Abstract
Human herpesvirus-6 (HHV-6) frequently reactivates after allogeneic hematopoietic stem-cell transplantation (HSCT); its most severe manifestation is the syndrome of post-transplantation acute limbic encephalitis (HHV-6-PALE). The epidemiology, risk factors, and characteristics of HHV-6-PALE after unrelated cord-blood transplantation (UCBT) are not well characterized. We analyzed 1,344 patients undergoing allogeneic HSCT between March 2003 and March 2010 to identify risk factors and characteristics of HHV-6-PALE. The cohort included 1,243 adult-donor HSCT and 101 UCBT recipients. All patients diagnosed with HHV-6-PALE had HHV-6 DNA in CSF specimens in addition to symptoms and studies indicating limbic encephalitis. 19 (1.4%) cases of HHV6-PALE were identified during this study; 10 after UCBT (9.9%) and 9 after adult-donor HSCT (0.7%), for an incidence rate of 1.2 cases/1000 patient-days compared to 0.08 cases/1000 patient-days (p<0.001), respectively. Risk factors for HHV-6-PALE on multivariable Cox modeling were UCBT (adjusted hazard ratio (aHR) 20.0; 95% confidence interval (CI), 7.3–55.0; p<0.001), time-dependent acute graft-versus-host disease grades II-IV (aHR 7.5; 95% CI, 2.8–19.8; p<0.001), and adult-mismatched donor (aHR 4.3; 95% CI, 1.1–17.3; p=0.04). Death from HHV-6-PALE occurred in 50% of affected patients undergoing UCBT and no recipients of adult-donor cells. Patients receiving UCBT have increased risk for HHV6-PALE and greater morbidity from this disease.
Classification: Clinical research, adult, infectious diseases
Introduction
Human herpesvirus-6 (HHV-6) is an opportunistic pathogen in patients undergoing allogeneic hematopoietic stem-cell transplantation (HSCT). Primary infection with this herpesvirus typically occurs during infancy (1). Following acute infection, HHV-6 is able to establish latency in a wide variety of host cells, although it replicates most efficiently in vitro in CD4+ T lymphocytes (2). There are two closely related variants of HHV-6, types A and B; HHV-6B is the more frequent cause of human disease. Antibodies to either or both variants are found in >95% of adults (2–5). HHV-6 DNA becomes detectable in plasma samples from approximately 40–50% of patients undergoing HSCT from adult donors and up to 80% of patients following unrelated umbilical cord blood HSCT (UCBT) within 6 weeks following transplantation, a phenomenon attributed most commonly to HHV-6 reactivation (6–11). The HHV-6B variant accounts for approximately 98% of these events (12–14). HHV-6 reactivation after HSCT has been associated with many complications including delayed engraftment, graft rejection, grade II–IV acute graft-versus-host disease (aGVHD), central nervous system (CNS) disease, and increased all-cause mortality (9, 11, 15–24).
One of the most debilitating and sometimes fatal consequences of HHV-6 reactivation following HSCT is the syndrome of post-transplantation acute limbic encephalitis (HHV-6-PALE) (9, 19–24). Risk factors for this disease are poorly understood and variably reported as younger age, mismatched or unrelated donor, sex mismatched donor, underlying malignancy other than hematologic malignancy in first remission or chronic myelogenous leukemia chronic phase, low pre-transplant anti-HHV-6 IgG titer, treatment with anti-T cell monoclonal antibodies or steroids, high-level plasma HHV-6 viremia, and aGVHD grades II–IV (7–10, 15, 23, 25–27).
HHV-6-PALE after HSCT is well described (9, 19–24). Several case reports and series of HHV-6-PALE after UCBT have been published (21, 28–30), but the epidemiology, risk factors, and characteristics of this syndrome in patients receiving UCBT are not well characterized. Given the increased incidence of HHV-6 reactivation and higher plasma viral loads in recipients of UCB (6, 7), these patients may be at risk for more frequent and severe manifestations of CNS disease. This study describes the epidemiology, risk factors, and characteristics of HHV-6-PALE in patients undergoing UCBT at our institution.
Patients and methods
Patients
All patients who underwent an initial allogeneic HSCT between March 2003 and March 2010 were identified through the clinical database at Dana-Farber Cancer Institute/Brigham and Women’s Hospital (DFCI/BWH) Hematopoietic Stem Cell Transplantation Program. This period was chosen to correspond with the introduction of UCBT at our institution and the availability of a standardized HHV-6 cerebrospinal fluid (CSF) polymerase chain reaction (PCR) assay for testing all samples at a single reference laboratory. A waiver of the requirement for informed consent was granted by the Office for Human Research Studies of Dana–Farber/Harvard Cancer Center.
A total of 1,367 patients underwent allogeneic HSCT during the study period. Twenty-three patients were excluded due to receiving an initial allogeneic HSCT before the start of the study period or during the study period at an outside institution. A final cohort of 1,344 patients undergoing initial allogeneic HSCT during the study period was used for this analysis: 725 were from adult unrelated donors (633 HLA-matched at 6/6 loci, 92 HLA-mismatched), 518 from adult related donors (508 HLA-matched, 10 HLA-mismatched), and 101 from mismatched UCB donors (Tables 1 and 2). Fifteen patients underwent a second HSCT procedure during the 100-day follow-up period from the date of the initial HSCT. In this group, 8 patients had 2 UCBT, 6 patients had 2 HSCT from adult donors, and 1 patient had an adult-donor HSCT followed by UCBT. Neither foscarnet nor ganciclovir were used for antiviral prophylaxis during this study period. Patients received preemptive therapy for cytomegalovirus (CMV) DNAemia primarily with ganciclovir or valganciclovir, based on a CMV hybrid capture assay (Digene, Gaithersburg, MD) or a real-time PCR assay (Qiagen, Germantown, MD).
Table 1.
Characteristics of allogeneic HSCT cohort according to stem cell source (DFCI/BWH March 2003–March 2010)
| Characteristics | UCBT (%)* | Adult-donor HSCT (%) | P |
|---|---|---|---|
| No. of patients | 101 | 1243 | — |
| Median age, years (IQR, range) | 48 (37–58, 19–67) | 51 (41–58, 18–74) | 0.10 |
| Male sex | 54 (53.4) | 721 (58) | 0.37 |
| Race | 0.002 | ||
| Nonwhite | 11 (10.9) | 43 (3.5) | |
| White | 90 (89.1) | 1200 (96.5) | |
| Primary disease | 0.06 | ||
| AML | 34 (33.7) | 456 (36.7) | |
| NHL | 23 (22.8) | 187 (15.0) | |
| MDS | 10 (9.9) | 146 (11.8) | |
| ALL | 7 (6.9) | 106 (8.5) | |
| CLL | 5 (5.0) | 102 (8.2) | |
| CML | 5 (5.0) | 82 (6.6) | |
| HD | 8 (7.9) | 54 (4.3) | |
| AA | 7 (6.9) | 38 (3.1) | |
| MM | 0 | 41 (3.3) | |
| MPD | 2 (2.0) | 31 (2.5) | |
| Conditioning regimen | <0.001 | ||
| Reduced-intensity | 76 (75.3) | 639 (51.4) | |
| Myeloablative | 25 (24.8) | 604 (48.6) | |
| HLA match† | <0.001 | ||
| Mismatched donor | 101 (100) | 102 (8.2) | |
| Matched donor | 0 (0) | 1141 (91.8) | |
| Donor relatedness | <0.001 | ||
| Unrelated donor | 101 (100) | 725 (58.3) | |
| Related donor | 0 | 518 (41.7) | |
| Conditioning Agents‡ | |||
| Fludarabine | 92 (91.1) | 620 (49.9) | <0.001 |
| IV Busulfan | 3 (4.0) | 650 (52.3) | <0.001 |
| Cyclophosphamide | 30 (29.7) | 609 (49.0) | <0.001 |
| Total body irradiation | 30 (29.7) | 544 (43.8) | 0.006 |
| ATG | 69 (68.3) | 81 (6.5) | <0.001 |
| Melphalan | 67 (66.3) | 8 (0.6) | <0.001 |
| Thiotepa | 0 | 17 (1.4) | 1 |
| Etoposide | 0 | 6 (0.5) | 1 |
| BCNU | 0 | 6 (0.5) | 1 |
| CMV recipient seropositivity§ | 0.03 | ||
| CMV seropositive | 41 (40.6) | 592 (47.9) | |
| CMV seronegative | 60 (59.4) | 644 (52.1) | |
| Acute GVHD** | 0.19 | ||
| Grades II–IV | 17 (16.8) | 279 (22.5) | |
| None-grade I | 84 (83.2) | 964 (77.6) | |
| Subsequent transplant before 100 days†† | <0.001 | ||
| Yes | 8 (7.9) | 7 (0.6) | |
| No | 93 (92.1) | 1236 (99.4) |
HSCT indicates hematopoietic stem cell transplantation; UCBT, unrelated cord blood stem cell transplantation; AML, acute myelogenous leukemia; NHL, non-Hodgkin lymphoma; MDS, myelodysplastic syndrome; ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; HD, Hodgkin disease; AA, aplastic anemia; MM, multiple myeloma; MPD, myeloproliferative disease; HLA, human leukocyte antigen; ATG, rabbit antithymocyte globulin; BCNU, carmustine; CMV, cytomegalovirus; GVHD, graft-versus-host disease.
