on food intake, body composition, blood glucose, and HDL-cholesterol levels, but it reduced animal body weight. Serum concentrations of total cholesterol and triglyceride were significantly greater in db/db mice than those in control mice. Dietary intake of EGCG suppressed total cholesterol and triglyceride in db/db mice (Supporting Information Table S1). Both systolic and diastolic blood pressures were significantly higher in db/db mice than those in the controls. However, intake of EGCG normalized systolic and diastolic blood pressures in db/db mice (Supporting Information Table S1).
3.4. Dietary supplementation of EGCG suppresses vascular inflammation in db/db mice
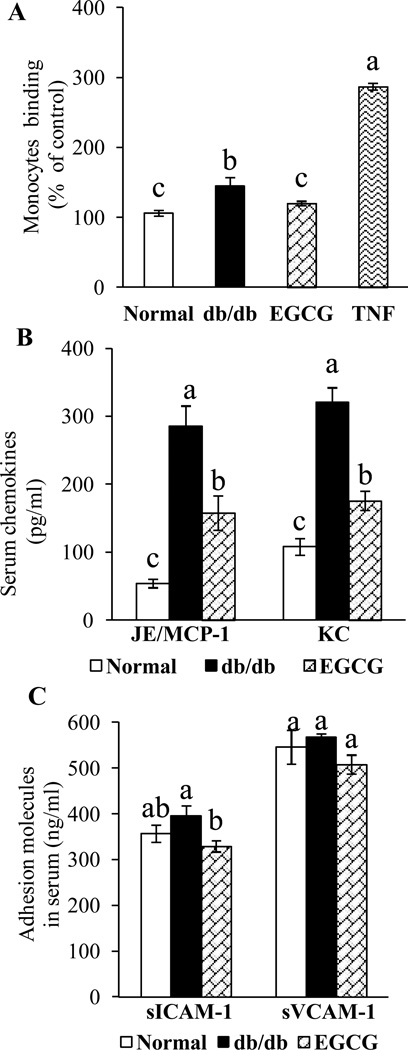
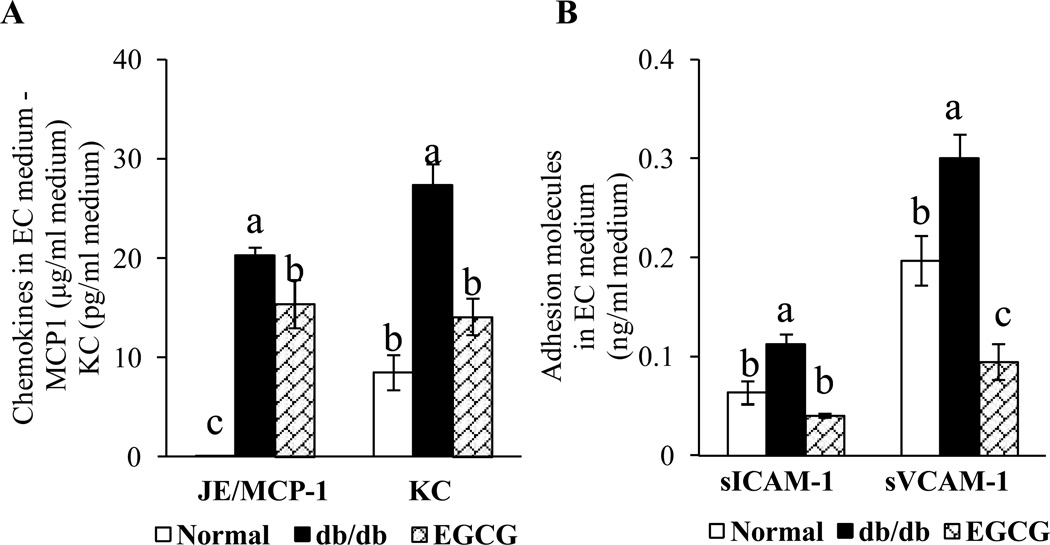
We further assessed whether EGCG has the potential to prevent vascular inflammation in diabetes in vivo. Mouse monocyte WEHI 78/24 cells had significantly higher binding to ECs isolated from diabetic db/db mice than those from control mice (Fig. 3A), indicating that the vessels in diabetic mice are activated and inflammatory [4]. However, dietary supplementation of EGCG reversed this adverse effect (Fig. 3A). MCP-1 and IL-8 are essential for firm adhesion of monocyte to ECs and subsequent transmigration into vascular tissue. The serum concentrations of MCP-1/JE and KC, the mouse homologues of human MCP-1 and IL-8, respectively, were significantly greater in db/db mice than those in control mice (Fig. 3B), similar to those observed in diabetic patients with vascular diseases [20]. Dietary ingestion of EGCG greatly suppressed the diabetes-induced