Percentages in parenthesis refer to the total number of patients in each column. Total numbers are the same as in Table 1.
Patients were considered HLA-matched if all 6/6 HLA A, B, and DR were identical.
Conditioning agents were used in a variety of combinations across the cohort. Since the agents are not mutually exclusive variables and patients received more than one, p value calculations are binary for the agent and HHV-6-PALE.
Seven adult-donor HSCT recipients had unknown CMV serostatus.
Patients were censored if acute GVHD occurred after HHV-6-PALE onset.
Subsequent transplants in UCB recipients were all UCBT. Subsequent transplants in adult-donor HSCT recipients were 6 peripheral blood HSCT and 1 UCBT.
Table 2.
Characteristics of allogeneic HSCT cohort, cases of human herpesvirus-6-associated post-transplantation acute limbic encephalitis (HHV-6-PALE), and crude incidence rates and incidence rate ratios within 100 days of transplantation (DFCI/BWH March 2003–March 2010)
| Characteristics | No. | HHV-6- PALE‡‡ |
% | P | Days at Risk§§ | IR (95% CI)*** | IRR (95% CI)††† |
|---|---|---|---|---|---|---|---|
| Cohort | 1344 | 19 | 1.4 | — | 125288 | 0.15 (0.09–0.2) | — |
| Recipient age, quartiles, y | 0.89 | ||||||
| 18–40 | 338 | 6 | 1.8 | 31405 | 0.19 (0.07–0.4) | 1 | |
| 40–51 | 339 | 4 | 1.2 | 31469 | 0.13 (0.03–0.3) | 0.67 (0.2–2.4) | |
| 51–58 | 330 | 5 | 1.5 | 30793 | 0.16 (0.05–0.4) | 0.85 (0.3–2.8) | |
| 58–74 | 337 | 4 | 1.2 | 31621 | 0.13 (0.03–0.3) | 0.66 (0.2–2.3) | |
| Recipient race | 0.04 | ||||||
| Nonwhite | 54 | 3 | 5.6 | 4964 | 0.60 (0.1–1.8) | 4.55 (1.3–15.6) | |
| White | 1290 | 16 | 1.2 | 120324 | 0.13 (0.08–0.2) | — | |
| Primary disease | 0.95 | ||||||
| AML | 490 | 5 | 1.0 | 45282 | 0.11 (0.04–0.3) | 1 | |
| NHL | 210 | 4 | 1.9 | 19823 | 0.20 (0.05–0.5) | 1.83 (0.5–6.8) | |
| MDS | 156 | 3 | 1.9 | 14331 | 0.21 (0.04–0.6) | 1.90 (0.5–7.9) | |
| ALL | 113 | 2 | 1.8 | 10476 | 0.19 (0.002–0.7) | 1.73 (0.3–8.9) | |
| CLL | 107 | 2 | 1.9 | 10204 | 0.20 (0.02–0.7) | 1.78 (0.3–9.1) | |
| CML | 87 | 1 | 1.2 | 8037 | 0.12 (0.002–0.7) | 1.13 (0.1–9.6) | |
| HD | 62 | 1 | 1.6 | 5915 | 0.17 (0.002–0.9) | 1.53 (0.2–13.1) | |
| AA | 45 | 0 | 0 | 4137 | — | — | |
| MM | 41 | 0 | 0 | 3945 | — | — | |
| MPD | 33 | 1 | 3.0 | 3138 | 0.32 (0.004–1.8) | 2.89 (0.3–24.7) | |
| Conditioning regimen | 0.33 | ||||||
| Myeloablative | 629 | 11 | 1.8 | 57353 | 0.19 (0.1–0.3) | 1.63 (0.7–4.05) | |
| Reduced-intensity | 715 | 8 | 1.1 | 67935 | 0.12 (0.05–0.2) | — | |
| Stem cell source | <0.001 | ||||||
| UCBT | 101 | 10 | 9.9 | 8406 | 1.20 (0.6–2.2) | 15.5 (6.3–38.0) | |
| Adult-donor HSCT | 1243 | 9 | 0.7 | 116882 | 0.08 (0.04–0.2) | — | |
| HLA match, adult cells‡‡‡ | 0.03 | ||||||
| Mismatched donor | 102 | 3 | 2.9 | 9070 | 0.33 (0.07–1.0) | 5.94 (1.5–23.8) | |
| Matched donor | 1141 | 6 | 0.5 | 107812 | 0.06 (0.02–0.1) | — | |
| Donor relatedness, adult cells§§§ | 0.09 | ||||||
| Unrelated donor | 725 | 8 | 1.1 | 67601 | 0.12 (0.05–0.2) | 5.83 (0.7–46.6) | |
| Related donor | 518 | 1 | 0.2 | 49281 | 0.02 (0.0003–0.1) | — | |
| Conditioning agents | |||||||
| Fludarabine | 712 | 10 | 1.4 | 0.98 | 67132 | 0.15 (0.07–0.3) | 1 |
| IV Busulfan | 653 | 2 | 0.3 | <0.001 | 62726 | 0.03 (0.004–0.1) | 0.21 (0.05–1.0) |
| Cyclophosphamide | 639 | 11 | 1.7 | 0.36 | 58169 | 0.19 (0.09–0.3) | 1.27 (0.5–3.0) |
| Total body irradiation | 574 | 11 | 1.9 | 0.18 | 52079 | 0.21 (0.1–0.4) | 1.42 (0.6–3.3) |
| ATG | 150 | 7 | 4.7 | 0.003 | 13852 | 0.51 (0.2–1.04) | 3.39 (1.3–8.9) |
| Melphalan | 75 | 6 | 8.0 | <0.001 | 6340 | 0.95 (0.4–2.06) | 6.35 (2.3–17.5) |
| Thiotepa | 17 | 0 | 0 | 1 | 1660 | — | — |
| Etoposide | 6 | 0 | 0 | 1 | 600 | — | — |
| BCNU | 6 | 0 | 0 | 1 | 600 | — | — |
| CMV recipient seropositivity | 0.82 | ||||||
| CMV seropositive | 652 | 10 | 1.5 | 60742 | 1.65 (0.79–3.03) | 1.17 (0.47–2.89) | |
| CMV seronegative | 685 | 9 | 1.3 | 64091 | 1.40 (0.64–2.67) | — | |
| Acute GVHD | 0.05 | ||||||
| Grades II–IV | 296 | 8 | 2.7 | 27039 | 0.30 (0.1–0.6) | 2.64 (1.06–6.6) | |
| None-grade I | 1048 | 11 | 1.1 | 98249 | 0.11 (0.06–0.2) | — |
HHV-6-PALE indicates HHV-6-associated post-transplantation acute limbic encephalitis; IR, incidence rate; IRR, incidence rate ratio; CI, confidence interval; ---, not applicable.
HHV-6-PALE was defined as a positive CSF PCR for HHV-6 DNA in addition to characteristic clinical findings within 100 days of transplantation.
Days at risk were censored at day of HHV-6-PALE symptom onset, death, or 100 days after transplantation.
IR indicates crude incidence rate of cases per 1,000 patient-days after HSCT. IR and CI were calculated using the Taylor series.
IRR indicates crude incidence rate ratio, when compared with other levels of the covariate. IRR and CI were calculated using the Byar method.
Only patients undergoing adult-donor HSCT are included given that all patients undergoing UCBT received mismatched-unrelated cells.
Only patients undergoing adult-donor HSCT are included given that all patients undergoing UCBT received mismatched-unrelated cells.
Covariates and definitions
Data on covariates of interest (Tables 1 and 2) were identified through the DFCI/BWH HSCT database, the Partners Healthcare System Research Patient Data Repository, and review of the electronic and paper medical records. Engraftment day was defined as the first of three consecutive days of an absolute neutrophil count greater than >500 cells/µL. Acute GVHD was defined according to the consensus criteria (31), and data were collected for day of onset, maximum overall grade, and drugs used for treatment.
Conditioning regimens were grouped as myeloablative or reduced-intensity (RIC). Myeloablative conditioning consisted of different combinations of chemotherapeutic agents, but a majority included cyclophosphamide and 1400 cGy total body irradiation (TBI) delivered in 7 fractions (32). A minority received high-dose busulfan and cyclophosphamide. RIC primarily consisted of fludarabine with low-dose busulfan or fludarabine with melphalan, combined with rabbit antithymocyte globulin (ATG, at a dose of 6 mg per kilogram of body weight) (33). Antithymocyte globulin use was primarily restricted to RIC UCBT and a few cases of RIC adult-donor HSCT (34, 35). Prophylaxis for GVHD in patients undergoing adult-donor HSCT consisted of tacrolimus with methotrexate and/or sirolimus in a majority of cases, as well as cyclosporine with mycophenolate mofetil (MMF) (32). In UCBT, GVHD prophylaxis consisted primarily of tacrolimus with sirolimus or cyclosporine with MMF (34–36). Patients participated in single-arm or randomized protocols or were treated with conditioning and aGVHD prophylaxis regimens at the discretion of treating physicians.
HHV-6-PALE was diagnosed in patients who had detectable HHV-6 DNA in their cerebrospinal fluid (CSF) in the context of acute-onset altered mental status, amnesia, seizures, or other evidence of medial temporal lobe disease involving the limbic system and no other identifiable etiology after extensive workup (19). Cases were reviewed in detail for day of HHV-6-PALE symptom onset, CSF results, EEG and MRI findings, antiviral and anticonvulsant treatments, concomitant clinical and laboratory findings, and patient outcomes.
HHV-6 testing
Prospective and routine monitoring of plasma HHV-6 DNA by PCR after HSCT was not performed in this patient cohort. Testing was performed at the discretion of the treating clinicians, often in the setting of fever workup, altered mental status, or other conditions raising suspicion for HHV-6 reactivation. However, CSF HHV-6 PCR testing was routinely performed on all CSF specimens obtained from patients after HSCT during the study period, and all patients suspected of having HHV-6-PALE underwent lumbar puncture. Testing for HHV-6 DNA was performed at Associated Regional and University Pathologists (ARUP, Salt Lake City, UT) using a PCR assay with a quantitative range between 1,000 and 999 × 106 copies/mL. Detectable HHV-6 DNA at levels <1,000 or >999 × 106 copies/mL is reported as such. The same assay was used for all CSF specimens in this study, which distinguishes between HHV-6A and B variants. ARUP personnel were unaware of the patient conditions prompting HHV-6 testing. Specific analysis for chromosomally integrated HHV-6 (ciHHV-6) was not performed.
Statistical analysis
Person time-at-risk was censored at day of HHV-6-PALE symptom onset, death, or 100 days after time of transplantation. Censoring after 100 days was chosen given the occurrence of HHV-6-PALE during this period in most reported cases (9, 19–24) and peak plasma HHV-6 reactivation 3–4 weeks after HSCT (7, 8). In addition, no cases of HHV-6-PALE have been diagnosed at our institution beyond 100 days after transplantation. Baseline patient characteristics were compared using the 2-sided Fisher exact test, chi-square test, or Wilcoxon rank-sum test as appropriate. HHV-6-PALE incidence rates (IR), incidence rate ratios (IRR), and their 95% confidence intervals were calculated with the Taylor series and Byar method, respectively, using OpenEpi version 2.3.1 (http://www.openepi.com; Atlanta, GA). Kaplan-Meier curves were generated for time-to-event analyses.
Characteristics associated with HHV-6-PALE were analyzed with Cox modeling; aGVHD was modeled as a time-varying covariate. Mismatched and unrelated donor variables were only compared in the adult donor cohort to avoid overestimating their association with HHV-6-PALE by including UCBT patients, who received mismatched and unrelated donor cells in all cases. We explored the potential diagnostic value of HHV-6 plasma DNA levels for HHV-6-PALE by generating receiver operating characteristic (ROC) curves. P values <0.05 were considered statistically significant. SAS version 9.2 (SAS institute, Cary, NC) was used for these analyses.
Results
HHV-6-PALE incidence and risk factors
HHV-6-PALE was diagnosed in 19 of 1,344 patients who underwent HSCT during the study period. The baseline characteristics of the cohort, along with stratified IR and crude IRR according to HSCT baseline covariates, are presented in Table 2. There were 125,288 patient-days of observation, and no patients were lost to follow-up before 100 days after transplantation. The cumulative incidence of HHV-6-PALE was 1.4% for an overall IR of 0.15/1,000 patient-days (95% confidence interval [CI], 0.09–0.24). HHV-6-PALE IR was higher among UCBT patients (10/101, IR 1.2/1,000 patient-days) compared with adult-donor HSCT recipients (9/1,243; IR 0.08/1,000 patient-days IRR 15.5; p<0.001; Table 2). Two of the cases occurred among 8 patients who underwent a second UCBT within 100 days; no cases occurred after a second adult-donor HSCT. Additional characteristics associated with HHV-6-PALE on univariable analysis are detailed in Table 2. Many of these variables were collinear with UCBT (Table 1). Although patient-level antiviral treatment for CMV DNAemia was not captured, there was no association between CMV recipient seropositivity and HHV-6-PALE.
Univariable and multivariable hazard ratios (HR) were calculated for possible risk factors as shown in Table 3. Significant covariates on univariable analyses and other covariates of interest were evaluated in multivariable models, accounting for the absolute number of events. These multivariable models maintained stable adjusted HR (aHR) with up to 3 variables, despite the low event rate of HHV-6-PALE. In the final multivariable Cox model, UCBT (aHR 20.0; 95% CI, 7.3–55.0; p<0.001), time-dependent aGVHD grades II–IV (aHR 7.5; 95% CI, 2.8–19.8; p<0.001), and adult mismatched donor (aHR 4.3; 95% CI, 1.1–17.3; p=0.04) remained predictive of HHV-6-PALE. To check the robustness of the data without including the 2 cases following a second HSCT, the same cohort was restricted to patients only receiving 1 HSCT with additional censoring at the day of second HSCT. The findings and estimates of risk were similar (data not shown).