increase in circulating concentrations of MCP-1/JE and KC in db/db mice (Fig. 3B). While the concentrations of serum sICAM-1 and sVCAM-1 were non-significantly increased in db/db mice, EGCG treatment significantly reduced the levels of sICAM-1 and non-significantly reduced sVCAM-1 in db/db mice (Fig. 3C). The concentrations of MCP-1/JE, KC, VCAM-1, and ICAM-1 were significantly higher in culture medium of ECs isolated from db/db mice than those in control mice. Dietary ingestion of EGCG greatly suppressed the amounts of MCP-1/JE, KC, sVCAM-1 and sICAM-1in the culture medium of ECs isolated from EGCG-treated db/db mice (Fig. 4A & 4B).
Figure 3.
EGCG treatment reduced (A) adhesion of monocytes to ECs, (B) serum levels of MCP-1/JE and KC, and (C) serum levels of soluble adhesion molecules sICAM-1 and cVCAM-1in diabetic db/db mice. Values are mean ± SEM, n=6 (means of quadruplicates) for A and n=8 (means of duplicate) for B and C. Means without a common letter differ, P < 0.05.
Figure 4.
Dietary EGCG reduced the secretion of (A) chemokines and (B) adhesion molecules from aortic ECs of diabetic db/db mice. Values are mean ± SEM, n=6 (means of duplicate).
Means without a common letter differ, P < 0.05.
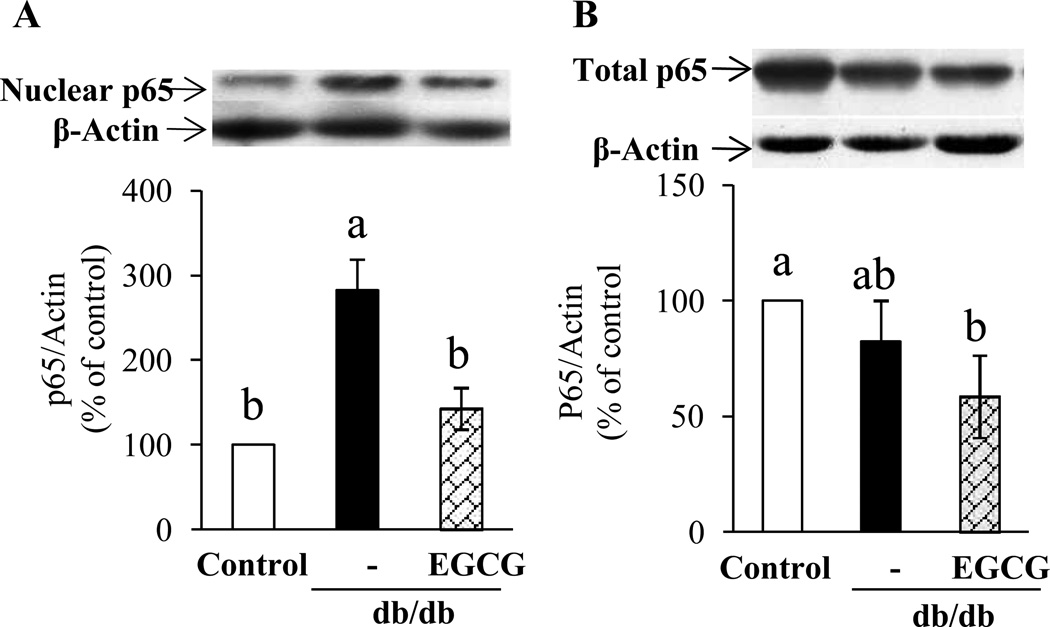
3.5. EGCG treatment reduces NFκB nuclear translocation in aorta of db/db mice
NFκB p65 nuclear translocation was significantly greater in the aorta of db/db mice than that of control mice, which was normalized by dietary supplementation of EGCG (Fig. 5A). However, no significant changes were detected in total NFκB p65 protein expression (Fig. 5B).