Table 3.
Proportional hazards modeling of risk of HHV-6-PALE after allogeneic HSCT
| Characteristics | Univariable HR (95% CI) | P | Multivariable HR (95% CI)**** | P |
|---|---|---|---|---|
| UCBT | 14.5 (5.9–35.8) | <0.0001 | 20.0 (7.3–55.0) | <0.0001 |
| Male | 1.61 (0.6–4.2) | 0.34 | — | — |
| Nonwhite | 4.63 (1.4–15.9) | 0.01 | — | — |
| Myeloablative conditioning | 1.60 (0.6–4.0) | 0.31 | — | — |
| Mismatched adult donor†††† | 2.38 (0.7–8.2) | 0.17 | 4.3 (1.1–17.3) | 0.04 |
| Unrelated adult donor | 0.62 (0.3–1.5) | 0.30 | — | — |
| ATG | 4.68 (1.8–11.9) | 0.001 | — | — |
| Acute GVHD: grades II–IV‡‡‡‡ | 8.07 (3.08–21.2) | <0.0001 | 7.5 (2.8–19.8) | <0.0001 |
Multivariable cox model analysis adjusting for UCBT, time-dependent acute GVHD grades II–IV, and mismatched donor.
To accurately calculate values for mismatched and unrelated donors, a dummy variable was made to separate the analysis into 3 groups (adult-donor HSCT mismatched or unrelated versus UCBT versus matched or unrelated HSCT) to avoid counting UCBT patients twice, given that all UCBT were mismatched-unrelated donors in this cohort.
Acute GVHD was modeled as a time-varying covariate.
Clinical features of HHV-6-PALE
All patients were infected with the HHV-6B variant. Comparison of the 19 patients who developed HHV-6-PALE revealed interesting differences. Ten of the cases occurred after UCBT. Nine of the cases followed adult-donor HSCT: 6 were from matched-unrelated donors; 3 from mismatched-unrelated donors; and 1 from a matched-related donor. Four patients never engrafted after their UCBT, whereas all adult-donor HSCT recipients engrafted. In those who engrafted, engraftment occurred at a median of 24 days (range, 16–59) in the UCBT cohort and 12 days (range, 4–14) in the adult-donor group. Encephalitis developed prior to engraftment in 7 recipients of UCB compared with 1 recipient of adult-donor cells. HHV-6-PALE symptom onset occurred at a median of 32 days (range, 16–67) in UCBT patients compared with 20 days (range, 7–37) in adult-donor HSCT patients (p=0.07).
The manifestations of HHV-6-PALE demonstrated previously were consistent with those observed in this study (19). Most patients had a similar array of symptoms notable for altered mental status, anterograde amnesia with marked deficit in short-term memory recall (but not registration), and intermittent agitation superimposed on lethargy. Neurologic examinations were otherwise unrevealing. Symptoms were acute in onset with waxing and waning courses. Visual hallucinations developed in 3 UCBT recipients but were not seen in adult-donor recipients. Concomitant conditions documented in a few patients from both groups included the syndrome of inappropriate antidiuretic hormone secretion and autonomic instability with labile blood pressures or hypothermia.
Magnetic resonance imaging (MRI) findings were significant for T2 hyperintense lesions on fluid attenuation inversion recovery (FLAIR) sequences of the medial temporal lobe in a limbic neuroanatomic distribution in 12/17 patients, primarily affecting the hippocampus, uncus, and amygdala. One recipient of UCB had extensive extralimbic abnormalities. Two patients did not have brain MRIs.
Electroencephalograms (EEG) obtained in 16/19 patients had similar findings, primarily revealing varying degrees of bilateral theta and delta slowing consistent with mild to severe encephalopathy. Among recipients of an adult-donor HSCT, 8/9 were treated with levetiracetam for seizures or seizure prophylaxis. Generalized tonic-clonic seizures were confirmed in 3 of these patients prior to starting an anti-epileptic drug (AED). Median time from symptom onset to treatment with an AED in this group was 3 days (range, 0–56). In the UCBT cohort, 6/10 patients were treated with levetiracetam for seizure prophylaxis a median of 5.5 days (range, 2–15) after symptom onset. No documented seizures occurred among UCBT recipients, although 2 patients demonstrated periodic epileptiform discharges on EEG.
The first lumbar puncture after symptom onset revealed elevated CSF total protein levels (median 53 mg/dL; range, 10–133) in most patients from both groups. Lymphocytic pleocytosis was found in a minority of patients and was less pronounced in the UCBT patients (median 3 cells/mm3; range, 0–16) than the adult-donor group (median 9 cells/mm3; range, 0–25). Red blood cell and glucose levels were generally within the expected limits. CSF HHV-6 viral loads were higher in HHV-6-PALE patients after UCBT (median 3.5 × 105 copies/mL; range, 5,980 – >106) than adult-donor HSCT (median 3,740 copies/mL; range, <1000 – >2 × 105; p=0.01).
Can HHV-6 plasma viral load predict HHV-6-PALE?
Of 142 adult-SCT patients tested within 100 days of transplantation, 43 (30.3%) had positive plasma HHV-6 PCR results. Of 68 UCBT patients tested within 100 days, 49 (72.1%) had detectable HHV-6 DNA at a median of 22 days (range, 9–69). Peak plasma viral loads were higher after UCBT (median 3.4 × 104 copies/mL; range, <1,000 – >106) than adult-donor HSCT (median 4.4 × 103 copies/mL; range, <1,000 – >106; p=0.01). All HHV-6-PALE patients who were tested (17/19) had concurrent HHV-6 viremia.
After UCBT, patients who developed HHV-6-PALE had higher viral loads (median 3.9 × 105 copies/mL; range, 3.1 × 104 – >106) than those who did not (median 1.9 × 104 copies/mL; range, <1,000 – >106; p=0.001). Peak values were detected a median of 1 day (range, −17–9) from HHV-6-PALE symptom onset, and treatment was started a median of 3 days (range, −3–23) from detection of plasma HHV-6 DNA.
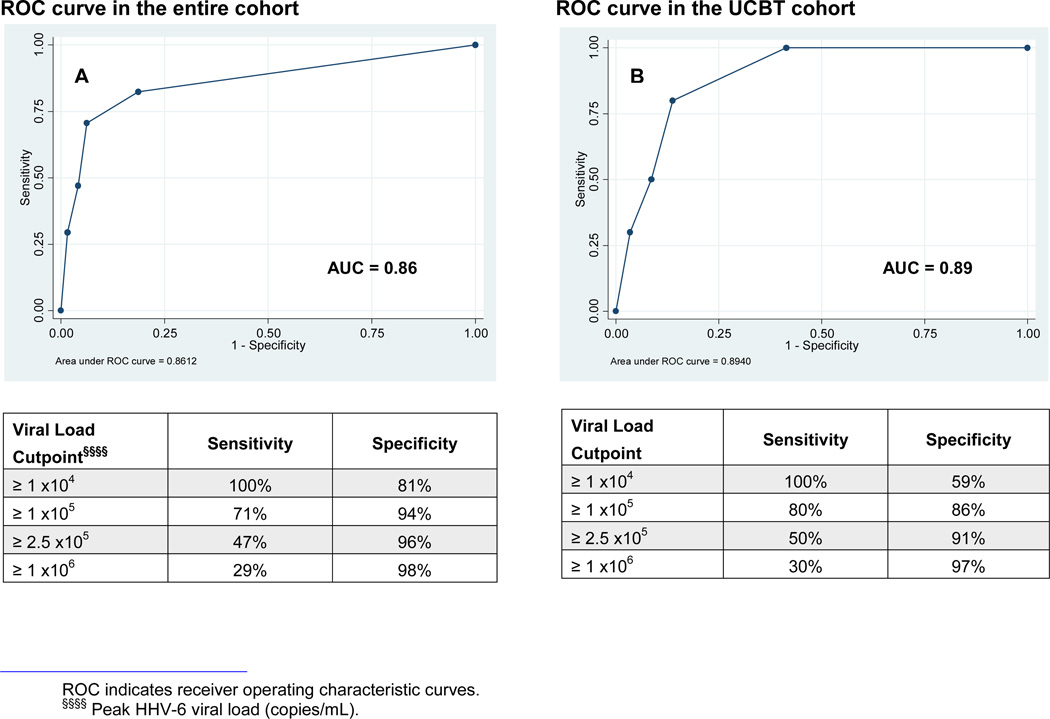
To explore the potential diagnostic performance of blood HHV-6 viral loads to identify cases of HHV-6-PALE, ROC curves were constructed to evaluate the sensitivity and specificity of peak plasma HHV-6 viral loads for HHV-6-PALE in our cohort (Figure 1). Among all tested patients, a plasma HHV-6 viral load ≥105 copies/mL was 71% sensitive and 94% specific for a diagnosis of HHV-6-PALE. Specificity increased to 98% for viral loads ≥106 copies/mL. Among tested UCBT patients, a plasma HHV-6 viral load ≥105 copies/mL was 80% sensitive and 86% specific for a diagnosis of HHV-6-PALE. Specificity increased to 97% for viral loads ≥106 copies/mL. Adult-donor HSCT recipients had slightly lower sensitivities and higher specificities at these viral load thresholds.
Figure 1. ROC curves plotting the sensitivity and specificity of peak plasma HHV-6 viral loads for the development of HHV-6-PALE.
A) 210 patients in the entire cohort had plasma HHV-6 testing, and 92 were positive. B) 68 patients in the UCBT cohort had plasma HHV-6 testing, and 49 were positive.
Treatment and outcomes
Intravenous foscarnet at a dose of 180 mg/kg/day was used off-label to treat 18/19 patients. All patients with high clinical suspicion for HHV-6-PALE or detection of HHV-6 DNA in CSF specimens were started on treatment during this study. Lumbar puncture was obtained before or within 24 hours of initiation of definitive treatment in all patients. Treatment was only continued for patients with detectable HHV-6 DNA in the CSF. Some early patients in the cohort were initially started on acyclovir or ganciclovir. Time to treatment after HHV-6-PALE symptom onset was a median of 6 days (range, 1–13) in UCBT patients and continued for a median of 16 days (range, 7–28). Time to treatment was a median of 3 days (range, 1–13) in adult-donor recipients and continued for a median of 21 days (range, 7–42). One patient did not receive antiviral treatment as this was not consistent with her goals of care at the time the diagnosis was made.
Most patients showed mild to moderate improvement in their symptoms shortly after the initiation of foscarnet therapy, but 9/13 patients surviving long enough to demonstrate recovery were left with residual fatigue and episodic memory impairment. While no adult-donor recipients died as a direct result of their encephalitis, 50% of UCBT patients died a median of 45 days (range, 35–74) after transplantation and 18 days (range, 7–26) after symptom onset due to complications directly attributable to HHV-6-PALE. Deaths occurred after similar courses punctuated by progressive encephalopathy and unresponsiveness requiring mechanical ventilation without return of consciousness. Of the 5 patients who died, 4 of the patients never engrafted, and 2 of these patients underwent subsequent UCBT due to failure of initial UCBT. There were no clear associations between morbidity and mortality and time to treatment; plasma or CNS HHV-6 viral loads; or other clinical, laboratory, or radiographic findings.
Discussion
UCBT is frequently used in patients who have no suitable or readily available matched or related stem-cell donor. However, UCBT is often associated with slower engraftment and impaired immune reconstitution relative to adult-donor HSCT, resulting in an increased risk for infectious complications. Moreover, there is no passively transferred immunity to bridge the period from conditioning to immunologic recovery. One of the most severe infections in the early period after transplantation is HHV-6-PALE, which engenders mortality or serious morbidity in the majority of affected patients.
In this study of a large cohort of HSCT recipients, the most significant and stable predictors of HHV-6-PALE were UCBT, time-dependent aGVHD grades II–IV, and adult-mismatched donor. The overall cumulative incidence of this syndrome was 1.4% with an IR of 0.15/1,000 patient-days. HHV-6-PALE occurred much more frequently after UCBT (9.9%) than after adult-donor HSCT (0.7%), and there was a 25% risk for HHV-6-PALE after a second UCBT. Mortality due to progressive HHV-6-PALE was 50% in UCBT recipients compared with no mortality after adult-donor HSCT.
Although it is possible that we did not identify all patients with HHV-6 reactivation in the CNS, any symptomatic patient with a likely diagnosis of HHV-6-PALE during this study period underwent CSF analysis before or within 24 hours of antiviral treatment for HHV-6. We think our definition for HHV-6-PALE captured only true cases of infection in this cohort. All patients diagnosed with HHV-6-PALE had detectable HHV-6 DNA in the CSF using a quantitative PCR assay in addition to typical symptoms of limbic encephalitis, and 12/17 patients who had brain MRIs showed objective signs of medial temporal lobe abnormalities affecting limbic structures. Although 5 patients did not have MRI evidence of limbic encephalitis, this is consistent with the findings of a review of 48 HHV-6-PALE cases in which approximately 30% of imaged patients had normal brain MRIs (9). Normal imaging may be more common early in the disease course. Repeat scanning, if obtained, may show interval development of disease, as seen in 2 patients from this study. Furthermore, the finding of HHV-6 DNA in CSF samples was unlikely to be incidental, as this is rarely demonstrated in CSF samples from HSCT patients undergoing lumbar puncture for neurologic symptoms due to diagnoses other than acute limbic encephalitis (19, 37).
Preemptive treatment of CMV DNAemia with ganciclovir or foscarnet could have lowered the risk for HHV-6-PALE by inadvertently treating HHV-6; however, there was no difference in the cumulative incidence of HHV-6-PALE among CMV seropositive recipients compared to CMV seronegative recipients. In addition, the median time to development of HHV-6 viremia after HSCT occurred earlier than the typical time to CMV viremia (38, 39), making preemptive antiviral treatment of CMV DNAemia unlikely to impact the risk for HHV-6-PALE.
Detection of HHV-6 DNA in clinical specimens does not always indicate active infection and requires careful interpretation, as recent research demonstrates that this virus may be chromosomally integrated in nucleated cells of up to 2% of the population (40–43). Individuals with ciHHV-6 can have persistently elevated viral loads in blood and CSF specimens that are typically >105.5 copies/ml. Transplant recipients with HHV-6 reactivation have transiently detectable HHV-6 DNA usually <105 copies/ml in blood samples, although higher viral loads have been reported. Seven patients diagnosed with HHV-6-PALE in this study had plasma viral loads >105.5 copies/ml that could be suggestive of ciHHV-6; 4 had objective radiographic abnormalities consistent with limbic encephalitis, 2 had non-specific brain MRIs, and 1 did not have brain imaging. Of the 3 patients without objective evidence of limbic disease, all had subsequent testing documenting decreases in HHV-6 plasma viral loads to <1,000 copies/mL. In addition, CSF samples from 6/7 patients with plasma HHV-6 viral loads >105.5 copies/ml had <5 nucleated cells, indicating that virus identification was unlikely due to ciHHV-6 detected in inflammatory cells. Thus, we think that all patients diagnosed with HHV-6-PALE had active HHV-6 infection.
A recent study exploring HHV-6-associated CNS disease after UCBT suggested a higher incidence than seen in our cohort. Mori and colleagues reported a 15.7% cumulative incidence rate of HHV-6-PALE or myelitis after UCBT, 2.8% after adult-donor HSCT, and 28.6% after 2 or more UCBT at their institution (21). Similar to our findings, logistic regression analysis of their data identified UCBT and UCBT re-transplantation as risk factors for HHV-6-PALE. The higher rate of HHV-6-associated CNS disease in their study was likely due to a less stringent case definition.