Figure 5.
(A) p65 nuclear translocation increased in aorta of db/db mice and EGCG supplementation greatly suppressed this translocation in db/db mice. (B) Total NFκB p65 protein expression in arotic vessels of the mice. Values are mean ± SE, n=5 (means of duplicate).
Means without a common letter differ, P < 0.05.
4. Discussion
The main finding of the present study is that EGCG at a concentration (1 µM) achievable in humans consuming green tea beverage can suppress high glucose-triggered EC inflammation ex vivo and reduce vascular inflammation in diabetic db/db mice. Hyperglycemia plays a significant role in atherogenesis and epidemiological studies have shown that diabetes is an independent risk factor for atherosclerosis-associated morbidity [1]. Endothelial activation and increased monocyte-EC interaction are the key early events in triggering vascular inflammation in diabetes [3]. Monocytes that firmly adhese to vessel wall transmigrate cross endothelial barrier. In subendothelial space, monocytes then transfom into macrophages and lipid-rich foam cells, which ultimately lead to the development of atherosclerotic lesions [21]. Indeed, monocytes are the primary inflammatory cell type that has been localized to human atherosclerotic plaques [4] and atherosclerosis fail to develop in models where monocytes are depleted [21]. Thus, protection of monocytes from adhesion to endothelium could be an useful strategy for the prevention of atherosclerosis. Previous studies demonstrate the vasculoprotective effect of green tea and EGCG [11, 13] but the underlying mechanism remains elusive and the protective role of green tea in diabetic vascular inflammation is unknown. In the present study, our findings suggest that EGCG may be a naturally occurring low-cost agent for prevention of diabetes-caused vascular inflammation and atherosclerosis.
In our study, exposure of ECs to high glucose significantly increased the adhesion of monocytes to ECs and EGCG (1 µM) suppressed this high glucose-induced inflammation both in static condition and under flow. The mean plasma EGCG level can reach 1 µM in humans drinking green tea [22], suggesting that this finding may have an important physiological relevance. While the exact structural features of EGCG responsible for this action is unknown, this unique effect of EGCG at physiological concentration could be due to the presence of the galloyl moiety and hydroxyl group at the 3' position on its molecule. Indeed, it has been reported that these structural features of EGCG play a major role in its anti-inflammatory properties [23]. Unless stimulated, ECs do not adhere to monocytes under normal conditions. Therefore, the significant increase in monocyte adhesion to db/db ECs suggest that ECs of diabetic mice are pre-activated to interact with monocytes [4]. However, dietary supplementation of EGCG normalized diabetes-induced binding of monocytes to aortic ECs. Given aformentioned role of increased interaction of monocytes-ECs in the pathogenesis of atherosclerosis, this may provide an explanation for a previous study showing that dietary intake of EGCG can reduce atherosclerotic lesions in ApoE-knockout mice [24].
Chemokines such as MCP-1 and IL-8 are implicated in inflammatory responses and the pathogenesis of atherosclerosis. They are essential for monocyte rolling, firm adhesion to ECs and their subsequent transmigration into vascular tissue [25]. Indeed, MCP-1 and IL-8 are found in human atheroma, and mice lacking receptors for these chemokines are less susceptible to atherosclerosis and have fewer monocytes in vascular lesions [25]. Mice do not express IL-8, but KC is considered to be the chemokine that is most closely related to human IL-8 [4]. KC triggers monocyte arrest in carotid arteries with early atherosclerotic lesions [26]. High glucose activates aortic ECs to produce IL-8 and thereby accelerates monocytes-ECs interaction, which may be a primary link between hyperglycemia and the mechanism leading to atherosclerotic plaque formation [7]. We found that serum MCP-1/JE and KC levels are significantly increased in db/db mice compared to those in controls, but dietary ingestion of EGCG greatly suppressed diabetes-caused increase in circulating MCP-1 and KC in db/db mice. Consistently, the secretion of these chemokines by aortic ECs isolated from EGCG treated db/db mice was also significantly lower as compared with db/db mice. These suggest the anti-inflammatory effect of EGCG given the critical role of these chemokines in vascular inflammation.
Chemokines accelerate the increased expression of adhesion molecules which play a pivotal role in attracting, binding and transmigration of monocytes into sites of inflammation [27]. Indeed, the adhesion molecules such as VCAM-1, ICAM-1, and E-selectin are elevated in type 2 diabetic patients with atherosclerosis [28]. ICAM-1 and VCAM-1 are widely expressed in different cell types. In addition to endothelium, VCAM-1 is expressed on a variety of cell types including fibroblasts, macrophages, and dendritic cells, while ICAM-1 can be released from ECs, epithelial cells, dendritic cells, lymphocytes, monocytes, eosinophils, keratinocytes, liver cells, and fibroblasts [29]. Hence the circulating adhesion molecules could be produced largely from sources other than ECs. In this study, we observed that the secretion of ICAM-1 and VCAM-1 from ECs were significantly increased in diabetic db/db mice compared with normal mice, which was reversed by treatment with EGCG. However, the circulating levels of these adhesion molecules were not altered in db/db mice and the level of sICAM-1 was moderately reduced in EGCG treated db/db mice. These results suggest that ECs are primarily activated in these diabetic mice, and that EGCG may primarily target vascular ECs for exerting this anti-inflammatory action, which are consistent with our ex vivo finding that EGCG suppresses hyperglycemia-induced inflammation of HAEC.
Proinflammatory chemokines and adhesion molecules are directly regulated by the activation of NFκB [8]. Hyperglycemia enhances the activation of NFκB which then induces the expression of inflammatory genes encoding a number of mediators of atherogenesis such as inflammatory chemokines, and adhesion molecules [1]. In un-stimulated cells, NFκB exists in an inactive form sequestered in the cytoplasm and exposure of cells to inflammatory stimuli results in rapid translocation of NFκB into the nucleus. In the nucleus, p50/p65 dimers bind to the promoters of NFκB-dependent inflammatory genes and regulate variety of genes that are induced in atherosclerosis, including genes encoding VCAM-1, ICAM-1 [9] and IL-8 [30]. NFκB activation and nuclear localization of p65 were detected in human atherosclerotic lesions in vascular smooth muscle cells, macrophages, and ECs [31]. High glucose was shown to increase p65 nuclear translocation and its activation [9, 32]. Indeed, activation of NFκB has been suggested to participate in diabetes-caused vascular complications [32]. In the present study, EGCG suppressed high glucose-induced NFκB activation in vascular ECs. Further, dietary supplementation of EGCG greatly suppressed the increased p65 nuclear translocation without altering protein expression of total p65 in the aortic vessels of db/db mice. To our knowledge, we are the first to show the increased p65 nucleus translocation in the aortic vessel of db/db mice, which was prevented by EGCG treatment.
Hypertension is prevalent in patients with type 2 diabetes [33] and we assessed whether EGCG can prevent the inflammation impaired vascular function in these diabetic mouse models. Similar to observations in humans, there were significant increases in systolic and diastolic blood pressures in db/db mice. Interestingly, dietary EGCG normalized blood pressures, suggesting that EGCG can ameliorate vascular function in diabetes. However, whether this EGCG effect is a direct result of its anti-inflammatory effect on vasculature remains to be investigated. In the present study, EGCG treatment didn’t alter blood glucose levels, although it moderately reduced body weight and improved lipid profiles in EGCG supplemented db/db mice. These results are inconsistent with data from a previous study showing that dietary EGCG supplementation can improve blood glucose and triglycerides in diabetic db/db mice [34]. This discrepancy could be due to the relative low dose used in the present study (0.1% EGCG in diet) as compared to the doses used in this previous study (0.25%, 0.5% and 1% EGCG in diet). Because the bioavailability of EGCG in rodents is low [34], it is possible that administration of 0.1% EGCG in the diet cannot achieve efficacious plasma concentrations for exerting an anti-diabetic effect as observed with higher doses in db/db mice [34]. Based on allometric scaling conversion [16], the dose of 1 g EGCG/kg diet used in the present animal study is equivalent to 2–3 cups of green tea (assuming a 200 mL cup and 200 mg of EGCG per cup) per day for a person requiring 8374 kJ (2000 kcal)/day [16]. However, it is questionable whether similar blood EGCG concentrations can be achieved in humans and mice exposed to the same dose of EGCG, as EGCG may be less bioavailable in humans as compared to mice [35].