The increased incidence and morbidity of HHV-6-PALE in patients undergoing UCBT is likely related to the higher frequency and degree of HHV-6 reactivation in this patient population. Aspects specific to UCBT that may account for this include the implicit use of mismatched and unrelated donor cells, absence of primed HHV-6-specific T-cells in the immature allograft, frequent treatment with T-cell depleting agents, and prolonged neutropenia, especially in patients who fail to engraft and require a second transplantation (8–10, 23). Among tested UCBT patients, 72% had detectable plasma HHV-6 DNA at greater levels compared with 30% of tested adult-donor recipients within 100 days of transplantation. We found that patients with higher viral loads were at increased risk for HHV-6-PALE. Plasma HHV-6 viral loads ≥105 copies/mL were 94% specific for a diagnosis of HHV-6-PALE among all tested patients undergoing allogeneic HSCT and 86% specific after UCBT; specificities were 98% and 97%, respectively, for viral loads ≥106 copies/mL. These values should be interpreted with caution, as plasma HHV-6 viral load testing was clinically driven when the treating clinician had concern for HHV-6 reactivation. However, data reported by Ogata and colleagues in a study of 111 patients who had weekly PCR surveillance for plasma HHV-6 DNA demonstrated similar sensitivities and specificities for these viral load thresholds (44).
Inflammatory conditions such as aGVHD grade II–IV and its treatment have also been associated with HHV-6-PALE (44). Indeed, time-dependent aGVHD grade II–IV was significantly associated with HHV-6-PALE in our study. Although this association was driven by the adult-donor cohort and did not reach statistical significance among UCBT recipients alone, this was likely a result of a small number of events due to the lower rate of aGVHD after UCBT (45). The incidence of aGVHD grade II–IV was relatively low in this cohort, likely attributable to the use of sirolimus-containing GVHD prophylaxis (32). Given our findings, one might expect an increased incidence of HHV-6-PALE in a patient population with a higher rate of aGVHD. Patients with significant aGVHD before HHV-6-PALE onset were treated with systemic glucocorticoids, so it is difficult to discern the contribution of each element separately in regards to the association with HHV-6-PALE.
Treatments that deplete T-lymphocytes, which probably play an important role in immune control of HHV-6 replication, may increase the risk for HHV-6 reactivation. Studies have demonstrated that treatment with steroids (9, 23), ATG (11), and anti-CD3 antibodies (such as BC3 (26) and OKT3 (46)) increase the risk for HHV-6 viremia. We had high suspicion that ATG use would increase the risk of HHV-6-PALE, especially given that UCBT patients were more likely to receive ATG in our cohort. However, ATG was not associated with HHV-6-PALE on adjusted Cox modeling. Perhaps increased risk from ATG was mitigated by its protective effect for aGVHD. RIC protocols that do not include ATG will be instructive in further evaluating its association with HHV-6-PALE after UCBT.
Patient-donor HLA mismatch is an additional cause of prolonged immune dysfunction after HSCT and has been associated with HHV-6 reactivation (8, 23, 47). Unsurprisingly, patient-donor mismatch was associated with HHV-6-PALE in our adult-donor recipients.
Some clinical features of HHV-6-PALE after UCBT deserve mention. Three UCB recipients developed visual hallucinations, and 1 patient had extensive extralimbic findings on MRI and autopsy studies. Interestingly, visual hallucinations and extralimbic disease are more commonly described in pediatric cases of HHV-6-PALE (48–50). The reasons for these similarities are unclear. HHV-6-PALE symptom onset occurred before engraftment in 7/10 UCBT patients compared to 1/9 adult-donor recipients. Symptoms also began later after UCBT, despite similar timing of blood HHV-6 reactivation (8). These findings may be due to a lack of or delayed engraftment and impaired immune control that is common following UCBT. They also suggest that HHV-6B has important neuropathic effects independent of immune system activation, as 4 patients died from this syndrome without ever engrafting.
Early treatment for HHV-6-PALE after HSCT, as well as prophylactic or preemptive measures, may improve outcomes and reduce the incidence of this disease. Foscarnet, cidofovir, and ganciclovir are available antiviral agents that demonstrate in vitro and in vivo activity against HHV-6, but there have been no controlled trials to study these agents for HHV-6 therapy (2, 25, 51, 52). A few studies evaluating the efficacy of preemptive or prophylactic ganciclovir or foscarnet to prevent HHV-6-PALE have been disappointing, perhaps due to the dynamic kinetics of HHV-6 viremia (17, 53, 54). However, based on the results of this and other studies on HHV-6 in HSCT, it would be reasonable to perform weekly HHV-6 surveillance in patients undergoing UCBT or mismatched adult-donor HSCT, as well as those who develop aGVHD grades II–IV or other conditions requiring treatment with T lymphocyte-depleting agents. Given the available data, we recommend a low threshold for starting treatment in patients with plasma HHV-6 viral loads ≥106 copies/mL or patients with viral loads ≥105 copies/mL in addition to findings concerning for CNS disease. Lumbar puncture should be performed as soon as it can be safely done and ideally prior to antiviral treatment. Treatment should be continued for 3–4 weeks only if HHV-6 DNA is detected in the CSF by PCR. Prospective screening for HHV-6 in high risk patients, as well as follow-up HHV-6 testing, will be important to evaluate for ciHHV-6 and response to treatment. We favor the use of foscarnet at a dose of 180 mg/kg/day given the limitations of ganciclovir use in this patient population due to bone marrow suppression (55), lower in vitro efficacy (56), and concern for HHV-6 resistance (57–59). Additional randomized controlled studies evaluating the efficacy of preemptive treatment of patients with high HHV-6 plasma viral loads will be important to further refining management of these patients.
In summary, we defined the incidence of HHV-6-PALE in the UCBT patient cohort at our institution and identified UCBT, time-dependent aGVHD grades II–IV, and adult-mismatched donor as risk factors for this syndrome. Detailed review of patient medical records highlighted the increased morbidity and mortality of HHV-6-PALE after UCBT compared to adult-donor HSCT. Strategies to minimize the impact of HHV-6-PALE in this population need to be further evaluated.
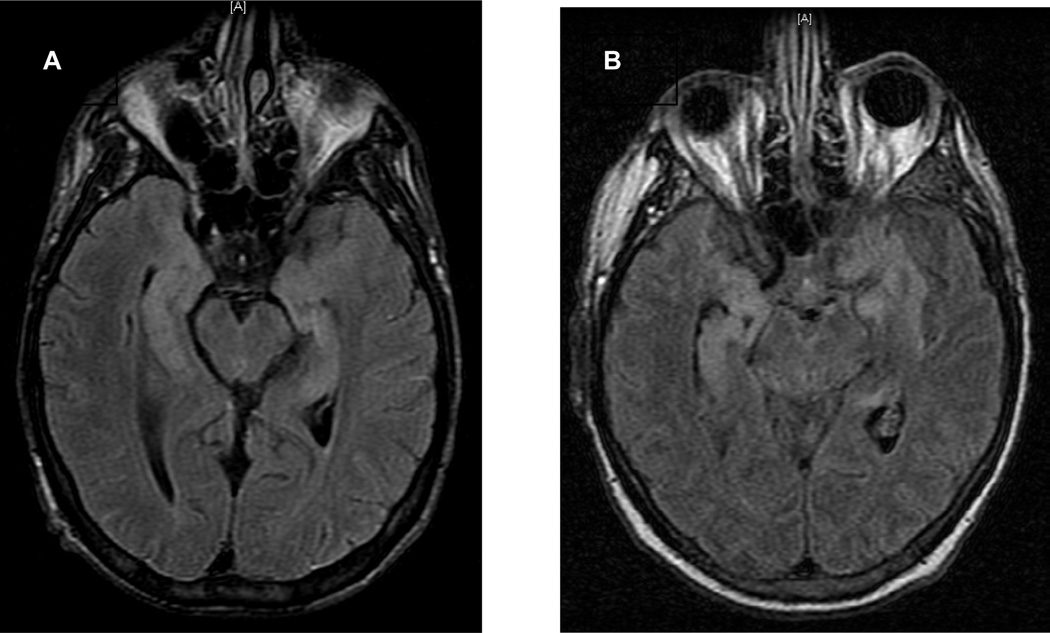
Figure 2. This figure demonstrates axial fluid attenuation inversion recovery (FLAIR) MR images from 2 patients with HHV-6-PALE.