Taken together, dietary supplementation of EGCG counteracts several adverse effects associated with vascular inflammation and atherosclerosis, including increased adhesion of ECs to monocytes, enhanced circulating chemokines, increased secretion of chemokines and adhesion molecules by ECs, as well as hypertension of diabetic db/db mice. However, whether EGCG can prevent or ameliorate the pathogenesis of atherosclerosis remains to be determined. In addition, the cellular and molecular mechanism by which EGCG protects diabetic vasculature from chronic inflammation needs to be further defined.
Supplementary Material
Acknowledgments
Supported by grants from the National Center for Complimentary and Alternative Medicine in the National Institutes of Health (R21AT004694, and 3R21AT004694-02S1 to D. Liu); the Diabetes Action Research and Education Foundation (To D. Liu); and a postdoctoral fellowship award from American Heart Association Mid-Atlantic (To P.V.A. Babu).
Abbreviations used
- DiO-Ac-LDL
3,3’-dioctadecyloxacarbocyanine perchlorate labeled acetylated low density lipoprotein
- BAEC
bovine aortic endothelial cells
- DMSO
dimethylsulfoxide
- EGCG
epigallocatechin gallate
- EC
endothelial cell
- E-selectin
endothelial-leukocyte adhesion molecule-1
- EC
epicatechin
- ECG
epicatechin gallate
- EGC
epigallocatechin
- FBS
fetal bovine serum
- HG
high glucose
- ICAM-1
intercellular adhesion molecule-1
- IL-8
interleukin-8
- MCP-1
monocyte chemotactic protein-1
- MAEC
mouse aortic endothelial cells
- NFκB
nuclear factor κB
- HAEC
human aortic endothelial cells
- TNFα
tumor necrosis factor α
- VCAM-1
vascular adhesion molecule-1.
Literature Cited
- 1.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Potenza MA, Gagliardi S, Nacci C, Carratu MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr. Med. Chem. 2009;16:94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan S, Bolick DT, Hatley ME, Natarajan R, et al. Glucose regulates interleukin-8 production in aortic endothelial cells through activation of the p38 mitogenactivated protein kinase pathway in diabetes. J. Biol. Chem. 2004;279:31930–31936. doi: 10.1074/jbc.M400753200. [DOI] [PubMed] [Google Scholar]
- 5.Kunt T, Forst T, Fruh B, Flohr T, et al. Binding of monocytes from normolipidemic hyperglycemic patients with type 1 diabetes to endothelial cells is increased in vitro. Exp. Clin. Endocrinol. Diabetes. 1999;107:252–256. doi: 10.1055/s-0029-1212108. [DOI] [PubMed] [Google Scholar]
- 6.Morigi M, Angioletti S, Imberti B, Donadelli R, et al. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J. Clin. Invest. 1998;101:1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan S, Yeh M, Danziger EC, Hatley ME, et al. Glucose regulates monocyte adhesion through endothelial production of interleukin-8. Circ. Res. 2003;92:371–377. doi: 10.1161/01.RES.0000061714.74668.5C. [DOI] [PubMed] [Google Scholar]
- 8.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin. Sci. (Lond) 2007;112:375–384. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- 9.Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48:855–864. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- 10.Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, et al. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007;292:E1378–E1387. doi: 10.1152/ajpendo.00698.2006. [DOI] [PubMed] [Google Scholar]
- 11.Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. J. Am. Coll. Nutr. 2007;26:373S–388S. doi: 10.1080/07315724.2007.10719626. [DOI] [PubMed] [Google Scholar]
- 12.Widlansky ME, Hamburg NM, Anter E, Holbrook M, et al. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Nutr. 2007;26:95–102. doi: 10.1080/07315724.2007.10719590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr. Med. Chem. 2008;15:1840–1850. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert JD, Lee MJ, Diamond L, Ju J, et al. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. 2006;34:8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- 15.Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H610–H619. doi: 10.1152/ajpheart.00236.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bose M, Lambert JD, Ju J, Reuhl KR, et al. The major green tea polyphenol, (−)- epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J. Nutr. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Si H, Liu D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J. Nutr. 2008;138:297–304. doi: 10.1093/jn/138.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan S, Hatley ME, Reilly KB, Danziger EC, Hedrick CC. Modulation of PPARalpha expression and inflammatory interleukin-6 production by chronic glucose increases monocyte/endothelial adhesion. Arterioscler. Thromb. Vasc. Biol. 2004;24:851–857. doi: 10.1161/01.ATV.zhq0504.2260. [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama T, Mishima K, Ide F, Yamada K, et al. Functional analysis of an established mouse vascular endothelial cell line. J. Vascul. Res. 2007;44:138–148. doi: 10.1159/000098520. [DOI] [PubMed] [Google Scholar]
- 20.de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- 21.Pittet MJ, Swirski FK. Monocytes link atherosclerosis and cancer. Eur. J. Immunol. 2011;41:2519–2522. doi: 10.1002/eji.201141727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MJ, Maliakal P, Chen L, Meng X, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 2002;11:1025–1032. [PubMed] [Google Scholar]
- 23.Andriamanalijaona R, Kypriotou M, Bauge C, Renard E, et al. Comparative effects of 2 antioxidants, selenomethionine and epigallocatechin-gallate, on catabolic and anabolic gene expression of articular chondrocytes. J. Rheumatol. 2005;32:1958–1967. [PubMed] [Google Scholar]
- 24.Chyu KY, Babbidge SM, Zhao X, Dandillaya R, et al. Differential effects of green tea-derived catechin on developing versus established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004;109:2448–2453. doi: 10.1161/01.CIR.0000128034.70732.C2. [DOI] [PubMed] [Google Scholar]
- 25.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 26.Huo Y, Weber C, Forlow SB, Sperandio M, et al. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J. Clin. Invest. 2001;108:1307–1314. doi: 10.1172/JCI12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin J, Collot-Teixeira S, McGregor L, McGregor JL. The dialogue between endothelial cells and monocytes/macrophages in vascular syndromes. Curr. Pharm. Des. 2007;13:1751–1759. doi: 10.2174/138161207780831248. [DOI] [PubMed] [Google Scholar]
- 28.Otsuki M, Hashimoto K, Morimoto Y, Kishimoto T, Kasayama S. Circulating vascular cell adhesion molecule-1 (VCAM-1) in atherosclerotic NIDDM patients. Diabetes. 1997;46:2096–2101. doi: 10.2337/diab.46.12.2096. [DOI] [PubMed] [Google Scholar]
- 29.Golias C, Tsoutsi E, Matziridis A, Makridis P, et al. Review. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo. 2007;21:757–769. [PubMed] [Google Scholar]
- 30.Bartchewsky W, Jr, Martini MR, Masiero M, Squassoni AC, et al. Effect of Helicobacter pylori infection on IL-8, IL-1beta and COX-2 expression in patients with chronic gastritis and gastric cancer. Scand. J. Gastroenterol. 2009;44:153–161. doi: 10.1080/00365520802530853. [DOI] [PubMed] [Google Scholar]
- 31.Brand K, Page S, Rogler G, Bartsch A, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin. Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 33.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 34.Wolfram S, Raederstorff D, Preller M, Wang Y, et al. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J. Nutr. 2006;136:2512–2518. doi: 10.1093/jn/136.10.2512. [DOI] [PubMed] [Google Scholar]
- 35.Lambert JD, Hong J, Kim DH, Mishin VM, et al. Piperine enhances the bioavailability of the tea polyphenol (−)-epigallocatechin-3-gallate in mice. J. Nutr. 2004;134:1948–1952. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.