A) This image from the recipient of an adult-donor HSCT shows characteristic well-demarcated high-intensity signal abnormalities in the bilateral medial temporal lobes involving the hippocampus and amygdala. B) This image is from the recipient of an UCBT and shows signal abnormalities extending beyond the limbic system.
Acknowledgements
The Jock and Bunny Adams Research and Education Endowment, NIH grant CA142106.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship:
Contribution: F.M.M. and J.A.H. conceived of and designed the study; J.A.H., F.M.M., V.T.H, and B.G.S. collected and assembled data; J.A.H. and F.M.M. analyzed and interpreted the data; J.A.H., F.M.M., and S.K. performed statistical analysis; J.A.H. drafted the article; F.M.M., S.K., V.T.H., C.C., J.K., P.A., E.P.A., L.R.B, J.H.A., and R.J.S. critically revised the article for important intellectual content.
Conflict-of-interest disclosure: F.M.M. has received research grant support from Astellas Pharma US and Chimerix for unrelated work. All other authors declare no potential financial interest.
References
- 1.Zerr DM, Meier AS, Selke SS, et al. A population-based study of primary human herpesvirus 6 infection. N Engl J Med. 2005;352:768–776. doi: 10.1056/NEJMoa042207. [DOI] [PubMed] [Google Scholar]
- 2.De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18:217–245. doi: 10.1128/CMR.18.1.217-245.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuno T, Takahashi K, Balachandra K, et al. Seroepidemiology of human herpesvirus 6 infection in normal children and adults. Journal of clinical microbiology. 1989;27:651–653. doi: 10.1128/jcm.27.4.651-653.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy S, Manna P. Quantitative detection and differentiation of human herpesvirus 6 subtypes in bone marrow transplant patients by using a single real-time polymerase chain reaction assay. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2005;11:530–541. doi: 10.1016/j.bbmt.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Cone RW, Huang ML, Ashley R, Corey L. Human herpesvirus 6 DNA in peripheral blood cells and saliva from immunocompetent individuals. Journal of clinical microbiology. 1994;32:2633. doi: 10.1128/jcm.31.5.1262-1267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevallier P, Hebia-Fellah I, Planche L, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone marrow transplantation. 2010;45:1204–1211. doi: 10.1038/bmt.2009.326. [DOI] [PubMed] [Google Scholar]
- 7.Sashihara J, Tanaka-Taya K, Tanaka S, et al. High incidence of human herpesvirus 6 infection with a high viral load in cord blood stem cell transplant recipients. Blood. 2002;100:2005–2011. [PubMed] [Google Scholar]
- 8.Yamane A, Mori T, Suzuki S, et al. Risk factors for developing human herpesvirus 6 (HHV-6) reactivation after allogeneic hematopoietic stem cell transplantation and its association with central nervous system disorders. Biol Blood Marrow Transplant. 2007;13:100–106. doi: 10.1016/j.bbmt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Zerr DM. Human herpesvirus 6 and central nervous system disease in hematopoietic cell transplantation. J Clin Virol. 2006;37(Suppl 1):S52–S56. doi: 10.1016/S1386-6532(06)70012-9. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa T, Asano Y, Ihira M, et al. Human herpesvirus 6 viremia in bone marrow transplant recipients: clinical features and risk factors. J Infect Dis. 2002;185:847–853. doi: 10.1086/339411. [DOI] [PubMed] [Google Scholar]
- 11.Wang LR, Dong LJ, Lu DP. Surveillance of active human herpesvirus 6 infection in chinese patients after hematopoietic stem cell transplantation with 3 different methods. Int J Hematol. 2006;84:262–267. doi: 10.1532/IJH97.A10607. [DOI] [PubMed] [Google Scholar]
- 12.Drobyski WR, Eberle M, Majewski D, Baxter-Lowe LA. Prevalence of human herpesvirus 6 variant A and B infections in bone marrow transplant recipients as determined by polymerase chain reaction and sequence-specific oligonucleotide probe hybridization. J Clin Microbiol. 1993;31:1515–1520. doi: 10.1128/jcm.31.6.1515-1520.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang FZ, Dahl H, Ljungman P, Linde A. Lymphoproliferative responses to human herpesvirus-6 variant A and variant B in healthy adults. J Med Virol. 1999;57:134–139. [PubMed] [Google Scholar]
- 14.Boutolleau D, Duros C, Bonnafous P, et al. Identification of human herpesvirus 6 variants A and B by primer-specific real-time PCR may help to revisit their respective role in pathology. J Clin Virol. 2006;35:257–263. doi: 10.1016/j.jcv.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, Boeckh M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:932–940. doi: 10.1086/428060. [DOI] [PubMed] [Google Scholar]
- 16.Zerr DM, Fann JR, Breiger D, et al. HHV-6 reactivation and its effect on delirium and cognitive functioning in hematopoietic cell transplantation recipients. Blood. 2011;117:5243–5249. doi: 10.1182/blood-2010-10-316083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Betts BC, Young JA, Ustun C, Cao Q, Weisdorf DJ. Human Herpesvirus 6 Infection after Hematopoietic Cell Transplantation: Is Routine Surveillance Necessary? Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dulery R, Salleron J, Dewilde A, et al. Early Human Herpesvirus Type 6 Reactivation After Allogeneic Stem Cell Transplantation: a Large-Scale Clinical Study. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.12.579. [DOI] [PubMed] [Google Scholar]
- 19.Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: Clinical features and relationship to HHV6. Neurology. 2007;69:156–165. doi: 10.1212/01.wnl.0000265591.10200.d7. [DOI] [PubMed] [Google Scholar]
- 20.Gewurz BE, Marty FM, Baden LR, Katz JT. Human herpesvirus 6 encephalitis. Curr Infect Dis Rep. 2008;10:292–299. doi: 10.1007/s11908-008-0048-1. [DOI] [PubMed] [Google Scholar]
- 21.Mori Y, Miyamoto T, Nagafuji K, et al. High incidence of human herpes virus 6-associated encephalitis/myelitis following a second unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:1596–1602. doi: 10.1016/j.bbmt.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Fujimaki K, Mori T, Kida A, et al. Human herpesvirus 6 meningoencephalitis in allogeneic hematopoietic stem cell transplant recipients. Int J Hematol. 2006;84:432–437. doi: 10.1532/IJH97.06072. [DOI] [PubMed] [Google Scholar]
- 23.Ogata M, Kikuchi H, Satou T, et al. Human herpesvirus 6 DNA in plasma after allogeneic stem cell transplantation: incidence and clinical significance. The Journal of infectious diseases. 2006;193:68–79. doi: 10.1086/498531. [DOI] [PubMed] [Google Scholar]
- 24.Muta T, Fukuda T, Harada M. Human herpesvirus-6 encephalitis in hematopoietic SCT recipients in Japan: A retrospective multicenter study. Bone Marrow Transplantation. 2009;43:583–585. doi: 10.1038/bmt.2008.359. [DOI] [PubMed] [Google Scholar]
- 25.Zerr DM, Gupta D, Huang ML, Carter R, Corey L. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:309–317. doi: 10.1086/338044. [DOI] [PubMed] [Google Scholar]
- 26.Zerr DM, Gooley TA, Yeung L, et al. Human herpesvirus 6 reactivation and encephalitis in allogeneic bone marrow transplant recipients. Clinical Infectious Diseases. 2001;33:763–771. doi: 10.1086/322642. [DOI] [PubMed] [Google Scholar]
- 27.Ljungman P, Wang FZ, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774–781. [PubMed] [Google Scholar]
- 28.Tanaka M, Taguchi J, Hyo R, et al. Human herpesvirus-6 encephalitis after unrelated cord blood transplantation. Leukemia and Lymphoma. 2005;46:561–566. doi: 10.1080/10428190400029882. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald SA, Taylor HL. Human herpesvirus 6-associated limbic encephalitis refractory to therapeutic plasma exchange in adult recipient of unrelated umbilical cord blood transplantation. Journal of Clinical Apheresis. 2009;24:89–90. [Google Scholar]
- 30.Chik KW, Chan PKS, Li CK, et al. Human herpesvirus-6 encephalitis after unrelated umbilical cord blood transplant in children. Bone Marrow Transplantation. 2002;29:991–994. doi: 10.1038/sj.bmt.1703596. [DOI] [PubMed] [Google Scholar]
- 31.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- 32.Antin JH, Kim HT, Cutler C, et al. Sirolimus, tacrolimus, and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102:1601–1605. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 33.Alyea EP, Kim HT, Ho V, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105:1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 34.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cutler C, Stevenson K, Kim HT, et al. Double umbilical cord blood transplantation with reduced intensity conditioning and sirolimus-based GVHD prophylaxis. Bone Marrow Transplant. 2011;46:659–667. doi: 10.1038/bmt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang FZ, Linde A, Hagglund A, Testa M, Locasciulli A, Ljungman P. Human herpesvirus 6 DNA in cerebrospinal fluid specimens from allogeneic bone marrow transplant patients: Does it wave clinical significance? Clinical Infectious Diseases. 1999;28:562–568. doi: 10.1086/515142. [DOI] [PubMed] [Google Scholar]
- 38.Marty FM, Bryar J, Browne SK, et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood. 2007;110:490–500. doi: 10.1182/blood-2007-01-069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 40.Ward KN, Leong HN, Thiruchelvam AD, Atkinson CE, Clark DA. Human herpesvirus 6 DNA levels in cerebrospinal fluid due to primary infection differ from those due to chromosomal viral integration and have implications for diagnosis of encephalitis. J Clin Microbiol. 2007;45:1298–1304. doi: 10.1128/JCM.02115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leong HN, Tuke PW, Tedder RS, et al. The prevalence of chromosomally integrated human herpesvirus 6 genomes in the blood of UK blood donors. J Med Virol. 2007;79:45–51. doi: 10.1002/jmv.20760. [DOI] [PubMed] [Google Scholar]
- 42.Ward KN, Leong HN, Nacheva EP, et al. Human herpesvirus 6 chromosomal integration in immunocompetent patients results in high levels of viral DNA in blood, sera, and hair follicles. J Clin Microbiol. 2006;44:1571–1574. doi: 10.1128/JCM.44.4.1571-1574.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pellett PE, Ablashi DV, Ambros PF, et al. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol. 2011 doi: 10.1002/rmv.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogata M, Satou T, Kawano R, et al. Correlations of HHV-6 viral load and plasma IL-6 concentration with HHV-6 encephalitis in allogeneic stem cell transplant recipients. Bone Marrow Transplant. 2010;45:129–136. doi: 10.1038/bmt.2009.116. [DOI] [PubMed] [Google Scholar]
- 45.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. The New England journal of medicine. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 46.Kikuta H, Lu H, Tomizawa K, Matsumoto S. Enhancement of human herpesvirus 6 replication in adult human lymphocytes by monoclonal antibody to CD3. The Journal of infectious diseases. 1990;161:1085–1087. doi: 10.1093/infdis/161.6.1085. [DOI] [PubMed] [Google Scholar]
- 47.Maury S, Mary JY, Rabian C, et al. Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. British journal of haematology. 2001;115:630–641. doi: 10.1046/j.1365-2141.2001.03135.x. [DOI] [PubMed] [Google Scholar]
- 48.Provenzale JM, van Landingham K, White LE. Clinical and Imaging Findings Suggesting Human Herpesvirus 6 Encephalitis. Pediatric Neurology. 2010;42:32–39. doi: 10.1016/j.pediatrneurol.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 49.Bosi A, Zazzi M, Amantini A, et al. Fatal herpesvirus 6 encephalitis after unrelated bone marrow transplant. Bone Marrow Transplantation. 1998;22:285–288. doi: 10.1038/sj.bmt.1701326. [DOI] [PubMed] [Google Scholar]
- 50.Provenzale JM, vanLandingham KE, Lewis DV, Mukundan S, White LE. Extrahippocampal Involvement in Human Herpesvirus 6 Encephalitis Depicted at MR Imaging. Radiology. 2008;249:955–963. doi: 10.1148/radiol.2492071917. [DOI] [PubMed] [Google Scholar]
- 51.Agut H, Aubin JT, Huraux JM. Homogeneous susceptibility of distinct human herpesvirus 6 strains to antivirals in vitro. The Journal of infectious diseases. 1991;163:1382–1383. doi: 10.1093/infdis/163.6.1382. [DOI] [PubMed] [Google Scholar]
- 52.Burns WH, Sandford GR. Susceptibility of human herpesvirus 6 to antivirals in vitro. The Journal of infectious diseases. 1990;162:634–637. doi: 10.1093/infdis/162.3.634. [DOI] [PubMed] [Google Scholar]
- 53.Ogata M, Satou T, Kawano R, et al. Plasma HHV-6 viral load-guided preemptive therapy against HHV-6 encephalopathy after allogeneic stem cell transplantation: a prospective evaluation. Bone Marrow Transplant. 2008;41:279–285. doi: 10.1038/sj.bmt.1705907. [DOI] [PubMed] [Google Scholar]
- 54.Ishiyama K, Katagiri T, Hoshino T, Yoshida T, Yamaguchi M, Nakao S. Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transplant. 2011;46:863–869. doi: 10.1038/bmt.2010.201. [DOI] [PubMed] [Google Scholar]
- 55.McGavin JK, Goa KL. Ganciclovir: an update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs. 2001;61:1153–1183. doi: 10.2165/00003495-200161080-00016. [DOI] [PubMed] [Google Scholar]
- 56.Akhyani N, Fotheringham J, Yao K, Rashti F, Jacobson S. Efficacy of antiviral compounds in human herpesvirus-6-infected glial cells. Journal of Neurovirology. 2006;12:284–293. doi: 10.1080/13550280600880772. [DOI] [PubMed] [Google Scholar]
- 57.Manichanh C, Olivier-Aubron C, Lagarde JP, et al. Selection of the same mutation in the U69 protein kinase gene of human herpesvirus-6 after prolonged exposure to ganciclovir in vitro and in vivo. J Gen Virol. 2001;82:2767–2776. doi: 10.1099/0022-1317-82-11-2767. [DOI] [PubMed] [Google Scholar]
- 58.Isegawa Y, Hara J, Amo K, et al. Human herpesvirus 6 ganciclovir-resistant strain with amino acid substitutions associated with the death of an allogeneic stem cell transplant recipient. J Clin Virol. 2009;44:15–19. doi: 10.1016/j.jcv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Baldwin K. Ganciclovir-resistant human herpesvirus-6 encephalitis in a liver transplant patient: a case report. J Neurovirol. 2011;17:193–195. doi: 10.1007/s13365-011-0019-4. [DOI] [PubMed] [Google Scholar]